Abstract
Background
The present study aimed to assess the role of C3435T polymorphism in drug-resistance in epilepsy by a meta-analysis.
Material/Methods
Databases were obtained from the Cochrane Library, MEDLINE, EMBASE, PubMed, Science Direct database, CNKI, and Wanfang up to October 2014. All the case-control association studies evaluating the role of ABCB1 C3435T in pharmacoresistance to anti-epileptic drug (AED) were identified. RevMan 5.0 software was utilized to perform quantitative analyses in an allele model (C vs. T) and a genotype model (CC vs. CT+TT).
Results
From the 189 potential studies, we included 28 articles for the meta-analysis, including 30 independent case-control studies involving 4124 drug-resistant epileptic patients and 4480 epileptic patients for whom drug treatment was effective. We excluded 164 studies because of duplication, lack of genotype data, and non-clinical research. We found that C3435T polymorphism was not significantly associated with drug resistance in epilepsy, either in allele model (C vs. T: OR=1.07; 95%CI: 0.95–1.19) or in genotype model (CC vs. CT+TT: OR=1.05; 95%CI: 0.89–1.24, P=0.55). Subgroup analyses suggested that in Caucasian populations there are significant differences between resistance group (NR) and control group (R) in both allele model (C vs. T: OR=1.09; 95%CI: 1.00–1.18, P=0.05) and genotype model (CC vs. CT+TT: OR=1.20; 95%CI: 1.04–1.40, P=0.01). However, we did not find this association in Asian populations.
Conclusions
We conclude that the ABCB1 C3435T polymorphism may be a genetic marker for drug resistance in epilepsy in Caucasian populations.
MeSH Keywords: Epilepsy, Absence; Meta-Analysis; Polymorphism, Genetic
Background
Epilepsy is a common and complex disease characterized by a predisposition to recurrent unprovoked seizures [1]. After treatment with anti-epileptic drugs (AEDs), most epileptic patients respond well to medications. However, about one-third of newly treated patients do not respond adequately to medications, because these patients exhibit drug resistance to AEDs [2]. P-glycoprotein (P-gp) was the first discovered human ABC (the ATP-binding cassette) transporter in drug-resistance ovarian cells several decades ago [3]. P-gp is the expression product of ABCB1 (the ATP-binding cassette, subfamily B, member 1 transporter gene), which is also known as MDR1 (multi-drug resistance gene 1). The ABCB1 gene is highly polymorphic and more than 50 variants reside in the coding region which can possibly cause altered function [4]. The C3435T polymorphism is one of the most common polymorphisms in the ABCB1 gene. Siddiqui et al. [5] first reported that among Caucasians, the C3435T single-nucleotide polymorphism (SNP) of ABCB1 was correlated with drug resistance in epilepsy. Following that study, more than 20 replication studies [6–27] were conducted to evaluate this hypothesis.
In 2010, Haerian et al. [28] performed a meta-analysis and did not find an association between ABCB1 polymorphism and drug resistance in epilepsy. In recent year, several large sample-size and well-designed studies related to this topic have been conducted [29–33]. However, the results remain contradictory. To clarify the association with ABCB1 gene C3435T polymorphism and drug resistance in epilepsy, we performed an updated meta-analysis to further explore the correlations between the ABCB1 C3435T polymorphism and drug resistance in epilepsy.
Material and Methods
Literature screening
We used the keywords “polymorphism”, “multi-drug resistance gene 1”, “C3435T”, “epilepsy”, “intractable epilepsy”, “antiepileptic drugs”, and “drug-resistant” to search the Cochrane Library, MEDLINE, EMBASE, PubMed, Science Direct database, China National Knowledge Infrastructure (CNKI) database, the China Biomedical Literature (CBM) database, the MedCH international medical abstract database, and Wanfang up to October 2014. These searches were supplemented by retrospective and manual searches of the literature by going to a library to read paper copies of scientific journals. The first report on the relationship between the ABCB1 C3435T polymorphism and drug resistance in epilepsy appeared in 2003, and the end date for the retrieval process was October 31, 2014.
Literature inclusion and exclusion criteria
Literature inclusion criteria
1) Chinese or English publication that addressed the association of the ABCB1 C3435T polymorphism with drug resistance in epilepsy; 2) reported complete data, including the number of examined individuals in the drug-resistant group and the therapeutically effective group, the frequency of the CC, CT, and TT genotypes at the 3435 locus of the ABCB1 gene; 3) the study subjects were epileptic patients who were treated with AEDs.
Exclusion criteria
Studies were excluded if: 1) they were duplicate publications from the same population and the same authors examined in another publication, in which case only the publication with the largest sample size was retained; or 2) they did not contain sufficient quantity or quality of data to analyze.
Data extraction
Data extraction was performed independently by 2 researchers (Li SX and Liu YY), and the extracted data were subsequently verified. The retrieved data included the author names, the date of publication, the nationality of the study population, and the allele and genotype frequency distributions. If genotype frequency distributions were expressed as percentages, then data were entered after converting these percentages into numbers of cases. If allele distributions were not provided, then these distributions were calculated from genotype distributions.
Statistical analysis
Meta-analysis was performed using the RevMan 5.0 software. Cochran’s Q test was used for the analysis of heterogeneity between the results of each study. When there was no heterogeneity between studies (I2<50%), a fixed-effects model was used for the meta-analysis. When there was heterogeneity (I2>50%), a random-effects model was used for the meta-analysis. The OR and 95% CI of each allele and genotype frequency were calculated for each study. The Hardy-Weinberg equilibrium of the control group was calculated. P<0.05 was considered statistically significant. Sensitivity analysis was conducted using the individual exclusion method. The overall effects were re-assessed and compared with the overall effects prior to exclusion. Begg’s test and Egger’s test were applied to determine whether there was publication bias in the studies.
Results
Search results and literature
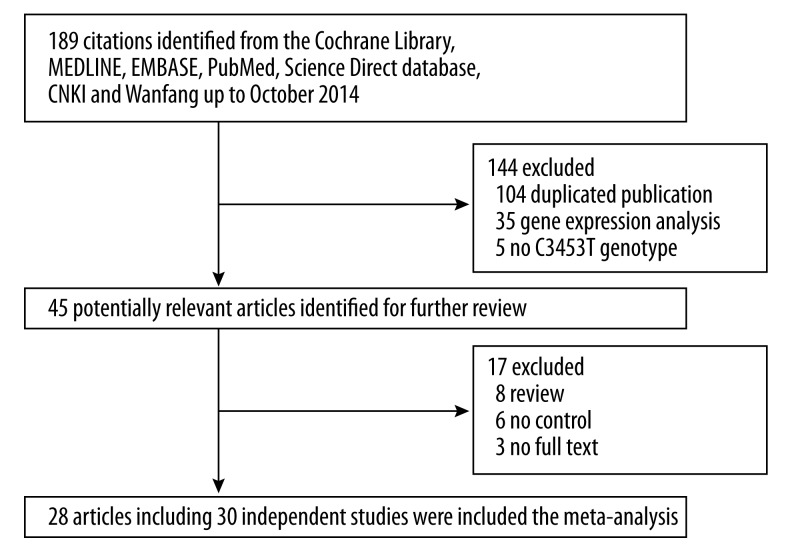
As shown in Figure 1, a total of 189 articles were retrieved after first search in the Cochrane Library, MEDLINE, EMBASE, PubMed, Science Direct database, China National Knowledge Infrastructure (CNKI) database, the China Biomedical Literature (CBM) database, the MedCH international medical abstract database, and Wanfang up to October 2014. Finally, there were 28 articles including 30 independent case-control studies [6–27,29–33] that fulfilled the inclusion criteria. The characteristics of each study are summarized in Table 1. These 30 studies involving 8604 subjects were ultimately analyzed in our meta-analysis. There were 17 studies carried out in Caucasian populations while the other 13 studies were performed in Asian populations. In the subgroup analysis, patients from Hong Kong, China were included in the Asian population, whereas patients from Australia and Scotland were included in the Caucasian population. Therefore, there were effectively a total of 13 studies examining Asian populations and a total of 17 studies that examined Caucasian populations (Table 1).
Figure 1.
The flow chart of literatures identification.
Table 1.
The characteristics of included studies.
| Authors | Publication year | Country | Number of Subjects | NR | R | NR | R | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NR | R | CC | CT | TT | CC | CT | TT | C | T | C | T | |||
| Alpman et al. | 2010 | Turkey | 39 | 92 | 6 | 20 | 12 | 26 | 37 | 24 | 32 | 44 | 89 | 85 |
| Haerian et al. | 2011 | Asian | 323 | 362 | 109 | 158 | 56 | 110 | 180 | 72 | 376 | 270 | 400 | 324 |
| Szoeke et al. | 2009a | Australia | 64 | 148 | 21 | 27 | 16 | 34 | 67 | 47 | 69 | 59 | 135 | 161 |
| Szoeke et al. | 2009b | Scotland | 133 | 152 | 20 | 69 | 44 | 34 | 72 | 46 | 109 | 157 | 140 | 164 |
| Szoeke et al. | 2009c | China | 11 | 34 | 1 | 8 | 2 | 13 | 20 | 1 | 10 | 12 | 46 | 22 |
| Tan et al. | 2004 | Australia | 401 | 208 | 75 | 193 | 133 | 37 | 115 | 56 | 343 | 459 | 189 | 227 |
| Chen L et al. | 2007 | China | 50 | 164 | 15 | 25 | 10 | 63 | 79 | 22 | 55 | 45 | 205 | 123 |
| Di Q et al. | 2011 | China | 91 | 79 | 44 | 37 | 10 | 32 | 30 | 17 | 125 | 57 | 94 | 64 |
| Dong et al. | 2011 | China | 157 | 193 | 64 | 75 | 18 | 82 | 83 | 28 | 203 | 111 | 247 | 139 |
| Hung et al. | 2007 | China | 114 | 213 | 40 | 55 | 19 | 39 | 107 | 67 | 135 | 93 | 185 | 241 |
| Kwan et al. | 2007 | China | 221 | 297 | 80 | 104 | 37 | 114 | 161 | 22 | 264 | 178 | 389 | 205 |
| Ufer et al. | 2009 | Germany | 188 | 103 | 44 | 85 | 59 | 20 | 46 | 37 | 173 | 203 | 86 | 120 |
| Grover et al. | 2010 | India | 87 | 125 | 13 | 44 | 30 | 14 | 55 | 56 | 70 | 104 | 83 | 167 |
| Kumaril et al. | 2011 | India | 125 | 260 | 12 | 67 | 46 | 42 | 120 | 98 | 91 | 159 | 204 | 316 |
| Takhan et al. | 2009 | India | 94 | 231 | 9 | 52 | 33 | 38 | 104 | 89 | 70 | 118 | 180 | 282 |
| Vahab et al. | 2009 | India | 113 | 54 | 3 | 61 | 49 | 3 | 8 | 43 | 67 | 159 | 14 | 94 |
| Sayyah et al. | 2011 | Iran | 132 | 200 | 34 | 55 | 43 | 32 | 80 | 88 | 123 | 141 | 144 | 256 |
| Shahwan et al. | 2007 | Ireland | 122 | 233 | 20 | 64 | 38 | 37 | 119 | 77 | 104 | 140 | 193 | 273 |
| Seo et al. | 2006 | Japan | 126 | 84 | 34 | 58 | 34 | 36 | 34 | 14 | 126 | 126 | 106 | 62 |
| Kim et al. | 2009 | Korea | 198 | 193 | 73 | 97 | 28 | 81 | 90 | 22 | 243 | 153 | 252 | 134 |
| Emich-Widera et al. | 2013 | Poland | 60 | 25 | 9 | 33 | 18 | 1 | 16 | 8 | 51 | 69 | 18 | 32 |
| Emich-Widera et al. | 2014 | Poland | 193 | 135 | 19 | 114 | 60 | 21 | 82 | 32 | 152 | 234 | 124 | 146 |
| Sills et al. | 2005 | Scotland | 230 | 170 | 41 | 112 | 77 | 32 | 82 | 56 | 194 | 266 | 146 | 194 |
| Sanchez et al. | 2010 | Spain | 111 | 178 | 40 | 49 | 22 | 52 | 81 | 45 | 129 | 93 | 185 | 171 |
| Dericiogl et al. | 2008 | Turkey | 89 | 100 | 26 | 34 | 29 | 25 | 49 | 26 | 86 | 92 | 99 | 101 |
| Ozgon et al. | 2008 | Turkey | 44 | 53 | 13 | 26 | 5 | 16 | 29 | 8 | 52 | 36 | 61 | 45 |
| Saygi et al. | 2014 | Turkey | 59 | 60 | 19 | 26 | 14 | 12 | 30 | 18 | 64 | 54 | 54 | 66 |
| Seven et al. | 2014 | Turkey | 69 | 83 | 17 | 30 | 22 | 22 | 38 | 23 | 64 | 74 | 82 | 84 |
| Siddiqui et al. | 2003 | UK | 200 | 115 | 55 | 106 | 39 | 18 | 63 | 34 | 216 | 184 | 99 | 131 |
| Soranzo et al. | 2004 | UK | 280 | 136 | 73 | 145 | 62 | 20 | 80 | 36 | 291 | 269 | 120 | 152 |
NR – anti-epileptic drug no response (case group); R – effective group (control group). a, b, and c represent independent studies from the same article.
Meta-analysis results
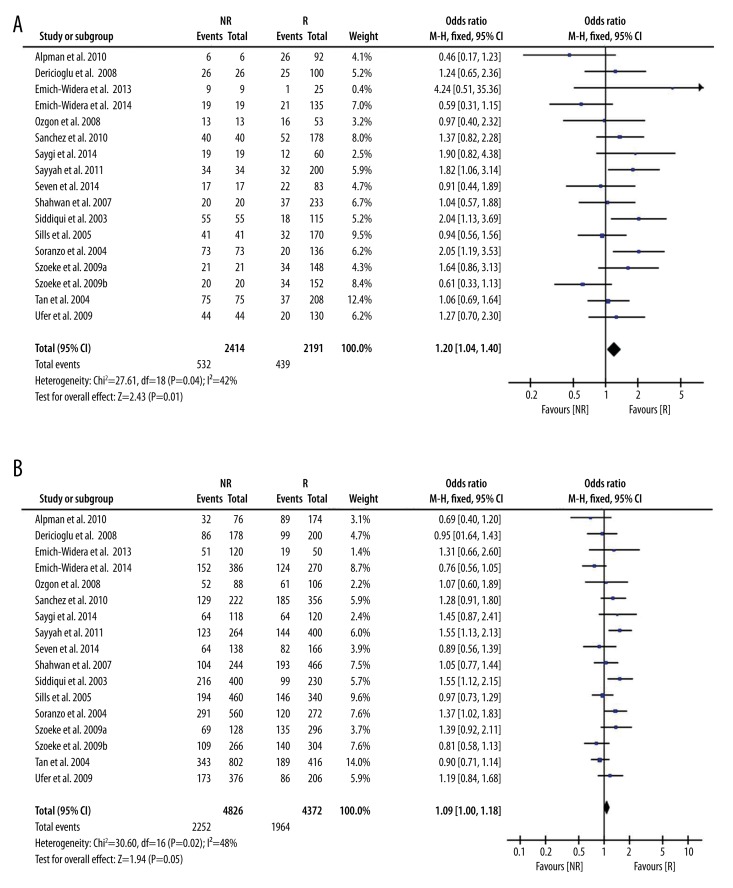
Analysis of the allele contrast model (C vs. T) for the overall population revealed that there was high heterogeneity among the included studies (I2=64%, P<0.001); therefore, a random-effects model was used to pool the OR values for the frequency of the 3435C allele. The pooled OR value was 1.07 (95% CI: 0.95–1.19, P=0.26) in allele model and 1.05 (95% CI: 0.89–1.24, P=0.55) in genotype model, indicating that the 3435C allele was not significantly correlated with drug resistance in epilepsy (Table 2). Subgroup analyses were performed in accordance with the race of the study subjects There was significant heterogeneity among the studies examining Asian populations (I2=−76%, P<0.001); therefore, a random-effects model was used to pool OR values, producing a pooled OR value of 1.03 (95% CI: 0.84–1.26, P=−0.77) in allele model and 0.90 (95% CI: 0.70–1.17, P=−0.43) in genotype model (Table 2). There was no heterogeneity among studies examining Caucasian populations (I2=42%, P=0.04); therefore a fixed-effects model was utilized to merge the OR values. We found in Caucasian populations there are significant differences between resistance group and control group in both allele model (C vs. T: OR=1.07; 95%CI: 0.95–1.19) and in genotype model (CC vs. CT+TT: OR=1.05; 95%CI: 0.89–1.24, P=0.55, Table 2 and Figure 2).
Table 2.
Meta-Analysis of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy.
| Genetic model | Sample size | Test of association | Test for heterogeneity | ||||
|---|---|---|---|---|---|---|---|
| Case | Control | OR | 95%CI | P | P | I2 | |
| Total | |||||||
| CC vs. (CT+TT) | 4124 | 4480 | 1.05 | 0.89–1.24 | 0.55 | 0.0003 | 54% |
| C vs. T | 8246 | 8950 | 1.07 | 0.95–1.19 | 0.26 | <0.001 | 64% |
| Caucasian | |||||||
| CC vs. (CT+TT) | 2414 | 2191 | 1.20 | 1.04–1.40 | 0.01 | 0.04 | 42% |
| C vs. T | 4826 | 4372 | 1.09 | 1.00–1.18 | 0.05 | 0.02 | 48% |
| Asian | |||||||
| CC vs. (CT+TT) | 1710 | 2289 | 0.90 | 0.70–1.17 | 0.43 | 0.002 | 61% |
| C vs. T | 3420 | 4578 | 1.03 | 0.84–1.26 | 0.77 | <0.001 | 76% |
OR – odds ratio, CI – confidence interval, vs. – versus.
Figure 2.
Forest plot of C3435T polymorphism of the ABCB1 gene and drug resistance in epilepsy in Caucasian population, the horizontal lines correspond to the study-specific OR and 95% CI, respectively. The area of the squares reflects the study-specific weight. The diamond represents the pooled results of OR and 95%CI. (A) C vs. T; (B) CC vs. CT+TT.
Quality analyses of the included studies
Sensitivity analysis
We deleted 1 study from the overall pooled analysis each time to check the influence of the removed data set on the overall ORs. The pooled ORs and 95% CIs were not significantly altered when any part of the study was omitted, which indicated that this study exhibited relatively good stability.
Analysis of publication bias
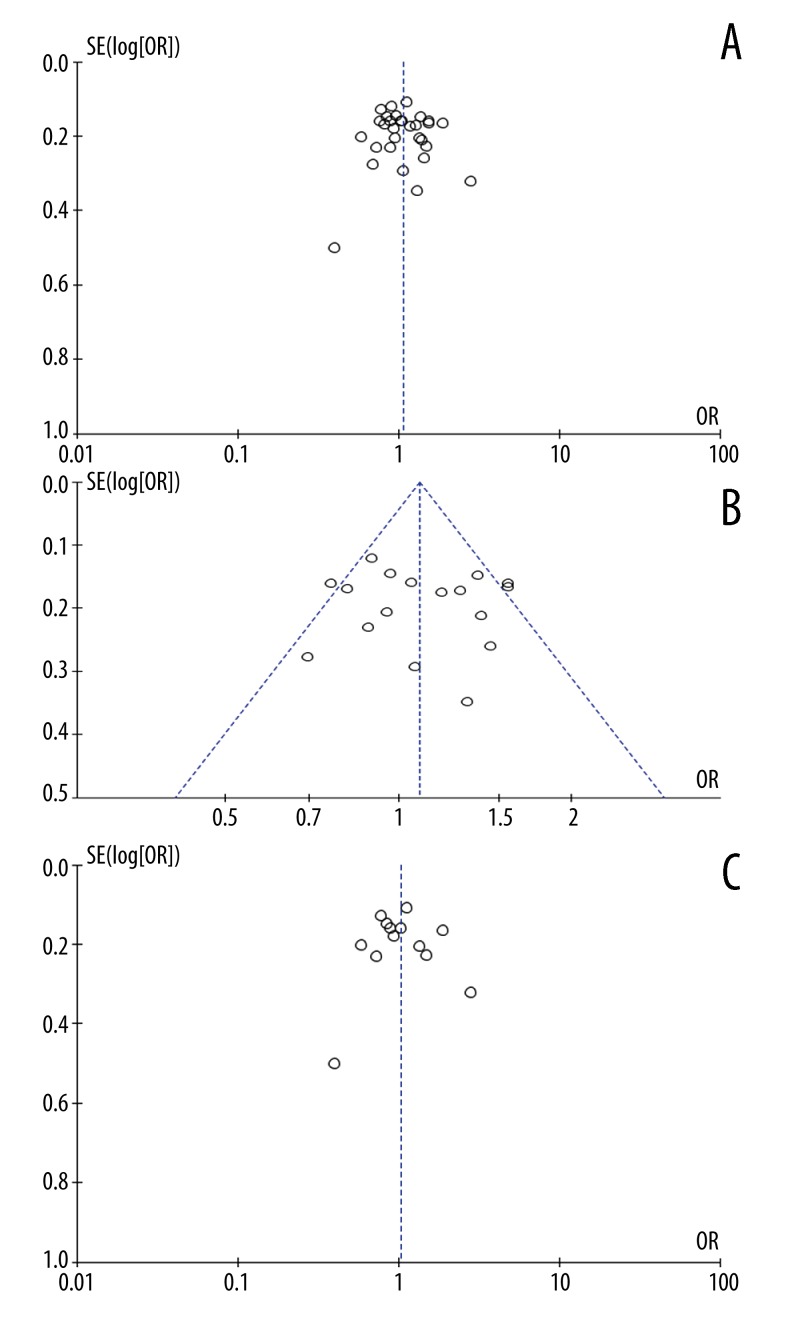
Funnel plot and Egger’s test were performed to assess the publication bias of the literatures. Symmetrical funnel plots were obtained in the SNP tested in all of the models. Egger’s test further confirmed the absence of publication bias in this meta-analysis (P>0.05) (Figure 3). Similarly, additional analyses of the studies included in the examined genetic models and subgroups revealed no significant publication bias, indicating that the study results were relatively creditable.
Figure 3.
Begg’s funnel plot for publication bias tests. Each point represents a separate study for the indicated association. Log or represents natural logarithm of OR. Vertical line represents the mean effects size. (A) In total; (B) in Caucasian population; (C) in Asian population.
Discussion
In the present study, we found that the C3435T polymorphism was associated with AEDs in Caucasian populations. This meta-analysis collected 28 publications addressing the relationship between the ABCB1 C3435T polymorphism and drug resistance in epilepsy. However, the results were contradictory. The C3435T polymorphism of ABCB1 gene was the first single-nucleotide polymorphism that was reported to be associated with drug resistance in epileptic patients [6]. In this report, the CC genotype of this polymorphism was found to be significantly higher in patients with drug-resistant epilepsy, whereas the TT genotype was significantly lower in the same group [6]. However, several studies failed to confirm the association between the C3435T polymorphism and drug-resistant epilepsy. In this meta-analysis, only 6 studies produced positive results [6–11], and in the remaining 24 studies no correlation was found between the C3435T polymorphism and drug resistance in epilepsy. Meta-analysis results showed no statistically significant correlation between the ABCB1 C3435T polymorphism and drug resistance in epilepsy in analyses of either the allele model or genetic model in the total population. Furthermore, subgroup analyses organized in accordance with subjects’ racial groups (Asian or Caucasian) revealed positive correlations between this polymorphism and drug resistance in epilepsy in Caucasian populations but not in Asian populations.
In the present study, we found significant heterogeneity among each study, primarily because of 3 factors. 1) The specific pathogenic gene loci that cause differences in ABCB1 function remain unclear; and 2) various included studies involved different uses of AEDs. For instance, certain included studies involved AED monotherapies, whereas others included investigations with combination therapies. Among the currently known AEDs, phenytoin, levetiracetam, lamotrigine, and phenobarbital are all transported by P-gp in the human body. In contrast, valproic acid is not transported by P-gp; thus, if valproic acid was administered to many of the examined patients, it may be difficult to accurately determine whether the ABCB1 C3435T polymorphism is truly correlated with drug resistance in epilepsy. 3) Currently, there is no universally accepted definition of drug resistance in epilepsy. Siddiqui et al. [6] defined drug resistance in epilepsy as the occurrence of at least 4 seizures during the year prior to a subject’s enrollment despite the use of at least 3 appropriately selected AEDs at these drugs’ maximum tolerated doses. Because different researchers used different criteria, certain patients who would have been classified into the therapeutically effective group by the aforementioned definition were instead classified into the drug resistance group in certain studies. This difference in patient categorization is also an important reason for the different results of various studies.
Conclusions
The current meta-analysis only confirmed the existence of significant correlations between this polymorphism and drug resistance in epilepsy in Caucasian populations. However, our results should be verified by a case-control study with larger sample size.
Footnotes
Source of support: Self financing
References
- 1.Wolf P. History of epilepsy: nosological concepts and classification. Epileptic Disord. 2014;16(3):261–69. doi: 10.1684/epd.2014.0676. [DOI] [PubMed] [Google Scholar]
- 2.Jose M, Banerjee M, Mathew A, et al. Pharmacogenetic evaluation of ABCB1, Cyp2C9, Cyp2C19 and methylene tetrahydrofolate reductase polymorphisms in teratogenicity of anti-epileptic drugs in women with epilepsy. Ann Indian Acad Neurol. 2014;17(3):259–66. doi: 10.4103/0972-2327.138475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potschka H, Fedrowitz M, Löscher W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport. 2001;12(16):3557–60. doi: 10.1097/00001756-200111160-00037. [DOI] [PubMed] [Google Scholar]
- 4.Brambila-Tapia AJ. MDR1 (ABCB1) polymorphisms: functional effects and clinical implications. Rev Invest Clin. 2013;65(5):445–54. [PubMed] [Google Scholar]
- 5.Siddiqui A, Kerb R, Weale ME, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348(15):1442–48. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 6.Soranzo N, Cavalleri GL, Weale ME, et al. Identifying candidate causal variants responsible for altered activity of the ABCBl multidrug resistance gene. Genome Res. 2004;14:1333–44. doi: 10.1101/gr.1965304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.See T, Ishitsu T, Ueda N, et al. ABCB1 polymorphisms influence the response to antiepileptic drugs in Japanese epilepsy patients. Pharmacogenomics. 2006;7:551–61. doi: 10.2217/14622416.7.4.551. [DOI] [PubMed] [Google Scholar]
- 8.Hung CC, Jen Tai J, Kao PJ, et al. Association of polymorphisms in NRI12 and ABCBl genes with epilepsy treatment responses. Pharmacogenomics. 2007;8:l151–58. doi: 10.2217/14622416.8.9.1151. [DOI] [PubMed] [Google Scholar]
- 9.Kwan P, Baurn L, Wong V, et al. Association between ABCB1 C3435T polymorphism and drug-resistant epilepsy in Han Chinese. Epilepsy Behav. 2007;11:112–17. doi: 10.1016/j.yebeh.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Sayyah M, Kamgarpour F, Maleki M, et al. Association analysis of intractable epilepsy with C3435T and G2677T/A ABCB1 gene polymorphisms in Iranian patients. Epileptic Disord. 2011;13:155–65. doi: 10.1684/epd.2011.0443. [DOI] [PubMed] [Google Scholar]
- 11.Tan NC, Heron SE, Scheffer IE, et al. Failure to confirm association of a polymorphism in ABCBl with multidrug-resistant epilepsy. Neurology. 2004;63:1090–92. doi: 10.1212/01.wnl.0000137051.33486.c7. [DOI] [PubMed] [Google Scholar]
- 12.Sills GJ, Mohanraj R, Butler E, et al. Lack of association between the C3435T polymorphism in the human multidrug resistance (MDRl) gene and response to antiepileptic drug treatment. Epilepsia. 2005;46:643–47. doi: 10.1111/j.1528-1167.2005.46304.x. [DOI] [PubMed] [Google Scholar]
- 13.Chen L, Liu CQ, Hu Y, et al. Research on the correlation between childhood epilepsy drug reactions of multidrug resistance gene MDRl C3435T polymorphism. Zhongguo Dang Dai Er Ke Za Zhi. 2007;9:11–14. [PubMed] [Google Scholar]
- 14.Shahwan A, Murphy K, Doherty C, et al. The controversial association of ABCBl polymorphisms in refiactory epilepsy: an analysis of multiple SNPs in an Irish population. Epilepsy Res. 2007;73:192–98. doi: 10.1016/j.eplepsyres.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Dericioglu N, Babaoglu MO, Yasar U, et al. Multidrug resistance in patients undergoing resective epilepsy surgery is not associated with C3435T polymorphism in the ABCBI (MDRI) gene. Epilepsy Res. 2008;80:42–46. doi: 10.1016/j.eplepsyres.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 16.Ozgon GO, Bebek N, Gul G, Cine N. Association of MDRl (C3435T) polymorphism and resistance to carbarnazepine in epileptic patients from Turkey. Eur Neurol. 2008;59:67–70. doi: 10.1159/000109264. [DOI] [PubMed] [Google Scholar]
- 17.Kim DW, Lee SK, Chu K, et al. Lack of association between BCBI, ABCG2, and ABCC2 genetic polymorphisms and multidrug resistance in partial epilepsy. Epilepsy Res. 2009;84:86–90. doi: 10.1016/j.eplepsyres.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Lakhan R, Misra UK, Kalita J, et al. No association of ABCBl Polymorphisms with drug-refractory epilepsy in a north Indian population. Epilepsy Behav. 2009;14:78–82. doi: 10.1016/j.yebeh.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Szoeke C, Sills GJ, Kwan P, et al. Multidrug-resistant genotype (ABCBl) and seizure recurrence in newly treated epilepsy: data from intemational pharmacogenetic cohorts. Epilepsia. 2009;50:1689–96. doi: 10.1111/j.1528-1167.2009.02059.x. [DOI] [PubMed] [Google Scholar]
- 20.Ufer M, Mosyagin I, Muhle H, et al. Non-response to antiepileptic pharmacotherapy is associated with the ABCC2-24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics. 2009;19:353–62. doi: 10.1097/fpc.0b013e328329940b. [DOI] [PubMed] [Google Scholar]
- 21.Vahab SA, Sen S, Ravindran N, et al. Analysis of genotype and haplotype effects of ABCBl (MDRl) polymorphisms in the risk of medically refractory epilepsy in an Indian population. Drug Metab Pharmacokinet. 2009;24:255–60. doi: 10.2133/dmpk.24.255. [DOI] [PubMed] [Google Scholar]
- 22.Grover S, Bala K, Sharma S, et al. Absence of a general association between ABCB1 Genetic variants and response to antiepileptic drugs in epilepsy patients. Biochimie. 2010;92:1207–12. doi: 10.1016/j.biochi.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 23.Sanchez MB, Herranz JL, Leno C, et al. Genetic factors associated with drug-resistance of epilepsy: Relevance of stratification by patient age and aetiology of epilepsy. Seizure. 2010;19:93–101. doi: 10.1016/j.seizure.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Kumari R, Lakhan R, Garg RK, et al. Pharmacogenomic association study on the role of drug metabolizing. drug transporters and drug target gene polymorphisms in drug-resistant epilepsy in a north Indian population. Indian J Hum Genet. 2011;17:S32–40. doi: 10.4103/0971-6866.80357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong L, Luo K, Tong Y, et al. Lack of association between ABCBl gene polymorphisms and pharmacoresistant epilepsy: an analysis in a western Chinese pediatric population. Brain Res. 2011;1391:114–24. doi: 10.1016/j.brainres.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Haerian BS, Lim KS, Mohamed EH, et al. Lack of association of ABCBl and PXR polymorphisms with response to treatment in epilepsy. Seizure. 2011;20:387–94. doi: 10.1016/j.seizure.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Di Q, Wang LL, Xu LG, et al. Refractory epilepsy and multidrug resistance gene 1 C3435T polymorphism correlation. Zhonghua Shen Jing Yi Xue Za Zhi. 2011;10:127–31. [Google Scholar]
- 28.Haerian BS, Roslan H, Raymond AA, et al. ABCB1 C3435T polymorphism and the risk of resistance to antiepileptic drugs in epilepsy: a systematic review and meta-analysis. Seizure. 2010;19(6):339–46. doi: 10.1016/j.seizure.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 29.Saygi S, Alehan F, Atac FB, et al. Multidrug resistance 1 (MDR1) 3435C/T genotyping in childhood drug-resistant epilepsy. Brain Dev. 2014;36(2):137–42. doi: 10.1016/j.braindev.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Alpman A, Ozkinay F, Tekgul H, et al. Multidrug resistance 1 (MDR1) gene polymorphisms in childhood drug-resistant epilepsy. J Child Neurol. 2010;25(12):1485–90. doi: 10.1177/0883073810368997. [DOI] [PubMed] [Google Scholar]
- 31.Emich-Widera E, Likus W, Kazek B, et al. CYP3A5*3 and C3435T MDR1 polymorphisms in prognostication of drug-resistant epilepsy in children and adolescents. Biomed Res Int. 2013;2013:526837. doi: 10.1155/2013/526837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seven M, Batar B, Unal S, et al. The drug-transporter gene MDR1 C3435T and G2677T/A polymorphisms and the risk of multidrug-resistant epilepsy in Turkish children. Mol Biol Rep. 2014;41(1):331–36. doi: 10.1007/s11033-013-2866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Emich-Widera E, Likus W, Kazek B, et al. Polymorphism of ABCB1/MDR1 C3435T in children and adolescents with partial epilepsy is due to different criteria for drug resistance – preliminary results. Med Sci Monit. 2014;20:1654–61. doi: 10.12659/MSM.890633. [DOI] [PMC free article] [PubMed] [Google Scholar]