Abstract
Swine flu is a multisystemic disease and can affect the gastrointestinal system. There are only three published reports of swine flu cases with acute appendicitis; two of them in children under 16 years of age. We present an unusual case of acute appendicitis in a child already diagnosed with swine flu infection. A 9½-year-old girl presented with febrile illness and mild abdominal pain. PCR (+) was positive for H1N1. 3 days after hospital admission she developed acute appendicitis and was operated on. On the fourth postoperative day she developed right upper lobe atelectasis; she was started on antiviral treatment to which she responded very well. She was discharged on day 7 without further consequences in her postoperative course. Children with swine flu may be susceptible to rapidly deteriorating and complicated acute appendicitis. This calls for more caution especially in periods of epidemics.
Background
Influenza virus infection is extremely common and raises global concern due to the increasing prevalence of pandemic H1N1 infection. In 2009, the first influenza pandemic of the 21st century started with an outbreak of swine origin influenza A (H1N1)2009 in the southwest of the USA and Mexico, rapidly followed by cases in Europe.1 2
The clinical presentation of influenza A (H1N1) is not different from seasonal influenza, with the majority of cases having mild disease.3 Patients most often present with the typical symptoms of an influenza-like illness: fever, cough, sore throat, rhinorrhoea, headache, myalgia and malaise.4 5 Gastrointestinal symptoms, such as diarrhoea, nausea and vomiting, have also been reported. Mostly in children and young to middle-aged adults, influenza A (H1N1)2009 can cause a rapidly progressive pneumonia frequently necessitating admission to the intensive care unit (ICU).5
The diagnosis of severe influenza A (H1N1)2009 is made on clinical grounds and laboratory confirmation. Reverse transcriptase PCR (RT-PCR) is the most appropriate detection method as it is rapid, sensitive, specific and scalable.5 6
Swine flu presenting as acute abdomen is not common. Three patients overall have been reported.7–9 Until now, only two cases have been reported in children aged under 16 years.8 9 Two patients had appendectomy performed.7 8 One was managed conservatively.9 We present another case of a young patient with verified H1N1 infection who presented with acute abdomen, and discuss aspects of the clinical presentation and management of this condition.
Case presentation
A 9½-year-old girl was admitted to the paediatric ward of Penteli Children's Hospital with a 3-day history of acute febrile illness. She presented with cough, headache, chills and general malaise. Her temperature reached 38°C. She was diagnosed with a viral upper respiratory infection. She was started on intravenous fluids and antipyretics. Because of fear of the ongoing H1N1 epidemic a sample was sent for testing. On the second day the patient started having mild abdominal pain with no diarrhoea or vomiting, which was attributed to her viral illness. She was only given clear fluids, by mouth. The pain started in the epigastrium but gradually intensified and 24 h later was mainly located on the right lower abdomen and was suggestive of acute appendicitis. A referral was made for paediatric surgical consultation. On examination, the patient had a sinus tachycardia of 151, blood pressure of 142/76 and a temperature of 38.2°C. There was muscle spasm on the right lower abdomen, diminished bowel sounds and rebound tenderness. There were no other abnormalities on cardiovascular and respiratory examination. An ultrasound scan demonstrated an enlarged appendix with thickened, inflamed wall and there was a significant volume of free fluid in the abdomen that was also confirmed intraoperatively (figure 1). White cell count was 14.16×109/L and C reactive protein was greater than 160 mg/L. Initial leucocytosis was followed by leucopenia soon after admission and before the operation. The clinical picture as well as the findings on the full blood count and ultrasound were suggestive of an acute appendicitis case. A decision was made to proceed to theatre. The girl's parents gave consent for an appendectomy.
Figure 1.
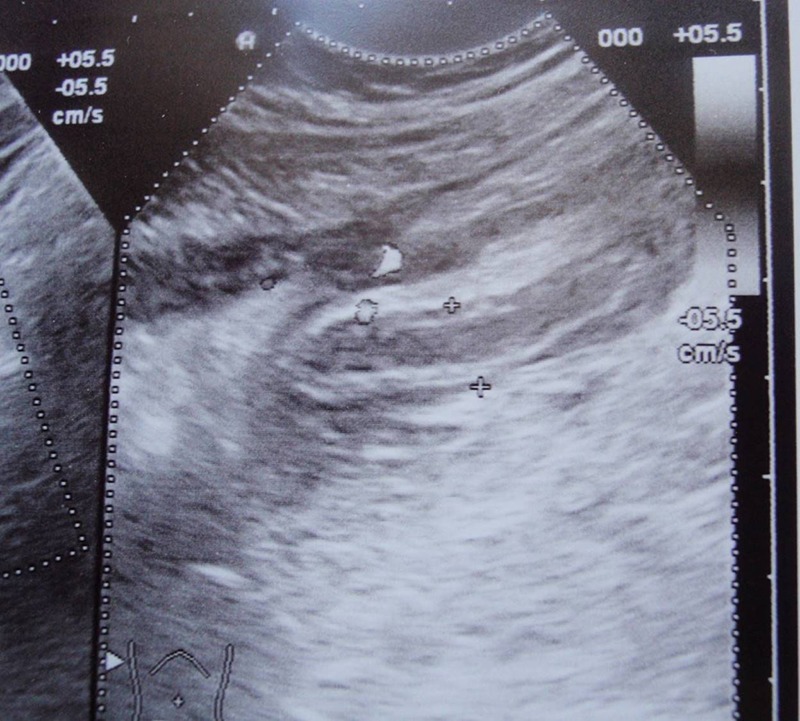
Abdominal ultrasound showed an enlarged appendix with thickened, inflamed wall.
Investigations
White cell count—14.16×109/L (day 1), then 4500×109 (day 3)
C reactive protein—>160 mg/L
PCR (+) was positive for Η1Ν1
Chest X-ray (day 1)—normal
Chest X-ray (day 4)—marked interstitial pattern, especially on the right (figure 2)
Abdominal ultrasound: abdominal ultrasound showed an enlarged appendix with thickened, inflamed wall and significant volume of free fluid in the abdomen (figure 1)
Histopathology report: inflamed appendix
Figure 2.
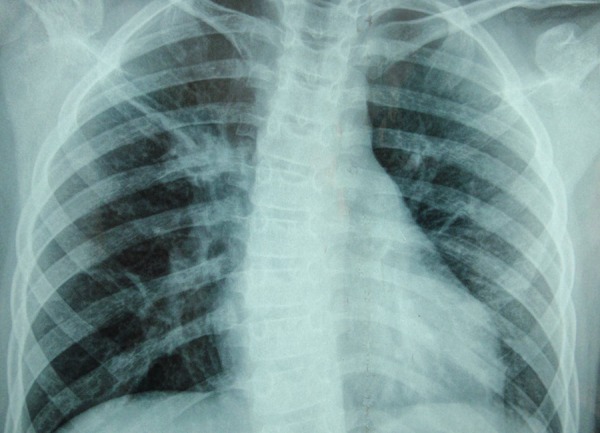
Chest X-ray on fourth postoperative day showed a marked interstitial pattern, especially on the right.
Differential diagnosis
Mesenteric lymphadenitis
Perforated appendix
Lower lobe pneumonia
Urinary tract infection
Treatment
Intraoperatively, we found an empyema of the appendix. Online appendix was severely inflamed, gangrenous at the tip with multiple adhesions to nearby intestinal loops. Appendectomy was performed. Postoperatively fever persisted. Chest X-ray on the fourth postoperative day showed a marked interstitial pattern, especially on the right (figure 2). Because of these findings and also as a response to the patient's postoperatively confirmed positive PCR, she was started on antiviral treatment in addition to her ongoing antibiotic treatment. Fortunately, the H1N1 infection subsided without further consequences in the patient's postoperative course. She was discharged on the seventh postoperative day well and with no remaining findings on chest X-ray.
Outcome and follow-up
One year after discharge from the hospital our patient is in excellent clinical condition. Her wounds healed nicely and on clinical examination there were no symptoms from gastrointestinal or respiratory system.
Discussion
As with any viral infection, there is the possibility of H1N1 being complicated by acute appendicitis. Therefore the diagnosis of H1N1 influenza virus should not restrict differential diagnosis in cases of persistent abdominal pain. Acute appendicitis is always a possibility, even though the virus mainly causes respiratory symptoms and the respiratory system is more severely compromised.
The first such case reported in the literature was of a 31-year-old man who presented with anorexia and lower abdominal pain for 12 h. He had tenderness and guarding in the right iliac fossa with a normal chest examination, and was diagnosed with acute appendicitis and taken to theatre for open appendicectomy.7 On day 3 postoperatively, his respiratory function declined markedly and a day later a nasal swab tested with real-time RT-PCR was positive for H1N1 infection. Treatment with high-dose oseltamivir was immediately added to the initial empirical antibacterial therapy. He rapidly progressed to acute respiratory distress syndrome with refractory hypoxaemia, and was transferred to the ICU for ventilation on an oscillator. He required mechanical ventilation for a total of 32 days and was discharged from ICU 54 days postoperatively. The histology of the appendix confirmed acute appendicitis. The authors raised certain interesting issues relating to diagnosis and management of their case. Even if H1N1 infection is suspected preoperatively, whether an appendicectomy should be safely put off until diagnosis is established should be questioned. Similarly, there is uncertainty as to whether the severe respiratory distress that accompanied this man’s H1N1 infection was in some way exacerbated by his general anaesthetic and the appendicectomy. General anaesthesia has been recognised to have negative impact on the course of influenza virus infections. However, in this case it would be difficult to justify not taking a patient with an obvious acute appendicitis to theatre on the basis of a low lymphocyte count. In conclusion, this case report highlights the impact that H1N1 virus can have on acute surgical emergencies and how it can complicate the postoperative course.7
Another case was of a 15-year-old girl with an influenza-like illness and right lower quadrant abdominal pain.8 Acute appendicitis was diagnosed by a CT scan and the patient underwent emergency appendectomy. Postoperatively, respiratory secretion samples were sent for swine influenza (H1N1) testing. Her respiratory fluorescent antibody (FA viral panel) was positive for influenza A, as was her RT-PCR for swine influenza (H1N1). The possibility of direct involvement of the appendix with swine influenza (H1N1) predisposing to early acute bacterial appendicitis was considered, but RT-PCR of the patient's appendix was negative. Therefore the authors suggested that the increased incidence of acute bacterial appendicitis during influenza epidemics/pandemics is not due to direct involvement of the appendix by the influenza virus. The authors suggested that acute appendicitis during an influenza epidemic/pandemic may be a secondary effect of either the immunosuppression by the influenza virus or the sequential effect of bacterial infection after the initial viral infection.8
The third patient was a 12-year-old boy who presented with a history of fever, cold and cough for 3 days.9 He had right lower quadrant abdominal pain and loose stools for 1 day. The abdominal pain was a dull ache localised to the right iliac fossa; it was non-radiating, non-colicky and was associated with vomiting. The patient had a history of appendicitis 4-year earlier that was managed conservatively. Ultrasonography of the abdomen was suggestive of appendicitis. The child was managed conservatively, started on intravenous fluids, intravenous antibiotics and oral oseltamivir. Gradually, after 2 days, the fever subsided; the cough and abdomen pain improved, and the child started accepting orally. A throat swab for H1N1 returned positive by RT-PCR. Intravenous antibiotics were stopped on day 3 of hospital stay and the 5-day course of oseltamivir was completed. The child was discharged on day 6 of hospital stay. Researchers suggest that many viral infections are associated with lymph tissue enlargement (hyperplasia of Peyer's patches), or that they can cause ulcerations resulting in bacterial infection. Appendicitis has been associated with a viral prodrome compatible with a viral illness preceding the first symptoms of appendicitis.9
In the literature review we also found a case of a 32-year-old primipara who presented at 25 weeks gestation with acute onset lower abdominal pain, vomiting and low-grade fever.10 The lower abdominal pain had persisted for 3 weeks and sudden increase in intensity during the last 1 day warranted her presentation. Examination revealed a pulse rate of 120 bpm and a temperature of 37.8°C; and severe right iliac fossa tenderness with marked guarding and rebound tenderness. The gravid uterus was soft with satisfactory fetal assessment. Serial haematological and biochemical investigations were normal. The general surgeons performed a diagnosis of acute appendicitis. At exploratory laparotomy, the appendix, pelvic and abdominal cavities looked normal and the patient underwent an appendicectomy. Postoperatively, she developed a non-productive cough and sore throat, with no improvement in her symptoms; she was finally diagnosed and treated for H1N1 infection.10 We only mention this case because the symptoms were suggestive of acute appendicitis but, as already stated, this was not proven intraoperatively. As a result, this patient should not be added to the other three previously reported cases.
In all these cases it is difficult to prove whether H1N1 infection caused the appendicitis or it allowed a bacterial secondary infection. In our case, testing for H1N1 of the appendix might have given some clue, but we believe that most likely this would be negative, as was seen in the second reported case. Unfortunately, we did not perform this test, because at the time of surgery we still did not have the results of our patient's PCR and the index of suspicion was not high. Moreover, we had issues of cost and availability of the test performed on the appendix, and it is questionable whether this knowledge would influence management of our patient. We believe that in our case, bacterial secondary infection seems more plausible. In our opinion, this seems most likely in all three reported cases. Moreover, our literature review did not reveal any other relationship between H1N1 infection and appendicitis.
In our case, it also seems that the course of the disease was more rapid and, in case of a delay in treatment, the appendix might have ruptured and a generalised peritonitis might have ensued. This stresses the importance of having a clinical suspicion and taking immediate action in case of worsening abdominal pain.
Postoperative care should take into account the possibility of worsening respiratory function. It seems reasonable that H1N1 infection makes a surgical patient more vulnerable to respiratory postoperative complications. In our case, the patient developed right upper lobe atelectasis and needed antiviral treatment.
Another issue is the preparation for this surgery. The paediatric surgeon has to strictly follow respiratory infection control protocols. Use of special protective devices such as face masks should be mandatory for all personnel in the surgical team and everyone who contacts the patient in the preoperation phase. If the hospital has a specific septic operating room, this is the situation to use it in; otherwise, a simple operating room can be used after it is specially prepared. As far as possible, disposable tools should be used. A special note should be given to the anaesthesiologist team to prepare special tools. After the surgical procedure, the operating room should be completely cleaned and disinfected.
In conclusion, during a swine influenza (H1N1) pandemic, clinicians should be alert to the possibility of an increased incidence/severity of acute bacterial appendicitis in patients with swine influenza (H1N1) infection.
Learning points.
Acute appendicitis is always a possibility, even though H1N1 virus mainly causes respiratory symptoms and the respiratory system is more severely compromised.
During a swine influenza (H1N1) pandemic, clinicians should be alert to the possibility of an increased incidence/severity of acute bacterial appendicitis in patients with swine influenza (H1N1) infection.
The paediatric surgeon has to strictly follow respiratory infection control protocols.
Footnotes
Contributors: EC identified and managed the case and also had the idea for the article. DB and ST performed the literature search, and selected and downloaded all relevant papers. CP wrote the article and is the guarantor of this paper.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Hsueh CW, Yu HM, Chen HS et al. Influenza-related postinfectious encephalomyelitis complicated by a perforated peptic ulcer. Pediatr Neonatol 2013;54:281–4. 10.1016/j.pedneo.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 2.Van Ierssel SH, Ieven M, Jorens PG. Severe influenza A (H1N1) 2009 infection: a single centre experience and review of the literature. Acta Clinica Belgica 2012;67: 1–6. [DOI] [PubMed] [Google Scholar]
- 3.Tang JW, Shetty N, Lam TT. Features of the new pandemic influenza A/H1N1/2009 virus: virology, epidemiology, clinical and public health aspects. Curr Opin Pulm Med 2010;16:235–41. 10.1097/MCP.0b013e3283375727 [DOI] [PubMed] [Google Scholar]
- 4.Venkata C, Sampathkumar P, Afessa B. Hospitalized patients with 2009 H1N1 influenza infection: the Mayo Clinic experience. Mayo Clin Proc 2010;85:798–805. 10.4065/mcp.2010.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organisation (WHO). Clinical management of human infections with pandemic (H1N1) 2009: revised guidance 2009. http://www.who.int/csr/resources/publications/swinefluclinical_management_h1n1.pdf [Google Scholar]
- 6.Boggild AK, McGeer AJ. Laboratory diagnosis of 2009 H1N1 influenza A virus. Crit Care Med 2010;38:e38–42. 10.1097/CCM.0b013e3181cd7bb2 [DOI] [PubMed] [Google Scholar]
- 7.Galbraith JG, Butler JS, Pead M et al. H1N1 infection in emergency surgery: a cautionary tale. Int J Surg Case Rep 2010;1:4–6. 10.1016/j.ijscr.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha BA, Pherez FM, Durie N. Swine influenza (H1N1) and acute appendicitis. Heart Lung 2010;39:544–6. 10.1016/j.hrtlng.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, Agarwala S, Jana M et al. Swine flu presenting as acute appendicitis. Indian J Pediatr 2014;81:208–9. 10.1007/s12098-013-1009-8 [DOI] [PubMed] [Google Scholar]
- 10.Ogah K, Munjuluri N, Hartis RJ. Swine flu mimicking acute abdomen in pregnancy. Obstet Gynaecol 2011;31:443 10.3109/01443615.2011.567341 [DOI] [PubMed] [Google Scholar]


