Abstract
Background
Although non-invasive positive pressure ventilation (NIPPV) for patients with acute decompensated heart failure (ADHF) was introduced almost 20 years ago, the variation in its use among hospitals remains unknown. We sought to define hospital practice patterns of NIPPV use for ADHF and their relationship with intubation and mortality.
Methods and Results
We conducted a cross-sectional study using a database maintained by Premier, Inc., that includes a date-stamped log of all billed items for hospitalizations at over 400 hospitals. We examined hospitalizations for ADHF in this database from 2005–2010 and included hospitals with annual average volume of greater than 25 such hospitalizations. We identified 384 hospitals that encompassed 524,430 hospitalizations (median annual average volume: 206). We used hierarchical logistic regression models to calculate hospital-level outcomes: risk-standardized NIPPV rate (RS-NIPPV), risk-standardized intubation rate (RSIR), and in-hospital risk-standardized mortality rate (RSMR). We grouped hospitals into quartiles by RS-NIPPV and compared RSMRs and RSIRs across quartiles. Median RS-NIPPV was 6.2% (interquartile range, 2.8–9.3%; 5th percentile, 0.2%; 95th percentile, 14.8%). There was no clear pattern of RSMRs across quartiles. The bottom quartile of hospitals had higher RSIR (11.4%) than each of the other quartiles (9.0%, 9.7%, and 9.1%; P<0.02 for all comparisons).
Conclusion
Substantial variation exists among hospitals in the use of NIPPV for ADHF without evidence for differences in mortality. There may be a threshold effect in relation to intubation rates, with the lowest utilizers of NIPPV having higher intubation rates.
Keywords: heart failure, mortality, ventilation
For almost 2 decades, physicians have used non-invasive positive pressure ventilation (NIPPV) in acute decompensated heart failure (ADHF), but its role remains unclear. Prior studies have been limited to the subset of patients with ADHF with acute cardiogenic pulmonary edema and have produced conflicting results.1–3 No studies have examined the role of NIPPV in a broad cohort of ADHF patients. Moreover, professional societies have disparate guideline recommendations regarding the use of NIPPV in patients with ADHF.4–7
In the face of tepid supporting evidence and a lack of professional consensus, understanding how hospitals have adopted NIPPV in treating patients with ADHF can help provide insight into the effectiveness of this therapy. Furthermore, investigating the relationship between the institutional use of NIPPV and hospital outcomes may highlight missed treatment opportunities. In particular, if hospitals that use NIPPV frequently have better outcomes compared with hospitals that do not, low-use hospitals might benefit from increasing their use of NIPPV in certain patients with ADHF. Therefore, in order to characterize hospital practice patterns and their relationship with hospital outcomes, we assessed the hospital variation in NIPPV use for patients with ADHF, the associations between NIPPV use and hospital characteristics, and the association between NIPPV use and hospital-level intubation rates and mortality rates for patients with ADHF.
Methods
Data Source
We conducted a retrospective study using a voluntary, fee-supported database developed by Premier, Inc. for measuring quality and health care utilization. As of 2011, this database contained more than 130 million cumulative hospital discharges from hospitals across the United States. Inpatient discharges in this database represent about 20% of all acute care inpatient hospitalizations nationwide. In addition to the information available in the standard hospital discharge file, this database contains a date-stamped log of all billed items at the hospitalization level, including medications and laboratory, diagnostic, and therapeutic services.
For this study, patient data were de-identified in accordance with the Health Insurance Portability and Accountability Act, and random, unique patient and hospital identifiers were applied to each record to facilitate analysis. The Yale University Human Investigation Committee exempted this study protocol from review by the Office of Human Research Protections.
Study Cohort
We constructed a cohort of hospitalizations between January 1, 2005, and December 31, 2010, that had a principal discharge diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes 402.01, 402.11, 402.91,404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 428.0, 428.1, 428.20, 428.21, 428.22, 428.23, 428.30, 428.31, 428.32,428.33, 428.40, 428.41, 428.42, 428.43, and 428.9) or a principal diagnosis of acute respiratory failure (ICD-9-CM code 518.81) combined with a secondary diagnosis of heart failure. A patient could contribute multiple hospitalizations to the cohort.
We excluded patients who were younger than 18 years of age at the time of admission or transferred to or from another acute care facility. We also excluded patients with a secondary diagnosis of obstructive sleep apnea (ICD-9-CM codes 327.2, 327.2x, 780.51, 780.53, and 780.57) because we felt it would not be feasible to determine whether NIPPV was administered for ADHF in these patients. We excluded all hospitalizations with a length of stay (LOS) of 1 or 2 days to ensure that each hospitalization in our cohort was at least 24 hours in duration, because we felt that admissions shorter than 24 hours did not likely represent true ADHF and their inclusion would reduce the specificity of our findings. At the institutional level, we excluded hospitals with an annual average of <25 qualifying hospitalizations over the 2005–2010 period in order to produce more stable estimates of hospital outcomes.
Independent Variables
For each hospitalization, in addition to age, sex, race and insurance status, we used the software (versions 3.0, 3.1, 3.2, 3.3, 3.4, 3.5 for federal fiscal years 2005, 2006, 2007, 2008, 2009, 2010, respectively) provided by the Healthcare Costs and Utilization Project of the Agency for Healthcare Research and Quality to classify comorbidities from the standard hospital discharge file based on methods described by Elixhauser.8 This tool provides a Diagnosis Related Group screen of ICD-9-CM secondary diagnoses.
At the hospital level, we included bed count, teaching status, geographic location, and urban/rural status, based on information collected from the American Hospital Association database. In addition, we created the following 4 hospital characteristics based on our cohort. First, yearly average volume of patients with ADHF at each hospital was defined as the total number of discharges in the standard ADHF inpatient cohort for 2005–2010 divided by the total number of years that the hospital was in the database. Second, fraction of patients with a cardiologist as attending physician at each hospital was defined as the fraction of patients in the study cohort for whom the attending physician’s specialty was cardiology. Third, cardiovascular procedure rate at each hospital was defined as the number of patients with ADHF receiving any of a predefined group of 228 cardiovascular procedures divided by the number of patients in the study cohort at that hospital. This group of procedures contains a wide variety of invasive cardiovascular procedures and surgeries (including percutaneous coronary intervention (PCI), coronary artery bypass graft surgery (CABG), and valve replacements) and has been previously used as a means of comparing hospital-level procedural intensity in treating patients with ADHF.9 Fourth, intensive care unit (ICU) admission rate at each hospital was defined as the number of patients with ADHF admitted to an ICU divided by the number of patients in the study cohort at that hospital.
Outcomes
Our main outcome was hospital-level use of primary NIPPV. In order to calculate this, we first determined the patient-level use of primary NIPPV at each institution. We defined NIPPV as having a standard charge code for either continuous positive airway pressure (CPAP) or bi-level positive airway pressure (BLPAP), and we defined primary NIPPV by 2 criteria: (1) NIPPV on hospital day 1 or 2, and (2) NIPPV without mechanical ventilation on a prior hospital day. We considered NIPPV on the same day as mechanical ventilation to be primary NIPPV provided that both therapies were given on hospital day 1 or 2. We felt that, so early in hospitalization, patients most likely received NIPPV first and were then intubated. Additional outcomes included in-hospital death, intubation, and LOS, defined as the number of calendar days spent in the hospital, inclusive of first and last.
Statistical Analysis
We used the patient-level data to calculate our hospital-level outcomes. First, we employed a hierarchical logistic regression model (HLRM) to estimate risk-standardized NIPPV rate (RS-NIPPV) for each hospital. This approach takes into account the hierarchical structure of the data to adjust for differences in case-mix among hospitals.10, 11 We considered patient age, gender, Elixhauser comorbidities as initial adjustment variables, and we fitted a logistic model to select the variables for the final model using a stepwise algorithm. In order to ensure that the magnitudes of the unadjusted NIPPV rates and RS-NIPPVs were comparable, we fit a volume-weighted linear regression model between the unadjusted rates and the adjusted rates, and then scaled the values for RS-NIPPV by the inverse of the slope of that regression. We then used HLRM to calculate in-hospital risk-standardized mortality rate (RSMR) and risk-standardized intubation rate (RSIR) for each hospital. For patients contributing multiple hospitalizations to the cohort, we used only a single randomly chosen hospitalization to calculate RSMR. We also determined each hospital’s median LOS.
We calculated summary statistics by using frequencies and percentages for categorical data and means, medians, and interquartile ranges (IQRs) for continuous variables. We used Kruskal-Wallis tests to examine relationships between RS-NIPPV and hospital characteristics. We then grouped the hospitals into quartiles by RS-NIPPV and compared RSMRs, RSIRs, and median LOS across quartiles using Kruskal-Wallis tests. Finally, we performed post hoc pairwise comparisons using Kruksal-Wallis tests with the Bonferroni correction (Supplement). To apply this correction, we calculated an adjusted P value for each comparison by multiplying the unadjusted P value by 6 (if this result was > 1, we replaced it with 1).
Statistical significance was set at P<0.05. All analyses were done with SAS version 9.3 (SAS Institute, Inc.) statistical software. Procedure GLIMMIX was used to estimate HLRM.
Results
Patient and Hospital Characteristics
We identified 813,783 hospitalizations for ADHF from 2005 to 2010. We excluded 289,353 hospitalizations—192,601 with fewer than 3 service days, 45,262 that were transfers in or out, 81,924 with obstructive sleep apnea, and 1,050 that occurred in the hospitals with fewer than 25 such annual admissions—leaving our final cohort with 524,430 hospitalizations (Figure 1). Median age was 77 years and 241,255 (46%) of hospitalizations were men. The 5 most common comorbidities were hypertension (66%), coronary atherosclerosis (58%), history of ventricular tachycardia or ventricular fibrillation (50%), cardiac dysrhythmias (50%), and chronic obstructive pulmonary disease (COPD) (40%). Table 1 contains detailed patient-level demographic and comorbidity data for the overall study cohort.
Figure 1.
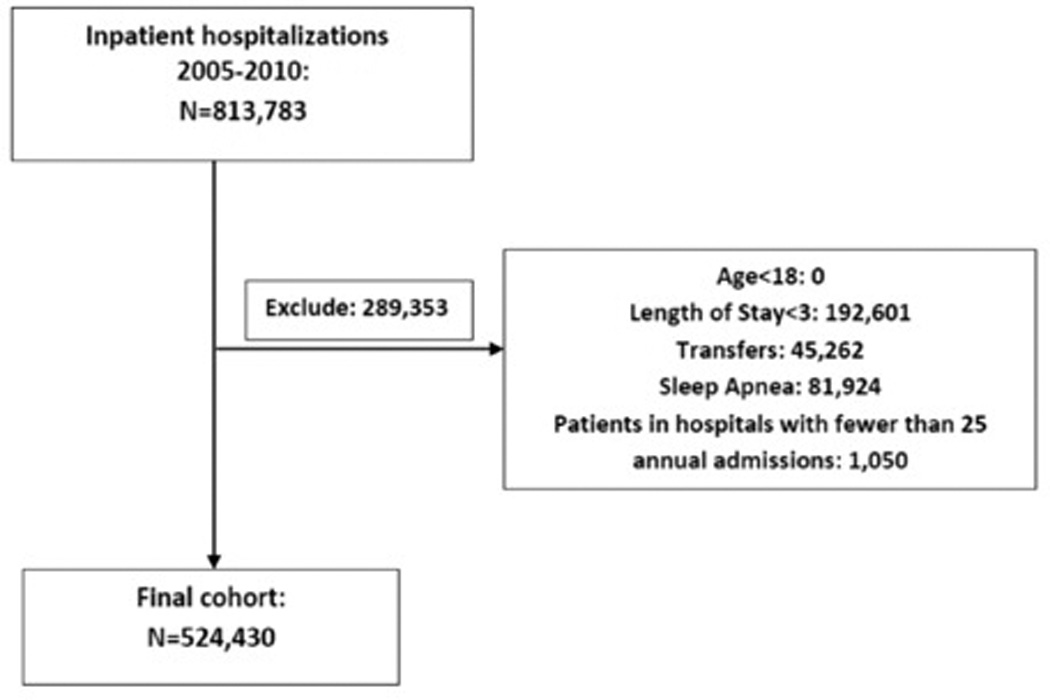
Study Cohort
Table 1.
Patient-level Characteristics
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|---|
| Number of patients | 524,430 | 110,000 | 126,285 | 142,888 | 145,257 |
| Percentage of Patients (%) | |||||
| Age (years) | |||||
| 18–24 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| 25–34 | 0.6 | 0.8 | 0.6 | 0.6 | 0.7 |
| 35–44 | 2.3 | 2.8 | 0.2 | 2.0 | 2.4 |
| 45–54 | 6.7 | 7.8 | 6.7 | 6.1 | 6.6 |
| 55–64 | 12.7 | 14.2 | 12.6 | 12.0 | 12.4 |
| 65–74 | 20.0 | 20.3 | 20.3 | 20.0 | 19.4 |
| 75+ | 57.5 | 54.1 | 57.5 | 59.2 | 58.4 |
| Gender | |||||
| Male | 46.0 | 45.9 | 46.3 | 46.0 | 45.8 |
| Female | 54.0 | 54.1 | 53.7 | 54.0 | 54.2 |
| Common Comorbidities* | |||||
| Hypertension | 65.9 | 65.6 | 65.3 | 66.9 | 65.6 |
| Coronary atherosclerosis | 58.2 | 56.9 | 58.9 | 59.2 | 57.4 |
| History of VT or VF | 50.1 | 47.6 | 51.5 | 50.0 | 50.8 |
| Cardiac dysrhythmias | 49.6 | 47.1 | 51.0 | 49.5 | 50.4 |
| COPD | 39.7 | 39.6 | 40.1 | 40.9 | 38.2 |
| Renal failure | 37.6 | 37.5 | 36.3 | 37.5 | 38.8 |
| Disorders of lipid metabolism | 35.6 | 34.2 | 35.7 | 35.7 | 36.6 |
| Diabetes without chronic complications | 33.3 | 33.3 | 32.4 | 34.2 | 33.1 |
| Fluid and electrolyte disorders | 32.1 | 31.3 | 31.7 | 31.6 | 33.4 |
| Deficiency anemias | 32.7 | 31.3 | 31.5 | 33.9 | 33.6 |
| Hypothyroidism | 16.4 | 15.6 | 16.5 | 16.7 | 16.6 |
| Peripheral vascular disease | 13.5 | 13.3 | 13.3 | 13.5 | 13.9 |
| History of peripheral vascular disease | 12.9 | 12.8 | 12.7 | 12.8 | 13.2 |
| Peripheral and visceral atherosclerosis | 11.4 | 11.3 | 11.1 | 11.3 | 11.8 |
| Diabetes with chronic complications | 10.7 | 10.4 | 10.6 | 10.8 | 10.9 |
| Obesity | 10.1 | 10.0 | 9.9 | 10.4 | 10.0 |
| Depression | 9.4 | 8.3 | 10.5 | 9.4 | 9.4 |
| Neurological disorders | 7.9 | 7.6 | 7.9 | 8.2 | 7.7 |
| Coagulopathy | 5.2 | 5.1 | 5.1 | 5.4 | 5.2 |
VT denotes ventricular tachycardia; VF, ventricular fibrillation; COPD, chronic obstructive pulmonary disease.
Comorbidities present in fewer than 5% of the overall cohort are omitted from this list.
Hospitalizations were distributed among 384 hospitals. Median (IQR) bed size was 273 (144–409), and median (IQR) yearly average volume of patients with ADHF was 206 (99–362). At the hospital-level, median (IQR) patient age was 78 years (75–81), and a median (IQR) of 45% (41–48%) of hospitalizations were men. Among the hospitals, 162 (42%) were located in the South, 105 (27%) were teaching, and 306 (80%) served urban populations (Table 2). Median (IQR) fraction of patients with a cardiologist as attending physician, cardiovascular procedure rate, and ICU admission rate were 8% (1–20%), 9% (3–14%), and 22% (17–30%), respectively.
Table 2.
RS-NIPPV by Hospital Characteristics
| Number of Hospitals (%) |
RS-NIPPV | P* | |
|---|---|---|---|
| Median (IQR) | |||
| Overall | 384 | 6.2% (2.8–9.3%) | – |
| Teaching Status† | |||
| Teaching | 105 (27%) | 6.7% (3.1–10.6%) | 0.3642 |
| Non-Teaching | 278 (73%) | 6.1% (2.7–9.2%) | |
| Population Served† | |||
| Urban | 306 (80%) | 6.4% (2.5–9.3%) | 0.9963 |
| Rural | 77 (20%) | 5.9% (3.4–8.3%) | |
| Geographic Region† | |||
| Midwest | 87 (23%) | 5.6% (1.9–9.4%) | 0.2696 |
| Northeast | 61 (16%) | 7.0% (3.4–10.1%) | |
| South | 162 (42%) | 6.0% (2.8–8.8%) | |
| West | 73 (19%) | 6.6% (3.4–9.9%) | |
| Number of Beds† | |||
| 1–100 | 60 (16%) | 4.6% (2.2–9.1%) | 0.1283 |
| 101–250 | 113 (30%) | 5.7% (2.6–8.2%) | |
| 251–400 | 110 (29%) | 6.9% (3.0–9.8%) | |
| >400 | 100 (26%) | 6.8% (3.1–10.1%) | |
| Yearly Average Volume of ADHF Patients | |||
| 1–100 | 100 (26%) | 5.6% (2.5–9.2%) | 0.0945 |
| 101–250 | 122 (32%) | 5.8% (2.6–8.9%) | |
| 251–400 | 84 (22%) | 6.3% (1.7–9.2%) | |
| >400 | 78 (20%) | 7.0% (4.5–10.3%) | |
| Fraction of Patients with a Cardiologist as Attending Physician | |||
| 0–5% | 152 (40%) | 6.0% (3.0–9.2%) | 0.6676 |
| 6–10% | 58 (15%) | 6.0% (2.3%–9.9%) | |
| 11–15% | 47 (12%) | 7.3% (3.4%–10.0%) | |
| >15% | 127 (33%) | 6.1% (2.3%–8.7%) | |
| Cardiovascular Procedure Rate | |||
| 0–5% | 132 (34%) | 6.0% (3.1–9.1%) | 0.8841 |
| 6–10% | 90 (23%) | 6.8% (2.3–9.6%) | |
| 11–15% | 91 (24%) | 6.4% (2.3–10.0%) | |
| >15% | 71 (18%) | 6.0% (3.0–8.5%) | |
| ICU Admission Rate | |||
| 0–15% | 69 (18%) | 6.6% (2.3–8.9%) | 0.9492 |
| 16–25% | 182 (47%) | 6.0% (3.1–9.6%) | |
| 26–35% | 74 (19%) | 6.7% (3.2–9.2%) | |
| >35% | 59 (15%) | 6.1% (2.0% –8.7%) | |
RS-NIPPV denotes risk-standardized non-invasive positive pressure ventilation rate; NIPPV, non-invasive positive pressure ventilation; IQR, interquartile range; ADHF, acute decompensated heart failure; ICU, intensive care unit.
P values calculated using Kruskal-Wallis tests.
One hospital did not have information from the American Hospital Association.
Distribution of RS-NIPPV among Hospitals
We found a wide distribution of RS-NIPPV across hospitals. The median (IQR) was 6.2% (2.8–9.3%), and hospitals ranged from 0.2% at the 5th percentile to 14.8% at the 95th percentile (Figure 2). A substantial proportion of hospitals (n=52, 14%) had RS-NIPPV less than 1%. On the other hand, nearly 5% of hospitals had RS-NIPPV greater than 15%. We found no significant relationship between RS-NIPPV and any hospital characteristic (Table 2).
Figure 2.
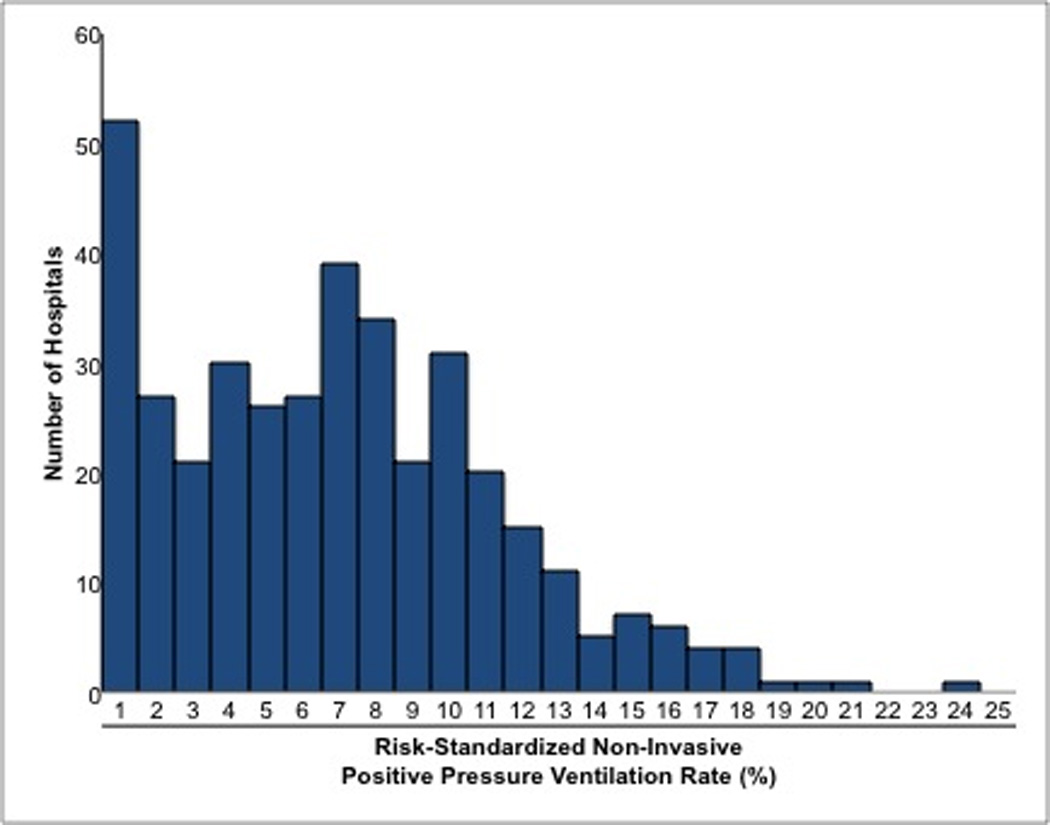
Distribution of Risk-Standardized Non-Invasive Positive Pressure Ventilation Rates among Hospitals
Hospital Outcomes by Quartile of RS-NIPPV
After grouping hospitals into quartiles by RS-NIPPV, quartiles 1, 2, 3, and 4 had median (IQR) RS-NIPPV of 0.9% (0.3–1.9%), 4.5% (3.6–5.6%), 7.4% (6.8–8.3%), and 11.6% (10.1–14.3%), respectively (Table 3). Patient-level characteristics across different quartiles are shown in Table 1.
Table 3.
Hospital Outcomes by Quartiles of RS-NIPPV
| Overall | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P* | |
|---|---|---|---|---|---|---|
| Median (IQR) | ||||||
| RS-NIPPV | 6.2% (2.8–9.3%) | 0.9% (0.3–1.9%) | 4.5% (3.6–5.6%) | 7.4% (6.8–8.3%) | 11.6% (10.1–14.3%) | – |
| RSMR | 5.5% (4.7–6.4%) | 5.4% (4.7–6.5%) | 5.6% (4.8–6.8%) | 5.6% (5.0–6.6%) | 5.2% (4.4–5.9%) | 0.0315 |
| RSIR | 9.8 (7.4–12.1%) | 11.4% (8.1–15.4%) | 9.0% (6.9–11.0%) | 9.7% (7.7–11.7%) | 9.1% (7.2–11.1%) | <0.0001 |
| Median LOS | 5 (5,6) | 5(5,5) | 5(5,6) | 5(5,6) | 5(5,5) | 0.1095 |
RS-NIPPV denotes risk-standardized non-invasive positive pressure ventilation rate; IQR, interquartile range; RSMR, risk-standardized mortality rate; RSIR, risk-standardized intubation rate; LOS, length of stay.
P values calculated using Kruskal-Wallis tests.
RSMRs
Median (IQR) RSMR among all hospitals was 5.5% (4.7–6.4%). We found a significant difference in RSMRs across quartiles of RS-NIPPV (P=0.0315) (Table 3). In post hoc analysis, the only significant pairwise comparison was between quartile 3 (median RSMR, 5.6%) and quartile 4 (median RSMR, 5.2%; adjusted P=0.0438) (Table 4).
Table 4.
Adjusted P-Values* for Pairwise Comparisons of RSMRs and RSIRs between Quartiles of RS-NIPPV
| Quartile 1 and Quartile 2 |
Quartile 1 and Quartile 3 |
Quartile 1 and Quartile 4 |
Quartile 2 and Quartile 3 |
Quartile 2 and Quartile 4 |
Quartile 3 and Quartile 4 |
|
|---|---|---|---|---|---|---|
| RSMR | 1 | 1 | 0.939 | 1 | 0.1122 | 0.0438 |
| RSIR | 0.0012 | 0.0198 | 0.0006 | 1 | 1 | 1 |
| Median LOS | 1 | 1 | 0.7938 | 1 | 0.249 | 0.1932 |
RS-NIPPV denotes risk-standardized non-invasive positive pressure ventilation rate; RSMR, risk-standardized mortality rate; RSIR, risk-standardized intubation rate; LOS, length of stay.
P values for all comparisons were calculated using Kruskal-Wallis tests and adjusted using the Bonferroni correction.
RSIRs
Median (IQR) RSIR among all hospitals was 9.8% (7.4%-12.1%). We found a significant difference in RSIRs across quartiles (P<0.0001) (Table 3). In post hoc pairwise comparisons, quartile 1 (median RSIR, 11.4%) was significantly higher than quartiles 2–4 (median RSIRs, 9.0%, 9.7%, and 9.1%, and adjusted P=0.0012, 0.0198, and 0.0006, for quartiles 2, 3, and 4, respectively), but quartiles 2–4 were not significantly different from one another (Table 4).
Median LOS
Median (IQR) LOS among all hospitals was 5 (5–6) days, (Table 3), and we found no significant differences across quartiles in median LOS (P=0.11), or in any post hoc pairwise comparison (Table 4).
Discussion
We found substantial variation across hospitals in the use of primary NIPPV in patients with ADHF. Although median RS-NIPPV was 6.2%, nearly 14% of hospitals had risk-standardized rates lower than 1%, and nearly 5% of hospitals had risk-standardized rates higher than 15%. Moreover, although we found no clear pattern of associations between RS-NIPPV and RSMR or median LOS, we found that the bottom quartile of hospitals by RS-NIPPV had significantly higher RSIRs than other quartiles. These results suggest that the lowest-use hospitals may be able to reduce the need for intubation in some patients with ADHF without increasing mortality by using NIPPV more frequently, but that otherwise we could not detect a benefit of higher rates.
Although heart failure is highly prevalent12 and ADHF hospitalizations are very common,13 patients with ADHF present heterogeneously and may therefore require heterogeneous treatment.7 Our results demonstrate that heterogeneity in treatment for ADHF also exists among hospitals, even after accounting for differences in case-mix. These results are consistent with prior findings demonstrating that hospital practice patterns in treating acute cardiovascular conditions can vary significantly even after risk-adjustment for patient characteristics.14, 15 However, we found no correlation between any measured hospital characteristic and NIPPV use. These included institutional characteristics (teaching status, number of beds, urban/rural status) as well as condition-specific characteristics (yearly average volume of patients, cardiovascular procedure rate, ICU admission rate, fraction of patients with a cardiologist as attending physician). These findings suggest not only that NIPPV use for patients with ADHF varies widely across hospitals, but also that practice patterns for this therapy may not parallel patterns of intensity for other cardiovascular procedures such as PCI and CABG.9 The lack of randomized controlled trials for NIPPV in ADHF may partially explain this difference in utilization of NIPPV relative to other cardiovascular procedures, which have a stronger evidence base. Alternatively, hospital-level factors—such as the presence of respiratory therapists, the number of NIPPV devices available, and the existence of hospital protocols regarding management of respiratory failure—may play the dominant role in determining which hospitals use NIPPV frequently.
Furthermore, there may be clinical consequences associated with hospital variation in NIPPV use. Although we found no clear pattern of RSMRs across quartiles of NIPPV use, we found that hospitals in the lowest quartile had higher RSIRs than hospitals in all other quartiles. These results suggest that there may be a threshold effect between institutional NIPPV use and intubation for patients with ADHF, such that there is little marginal benefit with increasing use of NIPPV beyond a fairly low point. Nevertheless, given that intubation and mechanical ventilation may cause a myriad of potential complications—including laryngeal injury, ventilator-associated pneumonia, and post-traumatic stress disorder—some patients with ADHF may benefit from greater use of NIPPV among hospitals that are currently in the lowest quartile of use. Randomized controlled trials are needed to evaluate the potential benefits of NIPPV in a broad cohort of patients with ADHF, in particular the potential for avoiding intubation.
Our findings should be interpreted in the context of several limitations. First, our risk adjustment only involved the use of administrative data containing patient demographic characteristics and comorbidities due to a lack of clinical information. However, prior studies have validated the use of such administrative data in risk-adjustment for patients with ADHF16–18 and in comparing rates of hospital mortality for heart failure.11 Second, because the log of billed items is stamped only with the date, for patients who received NIPPV and intubation on the same day, we could not definitively determine which therapy occurred first. Nevertheless, in the first 2 days of hospitalization, we considered this to be primary NIPPV because we believe that it is more likely that patients who received both therapies received NIPPV first. Third, despite risk-adjustment, there may be unmeasured confounders at the patient-level or hospital-level. Fourth, although the database we used represents a large and diverse sample of hospitals from across the country, all included hospitals participate voluntarily. Accordingly, our findings may not be generalizable to all hospitals nationwide due to unobserved differences between hospitals in the database and hospitals not in the database. Fifth, approximately 40% of patients in our cohort had COPD, and because some patients with COPD also receive NIPPV, it might be difficult to determine the reason for NIPPV in these patients. Nevertheless, we chose to include these patients in our cohort because only 4.5% of patients admitted with an acute COPD exacerbation receive NIPPV,19 and because COPD is such a common concomitant condition (31% in the Acute Decompensated Heart Failure National Registry)20 that omitting these patients would exclude an important subset of the heart failure population. Sixth, we made no attempt to differentiate CPAP and BLPAP. It is possible that CPAP may be more efficacious than BLPAP in patients with ADHF,21 and if so, some of the variation in practice patterns of NIPPV may reflect differential use of these 2 modalities. Seventh, we did not distinguish heart failure with preserved ejection fraction (HFpEF) from heart failure with reduced ejection fraction (HFrEF). If hospitals have substantially different case-mixes regarding these 2 phenotypes, and if NIPPV is used more commonly in a particular phenotype, our hierarchical models may not have been adequately adjusted. However, our models included age, gender, and comorbidities, all of which have been shown to differ between HFpEF and HFrEF.22 Finally, we were only able to study outcomes occurring during hospitalization, and it possible that longer-term hospital outcomes (such as 30-day mortality rate or 30-day readmissions rate) may also relate to NIPPV use.
In conclusion, we found that substantial variation exists among hospitals in the use of primary NIPPV in the treatment of ADHF, and that practice patterns for this therapy may not correspond with patterns of intensity for other cardiovascular procedures. Furthermore, hospitals in the lowest quartile of NIPPV use had higher intubation rates than other hospitals. Although clinical trials will be required to further elucidate the relationship between NIPPV and intubation, these results suggest that some hospitals may be able to reduce intubations without increasing mortality by increasing the use of NIPPV in certain patients with ADHF.
Supplementary Material
Acknowledgments
Sources of Funding
This research was supported by a grant from the Doris Duke Charitable Foundation to Yale University for Clinical Research Fellow Vivek T Kulkarni, by grant DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, CT, and by grant UL1 RR024139-006S1 from the National Center for Advancing Translational Sciences in Bethesda, MD. Dr. Dharmarajan is supported by grant T32 HL007854 from the National Heart, Lung, and Blood Institute; he is also supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale by the John A. Hartford Foundation and the American Federation for Aging Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Donaghue Foundation or the National Institutes of Health.
Dr. Krumholz receives support from a grant from Medtronic, Inc. through Yale University to develop methods of clinical trial data sharing and chairs a scientific advisory board for United Healthcare.
Footnotes
Disclosures
The other authors report no conflicts.
References
- 1.Gray A, Goodacre S, Newby DE, Masson M, Sampson F, Nicholl J. Noninvasive ventilation in acute cardiogenic pulmonary edema. N Engl J Med. 2008;359:142–151. doi: 10.1056/NEJMoa0707992. [DOI] [PubMed] [Google Scholar]
- 2.Peter JV, Moran JL, Phillips-Hughes J, Graham P, Bersten AD. Effect of non-invasive positive pressure ventilation (NIPPV) on mortality in patients with acute cardiogenic pulmonary oedema: a meta-analysis. Lancet. 2006;367:1155–1163. doi: 10.1016/S0140-6736(06)68506-1. [DOI] [PubMed] [Google Scholar]
- 3.Weng C, Zhao YT, Liu QH, Fu CJ, Sun F, Ma YL, Chen YW, He QY. Meta-analysis: noninvasive ventilation in acute cardiogenic pulmonary edema. Ann Intern Med. 2010;152:590–600. doi: 10.7326/0003-4819-152-9-201005040-00009. [DOI] [PubMed] [Google Scholar]
- 4.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 5.Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. Executive summary: HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.McMurray J, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Køber L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Rønnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Eur J Heart Fail. 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 7.Weintraub NLCS, Pang PS, Levy PD, Anderson AS, Arsianian-Engoren C, Gibler WB, McCord JK, Parshall MB, Francis GS, Gheorghiade M. Acute heart failure syndrome: emergency department presentation treatment, and disposition: approaches and future aims: a scientific statement from the American Heart Association. Circulation. 2010;122:1975–1996. doi: 10.1161/CIR.0b013e3181f9a223. [DOI] [PubMed] [Google Scholar]
- 8.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chen SI, Dharmarajan K, Kim N, Strait KM, Li SX, Safavi KC, Lindenauer PK, Krumholz HM, Lagu T. Procedure intensity and the cost of care. Circ Cardiovasc Qual Outcomes. 2012;5:308–313. doi: 10.1161/CIRCOUTCOMES.112.966069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 11.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SL. An adminstrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Stafford R, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 13.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Partovian C, Gleim SR, Mody PS, Li SX, Wang H, Strait LM, Allen LA, Lagu T, Normand SL, Krumholz HM. Hospital patterns of use of positive inotropic agents in partients with heart failure. J Am Coll Cardiol. 2012;60:1402–1409. doi: 10.1016/j.jacc.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Safavi K, Dharmarajan K, Kim N, Strait KM, Li SX, Chen SI, Lagu T, Krumholz HM. Variation exists in rates of admission to intensive care units for heart failure patients across hospitals in the United States: hospital variation in admission to the ICU. Circulation. 2013;127:923–929. doi: 10.1161/CIRCULATIONAHA.112.001088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee DS, Donovan L, Austin PC, Gong Y, Liu PP, Rouleau JL, Tu JV. Comparison of coding of heart failure and comorbidities in adminstrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Li B, Evans D, Faris P, Dean S, Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quan H, Parsons GA, Ghali WA. Validity of information on comorbiditiy derived from ICD-9-CCM administrative data. Med Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, Mannino D, Sciurba FC, Holguin F. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fonarow GC, Adams KF, Abraham WT, Yancy CW, Boscardin WJ. Risk stratification for in-hospital mortality in acute decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–580. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 21.Panacek EA, Kirk JD. Role of noninvasive ventilation in the management of acutely decompensated heart failure. Rev Cardiovasc Med. 2002;3(Suppl 4):S35–S40. [PubMed] [Google Scholar]
- 22.Sweitzer NK, Lopatin M, Yancy CW, Mills RM, Stevenson LW. Comparison of clinical features and outcomes of patients hospitalized with heart failure and normal ejection fraction (>or =55%) versus those with mildly reduced (40% to 55%) and moderately to severely reduced (<40%) fractions. Am J Cardiol. 2008;101:1151–1156. doi: 10.1016/j.amjcard.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


