Abstract
Introduction
Teriflunomide, indicated for the treatment of relapsing–remitting multiple sclerosis, is contraindicated in pregnancy based on signs of developmental toxicity in the offspring of rats and rabbits; developmental toxicity has also been observed in preclinical studies of other disease-modifying therapies. Despite the requirement to use reliable contraception in clinical trials evaluating the safety and efficacy of teriflunomide, a number of pregnancies have been reported. This work reports pregnancy outcomes in teriflunomide clinical trials.
Methods
Pregnancy outcomes were evaluated in a retrospective analysis of the global pharmacovigilance database. The following information was collected from the pharmacovigilance database or individual patient files: treatment allocation, pregnancy outcome, teriflunomide exposure, and use of the accelerated elimination procedure.
Results
At data cut-off, 83 pregnancies were reported in female patients and 22 pregnancies were documented in partners of male patients. All newborns were healthy and did not have any structural or functional abnormalities at birth.
Conclusion
Available data do not indicate any teratogenic signals in patients treated with teriflunomide.
Electronic supplementary material
The online version of this article (doi:10.1007/s40120-014-0020-y) contains supplementary material, which is available to authorized users.
Keywords: Clinical trials, Multiple sclerosis, Pregnancy, Teratogenicity, Teriflunomide
Introduction
In patients with multiple sclerosis (MS), diagnosis typically occurs during childbearing years [1, 2]. Consequently, it is important to understand potential risks during pregnancy posed by exposure to disease-modifying therapies (DMTs). MS itself does not appear to be associated with an increased risk of adverse pregnancy outcomes [1]. However, prenatal exposure to DMTs may adversely impact fetal development [3].
Teriflunomide is a once-daily, oral immunomodulator approved for treatment of relapsing–remitting MS. Teriflunomide is contraindicated in pregnant women, or women of childbearing potential not using reliable contraception, based on the occurrence of teratogenicity and embryo-lethality in the offspring of teriflunomide-treated rats and rabbits [4, 5]. Importantly, in vitro teriflunomide studies showed no evidence for mutagenicity, and teriflunomide was not clastogenic in vivo [4, 5]. Preclinical studies with other oral DMTs have also shown potential teratogenic signals in rodents [3].
Reliable contraception should be used throughout teriflunomide treatment, and women wishing to become pregnant must discontinue teriflunomide treatment [4, 5]. In the event of pregnancy, female patients must undergo an accelerated elimination procedure until teriflunomide plasma concentrations fall below 0.02 mg/L; a level, based on animal studies, predicted to have minimal risk to the fetus [4, 5].
Despite the requirement to use reliable contraception in teriflunomide clinical trials, a number of pregnancies have been reported. Here, we present the outcomes of these pregnancies.
Methods
Pregnancy Monitoring Procedures
Pregnant or lactating females were excluded from teriflunomide clinical trials. All patients entering trials were counseled on the potential risks of drug exposure in pregnancy and provided written consent not to become pregnant or to father a child, and to use reliable contraception. Where pregnancies were identified, patients were instructed to discontinue study treatment immediately and to start an accelerated elimination procedure [4, 5].
Analysis of Pregnancy Outcomes
A retrospective analysis of the global pharmacovigilance database was conducted to evaluate pregnancy outcomes identified in female study participants and female partners of male study participants in teriflunomide clinical trials. The following studies were included: monotherapy studies Phase 2 proof-of-concept (NCT01487096) and extension (NCT00228163), Phase 3 TEMSO (NCT00134563), TEMSO extension (NCT00803049), TOWER (NCT00751881), TOPIC (NCT00622700) and TENERE (NCT00883337); adjunctive therapy studies Phase 2 interferon beta (NCT00489489), glatiramer acetate (NCT00475865) and extensions (NCT00811395), and Phase 3 TERACLES (interferon beta; NCT01252355). The following information was collected from the pharmacovigilance database or individual patient files: treatment allocation, pregnancy outcome, teriflunomide exposure, and use of the accelerated elimination procedure.
Compliance with Ethics Guidelines
The data in this article are based on previously conducted studies, and do not involve any new studies, with human or animals subjects, performed by any of the authors.
Results
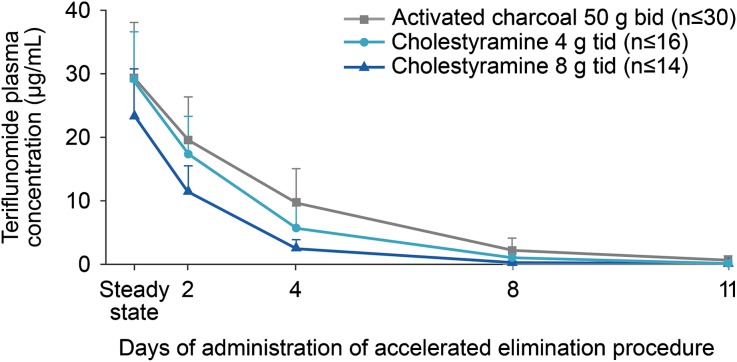
At data cut-off, 83 pregnancies were reported in female patients enrolled into teriflunomide clinical trials (Table 1). Of these, 13 pregnancies occurred in women exposed to interferon beta or placebo. Seventy pregnancies (including one twin pregnancy) were reported in women with known teriflunomide exposure; outcomes included 26 live births, 13 spontaneous abortions, and 29 induced abortions, including one abortion of two embryos. Subsequent to the last data cut-off, one ongoing pregnancy (Table 1) resulted in the delivery of a baby boy at 39 weeks of pregnancy. Maternal exposure in our analysis ranged from 12 days to approximately 4.5 years. The use of an accelerated elimination procedure with cholestyramine (8 or 4 g every 8 h) or activated charcoal (50 g every 12 h), both of which have been shown to decrease teriflunomide concentrations by ≥98% after 11 days of administration [4, 5] (Fig. 1), was also documented. Of the 26 pregnancies exposed to teriflunomide that resulted in live births, 10 patients discontinued teriflunomide treatment and nine completed the accelerated elimination procedure before becoming aware of their pregnancy. The remaining 16 patients discontinued teriflunomide treatment a few days to 11 weeks after becoming pregnant, and 13 of these underwent the accelerated elimination procedure. The use of the accelerated elimination procedure in the vast majority of patients exposed to teriflunomide minimized exposure to the developing fetus by rapidly reducing teriflunomide plasma levels below those predicted to have any fetal risk (<0.02 mg/L) [4, 5]. Median birth weight, documented for 18 newborns, was 3.3 kg, and mean gestational age, documented in 23 cases, was 39 weeks (range 36–44 weeks). All newborns were healthy and did not have any structural or functional abnormalities at birth. The spontaneous abortion rate in teriflunomide-exposed patients was 18.6%, within the range reported for the general population [6]. None of the induced abortions were performed due to defects or malformations.
Table 1.
Pregnancy outcomes among female patients and partners of male patients enrolled in teriflunomide clinical studies
| Pregnancy outcome | Teriflunomide | Placebo | Interferon beta | Total |
|---|---|---|---|---|
| Female patients | ||||
| Live birth | 26 | 2 | 2 | 30 |
| Induced abortion | 29 | 8 | 0 | 37 |
| Spontaneous abortion | 13 | 1 | 0 | 14 |
| Ongoing pregnancy | 1a | 0 | 0 | 1a |
| Unknown | 1 | 0 | 0 | 1 |
| Total | 70 | 11 | 2 | 83 |
| Female partners of male patients | ||||
| Live birth | 16 | 2 | – | 18 |
| Induced abortion | 2 | 1 | – | 3 |
| Spontaneous abortion | 1 | 0 | – | 1 |
| Total | 19 | 3 | – | 22 |
aFollowing data cut-off, the ongoing pregnancy resulted in the delivery of a baby boy at 39 weeks of pregnancy
Fig. 1.
Teriflunomide plasma concentrations following an accelerated elimination procedure. A loading dose of teriflunomide (70 mg/day for 3–4 days) was administered to achieve steady state rapidly; this was followed by a maintenance dose of teriflunomide 14 mg/day for 8–11 days. Cholestyramine [8 or 4 g, three times daily (tid)] or activated charcoal [50 g, two times daily (bid)] was administered orally for 11 days following teriflunomide treatment. Adapted with permission from Freedman [14]
Additionally, 22 pregnancies were documented in partners of male patients in the teriflunomide clinical program (Table 1). Of these, three pregnancies occurred in women whose partners were exposed to placebo. Of the 19 pregnancies with known paternal teriflunomide exposure, outcomes included 16 live births, one spontaneous abortion, and two induced abortions. No structural defects or functional abnormalities were reported in newborns with paternal teriflunomide exposure, and there were no reports of embryo–fetal abnormalities in either of the induced abortions.
Discussion
Preclinical studies of some oral DMTs have shown signals for some developmental toxicity in rodents [3]. Limited data on clinical outcomes of pregnancies associated with DMT exposure have also been reported in the literature [3, 7]. Findings from the teriflunomide clinical development program have thus far demonstrated no pregnancy safety concerns. All newborns with prenatal teriflunomide exposure were healthy and had no structural defects or functional deficits at birth. Teriflunomide treatment was not associated with a higher rate of spontaneous abortion (18.6%) compared with rates, including early pregnancy losses, reported in the general population (17–22%) [6]. These observations are supported further by evidence from prospective studies of the parent compound, leflunomide, approved for the treatment of rheumatoid arthritis (RA) since 1998 [8]. In a prospective study by the Organization of Teratology Information Specialists (OTIS), there were no significant differences in the rate of major structural defects, and no specific pattern of major or minor anomalies, in newborns of women with RA exposed to leflunomide relative to disease-matched or healthy comparator groups, with a similar rate to that in the general population [9]. This lack of a signal for teratogenicity was confirmed in a subsequent OTIS analysis [10]. In addition, leflunomide postmarketing surveillance of more than 2.4 million patient-years of exposure has not shown any teratogenic signal.
Teriflunomide has been detected at low levels in human semen [4]. However, animal studies show no evidence that teriflunomide adversely affects male fertility or damages sperm DNA [4, 5]. Although animal studies to evaluate the risk of male-mediated fetal toxicity have not been conducted, the risk of male-mediated embryo–fetal toxicity from teriflunomide treatment is considered low, with estimated plasma exposure in females from semen transfer from a male-treated patient expected to be ≥100 times lower than plasma exposure following oral dosing of teriflunomide 14 mg [5]. However, to minimize any potential risk, some regulatory authorities recommend that men wishing to father a child should also discontinue teriflunomide treatment and undergo the accelerated elimination procedure [4]; no such recommendation is made in the European labeling for teriflunomide [5].
It is important to collect as much data as possible to determine potential adverse outcomes following DMT exposure during pregnancy. In this regard, global pregnancy registries for teriflunomide will capture prospective data from pregnancies that occur worldwide in the postmarketing setting. In North America, the independent OTIS group has established a pregnancy registry to collect prospective data for women exposed to teriflunomide during pregnancy [11]. A second registry will collect pregnancy data from patients in Europe and Australia.
Conclusion
While this investigation was not powered to detect teratogenicity, available data do not indicate any teratogenic signals in patients treated with teriflunomide. Teriflunomide has been shown to be an effective therapeutic option for patients with MS [12, 13], including women of childbearing potential using effective contraception.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The data described in this manuscript were obtained from clinical studies funded by Genzyme, a Sanofi company, Cambridge, MA, USA. This manuscript was critically reviewed by Thierry Aupérin, PhD, of Genzyme. Editorial support was provided by Scott Chambers, of Fishawack Communications Ltd, Abingdon, UK, and was funded by Genzyme. No other funding or sponsorship was received for this study or publication of this article.
All named authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of this work as a whole, and have given final approval of the version to be published.
Conflict of interest
BK has received honoraria for lecturing, travel expenses for attending meetings, and financial support for research from Bayer Schering, Biogen Idec, Merck Serono, Novartis, Sanofi, and Teva Neuroscience. MB is an employee of Sanofi R&D.
Compliance with ethics guidelines
The data in this article are based on previously conducted studies, and do not involve any new studies, with human or animals subjects, performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Houtchens MK, Kolb CM. Multiple sclerosis and pregnancy: therapeutic considerations. J Neurol. 2013;260:1202–1204. doi: 10.1007/s00415-012-6653-9. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzi AR, Ford HL. Multiple sclerosis and pregnancy. Postgrad Med J. 2002;78:460–464. doi: 10.1136/pmj.78.922.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cree BA. Update on reproductive safety of current and emerging disease-modifying therapies for multiple sclerosis. Mult Scler J. 2013;19:835–843. doi: 10.1177/1352458512471880. [DOI] [PubMed] [Google Scholar]
- 4.AUBAGIO prescribing information. Cambridge: Genzyme Corporation, a Sanofi company; 2012. Available from: http://products.sanofi.us/aubagio/aubagio.pdf. Accessed Aug 28, 2014.
- 5.AUBAGIO Summary of Product Characteristics. Paris: Sanofi-aventis groupe; 2013. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002514/WC500148682.pdf. Accessed Aug 28, 2014.
- 6.Garcia-Enguidanos A, Calle ME, Valero J, Luna S, Dominguez-Rojas V. Risk factors in miscarriage: a review. Eur J Obstet Gynecol Reprod Biol. 2002;102:111–119. doi: 10.1016/S0301-2115(01)00613-3. [DOI] [PubMed] [Google Scholar]
- 7.Karlsson G, Francis G, Koren G, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurology. 2014;82:674–680. doi: 10.1212/WNL.0000000000000137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.ARAVA prescribing information. Bridgewater: Sanofi-aventis U.S. LLC; 2014. Available from: http://products.sanofi.us/arava/arava.html. Accessed Aug 28, 2014.
- 9.Chambers CD, Johnson DL, Robinson LK, et al. Birth outcomes in women who have taken leflunomide during pregnancy. Arthritis Rheum. 2010;62:1494–1503. doi: 10.1002/art.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassina M, Johnson DL, Robinson LK, et al. Pregnancy outcome in women exposed to leflunomide before or during pregnancy. Arthritis Rheum. 2012;64:2085–2094. doi: 10.1002/art.34419. [DOI] [PubMed] [Google Scholar]
- 11.Organization of Teratology Information Specialists (OTIS). MotherToBaby. Available from: www.pregnancystudies.org. Accessed Oct 31, 2014.
- 12.O’Connor P, Wolinsky JS, Confavreux C, et al. Randomized trial of oral teriflunomide for relapsing multiple sclerosis. N Engl J Med. 2011;365:1293–1303. doi: 10.1056/NEJMoa1014656. [DOI] [PubMed] [Google Scholar]
- 13.Confavreux C, O’Connor P, Comi G, et al. Oral teriflunomide for patients with relapsing multiple sclerosis (TOWER): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:247–256. doi: 10.1016/S1474-4422(13)70308-9. [DOI] [PubMed] [Google Scholar]
- 14.Freedman MS. Teriflunomide in relapsing multiple sclerosis: therapeutic utility. Ther Adv Chronic Dis. 2013;4:192–205. doi: 10.1177/2040622313492810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.