Abstract
The use of long-term antibiotics for deep-seated infections is very common, and is associated with many clinically significant side effects. In this report we describe the history of a 48-year-old man who attended West Suffolk Hospital with nausea and vomiting, and was subsequently found to have a deep-seated infection following his repeat aortic valve replacement. He completed a 7-week course of intravenous flucloxacillin and oral fusidic acid, however, prior to finishing this course a random blood test revealed a neutrophil count of zero. He was re-admitted to hospital with fever, and was treated accordingly as per the trust's neutropenic sepsis protocol with the addition of growth colony stimulating factor (GCSF). His neutrophil count recovered after 3 days and has remained within the normal range ever since.
Background
Neutropenia is defined as an absolute neutrophil count of less than 1500 cells/μL. Drug-induced neutropenia (DIN) is a rare phenomenon, sporadically reported. The most common drugs associated with this condition are antithyroid drugs, anticonvulsants and antibiotics. The use of long-term antibiotics, specially β-lactam is known to increase the risk of drug-induced neutropenia, and this bares potential risks for population groups commonly treated with long-term antibiotics (more than 10–14 days) for deep-seated infections, these include: patients with diabetes at risk of osteomyelitis and recurrent abscesses, those with prosthesis and patient groups who are immunosuppressed for a multitude of reasons. The underlying pathogenesis of DIN is poorly understood, and here we attempt to analyse the literature around this phenomenon, and conclude by recommending that clinicians should be very vigilant of blood cell counts in patients on long-term antibiotics.
Case presentation
A 48-year-old man, presented to West Suffolk Hospital (WSH), with rigour, diarrhoea and vomiting; this was on a background of a recently replaced mechanical aortic valve at Papworth Hospital, 4 weeks prior to admission. His medical history was remarkable for congenital aortic stenosis leading to mechanical aortic valve replacement in 1987, and AF for which he was on flecainide, and warfarin; other medications included pravastatin, omeprazole, bisoprolol and paracetamol. He was a non-smoker, and drank minimal alcohol. On examination he had a loud mechanical second heart sound, evident discharge from the site of recent sternotomy, and appeared generally septic.
Investigations
Initial bloods test revealed a C reactive protein of 2901–6 and white cell count of 8.5×103/µL. Blood cultures and wound swabs were taken and our patient was treated as per the local sepsis protocol (the antibiotic regime of choice in this protocol is tazocin 4.5 g three times per day).
Given this patient's recent cardiac surgery, infective endocarditis was suspected; although a transoesophageal echocardiogram did not reveal any cardiac vegetations, a CT scan of his chest revealed a collection situated between the sternotomy wound and the ascending aorta (figure 1). He was initially started on tazocin, however, blood cultures and wound profile were Staphylococcus aureus positive. As such he was treated with targeted antibiotic therapy against S. aureus (causing a deep-seated infection and bacteraemia) with a 7-weeks course of intravenous flucloxacillin, followed by the addition of 4 weeks of oral fusidic acid.
Figure 1.
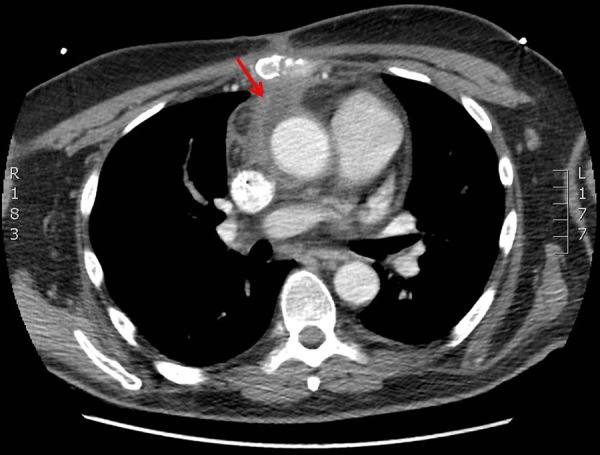
Located between the sternotomy scar and the ascending aorta, there is a small triangle of fluid attenuation with an enhancing wall, depicted by the red arrow, extending down to the anterior surface of the extending aorta.
Furthermore he was transferred to Papworth hospital for wound debridement and washout. After a 4-week stay at Papworth hospital, our patient was transferred back to WSH for continuation of his intravenous antibiotic therapy; a peripherally inserted central catheter (PICC) was inserted and he was discharged home with instructions on how to self-administer his antibiotics. As he approached the end of his antibiotic course, and due to have his PICC line removed, an outpatient random blood test was performed to check inflammatory markers. To our surprise the neutrophil count was reported as 0, with a total white cell count of 1.6×103/µL, confirmed on repeat testing; all other white cell type counts were within the normal range, as were renal and liver function tests.
Differential diagnosis
The differential diagnosis of neutropenia would include infectious diseases, nutritional deficiencies, immune disorders and congenital or chronic neutropenia; drugs are implicated in many cases.
Treatment
Our patient was contacted and invited to return to hospital immediately for further investigation and management. He notified us that he had been experiencing an upper-respiratory infection associated with fever, from which he did not seem to be recovering.
On admission to hospital with recurrent fever, he was treated as per the neutropenic sepsis protocol, however, instead of tazocin (first line in neutopenic sepsis at WSH), meropenem was chosen as the antibiotic of choice—this was following discussion with our microbiologists and in view of previous culture sensitivities to meropenem, available on the pathology system.
Further discussion with the haematology team, led to start of growth colony stimulating factor. Our patient's neutrophil count remained 0.0 for three consecutive days, and subsequently increased after three doses of GCSF (this was stopped after the third dose). Blood cultures were negative at 48 h, patient remained afebrile post day1 of admission, and a repeat transoesophageal echocardiogram was negative for any vegetations. Meropenem was subsequently stopped after 2 days, and our patient was discharge 5 days after admission, once his neutrophil count had stabilised on 19 March 2014 (figure 2).
Figure 2.
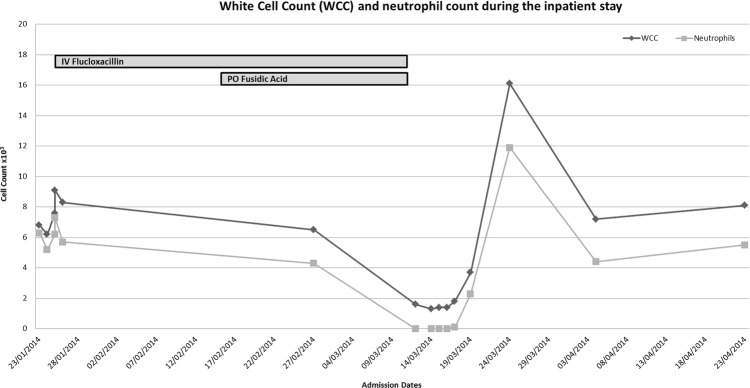
White cell count (WCC) and neutrophil count throughout admission.
Outcome and follow-up
This patient's neutrophil count has remained within the normal range since discharge. He was advised to mention his reaction to the combination antibiotic to future medical staff in case of the need for future antibiotic therapy with either agent.
Discussion
The management of S. aureus bacteraemia has been reviewed extensively; however there remains an uncertainty as to the most suitable antibiotic choice for treatment of this potentially life-threatening organism. Currently β-lactams, namely flucloxacillin (usually in combination with fusidic acid) remains a favourable first-line therapy,1 especially in the presence of concomitant deep-seated infection. Indeed Thwaites et al report that although glycopeptides and other β-lactams, namely cephalosporins are possible candidates for the treatment of S. aureus bacteraemia, convincing evidence to suggest equal efficacy to penicillin is lacking. Furthermore vancomycin has a less favourable side effect profile compared to β-lactams.2 In this review, authors also elude to the optimal treatment course of S. aureus bacteraemia as more than 10–14 days to ensure maximal clearance of infection.
Flocluxacillin and fusidic acid are used widely in deep-seated infections of soft tissues and bones where they are normally used for more than 2 weeks to allow effective clearance of the underlying infection. Here we have reported a case of antibiotic-associated neutropenia in a patient treated with both of the aforementioned antibiotics. The UK registry of drug adverse reactions, reports 45 and 33 incidents of flucloxacillin and fusidic acid-associated neutropenia, respectively, from 1963 to 2014. A search of Drugs Interaction Checker did not reveal any interactions between the two antibiotics used and our patient's usual medications.
A multicentre case–control study of 396 patients, affected by drug-induced neutropenia across 17 different hospitals, revealed an incidence of 5:1 million inhabitants per year—a rare entity in general. 12.01% of these cases were attributed to β-lactam antibiotics, with antithyroid drugs, sulphonamides, carbamazepine and ticlopidine responsible for the remaining cases. Authors report that β-lactam-induced neutropenia is indeed a rare occurrence with short-course treatments.3
Adverse side effects of parenteral treatment with penicillin is evident in cases treated for more than 2 weeks. In a prospective study of patients on treatment for infective endocarditis, on either intravenous penicillin or vancomycin for more than 10 days, the incidence of penicillin-associated neutropenia is reported as 14%, versus 4% in those treated with vancomycin, with penicillin G having the highest incidence compared to other β-lactams (namely cephalosporins).4 A further case series of 21 reports of drug-induced neutropenia, revealed 40% of these to be antibiotic related, 9 β-lactam-induced (duration 3–29 days)—all responsive to GCSF 300 µg/day.5 Data from eight children treated with long-term flucloxacillin for bone and joint infections, revealed a reversible neutropenia on stopping the medication.6
Fusidic acid has also been reported in association with neutropenia, in patients treated with both short-term and long-term antibiotics.7 8 Interestingly in two reported cases both patients were receiving concomitant treatment with flucloxacillin, raising the question of a synergistic pathway leading to neutropenia.
The exact mechanism by which flucloxacillin or fusidic acid may potentiate neutropenia is indeed not well understood. However, in a few reports penicillin/cephalosporin IgG antibody against penicillin/cephalosporin-induced neutrophils have been detected and might infer an idiosyncratic immune-mediated pathway for this adverse side effect.9 Indeed in the prospective case–control study by Olaison et al significant amounts of haemaglutinating antibodies were present in patients experiencing β-lactam associated adverse reaction, as long as 150 days after treatment withdrawal; these were of both IgM and IgG subtype directed against the benzylpenicilloyl determinant (BPO). Drug-specific antibodies against penecilloylated neutrophils may explain β-lactam-induced neutropenia, similar to the underlying mechanisms leading to penicillin-induced immune haemolytic anaemia. In the latter study authors have also reported a dose-dependent association with the incidence of neutropenia (35% in patients treated with high-dose penicillin G, compared to 8% in those treated with lower doses).4
In vivo analysis of bone marrow aspirates, from patients experiencing β-lactam-associated neutropenia, have revealed a reduction in the myeloblast population.10 In the same study, in vitro exposure of myeloid progenitor cells to β-lactam antibiotics, in the early stages of proliferation, is thought to induce inhibition of granulopoiesis; addition of serum from patients with penicillin-G-induced neutropenia to autologous bone marrow cultures did not alter the effects of β-lactam antibiotics. Authors therefore conclude that myelosupressive effects of β-lactam antibiotics are non-immunological in aetiology. This may explain the rise in neutrophils in patients experiencing antibiotic-induced neutropenia, treated with GCSF.5
Given the rise in the incidence of the antibiotic-induced neutropenia beyond 10 days, we recommend clinicians treating patients on long-term antibiotics, whether in hospital or community, to be vigilant of the potential for life-threatening neutropenia. We also recommend regular monitoring of full blood counts, caution with reintroducing such antibiotics in patients with previous adverse reactions, and discussion with microbiology and haematology should this phenomenon occur.
Learning points.
Flucloxacillin and fusidic acid could cause neutropenia—rendering the patient susceptible to further infection—this may be reversible on treatment withdrawal.
Full blood counts should be regularly monitored while on a long-term treatment regime (more than 10–14 days), specially, for deep-seated infections (eg, bone and joints).
There may be a role for the use of growth colony stimulating factor (GCSF) in improving neutrophil counts in drug-induced neutropenia (DIN).
Myelotoxicity and/or antibodies against antibiotic-induced neutrophils could explain the phenomenon of DIN seen in this report.
In patients with a history of flucloxacillin or fusidic acid-associated neutropenia, future use of these agents should be weighed against the potential risks of further DIN.
Acknowledgments
The authors would like to thank the microbiology and haematology teams at West Suffolk Hospital for their advice on the management of this case. They would also like to thank the patient for consenting to the write-up of this report.
Footnotes
Contributors: NW was the responsible consultant for the care of the patient and NDA looking after the patient (at the time of working at West Suffolk Hospital as a foundation year 1 trainee). The manuscript was written by NDA and commented on by NW.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Thwaites GE; United Kingdom Clinical Infection Research Group (UKCIRG). The management of Staphylococcus aureus bacteremia in the United Kingdom and Vietnam: a multi-centre evaluation. PLoS ONE 2010;5:e14170 10.1371/journal.pone.0014170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thwaites GE, Edgeworth JD, Gkrania-Klotsas E et al. Clinical management of Staphylococcus aureus bacteraemia. Lancet Infect Dis 2011;11:208–22. 10.1016/S1473-3099(10)70285-1 [DOI] [PubMed] [Google Scholar]
- 3.Ibanez L, Vidal X, Ballarin E et al. Population-based drug-induced agranulocytosis. Arch Intern Med 2005;165:869–74. 10.1001/archinte.165.8.869 [DOI] [PubMed] [Google Scholar]
- 4.Olaison L, Belin L, Hogevik H et al. Incidence of beta-lactam-induced delayed hypersensitivity and neutropenia during treatment of infective endocarditis. Arch Intern Med 1999;159:607–15. 10.1001/archinte.159.6.607 [DOI] [PubMed] [Google Scholar]
- 5.Andres E, Maloisel F. Antibiotic-induced agranulocytosis: a monocentric study of 21 cases. Arch Intern Med 2001;161:2619 10.1001/archinte.161.21.2619 [DOI] [PubMed] [Google Scholar]
- 6.van den Boom J, Kristiansen JB, Voss LM et al. Flucloxacillin associated neutropenia in children treated for bone and joint infections. J Paediatr Child Health 2005;41:48–51. 10.1111/j.1440-1754.2005.00535.x [DOI] [PubMed] [Google Scholar]
- 7.Christiansen K. Fusidic acid adverse drug reactions. Int J Antimicrob Agents 1999;12(Suppl 2):S3–9. 10.1016/S0924-8579(98)00068-5 [DOI] [PubMed] [Google Scholar]
- 8.Evans DI. Granulocytopenia due to fusidic acid. Lancet 1988;2:851 10.1016/S0140-6736(88)92814-0 [DOI] [PubMed] [Google Scholar]
- 9.No authors listed Antibiotic-induced neutropenia. Lancet 1985;2:814. [PubMed] [Google Scholar]
- 10.Neftel KA, Hauser SP, Muller MR. Inhibition of granulopoiesis in vivo and in vitro by beta-lactam antibiotics. J Infect Dis 1985;152:90–8. 10.1093/infdis/152.1.90 [DOI] [PubMed] [Google Scholar]


