Abstract
OBJECTIVE
To review studies on the readability of package leaflets of medicinal products for human use.
METHODS
We conducted a systematic literature review between 2008 and 2013 using the keywords “Readability and Package Leaflet” and “Readability and Package Insert” in the academic search engine Biblioteca do Conhecimento Online, comprising different bibliographic resources/databases. The preferred reporting items for systematic reviews and meta-analyses criteria were applied to prepare the draft of the report. Quantitative and qualitative original studies were included. Opinion or review studies not written in English, Portuguese, Italian, French, or Spanish were excluded.
RESULTS
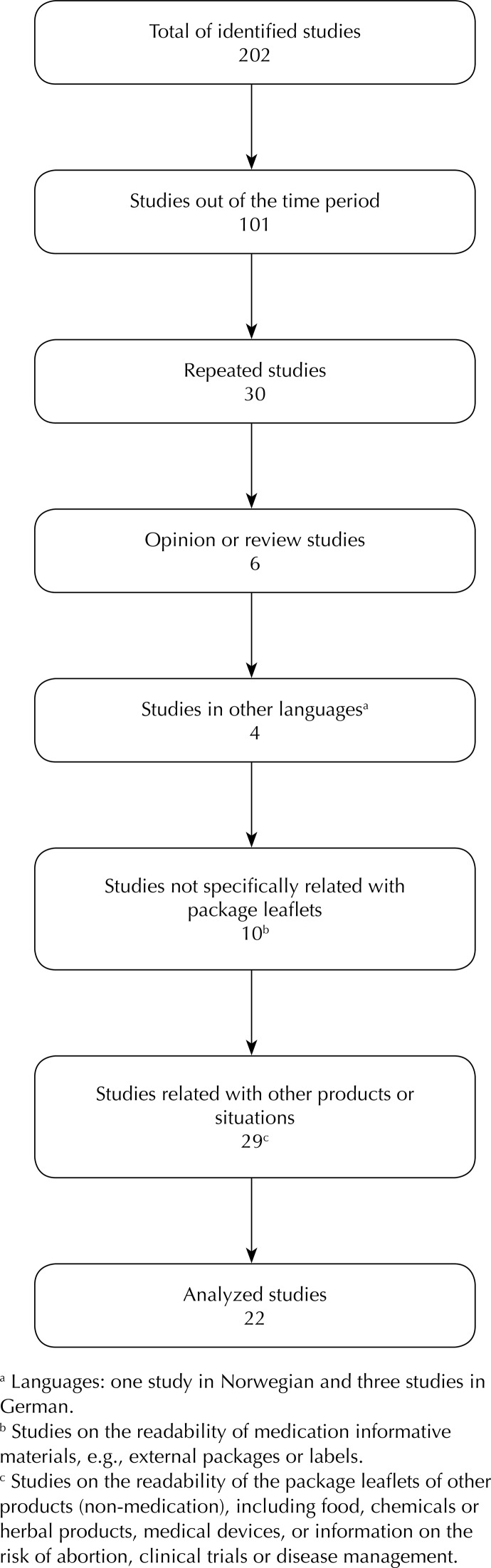
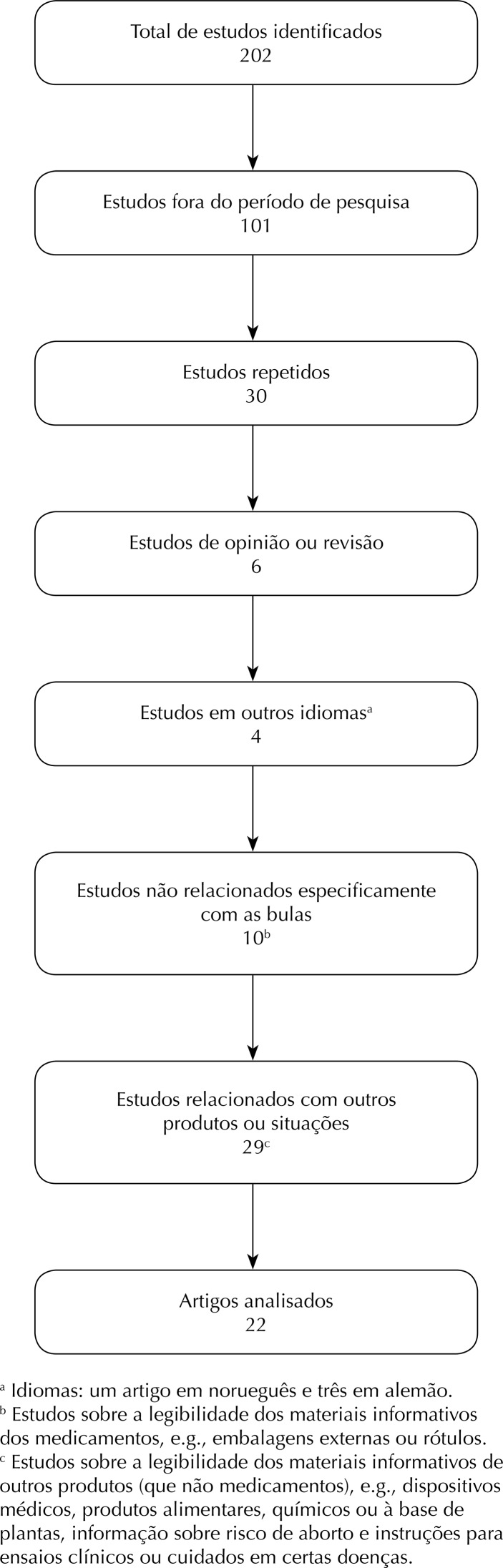
We identified 202 studies, of which 180 were excluded and 22 were enrolled [two enrolling healthcare professionals, 10 enrolling other type of participants (including patients), three focused on adverse reactions, and 7 descriptive studies]. The package leaflets presented various readability problems, such as complex and difficult to understand texts, small font size, or few illustrations. The main methods to assess the readability of the package leaflet were usability tests or legibility formulae. Limitations with these methods included reduced number of participants; lack of readability formulas specifically validated for specific languages (e.g., Portuguese); and absence of an assessment on patients literacy, health knowledge, cognitive skills, levels of satisfaction, and opinions.
CONCLUSIONS
Overall, the package leaflets presented various readability problems. In this review, some methodological limitations were identified, including the participation of a limited number of patients and healthcare professionals, the absence of prior assessments of participant literacy, humor or sense of satisfaction, or the predominance of studies not based on role-plays about the use of medicines. These limitations should be avoided in future studies and be considered when interpreting the results.
Keywords: Medicine Package Inserts, Comprehension, Consumer Health Information, Review
INTRODUCTION
The readability of the package leaflets is an essential issue for the safety and rational use of medicines after they are prescribed or dispensed in pharmacies. Patients may independently consult the package leaflets to clarify their doubts, such as information on medicine administration. 21 , 22 , 25
The inclusion of package leaflets inside all medicine packages is obligatory in the European Union. a In accordance with regulations, 29 the package leaflets must be organized in pre-defined sections b and written in a clear and comprehensible way. c
The European template on the content of the package leaflets is the Quality Review of Documents (QRD). b This template was updated several times since the first version was published (1996). 29 According to the 9th version of QRD, b the package leaflets should be organized as follows:
What X (X = name of the medicine) is and its indicated use;
What you need to know before you <take> <use> X;
How to <take> <use> X;
Possible side effects;
How to store X;
Contents of the pack and other information.
The results of legibility and usability c tests are used to prove the simplicity, clarity, and comprehensibility of the information on the package leaflets for the medicine users. 21 , 25 The guideline on the readability of the labeling and package leaflet of medicinal products for human use (European commission, 1998) was the first on this issue in Europe and is used by the European Medicine Agency. c According to the general principles of these guideline, a , c a questionnaire should be administered to at least 20 patients, preferentially from the population for which the medicinal product is intended. Healthcare professionals should not participate in legibility tests c , d so as to not bias the results. In contrast, it is advisable that geriatric and less proficient patients participate in these tests because these subjects usually present more difficulties in reading and interpreting documents. 14 The main topics of the package leaflets (indications or contraindications) are commonly selected to be examined. c The aims of these tests are to identify problems with the location and comprehension of the information on package leaflets, and if necessary, to optimize the package leaflets c and repeat the tests (retests). 21 , 25 The package leaflets are considered acceptable when the participants obtain at least 90.0% of answers. d
Although the medical authorities of each European country evaluate the legibility of package leaflets before their approval, 2 sometimes these documents are not adequately understood [e.g., dosage or adverse drug reactions (ADR)]. This is particularly bad for low-literate patients. 21 , 25
The objective of the present study was to review studies on the readability of package leaflets of medicinal products for human use.
METHODS
Systematic review. The preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria were used to organize the report on the selected studies. e The studies were conducted between January 1, 2008 and February 24, 2013 (five years and two months), with the aim of including recent investigations and pharmaceutical regulatory updates. c
The study keywords were “readability and package leaflet” or “readability and package insert” separated by the Boolean operator “and”. The selection of both designations followed from the fact that the designation “package leaflet” is more common in European countries c , d and “package insert” is used outside Europe. 20 , f
The search was performed using the academic search engine, Biblioteca do Conhecimento Online (b-on). g This tool allows access to thousands of scientific journals and concurrent searches in different databases and bibliographic databases, including BioMed Central, h BioOne, i Bioline International, j Directory of Open Access Journals, k Medical Literature Analysis and Retrieval System Online (Medline), l United States National Library of Medicine National Institutes of Health (PubMed), m Scientific Electronic Library Online (Scielo Global), n Elsevier o and SpringerLink. p Moreover, two complementary searches were performed. One used PubMed to confirm the existence of additional results, and the other used the Cochrane Collaboration Reviews q to confirm the existence of other reviews on this topic, which contributed to validate the interest and relevance of this review. All the review results are properly archived and available for future consultations. The inclusion and exclusion criteria are described in Table 1.
Table 1. Inclusion and exclusion criteria of studies on the package leaflets of medicinal products for human use.
| Criteria | |
|---|---|
| Inclusion | Exclusion |
|
| |
| Studies or abstracts | Studies or abstracts |
| Original | Out of the research period |
| Quantitative | Repeated |
| Qualitative | Review or opinion |
| Exploratory | Not directly related with the research topicaa |
| Descriptive | Other languagesb |
a Studies not specifically related with the package leaflets (e.g., studies on the readability of medicines labels) or medicines (e.g., studies on the readability of the package leaflets of medical devices or information on disease management).
b Original documents in languages other than English, Portuguese, Italian, French, or Spanish.
The repeated references were automatically identified using EndNoteWeb (a management references program). r The main findings of the selected studies were summarized and organized into a tabular format (objectives, methods, results, and conclusions). The selected studies were divided into the following two categories. The first category comprised exploratory studies (studies with the participation of health professionals or patients), and the second was descriptive studies or studies involving non-enrolling participants (studies using legibility formulas or investigating the linguistic characteristics of texts). In particular, the studies on the readability of ADR were described and analyzed because of the importance of this issue for patient safety. 29 The selected studies were classified as follows:
Exploratory studies specifically enrolling health professionals;
Exploratory studies enrolling patients (or potential users of medicines), such as studies on comprehension of ADR (readability/usability tests) by patients;
Descriptive studies (studies using non-enrolled participants, i.e., all the non-experimental studies) on the readability of package leaflets, including studies that evaluate the number of words, length of phrases, or letter type.
Overall, the selected studies were comparatively analyzed. The main findings, potential limitations, and the opportunity for future work were evaluated and registered.
RESULTS AND DISCUSSION
Twenty-two studies out of the 202 were selected and comprised 16 full papers, three brief communications, and three indexed abstracts. The number of included and excluded studies are presented in Table 2 in addition to different keywords and search tools. The flowchart is organized using the PRISMA f criteria, representing the exclusion reasons (Figure). None of the studies on the topics under review was found in the database of Cochrane Collaboration Reviews, confirming the interest in this review.
Table 2. Number of studies searched, included and excluded, pear search tools, keywords, and reasons for exclusion; period: January 1, 2008 February 24, 2013.
| Search tool | Keywords | Total | Repeated | Excluded/Reasons | Selecteda |
|---|---|---|---|---|---|
| PubMed | “Readability and Package Insert” | 17 | 1 | 14 | 2 |
| 10 - out of the period | |||||
| 1 - other languageb | |||||
| 1 - opinion studies | |||||
| 2 - other topics | |||||
| “Readability and Package Leaflet” | 67 | 6 | 58 | 3 | |
| 34 - out of the period | |||||
| 2 - other language | |||||
| 1 - opinion studies | |||||
| 5 - other topics | |||||
| 16 - other productsc | |||||
| B-on | “Readability and Package Insert” | 59 | 20 | 32 | 7 |
| 28 - out of period | |||||
| 1 - other language | |||||
| 3 - other products | |||||
| “Readability and Package Leaflet” | 59 | 3 | 46 | 10 | |
| 29 - out of period | |||||
| 4 - opinion studies | |||||
| 3 - other topics | |||||
| 10 - other productsc | |||||
| Total | 202 | 30 | 150 | 22 |
PubMed: United States National Library of Medicine National Institutes of Health; b-on: Biblioteca do Conhecimento Online
a Selected studies = Total - Repeated - Excluded.
b Original documents in languages other than English, Portuguese, Italian, French, or Spanish.
c Studies on the readability of the package leaflets of other products than medications.
Figure. Flowchart: exclusion reasons for the researched studies.
The main aspects (objectives, methods, results, and conclusions) of the selected studies are summarized in Table 3. The 22 selected studies were distributed as follows: two in Group A (exploratory studies enrolling health professionals), 12 in Group B (exploratory studies enrolling patients or potential patients who will use the medicines), and eight in Group C (descriptive studies).
Table 3. Informative summary of the selected studies on the readability of package leaflets of medicinal products and identified in search tools; period: January 1, 2008‒February 24, 2013.
| Reference | Objective(s) | Methods | Results | Conclusion |
|---|---|---|---|---|
|
A. Exploratory studies with the participation of health professionals | ||||
| Cavaco et al,5 2012 (resume) | Optimization of package leaflets | 2 groups: potential users and physicians An original and optimized package leaflet (diclofenac) was tested Questionnaires Opinion on technical terms (Likert scale) | 42 potential users 42 physicians Satisfaction on the original package leaflet: 0.0% good; 10.0% satisfactory Satisfaction on the technical terms (optimized package leaflet): 20.0% good; 65.0% satisfactory | Lexical modifications produced favorable results |
| March J et al,22 2009 | Opinion study | Interviews Flesch formula (25 package leaflets) | Participants: (40) patients, (6) physicians, (11) pharmacists and (13) from associations of patients Health professionals attributed more importance to the package leaflets in comparison to patients More difficult issues: dosage, ADR and contra-indications Flesch index: high | The real needs of health professionals and patients should be considered during the development of package leaflets The patients preferred to receive the direct opinion of health professionals |
|
B. 1. Exploratory studies with the participation of potential users of medicines: studies on patients’ comprehension of drug adverse reactions | ||||
| Knapp et al,17 2010 (brief communication) | Comprehension of ADR | ADR presented in different formats Opinion on the preferred format Imaginary scenario: opinion on the probability of ADR (if taking tamoxifen) | 134 participants The absolute frequencies (e.g., 48 persons in each 100) were considered more precise/clear than the interval of frequencies (e.g., affect more than one person in each 10) | The use of absolute frequencies to present ADR demonstrated to be more appropriate |
| Knapp P et al,18 2009 | Presentation of ADR | Classification of ADR: using verbal (e.g., rare) or numerical (e.g., 1 in 10) descriptors, or both Imaginary scenario: estimate the risk of 4 ADR and satisfaction (if taking tamoxifen) | 187 Participants Absolute frequencies were more favorable | Future studies are advisable |
|
B. 2. Exploratory studies with the participation of users or potential users of medicines: comprehension studies | ||||
| Symonds T et al,26 2010 | Participant comprehension (sildenafil package leaflet) | Two groups of participants: consultation versus hypothetical auto-administration Questionnaire Blind study | Participants: 113 healthy men and 70 with health problems (e.g., prostatic hypertrophy) The results between both groups were concordant in more than 73.9% | It may be necessary to optimize the indications |
| Shiffman S et al,25 2011 | Participant comprehension (antidepressant information) | Materials: medication guide and package leaflet Blind study 52 participants | A rare and dangerous ADR was identified by less than 20.0% of the participants | The information was not fully understood |
| Fuch et al,12 2010 | Text length (evaluation) | Crossover study: 1,105 participants (first phase), and 1,057 participants (second phase) Tested materials: 5 original package leaflets + 5 optimized package leaflets Questionnaire | The location of information was more difficult in the longer package leaflets Average of words: 2,505 (original) and 2,002 (optimized) The optimized package leaflets contained: less technical words (14 versus 86), less abbreviations (4 versus 17), and shorter phrases (7 versus 29) | The length of the package leaflets was related with participant comprehension The shorter package leaflets (1,500 words) were more adequate |
| Lee et al,19 2012 (abstract) | Legibility tests (comparison) | Two package leaflets: over the counter medicines (acetaminophen) Task: difficult words were underlined Questionnaire: the questions were based on imaginary scenarios and related with the topics of the package leaflets | 51 students Better scores (73.0% to 80.0%) on: indications, dosage, pregnancy information, contra-indications, and formulation 118 difficult words | Simplification of the package leaflets (friendlier package leaflets) |
| Maat HP et al,21 2010 | Readability (evaluation) | 3 original package leaflets + 3 optimized package leaflets (shorter phrases, simple text). Questionnaire | 154/164 potential users (original/modified package leaflets) Optimized package leaflets: higher proportion of correct answers and topics located | The use of more narrow criteria to conceive the package leaflets is advisable |
| Brosnan S et al,1 2012 | Readability (evaluation) | Patients with a prescription of clozapine A validated tool was used to evaluate patients’ literacy Optimized package leaflet: shorter phrases Questionnaire on comprehension | 40 patients Literacy: 29 (72.5%) adequate, 11 low Score of questionnaire: 72.5% (original package leaflet), 95.0% (optimized package leaflet) | It is important to consider patients’ literacy during the optimization of package leaflets |
| Cavaco A et al,6 2012 (Brief communication) | Literacy and readability | Clients of community pharmacies A validated tool was used to evaluate participant literacy Satisfaction with the readability of a diclofenac package leaflet (Likert scale) | 53 participants (40.0% higher education, 80.0% adequate literacy) The average satisfaction was scored slightly below the neutrality Less favorable issues: letter size, medical technical terms, and abbreviations | The readability issues were not related with the literacy level |
| Calamusa A et al,2 2012 | Quantifying knowledge | Questionnaire (drug store in large shopping areas) Topics: medicine use and specific terminology | 1,206 adults 42.0% participants mistook contraindications for ADR Lack of information on the long-term use of: laxatives (14.0%) or nasal decongestants (20.0%) | Advice on the use of medicines is recommended |
| Dowse R et al,8 2011 | Participant comprehension | Low-literate participants Package leaflet containing pictograms (anti-retroviral) Interview: locate and explain the information, and give opinion on the use of pictograms | 39 participants Average (comprehension): 60.0% The zones of text with pictograms were better understood All participants agreed with the use of pictograms | It is important to consider patient literacy in the development of package leaflets The use of pictograms is likely to increase the intelligibility of package leaflets |
| Franck J et al,11 2011 | Participant comprehension | 2 package leaflets (oxazepam and tetracycline) An informatics tool was used to optimize the package leaflets (brief explanation on medical terms) Legibility tests (in accordance to the guideline of European Medicine Agency) | Participants: 10/20 (original/ optimized package leaflets) Participant literacy: homogeneous Optimized package leaflets: more favorable results | The time and cost to optimize the package leaflets was reduced in consequence of using an informatics methodology |
|
C. Descriptive studies: evaluation of the linguistic characteristics | ||||
| Weiss SM et al,28 2010 | Adequacy of texts | Informative materials: approved/not approved by Food and Drug Administration Formula of Simple Measure of Gobbledygook (SMOG) | Index of SMOG: above the recommended | Simplification of the package leaflets, especially for the low educated patients |
| Fuch J et al,13 2010 | Information (characterization) | 271 package leaflets Quantification: number of words/difficult words Other topics identified: maximum daily dose, ADR, among other | Distribution of the information in the package leaflets: 29.5% maximum daily dosage; 54.6% ADR, and 24.2% frequency of ADR The more recent package leaflets were lengthier and comprised a higher proportion of difficult words | Simplification of the package leaflets, such as useful information to patients |
| Knapp P et al,3 2008 (brief communication) | Presentation of ADR | 50 Package leaflets Presentation of ADR: characterization and evaluation | 20 (40.0%) of the package leaflets gave no indication of the likelihood of the ADR 26 (42.0%) package leaflets included verbal descriptors, such as the general designation “common” 4 (8.0%) included data of frequency | In the majority of the cases ADR were not adequately presented |
| Pinero-Lopez MA et al,23 2011 (abstract) | Evaluation of text-readability | Package leaflets of biopharmaceutical medicines Formulas: SMOG and Flesch | 40 package leaflets Readability index: low (both formulas) Most difficult section: ADR | Simplification of the package leaflets |
| Roskos SE et al,24 2008 | Evaluation of text-readability | 7 package leaflets of nasal steroids Formula of Fry The size of letter and illustrations size were evaluated | On average, the package leaflets were classified as appropriate to people with seven years of schooling (instead of the five years recommended) Letter size: 9 instead of 11 (or the minimum recommended size) Only three pictures in the package leaflets | Readability problems were identified |
| Wallace et al,27 2007 | Adequacy of texts | 83 sample of tablets + package leaflets (hospital) Formula of Fry Letter size | Package leaflets: only in 19 samples The package leaflets were classified as appropriate to people with 10 years of schooling (formula values) | Ideally, samples should contain package leaflets Simplification of the package leaflets |
| Zite NB et al,30 2008 | Characteristics of texts | 8 package leaflets (contraceptive). Formula of Gobbledygook “User-Friendliness Toll” to evaluate: layout, graphical aspects and clarity of information | The package leaflets were classified as appropriate to people with 10 years of schooling (formula values) It was found dosage issues and different explanations on the ideal contraceptive effect | Simplification of the package leaflets (review of texts) |
| Cavaco A et al,4 2010 | Evaluation of text-readability | 4 package leaflets Translation: Portuguese to English Formulas of SMOG and Flesch-kincaid (English translations) | The package leaflets were classified as appropriate to people with 10 years of schooling (formula values) Correlation of Spearman between the results: high | Simplification of the package leaflets for less-educated people (adjustment/adaptation) |
ADR: Adverse drug reactions; SMOG: Formula of Simple Measure of Gobbledygook
Overall, few studies on the readability of the package leaflets were identified compared to a search in PubMed using the search term, “patient information”, which identified 6,357 search results on October 13, 2013.
Exploratory studies enrolling health professionals, patients or potential users of medicines
In the two exploratory studies (group A), 5 , 22 wherein healthcare professionals (physicians or pharmacists) participated, it was reported that the healthcare professionals were satisfied with the information in the leaflets. They considered the information in the package leaflets more important than did the actual patients (or potential patients) who required the medicines. In contrast, the patients (or potential patients) who would be using the medicines expressed their preferences for receiving personal explanations on the use of medicines during consultations. One reason for this was due to the high prevalence of technical terms in the package leaflets. Only two studies with healthcare professionals were identified in this revision, although these studies were important to validate the optimized package leaflets. 5 , 22
From the twelve exploratory studies with patients (or potential patients) receiving medicines (group B), ten were conducted to evaluate participant comprehension (usability and/or legibility tests, 1 , 2 , 6 , 8 , 11 , 12 , 19 , 21 , 25 , 26 or studies to specifically evaluate participant comprehension of the manner in which ADR were presented. 18 , 17
The main problems identified in these 10 studies were patient (or potential patient) comprehension issues as some topics were poorly understood; 2 , 11 , 19 , 25 , 26 too complex texts, indicating the necessity of optimizing and simplifying the package leaflets; 1 , 11 , 12 , 21 and package leaflets not properly adapted for the low-literate patients, indicating the need to use simpler language. 1 , 6 , 8 , 19
The majority of the reviewed readability studies used package leaflets of specific medicines, including sildenafil, clozapine, acetaminophen, and diclofenac. 1 , 6 , 19 , 26 It is likely that these package leaflets were selected for the following reasons: the straight therapeutic indices of some medicines such as clozapine, 1 over-the-counter medicines (no prescription necessary), such as diclofenac and acetaminophen, and highly utilized medicines such as acetaminophen. 6 , 19 The package leaflets of these medicines are more likely to be consulted. Therefore, it is difficult to generalize about or extend conclusions of these studies to the package leaflets of medicines with different active ingredients. The study on the automatic simplification of the technical terms was considered particularly relevant because an automatic methodology was used to simplify diverse package leaflets at the same time. In this study, an informatics tool was used, and the technical terms of the package leaflets were identified. More common and equivalent terms were then found in pre-defined lexical databases and finally, the original terms were automatically replaced by the more common terms. 11
Diverse limitations were identified in these studies 1 , 2 , 6 , 8 , 1 1 , 12 , 19 , 21 , 25 , 2 6 and were categorized as follows: high diversity of methods, limited number of participants, lack of certain assessments (such as the evaluation of participant literacy, humor, cognitive state, and satisfaction), lack of multicenter or longitudinal studies, lack of studies on specific topics such as contra-indications and precautions, study of the package leaflets from a limited number of medicines and active ingredients, 1 , 6 , 19 , 26 and lack of pictograms (useful for low-literate patients) or other illustrations. 8 , 24 A few authors reported that the study limitations were contrary to good clinical practices, increasing the difficulty of precisely analyzing the results.
Studies on comprehension of adverse drug reactions
The presentation of ADR was particularly relevant in two studies (group B) because the patient comprehension of ADR strongly depended on the way ADR were presented. 18 , 17 ADR were described in different manners in these studies. These ADR were described using qualitative descriptors (very common ADR) or quantitative descriptors (adverse reactions with a likelihood of 1.0%-10.0%). 18 Further, it was found that patients preferred numerically expressed ADR (using absolute frequencies) 18 , 17 and considered the use of fractions (≥ 1/100) to be difficult to understand when the ADR frequency 3 , 17 , 18 , 27 was presented in this manner.
In general, the section of the package leaflets on ADR was compliant with the recommendations of the QRD. 3 , 1 7 , 18 , 29 , b According to the requirements of the QRD template, ADR are presented in an ordered list of values (from the more to less frequent ADR). For example, a “common” ADR may affect up to 1 in 10 people, and “uncommon” ADR may affect up to 1 in 100 people. 29
The number of studies specifically concerned with the most appropriate way to present ADR and the number of participants enrolled in these studies were limited.
Descriptive studies
The eight descriptive studies 3 , 4 , 13 , 23 , 24 , 27 , 28 , 30 (or studies with non-enrolled participants) on the usability of the package leaflets (group C) focused on the following aspects: 3 , 4 , 13 , 23 , 24 , 27 , 28 , 30 (i) use of legibility formulas, such as Flesch-Kincaid or Fry to calculate values on the association between the linguistic characteristic of texts and the education level of patients (linguistic metrics), 4 , 23 , 24 , 27 , 28 , 30 (ii) identification of specific linguistic characteristics (e.g., number of difficult words or phrases) to obtain indirect indicators on the proper readability of texts; 3 and (iii) evaluation of graphical aspects that facilitate the understanding of information (e.g., letter size or presence of illustrations). 24 , 27 , 30
Overall, the application of the descriptive methodologies confirmed a low readability of the package leaflets and the need to simplify the texts. The factors that decrease the readability of the package leaflets were evident in some studies and included the following: too complex texts (e.g., some package leaflets were classified as appropriate for readers with 10 or more years of education, instead of the five years recommended by Food and Drug Administration); s omission of relevant technical information (e.g., maximum daily dose, 3 , 4 , 13 , 23 , 24 , 27 , 28 , 30 extensive use of technical words 13 or small letters (e.g., letters with a font size of < 11; 24 , 27 and lack of illustrations. 24 , 30
We believe that the lack of readability formulae or other alternative linguistic metrics to evaluate texts specifically written in Portuguese 7 , 9 , 10 , 15 , 16 constitutes a limitation. The legibility concepts developed in the 1920s and have been continued by writers such as Rudolf Flesch, 10 George Klare, 16 Edgar Dale, and Jeanne Chall. 7 The Gunning formula (1935) 15 was one of the first, and according to the equation of this formula (suitable for English texts), the education level is equal to 0.4*(average size of phrases in number of words + the number of words with more than two syllables per 100 words). 9 There are currently several legibility formulas for diverse languages, such as Spanish, French, German, Swedish, Russian, Hebrew, Hindi, Chinese, Vietnamese, and Korean. 9 However, it is not known if legibility formulas specifically developed for the Portuguese language exist. Similar to other languages, Portuguese presents a specific combination of linguistic characteristics; thus, the development of legibility formulae specifically developed to evaluate the readability of Portuguese texts is recommended. 4
In some studies, it was not possible to cross-check the results of different formulae (double verification) 24 , 28 , 30 due to the fact that only one legibility formula was used.
Summary of the methodological limitations
In the studies of this review, the principal limitations identified are listed:
The inclusion of a limited number of patients or health professional in the readability and/or usability studies, particularly in the non-confirmatory studies; 1 , 5 , 6 , 8 , 11 , 19 , 21 , 22 , 25 , 26
The lack of certain evaluations before the study, such as the evaluation of the participants’ cognitive state, humor, satisfaction in participating in the test or opinion on the use of medicines and their package leaflets. These factors are likely to influence the interpretation of the results (interpretation bias); 8
In majority of cases, participant literacy was also not evaluated, which probably influenced the accuracy of the study conclusions; 14
The non-use of the original packages of medicines or the absence of questions based on imaginary scenarios in several readability and/or usability tests, probably also influenced the accuracy of data collection; 21
The illustrations were scarcely used, namely pictograms, 10 despite these graphic elements favoring the readability;
The selection of packages leaflets based on the composition of the medicines, (type of active ingredients) 1 , 6 , 19 , 26 may influence their selection (selection bias) because in general, the package leaflets with the worst linguistic characteristics were not selected, such as the longer package leaflets or the those containing more sentences per paragraph, abbreviations, or acronyms;
Few studies on patient comprehension of ADR 3 , 17 , 26 and absence of studies on patient comprehension of specific topics, such as precautions, interactions, and contraindications;
The nonexistence of multicenter studies to study intra- and intercultural differences, such as dialectal differences;
The absence of longitudinal studies to investigate possible alterations over time, such as those caused by social changes, alterations on the pharmaceutical regulation or the appearance of new therapeutics;
Studies using only one legibility formula, which does not allow the comparison of different metrics. However, the results obtained through the application of legibility formulas are highly correlated according to some studies; 9
The lack of legibility formulae for Portuguese to calculate indicators on the simplicity of texts, similarly to the legibility formulas of other languages, such as English (e.g., Flesch formula).
Because of these methodological limitations, it is possible that the studied package leaflets were not accurately evaluated.
CONCLUSIONS
The studies on the readability of the package leaflets should be based on technical principles and be highly suitable with high quality scientific standards. Several points are strongly recommended for improving and standardizing the readability of package leaflets. These include minimizing or avoiding the previously discussed limitations, using larger and more varied samples of package leaflets, enrolling more participants, and development of new metrics and legibility formulas for specific languages (e.g., Portuguese).
In this review, diverse factors related with the readability of the package leaflets were highlighted (e.g., clear information, simple terms, and package leaflets with a proper design. The main methods for ensuring the intelligibility and comprehension of the package leaflets were the usability tests and the application of formulae and/or metrics to their texts.
The encountered readability/usability tests rely on the involvement of patients using the medication to test and confirm the readability of the informative materials. Ideally, these tests should also include patients with low literacy levels and health professionals to ensure the collection of reliable and efficient results.
The diverse methodological limitations identified should be avoided in future studies and considered in the assessment of results.
In general, the investigations on the readability of the package leaflets and their methods need more scientific contributions to assure the accuracy, reliability, and appropriateness of results in the social context and language of each country.
Funding Statement
Research supported by the Fundação para a Ciência e Tecnologia, Ministério da Educação e Ciência, Portugal (Process SFRH /BD/76531/2011 – Doctoral grant).
Footnotes
Research supported by the Fundação para a Ciência e Tecnologia, Ministério da Educação e Ciência, Portugal (Process SFRH /BD/76531/2011 – Doctoral grant).
European Parliament and the Council. Directive 2001/83/EC: community code relating to medicinal products for human use. Brussels; 6 Nov 2001 [cited 2013 Aug 12]. Available from: http://ec.europa.eu/health/files/eudralex/vol-1/dir_2001_83_consol_2012/dir_2001_83_cons_2012_en.pdf
European Medicine Agency. Quality review of documents human product-information annotated template. Version 9. London; 2013 [cited 2013 Oct 13]. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000134.jspp
European Medicine Agency. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. London; 2009 [cited 2013 Aug 12]. Available from: http://ec.europa.eu/health/files/eudralex/vol-2/c/2009_01_12_readability_guideline_final_en.pdf
The Heads of Medicines Agencies, Co-ordination Group for Mutual Recognition and Decentralised Procedures-Human. Position paper on user testing of package leaflet – consultation with target patient groups. 2011 [cited 2013 Oct 27]. Available from: http://www.hma.eu/fileadmin/dateien/Human_Medicines/CMD_h_/procedural_guidance/Consulation_PatientsGroups/CMDh_234_2011.pdf
Critérios Prisma - Transparent reporting of systematic reviews and meta-analysis; 2013 [cited 2013 Aug 16]. Available from: http://www.prisma-statement.org/
Australian Governament, Department of Health and Ageing, Therapeutic Good Administration. Mechanisms to maintain the currency of approved Product Information (PI) and Consumer Medicine Information (CMI): public consultation paper. Version 2013 [cited 2013 Aug 12]. Available from: https://www.google.pt/url?sa=t&rct=j&q=&esrc=s&source=web&cd=2&ved=0CDsQFjAB&url=http%3A%2F%2Fwww.tga.gov.au%2Fword%2Fconsult%2Fconsult-opr-currency-pi-cmi-130513.docx&ei=fwdtUvenA_Op7Ab7ooHoDg&usg=AFQjCNHhjdYSrmN2s0muwVNMjwVS6UEu0w&sig2=Jtp7FfAdBJYD2oaORlX4zg&bvm=bv.55123115,d.ZG4&cad=rjtg
B On: Biblioteca do conhecimento online. Lisboa: Fundação para a Computação Nacional; 2013 [cited 2013 Aug 12]. Available from: http://www.b-on.pt/
BMC: BioMed Central the open access publisher; 2014 [cited 2014 Dec 2]. Available from: http://www.biomedcentral.com
BioOne online journals; 2014 [cited 2014 Dec 2]. Available from: http://www.bioone.org/
Bioline International; 2014 [cited 2014 Dec 2]. Available from: http://www.bioline.org.br/
DOAJ: Directory of Open Access Journals; 2014 [cited 2014 Dec 2]. Available from: http://doaj.org/
MEDLINE: Medical Literature Analysis and Retrieval System Online. Bethesda (MD): US National Library of Medicine; [s.d.]. [cited 2014 Jul 28]. Available from: http://www.ncbi.nlm.nih.gov/IEB/ToolBox/SDKDOCS/MEDLINE.HTML
PubMed: the bibliographic database of the United States National Library of Medicine National Institutes of Health [Internet]. Bethesda (MD): National Library of Medicine. [1946] - [cited 2014 Jul 27]. Available from: http://www.ncbi.nlm.nih.gov/pubmed
SciELO: Scientific Electronic Library Online [Internet]. São Paulo (BR): Bireme/OPS/FAPESP/CNPq. [1998]. [cited 2014 Jul 28]. Available from: http://www.scielo.org/php/index.php?lang=en
Elsevier; 2014 [cited 2014 Dec 2]. Available from: http://www.elsevier.com/
SpringerLink; 2014 [cited 2014 Dec 2]. Available from: http://link.springer.com/
Cochrane collaboration reviews; 2014 [cited 2014 Dec 2]. Available from: http://www.cochrane.org/cochrane-reviews
EndnoteWeb. New York: Thomson Reuters; 2013 [cited 2013 Aug 12]. Available from: https://www.myendnoteweb.com/EndNoteWeb.html?SID=P2g6anKIJyF54XPliP4&returnCode=ROUTER.Success&SrcApp=CR&Init=Yes
Food and Drug Administration: Guidance for Industry – Label comprehension studies for Nonprescription Drug Products; 2014 [cited 2010 Dec 2]. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm143834.pdf
REFERENCES
- 1.Brosnan S, Barron E, Sahm L, Sahm LJ. Health literacy and the clozapine patient. 10.1177/1757913911431038Perspect Public Health. 2012;132(1):39–42. doi: 10.1177/1757913911431038. [DOI] [PubMed] [Google Scholar]
- 2.Calamusa A, Di Marzio A, Cristofani R, Arrighetti P, Santaniello V, Alfani S, et al. Factors that influence Italian consumers’ understanding of over-the-counter medicines and risk perception. 10.1016/j.pec.2011.10.003Patient Educ Couns. 2012;87(3):395–401. doi: 10.1016/j.pec.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Carrigan N, Raynor DK, Knapp P. Adequacy of patient information on adverse effects: an assessment of patient information leaflets in the UK. 10.114-5916/08/0004-0305/$548.00/0Drug Safety. 2008;31(4):305–312. doi: 10.2165/00002018-200831040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Cavaco AM, Várzea D. Contribuição para o estudo da leitura de folhetos informativos nas farmácias portuguesas. 10.1016/S0870-9025(10)70009-2Rev Port Saude Publica. 2010;28(2):179–186. [Google Scholar]
- 5.Cavaco A, Pires C. Improving package leaflet information: potential users and physicians opinions [abstract] 10.1016/j.sapharm.2012.08.117Res Soc Adm Pharm. 2012;8(6):e50–e51. [Google Scholar]
- 6.Cavaco A, Santos AL. Avaliação da legibilidade de folhetos informativos e literacia em saúde. 10.1590/S0034-89102012000500019Rev Saude Publica. 2012;46(5):918–922. doi: 10.1590/s0034-89102012000500019. [DOI] [PubMed] [Google Scholar]
- 7.Dale E, Chall J. A formula for predicting readability. Educ Res Bull. 1948;27(1):11–20. 37–54. [Google Scholar]
- 8.Dowse R, Ramela T, Browne SH. An illustrated leaflet containing antiretroviral information targeted for low-literate readers: development and evaluation. 10.1016/j.pec.2011.01.013Patient Educ Couns. 2011;85(3):508–515. doi: 10.1016/j.pec.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 9.DuBay WH. The principles of readability. Costa Mes (Ca): Impact Information; 2004. [2013 Oct 27;]. http://www.impact-information.com/impactinfo/readability02.pdf [Google Scholar]
- 10.Flesch R. Marks of a readable style: a study in adult education. New York: Columbia University Teachers College; 1943. (Contributions to education, 897) [Google Scholar]
- 11.Franck MCJ, Foulon V, Van Vaerenbergh L. ABOP, the automatic patient information leaflet optimizer: evaluation of a tool in development. 10.1016/j.pec.2011.04.025Patient Educ Couns. 2011;83(3):411–416. doi: 10.1016/j.pec.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs J. The way forward in package insert user tests from a CRO’s perspective. 10.1177/009286151004400203Drug Inf J. 2010;44(2):119–129. [Google Scholar]
- 13.Fuchs J, Werner S, Scheunpflug C, Götze EA, Elstermann K, Scheffel K, et al. Excessive medical information increase in package inserts. 10.5414/CPP48781Int J Clin Pharmacol Ther. 2010;48(12):781–790. doi: 10.5414/cpp48781. [DOI] [PubMed] [Google Scholar]
- 14.Gazmarariana JA, Williams MV, Peelc J, Bakerd DW. Health literacy and knowledge of chronic disease. 10.1016/S0738-3991(02)00239-2Patient Educ Couns. 2003;51(3):267–275. doi: 10.1016/s0738-3991(02)00239-2. [DOI] [PubMed] [Google Scholar]
- 15.Gunning R. The technique of clear writing. New York: McGraw-Hill; 1952. [Google Scholar]
- 16.Klare GR. Measures of the readability of written communication: an evaluation. J Educ Psychol. 1952;43(7):385–399. [Google Scholar]
- 17.Knapp P, Gardner PH, Raynor DK, Woolf E, McMillan B. Perceived risk of tamoxifen side effects: a study of the use of absolute frequencies or frequency bands, with or without verbal descriptors. 10.1016/j.pec.2009.10.002Patient Educ Couns. 2010;79(2):267–271. doi: 10.1016/j.pec.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Knapp P, Raynor DK, Woolf E, Gardner PH, Carrigan N, McMillan B. Communicating the risk of side effects to patients: an evaluation of UK regulatory recommendations. 10.2165/11316570-000000000-00000Drug Safety. 2009;32(10):837–849. doi: 10.2165/11316570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Lee IH, Lee HW, Je NK, Lee S. Examining the readability of two package inserts for self-medication in South Korea. 21410.1111/j.1399-5448.2012.03324.xPharmacoepidemiol Drug Saf. 2012;21(452) Suppl 3:1–481. [Google Scholar]
- 20.Leiderman DB. Risk management of drug products and the U.S. Food and Drug Administration: evolution and context. 10.1016/j.drugalcdep.2009.02.007Drug Alcohol Depend. 2009;105(Suppl 1):S9–13. doi: 10.1016/j.drugalcdep.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 21.Maat HP, Lentz L. Improving the usability of patient information leaflets. 10.1016/j.pec.2009.09.030Patient Educ Couns. 2010;80(1):113–119. doi: 10.1016/j.pec.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 22.March Cerdá JC, Prieto Rodríguez MA, Ruiz Azarola A, Lorda PS, Barrio Cantalejo I, Danet A. Mejora de la información sanitaria contenida en los prospectos de los medicamentos: expectativas de pacientes y de profesionales sanitarios. 10.1016/j.aprim.2009.04.006Aten Primaria. 2010;42(1):22–27. doi: 10.1016/j.aprim.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinero-Lopez MA, Modamio P, Lastra CF, Marino EL. Readability levels of patient package inserts for biopharmaceuticals [abstract] 10.1007/s11096-011-9523-0Int J Clin Pharm. 2011;33(4):715–716. [Google Scholar]
- 24.Roskos SE, Wallace LS, Weiss BD. Readability of consumer medication information for intranasal corticosteroid inhalers. 10.2146/ajhp070087Am J Health System Pharm. 2008;65(1):65–8 DOI. doi: 10.2146/ajhp070087. [DOI] [PubMed] [Google Scholar]
- 25.Shiffman S, Gerlach KK, Sembower MA, Rohay JM. Consumer understanding of prescription drug information: an illustration using an antidepressant medication. 10.1345/aph.1P477Ann Pharmacother. 2011;45(4):452–458. doi: 10.1345/aph.1P477. [DOI] [PubMed] [Google Scholar]
- 26.Symonds T, Dean J, Coyne KS, Margolis MK, Hackett G, Edwards D, et al. The ability of men to assess their suitability to take a phosphodiesterase type 5 inhibitor: an assessment of the comprehension of patient information materials. 10.1111/j.1743-6109.2010.01767.xJ Sex Med. 2010;7(6):2217–2225. doi: 10.1111/j.1743-6109.2010.01767.x. [DOI] [PubMed] [Google Scholar]
- 27.Wallace LS, Keenum AJ, Roskos SE, Blake GH, Colwell ST, Weiss BD. Suitability and readability of consumer medical information accompanying prescription medication samples. 10.1016/j.pec.2007.11.017Patient Educ Couns. 2007;70(3):420–425. doi: 10.1016/j.pec.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 28.Weiss SM, Smith-Simone SY. Consumer and health literacy: the need to better design tobacco-cessation product packaging, labels, and inserts. 10.1016/j.amepre.2009.11.020Am J Prev Med. 2010;38(3 Suppl):S403–S413. doi: 10.1016/j.amepre.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 29.Wolf A, Fuchs J, Schweim HG. QRD template texts intended for package inserts: development from the first QRD template up to the new draft of July 2012. Pharm Ind. 2012;74(9):1540–1549. [Google Scholar]
- 30.Zite NB, Wallace LS. Do instructions for over-the-counter pre-coital female contraceptives promote “perfect use”? 10.1016/j.contraception.2008.10.002Contraception. 2008;79(3):211–215. doi: 10.1016/j.contraception.2008.10.002. [DOI] [PubMed] [Google Scholar]