Abstract
Background
The accurate grading of malignant astrocytomas has significant prognostic and therapeutic implications. Traditional histopathological grading can be challenging due to regional tumor heterogeneity, especially in scenarios where small amounts of tissue are available for pathologic review. Here, we hypothesized that a critical tumor resection volume is needed for correct grading of astrocytomas by histopathology. For insufficient tissue sampling, IDH1 molecular testing can act as a complementary marker to improve diagnostic accuracy.
Methods
Volumetric analyses were obtained using preoperative and postoperative MRI images. Histological specimens were gathered from 403 patients with malignant astrocytoma who underwent craniotomy. IDH1 status was assessed by immunohistochemistry and sequencing.
Results
Patients with >20 cubic centimeters (cc) of the total tumor volume resected on MRI have higher rate of GBM diagnosis compared to <20cc (OR 2.57, 95% CI 1.6-4.06, P<0.0001). The rate of IDH1 status remained constant regardless of the tumor volume resected (OR 0.81, 95% CI 0.48-1.36, P<0.43). The rate of GBM diagnosis is 2-fold greater for individual surgical specimen >10cc than those of lower volume (OR 2.48, 95% CI 1.88-3.28, P<0.0001). Overall survival for AA patients with >20cc tumor resection on MRI is significantly better than those with <20cc tumor resected (P<0.05). No volume-dependent differences were observed in patients with GBM (P<0.4), IDH1 wild type (P<0.1) or IDH1 mutation (P<0.88).
Conclusions
IDH1 status should be considered when total resection volume is <20cc based on MRI analysis and for surgical specimen < 10cc to complement histopathologic diagnosis of malignant astrocytomas. In these specimens, under-diagnosis of GBM may occur when analysis is restricted to histopathology alone.
Keywords: glioblastoma, anaplastic astrocytoma, malignant astrocytoma, diagnosis, surgical resection, isocitrate dehydrogenase
INTRODUCTION
Malignant astrocytomas are the most common primary central nervous system tumors in adults, with an incidence of 5 per 100,000 in the United States [1]. Clinicopathologic features including the patient’s age, performance status, and histology dictate prognosis and therapeutic decisions, with histologic classification the most influential factor but prone to subjective variability. In addition, patients with anaplastic astrocytoma (AA) survive much longer than patients with glioblastoma (GBM) [2–4]. Since histology drives the management of these subgroups of malignant glioma, accurate classification is fundamentally important [5].
Here, we report a prospectively collected, retrospective study of malignant astrocytomas that investigates diagnostic accuracy as a function of tumor volume obtained for pathologic grading. We hypothesized that accuracy of histological diagnosis in malignant astrocytoma depends strongly on the volume of surgical specimen obtained, while accuracy of molecular testing for isocitrate dehydrogenase 1 (IDH1) status is volume-independent. Hence, we test here whether adding IDH1 molecular testing improves the accuracy of diagnosis and prognosis when pre-operative imaging features suggest malignant astrocytoma. The added molecular data may be useful in patients with non-GBM diagnosis from inadequate tissue samples.
MATERIALS AND METHODS
IRB statement and clinical database
This study was conducted under an M. D. Anderson Cancer Center (MDACC) IRB-approved protocol (LAB09-0987) using a prospectively collected database for all glioma patients. The database was queried for patients with a centrally reviewed diagnosis of lobar supratentorial AA, whose first therapeutic intervention was an open surgical resection at our institution from June 1993 - April 2009. To minimize histopathologic sampling differences, biopsy-only patients were excluded, unless they proceeded to debulking surgery and confirmed AA diagnosis within the subsequent 2 months without intervening treatment. All pathology specimens were centrally reviewed by a five experienced neuropathologists at M.D. Anderson. Patients with documented 1p/19q allelic loss characteristic of oligodendroglial histology, or concomitant secondary malignancy, were also excluded. For comparison, a convenience sample of 250 GBMs with available tissue for IDH1 scoring was used. There were no differences in the clinical characteristics (age, KPS, enhancement, tumor size) compared to all newly diagnosed GBMs (n=751) recorded at MDACC during the same time period.
Tumor blocks, immunohistochemistry, and DNA sequencing
Formalin-fixed, paraffin-embedded sections were scored using immunohistochemistry with an R132H IDH1mutation-specific antibody (clone H09, Dianova, Hamburg, Germany) [6]. Primers for PCR amplification of the IDH1 R132 mutation hotspot were: forward: 5’-CTCCTGATGAGAAGAGGGTTG-3’ and reverse: 5’-M13Forward-CACATTATTGCCAACATGAC-3’, and products were sequenced (Beckman Coulter Genomics, Beverly, MA). Tumors were categorized by multiple scoring runs using at least one method (130/156 AAs and 246/250 GBMs).
Tumor volume measurements
MRI volume calculations were performed using Vitrea2 3D volumetric software (Vital Images, Inc., Minnetonka, MN). Personnel scoring the tumor volumes were blinded to molecular stratification and patient survival. T1 post-gadolinium enhancing tumor and T1 non-enhancing tumor volumes were available on 157 AA cases and 246 GBM cases. Total tumor volume was calculated as equivalent to the T2/FLAIR volume, or to the sum of enhancing and non-enhancing T1 volume in cases where T2/FLAIR sequences were not available. For pathology specimen volume measurements, the dimensions of each specimen were measured and recorded. The volume was estimated assuming tissue samples in a rectangular conformation.
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics software package v21.0.0. The chi-square test and Fisher exact test, as appropriate, were used to establish associations between categorical variables; the independent samples T-test was used for continuous factors. Survival curves were generated using the Kaplan-Meier method and compared using the log-rank test. All tests were two-tailed. A p-value of < 0.05 was considered significant.
RESULTS
GBM diagnosis and IDH1 status by volumetric MRI analysis
Patients (n=403) with open surgical resection and a histopathological diagnosis of malignant astrocytoma were analyzed. These included those with histologically confirmed GBM (n=246 (61%) and AA (n=157 (39%)). IDH1 status was mutant in 121 patients (30%) and wild type (WT) in 255 (63%). 27 patients (7%) had indeterminate IDH1 status and excluded from the subgroup IDH1 analysis (Table 1).
Table 1.
Summary of patient demographics, and of tumor genetic and volumetric characteristics.
| Patient characteristics | All patients (N = 403) |
GBM (N = 246) |
AA (N = 157) |
P | |
|---|---|---|---|---|---|
| Age (year) | |||||
| Mean ± SD | 48.9 ± 15.7 | 54.7 ± 15.0 | 39.8 ± 12.1 | <0.001 | |
| Range | 14.3–87.8 | 14.3–87.8 | 18.3–72.1 | ||
| Gender | |||||
| Male | 244 | 149 | 95 | ||
| Female | 159 | 97 | 62 | ||
| KPS, median (range) | 90 (20–100) | 90 (20–100) | 90 (60–100) | ||
| IDH Status (N, %) | |||||
| Wild type | 255 (63) | 212 | 43 | <0.001 | |
| Mutant | 121 (30) | 34 | 87 | ||
| Indeterminate | 27 (7) | 0 | 27 | ||
| MRI analysis | |||||
| Preoperative volume (cc, mean) | 81.9 | 95.7 | 60.4 | <0.001 | |
| Postoperative volume (cc, mean) | 33.3 | 40.1 | 22.7 | <0.001 | |
| Resection volume > 20 cc (N, %) | 48.6 | 55.6 | 37.7 | <0.001 | |
| Resection volume < 20 cc (N, %) | 104 (26) | 46 (11) | 58 (15) | ||
| Histological analysis | |||||
| Sample volume (cc, mean ± SD | 15.9 ± 37.9 | 18.6 ± 37.7 | 17.4 ± 45.6 | <0.98 | |
| Follow-up analysis | |||||
| Median survival (wk) | 94.1 | 58.3 | 205.0 | <0.001 | |
| Median follow-up in survivors (week) | 209.0 | 213.6 | 205.0 | ||
To examine the relationship between tumor volume removed and the rate of GBM histopathologic diagnosis, we correlated final tumor histological diagnosis with radiographic volume of resection using the difference between the pre- and post-operative tumor volumes. Figure 1A shows the percentage of histologic diagnosis of GBMs and IDH1 status for different resection volume intervals as determined by MRI volumetric analysis. The rate of GBM diagnosis among all malignant astrocytomas demonstrated a significant drop from 80% to less than 50% as resection volumes decreased. In contrast, for all malignant astrocytomas analyzed, the detection rate of IDH1 WT remained relatively constant at approximately 70% regardless of the tumor tissue volume obtained for diagnosis. The rate of histopathologic GBM diagnosis begins to diverge from the molecular data with resection volumes below 20cc (P < 0.05).
Fig. 1. Tissue volume-dependent diagnosis of malignant astrocytomas.
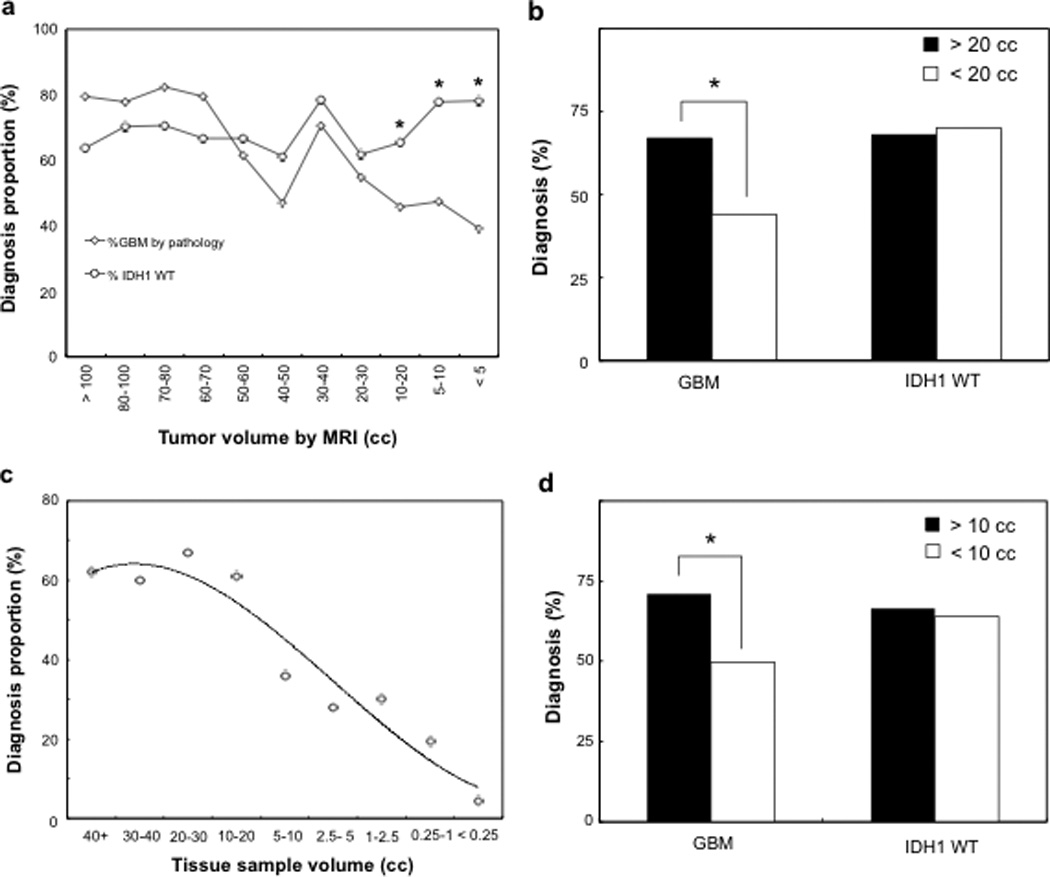
(A, B) The rate of GBM diagnosis is dependent on the MR volume of tumor resected. As the total tumor resection volume decreases, the percentage of GBM diagnosis also decreases. In contrast, the proportion of IDH1 WT tumors remained constant regardless of tumor resection volumes. The divergence between the rate of histopathological GBM diagnosis and IDH1 WT occurs at 20 cc. (C,D) The rate of histopathological GBM diagnosis is significantly reduced for tissue samples <10 cc. (C) The percentage of GBM diagnosis among all patient samples is inversely proportional to the tissue specimen volume. (D) For tissue samples > 10cc, there is a significantly higher likelihood that patient will have been diagnosed with GBM as compared to tissue samples < 10cc. No difference was observed for IDH1 WT status (* denotes P < 0.05).
Using this resection volume as a cut-off threshold, additional MRI volumetric analysis revealed that 299 patients had resection volumes greater than the 20cc cut-off, of which 200 patients (67%) were confirmed as histological GBMs and 99 patients (33%) as AAs. In contrast, 104 patients had resection volumes < 20cc, with 46 GBMs (44%) and 58 AAs (56%) diagnosed by histology (Figure 1B). Similarly, for patients with > 20cc of resection volumes, 190 patients had IDH1 WT tumors, representing 67% of all patients with known IDH1 status. For all patients with < 20cc of resection volume, 64 IDH1 WT samples were identified, accounting for 71% of the sample group (Figure 1B). The percentage of patients diagnosed with GBM by histology was highly dependent on tumor resection volume with a significantly higher rate of diagnosis for volumes < 20cc [Odds ratio (OR) 2.57, 95% confidence interval (CI) 1.6-4.06, P<0.0001], whereas no difference was observed in the percentage of IDH1 WT in these two volume subsets (OR 0.81, 95% CI 0.48-1.36, P<0.43).
GBM diagnosis by pathology sample volume
To assess the likelihood of GBM diagnosed as a function of the actual surgical specimen volume submitted to the neuropathologists, we reviewed all recorded tissue specimens with tissue dimension information. As a result, 1059 brain tumor fragments submitted for histologic analysis with specimen dimensions were identified for volume calculations. Of these samples, 599 (56.6 %) were diagnosed as GBM, 300 (28.3%) as AA, and 160 (15.1 %) as histological subtypes that were indeterminant. Figure 1C shows the percentage of GBMs diagnosed with respect to individual pathology sample volume. As the sample volume dropped to 5-10cc, a sharp decline in the percentage of GBM diagnosis was made from a mean of 71.2% to 51.7%. Using 10cc as the volume cut-off, further analysis showed that 330 samples had volume > 10cc, from which 235 GBM were diagnosed, representing a 71.2% GBM tissue diagnosis (Figure 1D). For specimen volume < 10cc, GBM diagnosis was confirmed in 364 of 729 tissue samples, giving a GBM diagnosis percentage of 49.9% (Figure 1D). This corresponds to a 30% reduction in the rate of GBM diagnosis in tissue samples < 10cc as compared to samples > 10cc. In contrast, IDH1 WT status remained consistent regardless of sample volume, with IDH1 WT percentages of 66.7% and 64.1% for tissue equal to or greater than, and < 10cc, respectively (Figure 1B). A significantly higher rate of pathological diagnosis in GBM among tissue specimens > 10cc was noted (OR 2.48, 95% CI 1.88-3.28, P < 0.0001), while no difference was observed for IDH1 WT status percentage (P < 0.45).
Effect on patient outcomes
To investigate whether the under-diagnosis of GBM by tissue pathology in smaller resection volumes correlates with patient outcome, we analyzed the overall survival of patients with respect to their MRI volumetric analysis. For patients diagnosed with GBM, we observed no significant difference between the groups with tumor resection volume > 20cc and those with < 20cc (P < 0.4) (Figure 2A). However, for patients diagnosed with AA by pathology, there is a divergence between the survival curves for resection volume > 20cc and those with < 20cc of tumor resected (P < 0.05) (Figure 2B). This is consistent with pathology sample volume of < 10cc, where patients with AA by pathology also showed worse survival (Supplemental Figure S3). In contrast, for both IDH1 WT and IDH1 mutant patients, there were no significant differences between groups with > 20cc and < 20cc resection volumes (P < 0.1 and P < 0.88, respectively) (Figure 2C, D). AA patients with > 20cc and < 20cc of resection volume but similar IDH1 status also showed no survival differences (Supplemental Figure S1.1).
Fig. 2. Survival analysis of malignant astrocytomas based on histopathology and IDH1 status.
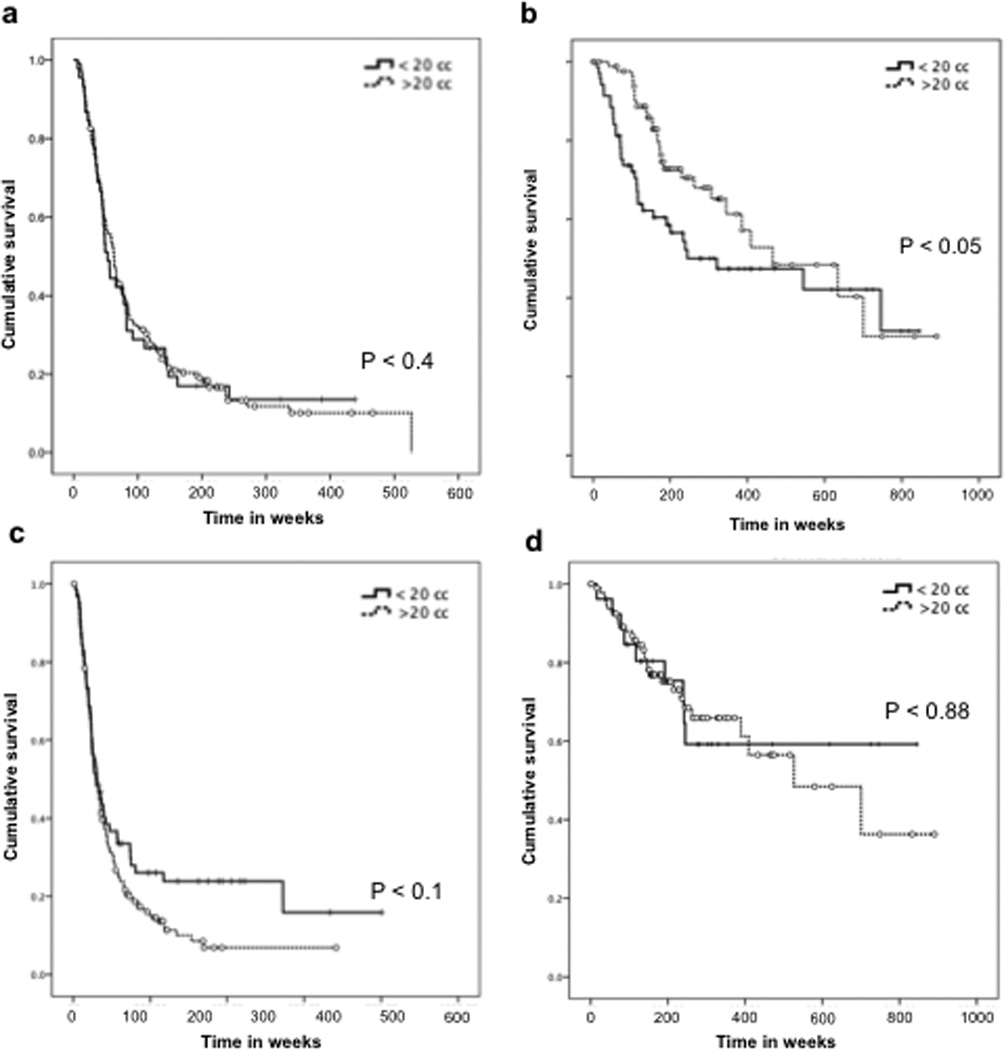
Kaplan-Meier curves show that in patients with histological diagnosis of GBM (A), the overall survival is similar with resection volumes > 20 cc or < 20 cc. However, in patients diagnosed with AA (B), resection volume < 20 cc correlated with worse prognosis (P < 0.05). Neither IDH1 WT (C) nor IDH1 mutants (D) demonstrated significant differences in patient survival between the two resection volume groups.
DISCUSSION
Reasons for under-grading in malignant gliomas
The accurate diagnosis and grading of malignant astrocytomas has significant clinical implications. Consistency in histopathological diagnosis of glioma grades is hindered by its overall reliance on the variable experience of pathologists and confounded by regional heterogeneity when small tissue volumes are collected. As a result, several studies have questioned the role of stereotactic biopsy for gliomas altogether [7–14].
The probability of detecting histological features described by the WHO grading system for GBM remains a diagnostic challenge in small volume tissue samples. Glantz and colleagues demonstrated that AA diagnoses frequently underestimate the true grade, and that stereotactic biopsy disproportionately over-diagnose AAs and under-diagnose GBMs as compared to open resection [8]. Stereotactic biopsy has become commonplace when the risk of craniotomy is deemed too high (when tumor is in deep or eloquent regions). However, its lower morbidity and mortality and shorter hospital stays must be balanced against the greater tendency for error in identifying tumor grade and type, and the higher chance of non-diagnosis. Stereotactic biopsy specimens are typically cylinders with dimensions of 1×2×5 mm. They are suitable for grading homogenous lesions but may be prone to diagnostic error in heterogeneous tumors [15].
IDH1 as a prognostic marker
A recent genome-wide sequencing study of GBMs identified mutations in a gene that encodes for IDH1 [16]. The IDH1 gene is located on chromosome 2q33 and encodes for cytoplasmic and peroxisomal proteins that catalyze the oxidative decarboxylation of isocitrate to α -ketoglutarate. The resulting production of nicotinamide adenine dinucleotide phosphate provides an antioxidant affecting tumor cell viability. IDH1 mutations are highly specific to gliomas and acute myeloid leukemia (AML), with up to 75% of mutations found in WHO II and WHO III gliomas [17–19]. IDH1 mutations have been associated with early genetic events in gliomagenesis although their exact role in glioma pathogenesis remains unclear. As they appear to inhibit the activity of IDH1 WT, mutation of this gene causes tumor suppression by increasing the enzymatic activity of the aberrant gene product. This in turn causes excess production of 2-hydroxyglutarate via NADPH-dependent reduction of α-ketoglutarate and other tumor-associated growth factors such as hypoxia-inducible factor subunit HIF-1α [20–21]. IDH1 mutations are more common in gliomas of lower grade and are associated with younger patient population and longer overall survival [17, 22–24]. The low rate of IDH1 mutations in GBM raises the question as to whether primary GBMs with IDH1 mutations may actually be secondary GBMs that arise from a lower grade malignant precursor tumor not detected initially [25]. Regardless, IDH1 mutation status has been suggested as a possible marker for improved segregation of primary GBMs from other malignant astrocytomas. Thus, its use would help resolve the current clinical dilemma that a subset of AA patients succumb to the disease within months and thus behave much like a GBM, while a minority of GBM patients with extended progression-free survival behave like tumors of lower grade [23–24, 26–27].
It is now clear that IDH1-mutated GBM patients have better prognosis and increased overall survival than those without IDH1 mutation [28, 29]. Survival in IDH1 WT AA patients is similar to that of IDH1 WT GBM patients [17, 28, 30–33]. Although the management of GBMs has been standardized according to the Stupp regimen [34], a unified treatment protocol has not yet been established for those with AA. At our institution, patients with IDH1 WT AA are managed more aggressively with adjuvant treatment than are IDH1 mutated patients.
In this study we had two aims. First, we examined the role of IDH1 mutation status in glioma samples and its relation to the sufficiency of pathological diagnosis in malignant astrocytomas. The importance of analyzing IDH1 status has not yet been well characterized with respect to tumor volumes obtained for pathological diagnosis. Second, no clearly defined tissue volume threshold has established the point at which such additional molecular data provide the greatest complement to histological analysis. Consistent with previously published reports, our data showed that the percentage of patients diagnosed with GBM varies significantly with tumor volume collected, and showed under-diagnosis of GBMs in smaller tissue samples [7]. However, the incidence of IDH1 mutation was consistent and independent of the tissue volumes collected across all ranges. This result suggests that in cases of insufficient tumor tissue collection, GBM may be under-diagnosed. When a negative pathologic analysis in a small biopsy specimen is insufficient to truly rule out GBM, IDH1 mutational status may provide an independent and complementary diagnostic and prognostic marker. Our analyses suggest that IDH1 mutational status would provide the greatest benefit for total resection < 20cc based on MR imaging analysis, or for individual tissue specimens < 10cc.
The discrepancy between the extent of resection volume based on MR imaging and the actual tissue volume collected occurs for several reasons. First, post-operative brain tissue swelling and expansion after the removal of tumor can cause the surgical cavity to appear smaller when visualized on the post-operative MRI, thus artificially inflating the true resection volume. Intraoperatively, the inadvertent suctioning of tumor fragments or partial submission of tumor resected may account for the volume differences. GBMs may be more prone to these intraoperative factors due to the presence of necrotic areas more easily removed by suctioning and less likely to be saved for specimen analysis. We observed significantly worse overall survival of histologically diagnosed AA patients with total resection volume of <20cc based on imaging analysis as compared to those with total resection volume >20cc. While it is worth noting that increased tumor tissue resection itself would provide certain benefit to survival. We have found no significant differences in histologically diagnosed AA patients with resection volumes greater than the threshold of 20cc (Supporting Figure S2). This suggests that the worse survival we observed in AA patients with resection volume less than 20cc is mainly due a portion of patients diagnosed with AA may in fact harbor under-diagnosed lesions of higher grade. Consistent with previous studies [29–30, 33], we found that IDH1 WT AA patients had similar survival to those with IDH1 mutant GBM (p=0.95). As such, we suggest that for histologically diagnosed AA with total resection < 20cc based on MR imaging, additional IDH1 testing should be performed, with WT AA treated as aggressively as GBM. However, it remains a matter of debate whether IDH1 WT AA should be more classified as a true GBM under-diagnosed by histology, or as a unique subclass of AA with similar prognostic outcomes to GBM.
CONCLUSION
Here, we have shown that traditional histopathological diagnosis of malignant astrocytomas can result in inaccurate diagnosis, especially in surgical specimens of smaller volume. When total resection volume is < 20cc by radiologic analysis or < 10cc for individual pathology specimen, the probability of diagnosing GBM decreases, making a diagnosis of AA more likely. This discrepancy led to worsening of overall survival in patients with histological AA, as they were more likely to have GBM missed by histopathological analysis. Similarly, when surgical specimen volume was < 10cc, a tendency to under-diagnose GBM by histology was observed. By using IDH1 mutational status as an additional marker, the diagnostic discrepancy at different resection or tissue specimen volumes can be minimized, as IDH status is tissue volume-independent. When tumor specimen collected for histological analysis is of insufficient volume, IDH1 molecular testing provides an independent and complementary marker for diagnosing malignant astrocytomas.
Supplementary Material
ACKNOWLEDGMENTS
The authors also wish to thank Dr. Dima Suki for helpful discussions related to statistical analysis. Financial support was provided by the Detweiler Travelling Fellowship from the Royal College of Physicians and Surgeons of Canada (B.Y.S.K.), Burroughs-Wellcome Career Award in Medical Sciences and James S. McDonnell Foundation (D.P.C.).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Stupp R, Tonn J, Brada M, Pentheroudakis G. High-grade malignant glioma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21:190–193. doi: 10.1093/annonc/mdq187. [DOI] [PubMed] [Google Scholar]
- 2.Gupta T, Sarin R. Poor-prognosis high-grade gliomas: evolving an evidence-based standard of care. Lancet Oncol. 2002;3:557–564. doi: 10.1016/s1470-2045(02)00853-7. [DOI] [PubMed] [Google Scholar]
- 3.Ryken T, Frankel B, Julien T, Olson JJ. Surgical management of newly diagnosed glioblastoma in adults: role of cytoreductive surgery. J Neurooncol. 2008;89:271–286. doi: 10.1007/s11060-008-9614-5. [DOI] [PubMed] [Google Scholar]
- 4.Tortosa A, Vinolas N, Villa S, Verger E, Gil JM, Brell M, Caral L, Pujol T, Acebes J, Ribalta T, Ferrer I, Graus F. Prognostic implication of clinical, radiologic and pathologic features in patients with anaplastic gliomas. Cancer. 2003;97:1063–1071. doi: 10.1002/cncr.11120. [DOI] [PubMed] [Google Scholar]
- 5.Scott C, Nelson J, Farnan N, Curran W, Murray K, Fischbach A, Gaspar L, Nelson D. Central pathology review in clinical trials for patients with malignant glioma. A report of radiation therapy oncology group. Cancer. 1995;767:307–313. doi: 10.1002/1097-0142(19950715)76:2<307::aid-cncr2820760222>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Capper D, Weissert S, Balss J, Habel A, Meyer J, Jager D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H, Hartmann C, von Deimling A. Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol. 2010;20:245–254. doi: 10.1111/j.1750-3639.2009.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson R, Fuller G, Abi-Said D, Lang F, Gokaslan Z, Shi W, Wildrick D, Sawaya R. Limitation of stereotactic biopsy in the initial management of gliomas. Neuro-Oncol. 2001;3:193–200. doi: 10.1093/neuonc/3.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glantz M, Burger P, Herndon J, Friedman A, Cairncross J, Vic N, Schold S. Influence of the type of surgery on the histologic diagnosis in patients with anaplastic gliomas. Neurology. 1991;41:1741–1744. doi: 10.1212/wnl.41.11.1741. [DOI] [PubMed] [Google Scholar]
- 9.Woodworth G, McGirt M, Samdani A, Garonzik I, Olivi A, Weingart J. Accuracy of frameless and frame-based image-guided stereotactic brain biopsy in the diagnosis of glioma: comparison of biopsy and open resection specimen. Neurological Research. 2005;27:358–362. doi: 10.1179/016164105X40057. [DOI] [PubMed] [Google Scholar]
- 10.La Fougere C, Suchorska B, Bartenstein P, Kreth F, Tonn J. Molecular imaging of gliomas with PET: Opportunities and limitations. Neuro-Oncology. 2011;13:806–819. doi: 10.1093/neuonc/nor054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brainard J, Prayson R, Barnett G. Frozen section evaluation of stereotactic brain biopsies: diagnostic yield at the stereotactic target position in 188 cases. Arch Pathol Lab Med. 1997;121:481–484. [PubMed] [Google Scholar]
- 12.Reithmeier T, Lopez W, Doostkam S, Machein M, Pinsker M, Trippel M, Nikkhah G. Intraindividual comparison of histopathological diagnosis obtained by stereotactic serial biopsy to open surgical resection specimen in patients with intracranial tumours. Clin Neurol Neurosurg. 2013 doi: 10.1016/j.clineuro.2013.05.019. S0303-8467(13)00175-3. [DOI] [PubMed] [Google Scholar]
- 13.Bruner J, Inouye L, Fuller GN, Langford LA. Diagnostic discrepancies and their clinical impact in a neuropathology referral practice. Cancer. 1997;79:796–803. doi: 10.1002/(sici)1097-0142(19970215)79:4<796::aid-cncr17>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Coons S, Johnson P, Scheithauer B, Yates A, Pearl D. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Owen C, Linskey M. Frame-based stereotaxy in a frameless era: current capabilities, relative role, and the postivie- and negative predictive values of blood through the needle. J Neurooncol. 2009;93:139–149. doi: 10.1007/s11060-009-9871-y. [DOI] [PubMed] [Google Scholar]
- 16.Parsons D, Jones S, Zhang X, Lin J, Leary R, Angenendt P, Mankoo P, Carter H, Siu I, Gallia G, Olivi A, McLendon R, Rasheed A, Keir S, Nikolskaya T, Nikolsky Y, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan H, Parsons W, Jin G, McLendon R, Rahseed A, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins G, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler K, Velculescu V, Vogelstein B, Bigner D. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kloosterhof N, Bralten L, Dubbink H, French P, van den Bent M. Isocitrate dehydrogenase-1: a fundamentally new understanding of diffuse glioma? Lancet Oncol. 2011;12:83–91. doi: 10.1016/S1470-2045(10)70053-X. [DOI] [PubMed] [Google Scholar]
- 19.Weller M, Wick W, von Deimling A. Isocitrate dehydrogenase mutations: A challenge to traditional views on the genesis and malignant progression of gliomas. Glia. 2011;8:1200–1204. doi: 10.1002/glia.21130. [DOI] [PubMed] [Google Scholar]
- 20.Bleeker F, Atai N, Lamba S, Jonker A, Rijkeboer D, Bosch K, Tigchelaar W, Troost D, Vandertop W, Bardelli A, Van Noorden C. The prognostic IDH1( R132 ) mutation is associated with reduced NADP+dependent IDH activity in glioblastoma. Acta Neuropathol. 2010;119:487–494. doi: 10.1007/s00401-010-0645-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao S, Lin Y, Xu W, Jiang W, Zha Z, Wang P, Yu W, Li Z, Gong L, Peng Y, Ding J, Lei Q, Guan K, Xiong Y. Glioma-Derived Mutations in IDH1 Dominantly Inhibit IDH1 Catalytic Activity and Induce HIF-1α. Science. 2009;324:261–265. doi: 10.1126/science.1170944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurian K, Haynes H, Crosby C, Hopkins K, Williams M. Isocitrate dehydrogenase mutation analysis in gliomas as a diagnostic and prognostic biomarker. The Lancet. 2013;381:S61. doi: 10.3109/02688697.2013.771139. [DOI] [PubMed] [Google Scholar]
- 23.Jansen M, Yip S, Louis D. Molecular pathology in adult gliomas: diagnostic, prognostic, and predictive markers. Lancet Neurol. 2010;9:717–726. doi: 10.1016/S1474-4422(10)70105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nobusawa S, Watanabe T, Kleihues P. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15:6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 25.Coombs SE, Rieken S, Wick W, Abdollahi A, von Deimling A, Debus J, Hartmann C. Prognostic significance of IDH-1 and MGMT in patients with glioblastoma: One step forward and one step back? Radiation Oncology. 2011;6:115–120. doi: 10.1186/1748-717X-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Preusser M, Wohrer A, Stary S, Hoftberger R, Streuber B, Hainfellner J. Value and limitations of immunohistochemistry and gene sequencing for detection of IDH1-R132H mutation in diffuse glioma biopsy specimen. J Neuropathol Exp Neurol. 2011;70:715–723. doi: 10.1097/NEN.0b013e31822713f0. [DOI] [PubMed] [Google Scholar]
- 27.Sanson M, Marie Y, Paris S, Idbaih A, Laffaire J, Ducray F, Hallani SE, Boisselier B, Mokhtari K, Hoang-Xuan K, Delattre JV. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27:4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 28.Hartman C, Hentschel R, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T, Reifenberger G, Weller M, Loeffler M, von Deimling A. Patients with IDH1 wildtype anaplastic astrocytoma exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavourable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120:707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 29.Nutt C, Mani D, Betensky R, Tamayo P, Cairncross J, Ladd C, Pohl U, Hartmann C, McLaughlin M, Batchelor T, Black P, von Deimling A, Pomeroy S, Golub T, Louis D. Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res. 2003;63:1602–1607. [PubMed] [Google Scholar]
- 30.Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, Sabel M, Koeppen S, Ketter R, Meyermann R, Rapp M, Meisner C, Kortmann R, Pietsc T, Wiestler O, Ernemann U, Bamberg M, Reifenberger G, von Deimling A, Weller M. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27:5874–5880. doi: 10.1200/JCO.2009.23.6497. [DOI] [PubMed] [Google Scholar]
- 31.Olar A, Aldape K. Biomarkers Classification and Therapeutic Decision-Making for Malignant Gliomas. Curr Treat Options Oncol. 2012;13:417–436. doi: 10.1007/s11864-012-0210-8. [DOI] [PubMed] [Google Scholar]
- 32.Weller M, Felsberg J, Hartmann C, Berger H, Steinbach J, Schramm J, Westphal M, Schackert G, Simon M, Tonn J, Heese O, Krex D, Nikkhah G, Pietsch T, Wiestler O, Reifenberger G, von Deimling A, Loeffler M. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol. 2009;27:5743–5750. doi: 10.1200/JCO.2009.23.0805. [DOI] [PubMed] [Google Scholar]
- 33.Stupp R, Mason W, van den Bent M, Weller M, Fisher B, Taphoorn J, Belanger K, Brandes A, Marosi C, Bogdahn U, Jurgen M, Eisenhauer E, Miriamnoff R. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


