Abstract
OBJECTIVE
To analyze the coverage of a cervical cancer screening program in a city with a high incidence of the disease in addition to the factors associated with non-adherence to the current preventive program.
METHODS
A cross-sectional study based on household surveys was conducted. The sample was composed of women between 25 and 59 years of age of the city of Boa Vista, RR, Northern Brazil who were covered by the cervical cancer screening program. The cluster sampling method was used. The dependent variable was participation in a women’s health program, defined as undergoing at least one Pap smear in the 36 months prior to the interview; the explanatory variables were extracted from individual data. A generalized linear model was used.
RESULTS
603 women were analyzed, with an mean age of 38.2 years (SD = 10.2). Five hundred and seventeen women underwent the screening test, and the prevalence of adherence in the last three years was up to 85.7% (95%CI 82.5;88.5). A high per capita household income and recent medical consultation were associated with the lower rate of not being tested in multivariate analysis. Disease ignorance, causes, and prevention methods were correlated with chances of non-adherence to the screening system; 20.0% of the women were reported to have undergone opportunistic and non-routine screening.
CONCLUSIONS
The informed level of coverage is high, exceeding the level recommended for the control of cervical cancer. The preventive program appears to be opportunistic in nature, particularly for the most vulnerable women (with low income and little information on the disease). Studies on the diagnostic quality of cervicovaginal cytology and therapeutic schedules for positive cases are necessary for understanding the barriers to the control of cervical cancer.
Keywords: Uterine Cervical Neoplasms, prevention & control; Cervix Neoplasms Prevention; Papanicolaou Test; Health Services Coverage; Mass Screening
INTRODUCTION
Although cervical cancer (CC) is a neoplasm with great potential for prevention, it is still an important public health problem in Brazil, leading to the highest number of deaths in young women (15-44 years old). a Until 2013, the Ministry of Health’s efforts to control CC were exclusively focused on using the vaginal smear (Pap smear) for screening the sexually active female population (25-64 years). b Nearly 16,000 new cases of CC have been estimated in Brazil in 2014 (15.3/100,000). c
In Northern Brazil, CC is an even bigger problem. The National Cancer Institute estimates an incidence of 26.6/100,000 in 2014 for the city of Boa Vista, RR, Northern Brazil, which has remained stable in recent years. However, a population-based study conducted in the state in 2010 7 revealed a higher cervical cancer incidence than official estimates (46/100,000).
Several factors, such as the cultural characteristics of the native people, geographic isolation, limitations of the Pap smear test, failures in the follow-up of pre-malignant lesions, and adoption of improper conduct, may contribute toward explaining the partial success of screening programs in Northern Brazil. 8 , 12 The population coverage of the preventive strategy is a crucial factor in this process. In Brazil, aspects associated with availability and access to the health systems have been widely studied and identified as a limitation in controlling CC in several regions. 1 , 6
Regions with a high incidence of CC usually present predominantly opportunistic screening programs (contrary to systematic and organized programs) that provide limited coverage, generate multiple tests for the same individual, and tend to neglect the women who would benefit the most from the screening test. 11 An awareness of the scope of preventive programs and factors associated with poor adherence to the proposed model can help in drafting public policies that are more efficient and better aligned with the status of each region.
The objective of this study was to analyze the coverage of a cervical cancer screening program in a city with a high incidence of the disease as well as the factors associated with non-adherence to the current preventive program.
METHODS
This cross-sectional study using household surveys was conducted in Boa Vista, Roraima, a city with a population of 285,000 inhabitants located in Legal Amazonia in Northern Brazil. Approximately 65.0% of the state’s population is concentrated in Boa Vista, d and the Family Health Strategy covers 75.0% of its population. The target population of the study included women aged 25-59 years living in the city for at least three years. Although the Ministry of Health has recently extended the age range of the target population to 64, the cut-off of 59 years was used as this study is retroactive to the period when the previous limit was applicable. Considering the estimated prevalence of 80.0% coverage for CC screening based on a national survey b conducted in 2008 by the Brazilian Institute for Geography and Statistics (IBGE) and assuming normal distribution for 95% confidence interval (95%CI) and an acceptable error of 5%, a minimum sample size of 550 participants was obtained, assuming a 10.0% loss. To evaluate the risk factors, the sample size has a 90.0% power for detecting an adjusted odds ratio of > 1.5 with a 95%CI, assuming a rejection rate of 10.0%.
The random cluster sampling method was used. The neighborhoods in Boa Vista’s urban area comprise 4,902 blocks. These were numbered and selected by a random number generator software. The selected blocks were adjusted (weighted) for the population in each macro area of the municipality. On weekends between June and August 2013, the researchers visited the blocks in the order of random selection to investigate the target samples. All the women living in the selected block were approached in their homes and invited to participate. Of these, 208 women were excluded for being present but not residing in their homes, not fulfilling the age criteria, having resided in another municipality in the last three years, or refusing to participate in the study. A previously tested form was used in a 30-min personal interview at the interviewee’s residence, preferably in the absence of any co-residents.
The primary outcome assessed was participation in a women’s health program, defined as undergoing at least one Pap smear test in the 36 months prior to the interview, regardless of the outcome and location of the test. Data collected were sociodemographic data, educational level (primary education being the cut-off), awareness of human papilloma virus and CC, personal and family clinical data, health worker visit, history of medical visits, and personal reasons for not being tested.
After the survey, 10.0% of the forms from each interviewer were randomly selected for quality control. These selected women were reinterviewed on telephone regarding what were considered to be “key” questions. The answers to these questions were compared with those obtained in the first phase. No questionnaires were discarded.
Descriptive statistical analysis included frequency distribution for categorical variables and means (standard deviation) and medians (with interquartile ranges) for continuous variables with normal and abnormal distribution, respectively. Prevalence was defined as the number of women screened at least once in the last three years per 100 interviewed volunteers, and it was adjusted to the age structure of the municipality. Further, 95%CI was estimated based on binomial distribution. To compare the sampling means, Student’s t test was used for the normal distribution of variables and homogeneity of sample variances. For the other variables, the Mann-Whitney test was used. The Chi-square test was used to compare differences in the proportions of categorical variables. Odds ratio (OR) and 95%CI were calculated using bivariate analysis and adjusted odds ratio (ORa) in multivariate logistic regression analysis. The criterion for selecting explanatory variables for entry into the multivariate analysis was the critical value of p < 0.15 in the bivariate analysis. Data were tabulated using double entry and analyzed using the EpiInfo version 7.1.3 software (CDC, Atlanta, USA).
This study was approved by the Ethics Committee of Universidade Federal de Roraima (Process 111.007 – CEP/UFRR). All study participants signed the informed consent form.
RESULTS
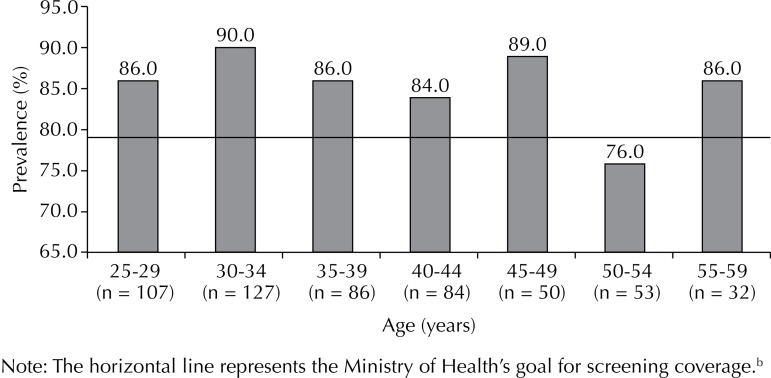
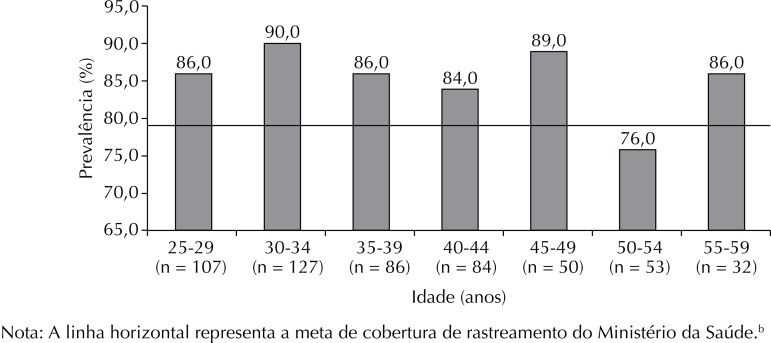
Of the 603 study participants (mean age), 54.8% were married or in a stable relationship. Almost half (46.0%) had completed high school, with only 1.6% being illiterate (1.6%) and 28.6% having complete or incomplete higher education (Table 1). Furthermore, 517 had undergone a screening test in the last three years, with an adjusted prevalence of adherence of 85.6% (95%CI 82.5;88.5), and 443 had been tested in the last year, with a prevalence of 72.8% (95%CI 68.6;77.0). The highest prevalence of adherence (90.0%) was observed in the 20-34-year age group, and the lowest in the 50-54-year age group (76.0%) (Figure 1). Periodic routine was the main reported reason for undergoing the preventive screening (n = 411; 79.5%). Other reasons provided by 20.5% of the women included pregnancies, gynecological complaints, and visits to the clinic for other reasons. Table 2 describes the characteristics of the women who did not adhere to the preventive screening.
Table 1. Socioeconomic data, knowledge about the disease and lifestyle in women aged 25-59 years. Boa Vista, RR, Northern Brazil, 2013. (N = 603).
| Personal and demographic data | n | % |
|---|---|---|
| Conjugal status | ||
| Married/Stable union | 330 | 54.8 |
| Single | 212 | 35.3 |
| Widowed/Separated | 60 | 9.9 |
| Education | ||
| Illiterate | 10 | 1.6 |
| Primary education | 144 | 23.8 |
| High school | 277 | 46.0 |
| Higher education | 136 | 22.6 |
| Postgraduate education | 36 | 6.0 |
| Have a health plan or health insurance | ||
| Yes | 85 | 14.1 |
| No | 509 | 84.4 |
| No response | 9 | 1.5 |
| Family income per capita | ||
| ≤ R$1,000.00 | 464 | 77.0 |
| > R$1,000.00 | 119 | 19.7 |
| No response | 20 | 3.3 |
| Receive government financial assistance | ||
| Yes | 260 | 43.1 |
| No | 343 | 56.9 |
| Number of inhabitants in the household | ||
| > 5 people | 114 | 18.9 |
| ≤ 5 people | 489 | 81.1 |
| Smoking | ||
| Yes | 166 | 27.5 |
| No | 437 | 72.5 |
| Regular use of alcohol | ||
| Yes | 26 | 4.3 |
| No | 577 | 95.7 |
| Family history of cervical cancer | ||
| Yes | 96 | 15.9 |
| No | 507 | 84.1 |
| Medical visit in the last year | ||
| Yes | 476 | 79.0 |
| No | 127 | 21.0 |
| Received home visit from health professionals during the study period | ||
| Yes | 155 | 25.7 |
| No | 447 | 74.3 |
| Know that cervical cancer is caused by a virus | ||
| Yes | 100 | 16.6 |
| No | 503 | 83.4 |
| Know what virus causes cervical cancer | ||
| Yes | 100 | 16.6 |
| No | 503 | 83.4 |
| Know what test detects cervical cancer | ||
| Yes | 565 | 93.7 |
| No | 38 | 6.3 |
| Know when screening begins | ||
| Yes | 329 | 54.6 |
| No | 274 | 45.4 |
| Know how frequently screening is done | ||
| Yes | 313 | 51.9 |
| No | 290 | 48.1 |
Note: The horizontal line represents the Ministry of Health’s goal for screening coverage.b
Figure. Prevalence of women non-adherent to preventive screening for cervical cancer over a successive three-year period by age group (N = 517). Boa Vista, RR, Northern Brazil, 2013.
Table 2. Personal characteristics of the women (N = 517) who underwent the screening test in a successive three-year period. Boa Vista, RR, Northern Brazil, 2013.
| Characteristic | n | % |
|---|---|---|
| When was the last preventative screening test? | ||
| Less than 1 year ago | 443 | 85.7 |
| 2 years ago | 73 | 14.3 |
| 3 years ago | 0 | 0.0 |
| What was the reason for the test? | ||
| Gynecological complaint | 57 | 11.0 |
| Prenatal care | 20 | 3.9 |
| Prevention campaigns | 12 | 2.3 |
| Undergo periodically | 411 | 79.5 |
| Opportunistic medical appointment | 13 | 2.5 |
| How often are they tested? | ||
| Once per year | 398 | 77.0 |
| Every 2 years | 44 | 8.5 |
| Every 3 years | 6 | 1.2 |
| 5-10 years | 4 | 0.8 |
| Not sure | 65 | 12.5 |
| Did a health care professional direct them to undergo testing? | ||
| Yes | 253 | 49.0 |
| No | 264 | 51.0 |
| After being tested. did they return to get the results? | ||
| Yes | 495 | 95.7 |
| No | 20 | 3.9 |
Among the 86 volunteers who had never undergone the screening test, 75.6% did not explain not getting tested, 10.5% reported shame or fear, 5.8% stated they did not think it was necessary, 2.4% reported difficulty scheduling an appointment or finding time for it, and 2.4% reported other personal reasons. The lack of time, interest, and medical recommendation were the reasons reported by three other women (1.1% each).
The prevalence of adherence to the preventive program was similar among women under 35 years of age and older than 35 years (12.0% versus 16.0%, respectively). The marital status and educational level did not change the rate of non-adherence to the screening. Table 3 shows individual variables and variables of awareness of the disease with non-adherence to the program for preventing CC.
Table 3. Correlation between individual variables, knowledge among women, and non-completion of Pap smear in a successive three-year period in univariate and multivariate analyses. Boa Vista, RR, Northern Brazil, 2013.
| Explanatory variables | Did not undergo screening in the last 3 years (%) | p | ORunivariate | ORadjusted |
|---|---|---|---|---|
| Household income per capita ≤ R$1,000.00 | 16.6 | < 0.001 | 3.1 (1.4-7.0) | 2.8 (1.2-6.7) |
| Household income per capita > R$1,000.00 | 5.9 | 1 | 1 | |
| Had a medical visit in the last year | 34 | < 0.0001 | 0.2 (0.1-0.3) | 0.4 (0.1-0.7) |
| No visit in the last year | 8.8 | 1 | 1 | |
| Know the name of the virus that causes CC | 6.0 | < 0.01 | 0.3 (0.1-0.8) | 0.5 (0.2-0.8) |
| Do not know | 16.9 | 1 | 1 | |
| Know which test detects CC | 13.2 | 0.02 | 0.3 (0.1-0.8) | 0.5 (0.2-0.9) |
| Do not know | 29.0 | 1 | 1 | |
| Know when screening begins | 9.7 | < 0.001 | 0.4 (0.2-0.7) | 0.6 (0.2-0.8) |
| Do not know | 19.7 | 1 | 1 | |
| Know that a vaccine to prevent CC exists | 5.4 | < 0.0001 | 0.2 (0.1-0.5) | 0.6 (0.2-0.9) |
| Do not know | 17.1 | 1 | 1 | |
| Age > 35 years | 16.0 | ns | 0.7 (0.4-1.1) | – |
| Age ≤ 35 years | 12.0 | 1 | ||
| Schooling till primary education | 22.5 | ns | 2 (1.2-3.4) | – |
| High school or beyond | 12.4 | 1 | ||
| Unmarried/Widowed/Separated status | 15.7 | ns | 0.8 (0.5-1.2) | – |
| Married or in a stable union | 13.0 | 1 | ||
| Receive government assistance | 15.4 | ns | 1.7 (0.7-1.8) | – |
| Do not receive government assistance | 13.4 | 1 | ||
| More than 5 family members in household | 14.0 | ns | 0.9 (0.5-1.7) | – |
| Up to 5 family members in household | 14.3 | 1 | ||
| Smoker | 15.6 | ns | 1.1 (0.7-1.9) | – |
| Non-smoker | 13.7 | 1 | ||
| Use alcohol | 15.4 | ns | 1.1 (0.3-3.2) | – |
| Do not use alcohol | 14.2 | 1 | ||
| Family history of CC | 17.7 | ns | 1.3 (0.7-2.4) | – |
| No family history of CC | 13.6 | 1 | ||
| Do not receive a home visit from health professional in the last year | 14.1 | ns | 1.0 (0.6-1.8) | – |
| Received a visit | 14.8 | 1 | ||
| Know how frequently screening is done | 13.7 | ns | 0.9 (0.5-1.4) | – |
| Do not know frequency | 14.8 | 1 |
CC: cervical cancer; NS: not significant (p > 0.15) for univariate analysis
Two individual variables were shown to influence the frequency of non-adherence to the screening in univariate analysis, and these were re-evaluated in multivariate analysis, namely (i) medical visit in the last year compared with women without medical visit (8.8% versus 34.6%; p < 0.0001) and (ii) household income per capita of > R$1,000.00 in association with household income per capita of < R$1,000.000 (5.9% versus 16.6%; p < 0.001). Of these, medical visits in the previous year had the greatest influence on the outcome, reducing the chance of non-adherence by approximately 60.0% (ORa = 0.4, 95%CI 0.1;0.7). Of the five variables regarding disease awareness and prevention, four influenced the outcome. Being aware of the causative virus reduced the prevalence of non-adherence by 10 percentage points compared to the rate among women who were unaware (6.0% versus 16.9%, respectively; ORa = 0.5; 95%CI 0.2;0.8). Information of the test that detects CC led to a lower prevalence of non-adherence (13.2% versus 29.0%, respectively; ORa = 0.5; 95%CI 0.2;0.9). The other assessed variables were not statistically significant.
DISCUSSION
The prevalence of the coverage of the preventive screening in Boa Vista was 85.6%. Two variables were associated with non-adherence to screening: per capita income exceeding R$1,000.00 and having received medical consultation in the last year.
This study assessed the coverage of a preventive program based on Pap smear testing in the city of Boa Vista, Roraima, Northern Brazil as a function of the high incidence rate of CC in this location, according to a recent population-based study. 7 In 2008, IBGE released the results of the Pesquisa Nacional por Amostra de Domicílios (PNAD – National Household Sample Survey) e referring to the access to and use of health services, among other data. In this survey, 72.7% of the female residents of Roraima aged 25-60 years claimed to have undergone the screening test, while this number was 76.9% for the Northern region and 78.4% for the country as a whole. Data from the 2012 Sistema de Vigilância de Fatores de Risco e Proteção para Doenças Crônicas por Inquérito Telefônico (VIGITEL – Chronic Disease Risk Factor Monitoring and Protection System Telephone Survey) f conducted by the Ministry of Health has revealed that 79.6% of women in Roraima stated undergoing screening in the last three years, and this prevalence of adherence exceeds the national average.
In this study, the prevalence of women tracked over the last three years was 85.6%, similar to that in the VIGITEL data. f The prevalence of women who reported undergoing the screening test in the last year was similar to that obtained from IBGE (72.7%). According to the World Health Organization (WHO), 80.0% population coverage is sufficient for markedly reducing the incidence of and mortality from CC, a as registered in the Brazilian state of Paraná, wherein mortality from CC was reduced by 50.0% after a task force expanded the screening coverage from 60.0% to 86.0% between 1997 and 2002. 3 However, a different scenario is observed in this study because Boa Vista still has a high incidence of the disease despite a high coverage rate.
Some factors may explain this paradox. For the women who were adherent to the screening strategy, more than 20.0% underwent testing for opportunistic reasons (pregnancy, medical appointment, or particularly, gynecological complaints). When women have complaints, such as itching and leukorrhea, they seek medical attention and are advised to undergo preventive testing. 12 This model is associated with women’s perception that the test is only necessary in case of illnesses or symptoms; however, it should be routinely performed in asymptomatic women. In this study, receiving medical consultation in the last year reduced the chances of non-adherence with the screening by 60.0%. One characteristic of opportunistic screening programs is the number of tests conducted on the same woman, in the same year, and at the expense of the exclusion of others who would probably benefit from testing. 11
Other factors may explain the inefficiency of the tracking program in Roraima, such as difficulties in the diagnostic confirmation, monitoring, and the treatment of intraepithelial and malignant lesions. Adequate screening program coverage only helps in controlling CC if the subsequent steps are followed. Data indicate the low capacity of the Brazilian Unified Health System (SUS) laboratory network for identifying intraepithelial lesions, 10 failures to follow-up positive cases, 9 and the lack of adequate human resources, particularly in the less-developed regions of the country. 6 , 12 The quality of these processes has not been investigated in Roraima, indicating the need for studies regarding these steps to guide policies and interventions for effectively controlling CC in this state.
The individual factors, such as age, education, and marital status did not demonstrate influence on the outcome. Low income was the main socioeconomic factor associated with non-adherence in this study. Albuquerque et al 2 have revealed that in Pernambuco, Northeastern Brazil, characteristics associated with the non-completion of testing were incomplete primary education, being single, and not receiving medical consultation in the last year. In Rio Grande do Sul, Southern Brazil, the most important factor for a low adherence to CC prevention was a low education level, 5 with a strong association between the presence of epithelial cell abnormality and education less than primary school. Borges et al 4 conducted a population-based study in 2012 in the city of Rio Branco, AC, Northern Brazil and revealed that women without a stable relationship, with low income, and schooling only till the primary section presented a higher estimate of risk of not being tested.
Variables related to awareness of CC in women strongly correlated with adherence to the preventive program in this study. Although most respondents have correctly stated that the Pap test can prevent CC, only half have correctly stated the frequency of screening and when screening begins; only 16.6% were able to correlate HPV to CC. These data suggest that governmental actions aimed at improving the public’s awareness of CC can result in a more comprehensive, systematic, and effective model of care.
The cluster sampling method used is one of the limitations of this study because it can fail to provide the appropriate representative of the population. Further, the cross-sectional design did not allow the use of temporality as a criterion for causality as risk factors, and outcome were measured at the same time and the bias of reverse causality cannot be eliminated. The issue is personal and intimate in nature, associated with the reproductive health of women, and may have influenced the results. However, the adopted sample size and correct survey procedures strengthen the reliability of the data.
It is concluded that the coverage of the Boa Vista CC screening program was 85.6%, surpassing the WHO target for the control of this disease. However, special attention should be paid to the diagnostic quality of cervical cytology slides and therapeutic schedules of the positive cases in order to clarify barriers and lead to a more effective control of CC. The data also show that ignorance of the disease and prevention mechanisms are risk factors for non-adherence to the preventive programs for a considerable portion of the population, particularly for low-income patients. Screening should be expanded to the society’s most vulnerable groups, and broad, effective, and realistic preventative strategies should be designed.
ACKNOWLEDGMENTS
To people of Coopebras/Roraima for their assistance with transportation logistics during the field survey.
Footnotes
World Health Organization. ICO Information Centre on HPV and Cervical Cancer (HPV Information Centre). Summary report on HPV and cervical cancer statistics in Brazil. Geneva; 2014 [cited 2014 Jan 1]. Available from: http://www.hpvcentre.net
Ministério da Saúde. Instituto Nacional de Câncer. Coordenação Geral de Ações Estratégicas. Divisão de Apoio à Rede de Atenção Oncológica. Diretrizes brasileiras para o rastreamento do câncer do colo de útero. Brasília (DF); 2014 [cited 2014 Jan 1]. Available from: http://www1.inca.gov.br/inca/Arquivos/PROGRAMA_UTERO_internet.PDF
Ministério da Saúde. Incidência de Câncer no Brasil: estimativa 2014. Brasília (DF); 2014 [cited 2014 Jan 1]. Available from: http://www.inca.gov.br/estimativa/2014
Instituto Brasileiro de Geografia e Estatística. Censo 2010. Brasília (DF); 2010 [cited 2014 Jan 1]. Available from: http://www.ibge.gov.br/english/estatistica/populacao/censo2010/caracteristicas_da_populacao/resultados_do_universo.pdf
Instituto Brasileiro de Geografia e Estatística. Pesquisa Nacional por Amostra de Domicílio: PNAD 2008. Um Panorama da Saúde no Brasil - Acesso e utilização dos serviços, condições de saúde e fatores de risco e proteção à saúde 2008. Brasília (DF); 2008 [cited 2014 Jan 1]. Available from: http://www.ibge.gov.br/home/estatistica/populacao/panorama_saude_brasil_2003_2008/PNAD_2008_saude.pdf
Ministério da Saúde. Secretaria de Vigilância em Saúde. Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção de Saúde. Vigitel Brasil 2012: vigilância de fatores de risco e proteção para doenças crônicas por inquérito telefônico / Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância de Doenças e Agravos não Transmissíveis e Promoção de Saúde. Brasília (DF): Ministério da Saúde, 2013 [cited 2014 Jan 1]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/vigitel_brasil_2012.pdf
Based on the master’s thesis by Navarro C, titled: “Avaliação da cobertura do exame de Papanicolaou e seus fatores determinantes em capital brasileira de elevada incidência de câncer de colo de útero”, presented to the Universidade Federal de Roraima in 2014.
REFERENCES
- 1.Albuquerque CLF, Costa MP, Nunes FM, Freitas RWJF, Azevedo PRM, Fernandes JV, et al. Knowledge, attitudes and practices regarding the Pap test among women in northeastern Brazil. 10.1590/1516-3180.2014.1321551Sao Paulo Medical J. 2014;132(1):3–9. doi: 10.1590/1516-3180.2014.1321551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albuquerque KM, Frias PG, Andrade CLT, Aquino EML, Menezes G, Szwarcwald CL. Cobertura do teste de Papanicolaou e fatores associados à não-realização: um olhar sobre o Programa de Prevenção do Câncer do Colo do Útero em Pernambuco, Brasil. 10.1590/S0102-311X2009001400012Cad Saude Publica. 2009;25(2):301–309. doi: 10.1590/s0102-311x2009001400012. [DOI] [PubMed] [Google Scholar]
- 3.Bleggi Torres LF, Werner B, Totsugui J, Collaco LM, Araujo SR, Huculak M, et al. Cervical cancer screening program of Parana: cost-effective model in a developing country. Diagn Cytopathol. 2003;29(1):49–54. doi: 10.1002/dc.10269. [DOI] [PubMed] [Google Scholar]
- 4.Borges MFSO, Dotto LMG, Koifman RJ, Cunha MA, Muniz PT. Prevalência do exame preventivo de câncer do colo do útero em Rio Branco, Acre, Brasil, e fatores associados à não-realização do exame. 10.1590/S0102-311X2012000600014Cad Saude Publica. 2012;28(6):1156–1166. doi: 10.1590/s0102-311x2012000600014. [DOI] [PubMed] [Google Scholar]
- 5.Cesar JA, Horta BL, Gomes G, Houlthausen RS, Willrich RM, Kaercher A, et al. Fatores associados à não realização de exame citopatológico de colo uterino no extremo Sul do Brasil. 10.1590/S0102-311X2003000500014Cad Saude Publica. 2003;19(5):1365–1372. doi: 10.1590/s0102-311x2003000500014. [DOI] [PubMed] [Google Scholar]
- 6.Corrêa DAD, Villela WV. O controle do câncer do colo do útero: desafios para implementação de ações programáticas no Amazonas, Brasil. 10.1590/S1519-38292008000400015Rev Bras Saude Mater Infant. 2008;8(4):491–497. [Google Scholar]
- 7.Fonseca AJ, Ferreira LP, Dalla-Benetta AC, Navarro C, Ferreira ML. Epidemiologia e impacto economico do cancer de colo de utero no Estado de Roraima: a perspectiva do SUS. 10.1590/S0100-72032010000800005Rev Bras Ginecol Obstet. 2010;32(8):386–392. doi: 10.1590/s0100-72032010000800005. [DOI] [PubMed] [Google Scholar]
- 8.Gontijo RC, Derchain SFM, Montemor EBL, Sarian LOZ, Serra MMP, Zeferino LC, et al. Citologia oncológica, captura de híbridos II e inspeção visual no rastreamento de lesões cervicais. 10.1590/S0102-311X2005000100016Cad Saude Publica. 2005;21(1):141–149. doi: 10.1590/s0102-311x2005000100016. [DOI] [PubMed] [Google Scholar]
- 9.Santos RS, Melo ECP, Santos KM. Análise espacial dos indicadores pactuados para o rastreamento do câncer do colo do útero no Brasil. 10.1590/S0104-07072012000400010Texto Contexto - Enferm. 2012;21(4):800–810. [Google Scholar]
- 10.Thuler LCS, Zardo LM, Zeferino LC. Perfil dos laboratórios de citopatologia do Sistema Único de Saúde. 10.1590/S1676-24442007000200006J Bras Patol Med Lab. 2007;43(2):103–114. [Google Scholar]
- 11.Vale DBAP, Morais SS, Pimenta AL, Zeferino LC. Avaliação do rastreamento do câncer do colo do útero na Estratégia Saúde da Família no Município de Amparo, São Paulo, Brasil. 10.1590/S0102-311X2010000200017Cad Saude Publica. 2010;26(2) doi: 10.1590/s0102-311x2010000200017. [DOI] [PubMed] [Google Scholar]
- 12.Zeferino LC. The challenge of reducing mortality due to cervical cancer. 10.1590/S0100-72032008000500001Rev Bras Ginecol Obstet. 2008;30(5):213–215. [PubMed] [Google Scholar]