Abstract
Objectives. We have described the characteristics of South Asian-born tuberculosis (TB) patients living in New York City (NYC) and compared them with other foreign-born patients to explore possible explanations for the disproportionate burden of TB in the South Asian population.
Methods. We used data on demographic and clinical characteristics for TB patients identified by the NYC Bureau of Tuberculosis Control from 2001 to 2010 to compare South Asian patients with other Asian and other foreign-born patients. We reviewed genotyping and cluster investigation data for South Asian patients to assess the extent of genotype clustering and the possibility of local transmission in this population.
Results. The observed disparity in TB rates and burden among South Asians was not explained by social or clinical characteristics. A large amount of TB strain diversity was observed among South Asians, and they were less likely than other foreign-born patients to be infected with the same TB strain as another NYC patient.
Conclusions. The majority of South Asians were likely infected with TB abroad. South Asians represent a meaningful foreign-born subpopulation for targeted detection and treatment of TB infection in NYC.
At the peak of the New York City (NYC) tuberculosis (TB) epidemic in 1992, there were 3811 cases of TB reported to the NYC Department of Health and Mental Hygiene, resulting in an incidence rate of 51 cases per 100 000 persons.1 Since 1992, the number of TB cases and rate of TB in NYC have steadily declined, falling to 711 reported cases in 2010 (8.7 cases per 100 000 persons).1 However, beginning in 1997, the number of foreign-born TB cases exceeded the number of US-born cases, and this trend has continued. In 2010, 80% of TB cases in NYC were foreign-born.1 Although TB incidence rates have fallen for both groups, declines have been much larger among the US-born, and the difference in incidence rates between the 2 groups remains persistently high. This ongoing disparity suggests a need to better understand TB in foreign-born individuals living in NYC, a city of 8 million people, of whom approximately 40% are foreign-born.2
Previous work has found substantial heterogeneity in the risk of TB among foreign-born people in NYC by country of birth.3 Declining resources for TB control necessitate the identification of meaningful groupings of foreign-born populations to prioritize and target for interventions. The NYC Bureau of Tuberculosis Control (BTBC) has undertaken several projects to better understand and identify high TB burden subpopulations in NYC.3 These efforts have identified South Asians living in NYC as a particular group of interest. In 2010, more than one third of the world’s TB patients lived in South Asia, and 4 of the World Health Organization’s top 22 high TB burden countries are in South Asia.4 In NYC, several South Asian countries were among the top 10 most common countries of birth for foreign-born TB patients from 2001 to 2010. Furthermore, although 7% of all foreign-born residents of NYC originated from South Asia in 2010, this group accounted for 13% of new foreign-born TB patients.5 In this analysis, we have described the characteristics of South Asian TB patients and compared them with other foreign-born patients living in NYC to explore the disproportionate burden of TB in the South Asian population.
METHODS
We included all foreign-born patients in NYC who were verified as having active TB disease from 2001 to 2010. US-born patients included those born in the United States or a US territory; all others with known country of birth were considered foreign-born. Previous work by the BTBC identified a very high burden of TB, including multidrug-resistant TB, in the Tibetan population of NYC, prompting a community needs assessment and tailored interventions.6 We excluded patients known to be of Tibetan origin from this analysis to avoid inflating estimates of South Asian TB incidence and potentially misrepresenting the characteristics of South Asian patients.
There is no consistent definition of South Asia in the public health literature. For the purposes of this study, we defined South Asians based on the World Bank South Asia region, which includes the following countries: Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka.7 We chose the World Bank definition because it was consistent with the definition used in a US national study of TB in South Asians and allowed us to easily identify comparison groups.8 We compared South Asians to 2 other groups: other Asians, which we defined based on the World Bank East Asia and Pacific region (Cambodia, China, East Timor, Fiji, Indonesia, Kiribati, North Korea, Laos, Malaysia, Marshall Islands, Micronesia, Mongolia, Myanmar, Palau, Papua New Guinea, Philippines, Samoa, Solomon Islands, South Korea, Thailand, Tonga, Tuvalu, Vanuatu, and Vietnam), and other foreign-born, which we defined as all other foreign-born patients.7
Descriptive Analysis
We extracted data from the BTBC electronic surveillance and case management system. We estimated incidence rates for each group and by South Asian country of birth using denominator data from the US Census Bureau American Community Surveys and Decennial Census, and detected significant trends using the Cochran–Armitage test.9,10 For demographic and clinical characteristics measured as categorical variables, we calculated unadjusted odds ratios (ORs) and 95% confidence intervals (95% CIs) using the Pearson χ2 test, excluding the missing values, to compare South Asians with other Asians and other foreign-born individuals. For quantitative data, we compared medians using the Wilcoxon rank-sum test. We defined patients with a history of TB infection as those with a self-reported or documented diagnosis of TB infection or disease 12 or more months before the current TB diagnosis.
We measured socioeconomic status by neighborhood-level poverty per NYC Department of Health and Mental Hygiene guidelines, using the US Census Bureau data on the percentage of residents within a census tract living below the federal poverty limit.11 Patients were geocoded to their address at diagnosis and assigned to a census tract. We used poverty data from the 2000 Decennial Census for patients verified as having active TB disease before 2005, and we used the American Community Survey 5-year sample poverty data for patients identified from 2005 to 2010. We divided neighborhood-level poverty into 4 categories representing the percentage of neighborhood residents living below the federal poverty limit: low (< 10%), medium (10% to < 20%), high (20% to < 30%), and very high (≥ 30%). To characterize the geographic distribution of South Asian cases, we calculated counts of cases by country of birth and United Hospital Fund neighborhood. United Hospital Fund neighborhoods consist of adjoining zip codes that approximate NYC Community Planning Districts.12
Genotyping Analysis
Since 2001, NYC has routinely genotyped the initial Mycobacterium tuberculosis complex isolate for all culture-positive TB patients. Genotyping is conducted using both IS6110-based restriction fragment length polymorphism (RFLP) and spacer oligonucleotide typing (spoligotyping) analysis.13 We defined a genotype cluster as 2 or more NYC patients who were identified during the study period and who had isolates with exactly matching genotypes based on both RFLP and spoligotype results.
Genotype clusters are routinely investigated in NYC to identify epidemiological links between patients and to better understand transmission within clusters.13 From 2001 to 2008, all clustered patients were eligible to be investigated. Beginning in 2009, cluster investigations were prioritized based on factors such as HIV infection, presence of children in the cluster, and the time between the identification of cluster cases. Cluster investigations include reviewing case management notes, and when possible, re-interviewing patients. Strong epidemiological links among clustered patients can support hypotheses of local transmission.14 Two patients were determined to have a definite epidemiological link if they named each other as contacts, had a shared contact, or spent time in the same location during the infectious period of at least 1 of the patients. If patients lived or spent time in areas within approximately 0.5 miles of each other, we characterized this as a possible link. We reviewed genotyping and cluster investigation data for South Asian patients to assess the extent of clustering and the possibility of local transmission in this population.
RESULTS
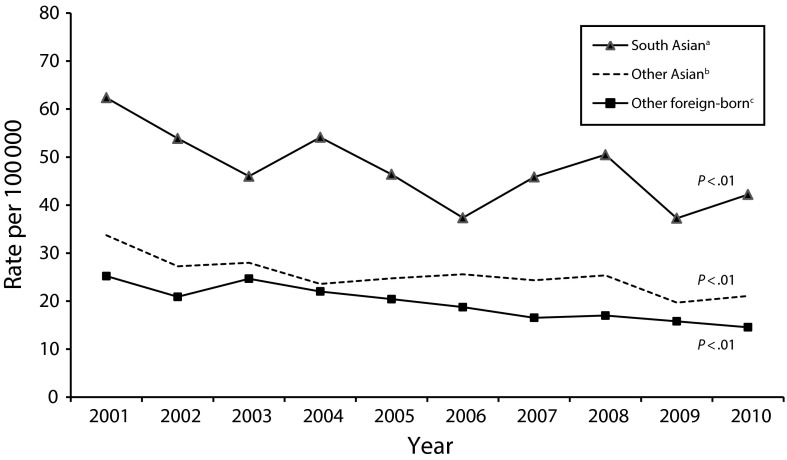
Between 2001 and 2010, there were 9657 verified cases of TB in NYC. Of these, 795 patients (8%) were South Asian, 1470 (17%) were other Asian, and 4360 (45%) were other foreign-born (Table 1). Among South Asian TB patients, 90% of patients (n = 707) originated from Bangladesh, India, or Pakistan. Although TB incidence rates for all 3 groups declined significantly over the study period (P < .01), the rates for South Asians were higher than the rates for other Asians and nearly double the rates for the other foreign-born population in every year (Figure 1).
TABLE 1—
Characteristics of Tuberculosis Patients Born in South Asia Compared With Other Asian and Other Foreign-Born Patients: New York City, 2001–2010
| Characteristic | South Asiana No. (%) or Median (Range) | Other Asianb No. (%) or Median (Range) | Unadjusted OR (95% CI)c | Other Foreign-Bornd No. (%) or Median (Range) | Unadjusted OR (95% CI)c |
| Total | 795 | 1470 | 4360 | ||
| Demographics | |||||
| Country of birth | |||||
| India | 317 (40) | ||||
| Bangladesh | 211 (27) | ||||
| Pakistan | 179 (23) | ||||
| Nepal | 65 (8) | ||||
| Sri Lanka | 11 (1) | ||||
| Afghanistan | 10 (1) | ||||
| Bhutan | 2 (0) | ||||
| Sex: female | 293 (37) | 567 (39) | 1.1 (0.9, 1.3) | 1656 (38) | 1.0 (0.9, 1.2) |
| Age group, y | |||||
| 0–5 | 5 (1) | 0 (0) | . . . | 32 (1) | 0.9 (0.3, 2.2) |
| 6–18 | 34 (4) | 26 (2) | 2.5 (1.5, 4.2) | 179 (4) | 1.0 (0.7, 1.5) |
| 19–44 | 450 (57) | 613 (42) | 1.8 (1.5, 2.2) | 2486 (57) | 1.0 (0.8, 1.1) |
| 45–64 | 206 (26) | 455 (31) | 0.8 (0.6, 0.9) | 1095 (25) | 1.0 (0.9, 1.2) |
| ≥65 | 100 (13) | 376 (26) | 0.4 (0.3, 0.5) | 568 (13) | 1.0 (0.8, 1.2) |
| Median age (interquartile range) | 38 (27 to 53) | 49 (33 to 65) | < 0.01 | 39 (28 to 53) | 0.21 |
| Years in the United States before diagnosis | |||||
| < 5 | 397 (50) | 478 (33) | 2.0 (1.8, 2.5) | 1560 (36) | 1.8 (1.5, 2.1) |
| 5 to < 10 | 136 (17) | 275 (19) | 0.9 (0.7, 1.1) | 815 (19) | 0.9 (0.7, 1.1) |
| 10 to < 15 | 97 (12) | 213 (14) | 0.8 (0.6, 1.1) | 527 (12) | 1.0 (0.8, 1.3) |
| 15 to < 20 | 64 (8) | 175 (12) | 0.6 (0.5, 0.9) | 414 (10) | 0.8 (0.6, 1.1) |
| ≥ 20 | 84 (11) | 300 (20) | 0.5 (0.4, 0.6) | 919 (21) | 0.4 (0.3, 0.6) |
| Median (range) | 4 (0 to 52) | 9 (−1 to 71) | < 0.01 | 8 (−1 to 86) | < 0.01 |
| Neighborhood povertye | |||||
| Low (< 10%) | 138 (17) | 226 (16) | 1.1 (0.9, 1.4) | 610 (14) | 1.3 (1.0, 1.6) |
| Medium (10% to < 20%) | 365 (46) | 476 (33) | 1.8 (1.5, 2.1) | 1212 (28) | 2.2 (1.9, 2.5) |
| High (20% to < 30%) | 209 (26) | 438 (30) | 0.8 (0.7, 1.0) | 1259 (30) | 0.9 (0.7, 1.0) |
| Very high (≥ 30%) | 74 (9) | 305 (21) | 0.4 (0.3, 0.5) | 1168 (27) | 0.3 (0.2, 0.4) |
| Employed in the 24 mo before diagnosisf | 384 (50) | 626 (43) | 1.3 (1.1, 1.6) | 2174 (51) | 0.9 (0.8, 1.1) |
| Ever employed as a health care workerf | 53 (7) | 87 (6) | 1.2 (0.8, 1.7) | 221 (5) | 1.4 (1.0, 1.9) |
| Ever homeless | 9 (1) | 30 (2) | 0.5 (0.3, 1.2) | 160 (4) | 0.3 (0.1, 0.6) |
| Ever incarcerated | 0 (0) | 6 (0) | . . . | 58 (1) | . . . |
| Ever used alcohol | 47 (6) | 86 (6) | 1.1 (0.7, 1.5) | 603 (14) | 0.4 (0.3, 0.5) |
| Ever used tobaccog | 113 (21) | 319 (32) | 0.6 (0.4, 0.7) | 612 (22) | 1.0 (0.8, 1.2) |
| Ever used illegal drugs | 6 (1) | 18 (1) | 0.6 (0.2, 1.6) | 179 (4) | 0.2 (0.1, 0.4) |
| Clinical characteristics | |||||
| HIV positive | 13 (2) | 20 (1) | 1.2 (0.6, 2.4) | 500 (11) | 0.1 (0.1, 0.2) |
| HIV negative | 513 (65) | 803 (55) | 1.5 (1.3, 1.8) | 2840 (65) | 1.0 (0.8, 1.1) |
| HIV not offered/unknown/missing | 56 (7) | 104 (7) | 1.0 (0.7, 1.4) | 291 (7) | 1.1 (0.8, 1.4) |
| HIV refused | 213 (27) | 543 (37) | 0.6 (0.5, 0.8) | 729 (17) | 1.8 (1.5, 2.2) |
| Female | 103 (48) | 219 (40) | 0.7 (0.5, 1.0) | 351 (48) | 1.0 (0.7, 1.3) |
| Aged 0–5 y | 1 (0) | 0 (0) | . . . | 10 (1) | 0.3 (0.0, 2.7) |
| Aged 6–18 y | 15 (7) | 8 (1) | 5.1 (2.1, 12.1) | 36 (5) | 1.5 (0.8, 2.7) |
| Aged 19–44 y | 92 (43) | 147 (27) | 2.0 (1.5, 2.9) | 255 (35) | 1.4 (1.0, 1.9) |
| Aged 45–64 y | 60 (28) | 181 (33) | 0.8 (0.6, 1.1) | 209 (29) | 1.0 (0.7, 1.4) |
| Aged ≥ 65 y | 45 (21) | 207 (38) | 0.4 (0.3, 0.6) | 219 (30) | 0.6 (0.4, 0.9) |
| Clinical characteristics | |||||
| History of TB infectionh | 64 (8) | 145 (10) | 0.8 (0.6, 1.1) | 448 (10) | 0.8 (0.6, 1.0) |
| Site of disease | |||||
| Extrapulmonary only | 285 (36) | 282 (19) | 2.4 (1.9, 2.9) | 1080 (25) | 1.7 (1.4, 2.0) |
| Pulmonary only | 435 (55) | 1063 (72) | 0.5 (0.4, 0.6) | 2830 (65) | 0.7 (0.6, 0.8) |
| Both | 75 (9) | 125 (9) | 1.1 (0.8, 1.5) | 450 (10) | 0.9 (0.7, 1.2) |
| Extrapulmonary sites of diseasei | |||||
| Lymphatic | 172 (60) | 135 (48) | 1.7 (1.2, 2.3) | 476 (44) | 1.9 (1.5, 2.5) |
| Bone/joint | 36 (13) | 43 (15) | 0.8 (0.5, 1.3) | 141 (13) | 1.0 (0.7, 1.4) |
| Pleural | 29 (10) | 58 (21) | 0.4 (0.3, 0.7) | 177 (16) | 0.6 (0.4, 0.9) |
| Peritoneal | 13 (5) | 13 (5) | 1.0 (0.5, 2.2) | 58 (5) | 0.8 (0.5, 1.6) |
| Meningeal | 7 (2) | 6 (2) | 1.2 (0.4, 3.5) | 70 (6) | 0.4 (0.2, 0.8) |
| Any pulmonary involvement | 510 (64) | 1188 (81) | 0.4 (0.3, 0.5) | 3280 (75) | 0.6 (0.5, 0.7) |
| AFB positive respiratory smear | 252 (49) | 639 (54) | 0.8 (0.7, 1.0) | 1883 (57) | 0.7 (0.6, 0.9) |
| Abnormal initial chest x-ray result | 487 (95) | 1153 (97) | 0.6 (0.3, 1.0) | 3088 (94) | 1.2 (0.7, 1.9) |
| Abnormal: cavitiesj | 116 (24) | 191 (17) | 1.5 (1.2, 2.0) | 670 (22) | 1.1 (0.9, 1.4) |
| Culture positive | 612 (77) | 1200 (82) | 0.8 (0.6, 1.0) | 3328 (76) | 1.1 (0.9, 1.3) |
| Drug susceptibility testing performed | 604 (99) | 1177 (98) | 1.2 (0.5, 2.8) | 3286 (99) | 0.8 (0.4, 1.7) |
| Susceptible | 449 (74) | 905 (77) | 0.9 (0.7, 1.1) | 2535 (77) | 0.9 (0.7, 1.0) |
| Multidrug resistance (MDR)k | 12 (2) | 18 (2) | 1.3 (0.6, 2.7) | 63 (2) | 1.0 (0.6, 1.9) |
| Extensive drug resistance (XDR)l | 1 (8) | 1 (6) | 1.5 (0.1, 27.4) | 5 (8) | 1.1 (0.1, 9.9) |
| Other resistance | 143 (24) | 254 (22) | 1.1 (0.9, 1.4) | 688 (21) | 1.2 (1.0, 1.4) |
| Genotyping | |||||
| Complete genotypem | 561 (92) | 1103 (92) | 0.7 (0.4, 1.1) | 3187 (96) | 0.5 (0.3, 0.8) |
| Total number of TB strains | 381 | ||||
| Clusteredn | 111 (20) | 243 (22) | 0.9 (0.7, 1.1) | 1310 (41) | 0.4 (0.3, 0.4) |
| No. of clusters patients fall into | 49 | ||||
| Median cluster size (range) | 3 (2 to 185) | ||||
| RFLP with < 6 bands | 70 (63) | ||||
| RFLP with 1 bando | 53 (76) | ||||
| No. of patients investigated | 105 (95) | ||||
| Epidemiological links identifiedp | 24 (23) | ||||
| Definite linksq | 15 (63) | ||||
| Nonhousehold linkq | 3 (20) | ||||
Note. AFB = acid-fast bacilli; CI = confidence interval; OR = odds ratio; RFLP = restriction fragment length polymorphism; TB = tuberculosis.
South Asia included India, Pakistan, Bangladesh, Sri Lanka, Nepal, Bhutan, the Maldives, and Afghanistan (World Bank Definition). Patients identified as Tibetan were excluded.
Other Asian countries included Cambodia, China, East Timor, Fiji, Indonesia, Kiribati, North Korea, Laos, Malaysia, Marshall Islands, Micronesia, Mongolia, Myanmar, Palau, Papua New Guinea, Philippines, Samoa, Solomon Islands, South Korea, Thailand, Tonga, Tuvalu, Vanuatu, and Vietnam.
Unadjusted ORs and 95% CIs were calculated excluding missing values and comparing South Asians with other Asian/other foreign-born.
Other foreign-born was defined as all non–US-born individuals, excluding those classified as South Asian or other Asian.
Neighborhood poverty was measured using US Census Data on the percentage of residents in a census tract living below 100% of the federal poverty limit. For patients identified before 2005, poverty data were taken from the 2000 Decennial Census. American Community Survey 5-year sample poverty data were used for patients identified from 2005 to 2010. One hundred twenty patients who were homeless at reporting and 25 patients with addresses that could not be geocoded were excluded.
Individuals younger than 16 years (n = 125) were excluded.
Tobacco use data were available for 2004–2010 only.
Patients with a self-reported or documented history of a diagnosis of TB infection or disease ≥ 12 months before the current TB diagnosis were defined as having a history of TB infection.
Percentage is among patients with extrapulmonary disease only. Categories are not exclusive because patients may have multiple extrapulmonary sites of disease.
Percentage is among those with an abnormal initial chest x-ray result.
Multidrug resistance was defined as resistance to at least isoniazid and rifampin.
Extensive drug resistance was defined as resistance to at least isoniazid and rifampin plus a fluoroquinolone and a second-line injectable TB medication. Percentage is among those with multidrug resistance.
Complete genotype was defined as a complete RFLP and spoligotype.
Patients with exactly matching RFLP and spoligotype results were considered clustered.
Percentage is among those with < 6 bands.
Percentage is among no. of patients investigated.
Percentage is among epidemiological links identified.
FIGURE 1—
Comparison of tuberculosis incidence rates by region of birth: New York City, 2001–2010.
Note. Rates are based on the 2000 Decennial United States Census and American Community Survey 1-year population estimates for New York City by country of birth. Population data were unavailable for all years for Bhutan. No patients born in the Maldives were reported in New York City during the study period. P values were calculated using the Cochran-Armitage test for trend.
aSouth Asia includes India, Pakistan, Bangladesh, Sri Lanka, Nepal, Bhutan, the Maldives, and Afghanistan (World Bank Definition). Patients identified as Tibetan were excluded.
bOther Asian countries include Cambodia, China, East Timor, Fiji, Indonesia, Kiribati, North Korea, Laos, Malaysia, Marshall Islands, Micronesia, Mongolia, Myanmar, Palau, Papua New Guinea, Philippines, Samoa, Solomon Islands, South Korea, Thailand, Tonga, Tuvalu, Vanuatu, and Vietnam.
cOther foreign-born are defined as all non–US-born individuals, excluding those classified as South Asian or other Asian.
The age distribution of South Asian TB patients differed from that of other Asian patients; 57% of South Asian patients were aged 19 to 44 years compared with 42% of other Asian patients (OR = 1.8; 95% CI = 1.5, 2.2), whereas only 13% of South Asian patients were aged 65 years or older compared with 26% of other Asian patients (OR = 0.4; 95% CI = 0.3, 0.5; Table 1). No significant differences in age distribution were observed when South Asian patients were compared with other foreign-born patients. The median age at diagnosis for South Asians (38 years; interquartile range = 27–53 years) was 11 years less than the median age of other Asian TB patients (P < .01).
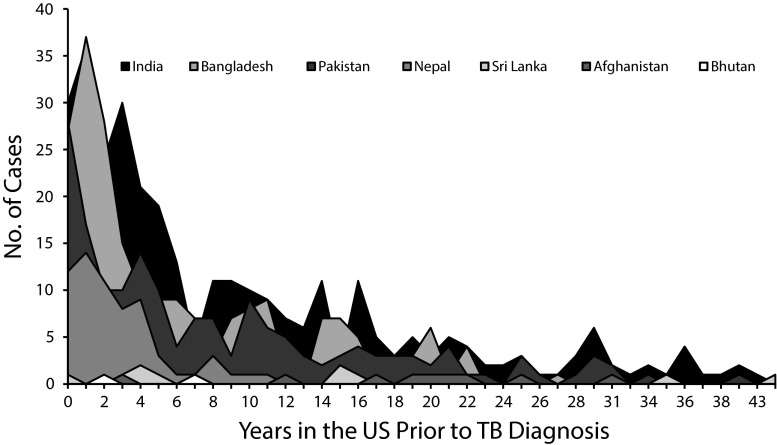
Half of the South Asian patients and approximately two thirds of other Asian and other foreign-born patients were diagnosed more than 5 years after US entry. Among South Asian patients, the time from arrival in the United States to TB diagnosis differed by country of birth and ranged from 0 to 43 years (Figure 2). TB patients born in Nepal (n = 65) had a median time to diagnosis of 2 years, compared with a median time of 20 years for patients from Afghanistan (n = 10).
FIGURE 2—
Years in the United States before tuberculosis diagnosis among patients born in South Asia: New York City, 2001–2010.
Note. TB = tuberculosis. South Asia includes India, Pakistan, Bangladesh, Sri Lanka, Nepal, Bhutan, the Maldives, and Afghanistan (World Bank Definition). Patients identified as Tibetan were excluded. No patients born in the Maldives were reported in New York City during the study period.
South Asian patients were less likely to report ever being homeless or incarcerated, ever having used alcohol, and ever having used illegal drugs compared with other foreign-born patients (Table 1). Fifty percent of South Asian patients were employed in the 24 months before diagnosis compared with 43% of other Asian patients (OR = 1.3; 95% CI = 1.1, 1.6). No differences in being employed as a health care worker were observed among the 3 groups. Few differences were observed in the prevalence of TB risk factors by country of birth among South Asian patients; 1 exception was the proportion of patients who reported ever using tobacco, which varied from 16% for patients born in India (n = 317) to 44% of patients from Sri Lanka (n = 11; data available as a supplement to the online version of this article at http://www.ajph.org).
Forty-six percent of South Asian patients lived in a medium-poverty neighborhood at diagnosis. Nine percent of South Asians lived in a very high-poverty neighborhood compared with 21% of other Asians (OR = 0.4; 95% CI = 0.3, 0.5) and 27% of other foreign-born TB patients (OR = 0.3; 95% CI = 0.2, 0.4; Table 1). The majority of all South Asian patients (59%) lived in the West Queens United Hospital Fund neighborhood at diagnosis, and this geographic concentration included patients from several different countries of birth (data not shown).
Clinical Characteristics
Two percent of South Asian TB patients were known to be HIV-infected (Table 1). South Asian patients were less likely to be HIV-infected compared with other foreign-born patients (OR = 0.1; 95% CI = 0.1, 0.2), but we found no significant differences compared with other Asian patients. Twenty-seven percent of South Asian patients and 37% of other Asian patients refused HIV testing compared with 17% of other foreign-born patients. The majority of all individuals refusing HIV testing were male. South Asian patients who refused HIV testing were predominantly aged 19 to 44 years and were less likely to be aged 65 years or older compared with other Asian and other foreign-born patients who refused testing (Table 1). This pattern was maintained by individual country of birth among South Asian patients (data available as a supplement to the online version of this article at http://www.ajph.org).
More than one third of South Asian patients had only an extrapulmonary site of TB disease, and this proportion was significantly higher compared with both other Asian (19%; OR = 2.4; 95% CI = 1.9, 2.9) and other foreign-born patients (25%; OR = 1.7; 95% CI = 1.4, 2.0; Table 1). Compared with South Asians with no extrapulmonary involvement, South Asian patients with extrapulmonary disease were less likely to be female (OR = 0.5; 95% CI = 0.4, 0.7), aged 65 years or older (OR = 0.6; 95% CI = 0.4, 0.9), or diagnosed within 5 years of arrival to the United States (OR = 0.6; 95% CI = 0.5, 0.8; data not shown). We observed no significant association between extrapulmonary TB and HIV infection among South Asians (OR = 0.5; 95% CI = 0.1, 1.9; data not shown). South Asian patients were more likely to have a lymphatic site of disease and less likely to have pleural TB compared with other Asians and other foreign-born patients (Table 1).
Genotyping and Clustering
South Asian patients were infected with 381 distinct TB strains (Table 1). Of 561 South Asian patients with a RFLP and spoligotype result, 111 (20%) were clustered to another NYC patient within the study period. This was significantly lower than the 41% of other foreign-born patients who were clustered (OR = 0.4; 95% CI = 0.3, 0.4), but was comparable to other Asian patients (22%; OR = 0.9; 95% CI = 0.7, 1.1). Clustered South Asian patients belonged to 49 genotype clusters. These clusters ranged in size from 2 to 185 patients, with a median value of 3 patients in each cluster. Twenty-three of the 49 clusters contained more than 1 South Asian patient, and of these, 14 clusters contained only South Asian patients. Sixty-three percent of clustered South Asian patients were infected with a strain that had an RFLP pattern with fewer than 6 bands, and 76% of these had only 1 band. Ninety-five percent (n = 105) of South Asians who were clustered to another NYC patient were investigated. Epidemiological links were found for 24 patients, of whom 15 (63%) were definite links and 14 (58%) involved another South Asian patient. The majority of definite links involved household contacts, but 3 definite links were found in non-household settings, including 2 in work sites and 1 in a homeless shelter.
Clustered South Asian patients were less likely to be aged 65 years or older or born in Bangladesh or Nepal compared with South Asians who did not cluster (Table 2). We did not observe differences in years in the United States before diagnosis, borough at diagnosis, or clinical characteristics between clustered and not clustered South Asian patients.
TABLE 2—
Comparison of Tuberculosis Patients Born in South Asia by Genotype Clustering: New York City, 2001–2010
| Characteristic, n (%) | Clustered | Not Clustered | Unadjusted OR (95% CI)a |
| Total | 111 (20) | 450 (80) | |
| Age group, y | |||
| 0–5 | 0 (0) | 0 (0) | — |
| 6–18 | 2 (2) | 13 (3) | 0.6 (0.1, 2.8) |
| 19–44 | 63 (57) | 249 (55) | 1.1 (0.7, 1.6) |
| 45–64 | 38 (34) | 112 (25) | 1.6 (1.0, 2.5) |
| ≥65 | 8 (7) | 76 (17) | 0.4 (0.2, 0.8) |
| Years in the United States before diagnosis | |||
| <5 | 56 (50) | 215 (48) | 1.1 (0.7, 1.7) |
| 5 to < 10 | 21 (19) | 76 (17) | 1.1 (0.7, 1.9) |
| 10 to < 15 | 8 (7) | 63 (14) | 0.5 (0.2, 1.0) |
| 15 to < 20 | 12 (11) | 34 (8) | 1.5 (0.7, 2.9) |
| ≥20 | 13 (12) | 53 (12) | 1.0 (0.5, 1.9) |
| Country of birth | |||
| India | 54 (49) | 185 (41) | 1.4 (0.9, 2.1) |
| Bangladesh | 17 (15) | 111 (25) | 0.6 (0.3, 1.0) |
| Pakistan | 18 (16) | 113 (25) | 0.6 (0.3, 1.0) |
| Nepal | 18 (16) | 31 (7) | 2.6 (1.4, 4.9) |
| Sri Lanka | 3 (3) | 3 (1) | 4.1 (0.8, 20.8) |
| Afghanistan | 1 (1) | 6 (1) | 0.7 (0.1, 5.6) |
| Bhutan | 0 (0) | 1 (0) | — |
| Borough at diagnosis | |||
| Bronx | 6 (5) | 30 (7) | 0.8 (0.3, 2.0) |
| Brooklyn | 21 (19) | 108 (24) | 0.7 (0.4, 1.2) |
| Manhattan | 13 (12) | 37 (8) | 1.5 (0.8, 2.9) |
| Queens | 68 (61) | 265 (59) | 1.1 (0.7, 1.7) |
| Staten Island | 3 (3) | 10 (2) | 1.2 (0.3, 4.5) |
| Site of disease | |||
| Extrapulmonary only | 25 (23) | 141 (31) | 0.6 (0.4, 1.0) |
| Pulmonary only | 72 (65) | 261 (58) | 1.3 (0.9, 2.1) |
| Both | 14 (13) | 48 (11) | 1.2 (0.6, 2.3) |
| AFB positive respiratory smearb | 47 (55) | 179 (58) | 0.9 (0.5, 1.4) |
Note. AFB = acid-fast bacilli; CI = confidence interval; OR = odds ratio. South Asia includes India, Pakistan, Bangladesh, Sri Lanka, Nepal, Bhutan, the Maldives, and Afghanistan (World Bank Definition). Patients identified as Tibetan were excluded. Patients with a positive culture, complete genotyping results, and exactly matching restriction fragment length polymorphism and spoligotype results were considered clustered.
Unadjusted OR and 95% CIs were calculated excluding missing values.
Percentage is among patients with any pulmonary involvement.
DISCUSSION
Although TB incidence rates among South Asians have declined since 2001, rates in this group remain elevated and are higher than rates seen for both other Asians and other foreign-born individuals living in NYC. The increased burden of TB in the South Asian population was not explained by social or clinical characteristics, such as age, poverty, and HIV infection, nor traditional TB risk factors like homelessness or drug use. We observed differences in social, clinical, and genotypic characteristics when South Asians were compared with other Asian and other foreign-born patients, whereas these characteristics appeared consistent among South Asians when compared by individual country of birth. This suggests that South Asians represent a meaningful foreign-born subpopulation for further study and intervention.
We chose to exclude Tibetans from this analysis because they were previously identified as a unique, high-burden population in NYC.6 Sensitivity analyses including Tibetans increased estimated TB incidence for South Asians from 2001 to 2010 by 18%, from 48 to 56 cases per 100 000 persons. These results support the exclusion criteria and further highlight the importance of understanding differences in TB burden and risk factors within the foreign-born population.
We found that South Asian patients were less likely than other foreign-born patients to cluster with another NYC patient, and we observed a large amount of strain diversity among South Asians. As such, the majority of TB infections in this population likely occurred abroad, and the disproportionate burden of TB among South Asian-born individuals living in NYC might be explained by the high prevalence of TB in their countries of origin. Systematic collection of information regarding the frequency of travel to patients’ home countries might improve understanding of the elevated TB burden in this population.
We found that half of all South Asian and two thirds of other Asian and foreign-born patients were diagnosed more than 5 years after arriving in the United States. Although immigration patterns influence the timing of diagnosis, these findings suggest that current recommendations, which only consider individuals from high prevalence countries who arrived within the last 5 years to be at increased risk for progression to active TB disease, might result in missed opportunities for prevention.15–17
The total number of foreign-born individuals living in NYC increased over the study period for all 3 comparison groups, with the largest growth (30%) observed for other Asians.9 At the same time, the number of TB cases for all 3 groups fell, with the largest declines occurring among South Asians. As such, the observed elevated annual TB rates for South Asians were not simply an artifact of the denominators used nor could they be attributed to a disproportionate increase in emigration from South Asia. The decreasing overall burden of TB in NYC might suggest that TB control efforts are keeping pace with increasing immigration. However, as observed, there can be long lags between individuals’ entry into the United States and the onset of active TB disease. As the foreign-born population continues to grow in both NYC and the United States, a greater focus on the detection and treatment of TB infection might be needed to maintain current levels of TB control.18,19
The results of our genotyping analysis suggest that we might have overestimated clustering in this population because more than 60% of clustered South Asian patients were infected with a TB strain that had a RFLP pattern with fewer than 6 bands, which is indicative of low discriminatory power.20 Clustering based on spoligotype and RFLP alone might not be a good proxy for transmission in this population. Genotyping using 24-locus mycobacterial interspersed repetitive unit variable number tandem repeat analysis has been available for all NYC cases through the Centers for Disease Control and Prevention since 2009; these results might help to better characterize TB strains for South Asians. Despite the low amount of clustering, epidemiological links were identified for 23% of clustered South Asian patients who were investigated, and some of these links involved contacts outside of the household. This might indicate some unexpected local transmission, which warrants further study.
We observed a very high prevalence of extrapulmonary TB disease among South Asians living in NYC. Several previous studies also found an elevated prevalence of extrapulmonary disease in this population, although this relationship remains poorly understood.8,21,22 In a national study, South Asians were more likely to have extrapulmonary TB disease than other foreign-born and US-born TB patients. This increase was not explained by common risk factors for disseminated disease, including female sex, young or old age, immunodeficiency, infection with Mycobacterium bovis, or socioeconomic factors.8,23,24 We similarly did not see an association between several of these risk factors and extrapulmonary disease among South Asians. Previous work found a relationship between ethnicity and site of disease, suggesting that other, nonclinical factors associated with country of birth might contribute to the risk of extrapulmonary disease; however, the biological mechanism by which this might occur is unknown.25
Our analysis of the geographic distribution of South Asian TB patients revealed a concentration in the West Queens United Hospital Fund neighborhood, an area that has one of the highest rates of TB in NYC.1 South Asian patients in this neighborhood originated from several different countries. A study modeling TB transmission in Rio de Janeiro, Brazil, demonstrated the potential impact of “TB hotspots” on overall disease transmission.26 Because we observed some genotype clustering involving South Asian TB patients, an analysis of the West Queens neighborhood might further illuminate transmission dynamics and the extent to which targeted interventions might help to reduce the incidence of TB in NYC as a whole.
A large percentage of South Asian TB patients in our study refused testing for HIV; similar results were observed in a national study.8 The majority of those who refused HIV testing were men and aged 19 to 44 years. Previous work showed that sex strongly influences HIV risk perceptions, and thus, the tendency to seek testing.27 Stigma and fear surrounding HIV infection also strongly influenced acceptance of testing, and the strength of these factors varied by ethnicity.28 Given the high proportion of South Asian TB patients refusing testing, we might have underestimated the prevalence of HIV infection and its contribution to the increased burden of TB in this population. Although HIV prevalence in the region is generally low, there are 4 million people living with HIV in South Asia and HIV incidence increased by more than 25% in Bangladesh and Sri Lanka from 2001 to 2011.29 As such, a better understanding of the reasons for refusing HIV testing among South Asians is needed.
Study Limitations
We used data that were originally collected for the purposes of TB case management and not for research; as such, we were limited by changes over time in some of the questions asked of TB patients and by missing or incomplete information for some demographic characteristics. Under NYC Executive Order 41, city agencies and employees are not permitted to question individuals about their immigration status. As a result, we could not explore the immigration status of TB patients or whether patients were eligible for TB screening before entry into the United States. Because of changes in protocol, not every clustered patient was investigated, so the reported frequency of epidemiological links involving South Asian patients might be slightly underestimated.
Conclusions
The study benefited from a very large and detailed data set that covered many years of observation and included demographic, social, and genotyping characteristics of TB patients. The large sample size also allowed us to disaggregate by country of birth and consider several comparison groups without compromising our ability to detect statistical significance. In contrast to the national study of TB among South Asians, our study also included data on genotype clustering and epidemiological links, allowing us to better evaluate the role of reactivation versus recent transmission in this population.
The results of this study emphasize the heterogeneity that exists within the foreign-born population of NYC while also suggesting that South Asians might represent a meaningful subgroup. Although the majority of TB infections in the South Asian population of NYC likely occurred abroad, reactivation and further transmission can only be avoided through the identification and treatment of TB infection. Because of the high TB disease burden among South Asians living in NYC, targeted testing and treatment of TB infection in this population might be warranted.
Acknowledgments
N. Stennis was supported by an appointment to the Applied Epidemiology Fellowship Program administered by the Council of State and Territorial Epidemiologists and funded by the Centers for Disease Control and Prevention Cooperative Agreement Number 5U38HM000414.
We thank Yelena Shuster for her assistance with compiling the data, Jeanne Sullivan Meissner for her help with the analysis of genotyping and cluster investigation data, and the New York City Bureau of Tuberculosis Control field staff for their contributions to data collection. We also thank our lab partners: New York City Public Health Laboratory, Public Health Research Institute, New York State Department of Health Wadsworth Center, and the Centers for Disease Control and Prevention.
Human Participant Protection
This study was approved by the New York City Department of Health and Mental Hygiene institutional review board.
References
- 1.Bureau of Tuberculosis Control. Three-year Summary: 2009, 2010, 2011. New York, NY: New York City Department of Health and Mental Hygiene; 2012. [Google Scholar]
- 2.United States Census Bureau. B05002: Place of Birth by Citizenship Status. American Community Survey 1-Year Estimates. Suitland, MD: United States Census Bureau; 2012. [Google Scholar]
- 3.Ahuja S. Differential incidence of tuberculosis and patient characteristics by country of birth in New York City, 2001–2008. American Thoracic Society International Conference Abstract Issue. Am J Respir Crit Care Med. 2012;185 A3263. [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Control: WHO Report 2011. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 5.United States Census Bureau. B05006: Place of Birth for the Foreign-Born Population in the United States. American Community Survey 1-Year Estimates. Suitland, MD: United States Census Bureau; 2010. [Google Scholar]
- 6.Lee Y-A, Munsiff SS, Li J, Driver C, Mathema B, Kreiswirth BN. Rising number of tuberculosis cases among Tibetans in New York City. J Immigr Health. 2001;3(4):173–180. doi: 10.1023/A:1012223510638. [DOI] [PubMed] [Google Scholar]
- 7.World Bank. Projects & Operations, by Country/Area. Available at: http://www.worldbank.org/projects/country?lang=en. Accessed June 26, 2013.
- 8.Asghar RJ, Pratt RH, Kammerer JS, Navin TT. Tuberculosis in South Asians living in the United States, 1993-2004. Arch Intern Med. 2008;168(9):936–942. doi: 10.1001/archinte.168.9.936. [DOI] [PubMed] [Google Scholar]
- 9.United States Census Bureau. B05006: Place of Birth for the Foreign-Born Population in the United States. American Community Survey 1-Year Estimates. Suitland, MD: United States Census Bureau; 2001-2010. [Google Scholar]
- 10.United States Census Bureau. PCT019: Place of Birth for the Foreign-Born Population in the United States. Suitland, MD: United States Census Bureau; 2000. [Google Scholar]
- 11.Toprani A, Hadler J. Epi Research Reports. New York, NY: New York City Department of Health and Mental Hygiene; 2013. Selecting and applying a standard area-based socioeconomic status measure for public health data: analysis for New York City; pp. 1–12. [Google Scholar]
- 12.Buchholz N, Resnick S, Konty K. The New York City Community Health Survey Atlas, 2010. New York, NY: New York City Department of Health and Mental Hygiene; 2012. [Google Scholar]
- 13.Clark CM, Driver CR, Munsiff SS et al. Universal genotyping in tuberculosis control program, New York City, 2001–2003. Emerg Infect Dis. 2006;12(5):719–724. doi: 10.3201/eid1205.050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perri BR, Proops D, Moonan PK et al. Mycobacterium tuberculosis cluster with developing drug resistance, New York, New York, USA, 2003–2009. Emerg Infect Dis. 2011;17(3):372–378. doi: 10.3201/eid1703.101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.New York City Department of City Planning. The Newest New Yorkers: Characteristics of the City’s Foreign-born Population. New York, NY: New York City Department of City Planning; 2013. [Google Scholar]
- 16.American Thoracic Society, Centers for Disease Control. Targeted tuberculin testing and treatment of latent tuberculosis infection. MMWR Recomm Rep. 2000;49(RR06):1–54. [PubMed] [Google Scholar]
- 17.Slopen ME, Laraque F, Piatek AS, Ahuja S. Missed opportunities for tuberculosis prevention in New York City, 2003. J Public Health Manag Pract. 2011;17(5):421–426. doi: 10.1097/PHH.0b013e31820759b8. [DOI] [PubMed] [Google Scholar]
- 18.New York City Department of City Planning. Current Estimates of New York City’s Population for July 2012. Available at: http://www.nyc.gov/html/dcp/html/census/popcur.shtml. Accessed September 17, 2013.
- 19.United States Census Bureau. America’s Foreign Born in the Last 50 Years. Available at: http://www.census.gov/how/infographics/foreign_born.html. Accessed September 17, 2013.
- 20.Cowan LS, Crawford JT. Genotype analysis of Mycobacterium tuberculosis isolates from a sentinel surveillance population. Emerg Infect Dis. 2002;8(11):1294–1302. doi: 10.3201/eid0811.020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finch PJ, Millard FJC, Maxwell JD. Risk of tuberculosis in immigrant Asians: culturally acquired immunodeficiency? Thorax. 1991;46(1):1–5. doi: 10.1136/thx.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nisar M, Williams CS, Davies PD. Experience of tuberculosis in immigrants from South East Asia—implications for the imminent lease back of Hong Kong. Respir Med. 1991;85(3):219–222. doi: 10.1016/s0954-6111(06)80083-1. [DOI] [PubMed] [Google Scholar]
- 23.García-Rodríguez JF, Alvarez-Diaz H, Lorenzo-Garcia MV, Marino-Callejo A, Fernandez-Rial A, Sesma-Sanchez P. Extrapulmonary tuberculosis: epidemiology and risk factors. Enferm Infecc Microbiol Clin. 2011;29(7):502–509. doi: 10.1016/j.eimc.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Cailhol J, Decludt B, Che D. Sociodemographic factors that contribute to the development of extrapulmonary tuberculosis were identified. J Clin Epidemiol. 2005;58(10):1066–1071. doi: 10.1016/j.jclinepi.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 25.Pareek M, Evans J, Innes J et al. Ethnicity and mycobacterial lineage as determinants of tuberculosis disease phenotype. Thorax. 2013;68(3):221–229. doi: 10.1136/thoraxjnl-2012-201824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dowdy DW, Golub JE, Chaisson RE, Saraceni V. Heterogeneity in tuberculosis transmission and the role of geographic hotspots in propagating epidemics. Proc Natl Acad Sci U S A. 2012;109(24):9557–9562. doi: 10.1073/pnas.1203517109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obermeyer CM, Osborn M. The utilization of testing and counseling for HIV: a review of the social and behavioral evidence. Am J Public Health. 2007;97(10):1762–1774. doi: 10.2105/AJPH.2006.096263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong LP. Multi-ethnic perspective of uptake of HIV testing and HIV-related stigma: a cross-sectional population based study. AIDS Care. 2013;25(11):1356–1369. doi: 10.1080/09540121.2013.766302. [DOI] [PubMed] [Google Scholar]
- 29.Joint United Nations Programme on HIV/AIDS. UNAIDS Report on the Global AIDS Epidemic. Geneva, Switzerland: United Nations; 2012. [Google Scholar]