Abstract
Elicitation of high-amplitude oscillations in the cardiovascular system may serve to dampen psychophysiological reactivity to emotional and cognitive loading. Prior work has used paced breathing to impose clinically valuable high-amplitude ~0.1Hz oscillations. In this study, we investigated whether rhythmical sighing could likewise produce high-amplitude cardiovascular oscillations in the very low frequency range (0.003–0.05Hz). ECG, respiration, skin conductance, and beat-to-beat blood pressure were collected in 24 healthy participants during baseline, 0.1Hz paced breathing, and 0.02Hz paced sighing (1 sigh every 50 seconds, with normal breathing interspersed). Results showed that each sigh elicited a strong, well-defined reaction in the cardiovascular system. This reaction did not habituate when participants repeatedly sighed for 8.5 minutes. The result was a high-amplitude 0.02Hz oscillation in multiple cardiovascular parameters. Thus, paced sighing is a reliable method for imposing very low frequency oscillations in the cardiovascular system, which has research and clinical implications that warrant further study.
1. Introduction
The influence of respiration on the cardiovascular system is well-established. The phenomenon of respiratory sinus arrhythmia has been extensively studied (e.g., Ritz, 2009; Grossman & Taylor, 2007; Berntson, Cacioppo, & Quigley, 1993). The phenomenon of HR baroreflex resonance in the cardiovascular system, which is observed as high-amplitude low-frequency oscillations in heart rate, blood pressure, and vascular tone when breathing is paced at a frequency of ~0.1 Hz (6 breaths per minute), further illustrates the impact of respiration on the cardiovascular system (Vaschillo, Vaschillo, Buckman, Pandina, & Bates, 2011). Both of these respiratory phenomena are essential sources of variability in heart rate, vascular tone, stroke volume, and blood pressure (Grossman & Taylor, 2007; Vaschillo et al., 2011).
Greater cardiovascular variability is associated with better physical and emotional health (Thayer, Hansen, Saus-Rose & Johnsen, 2009). Moreover, amplifying cardiovascular variability through therapeutic techniques such as HR variability (HRV) biofeedback, which trains people over the course of several weeks to rhythmically breath at 0.1 Hz (6 breaths per minute), can improve symptoms of asthma (Lehrer, Vaschillo, Vaschillo, Lu, Scardella, Siddique, & Habib, 2004), fibromyalgia (Hassett, Radvanski, Vaschillo, Vaschillo, Sigal, Karavidas, Buyske, & Lehrer, 2007), post traumatic stress disorder (Zucker, Samuelson, Muench, Greenberg, & Gevirtz, 2009), major depression (Karavidas, Lehrer, Vaschillo, Vaschillo, Marin, Buyske, Radvanski, & Hasset, 2007), neurosis (Chernigovskaia, Vaschillo, Petrash, & Rusanovski, 1990), and hypertension (McCraty, Atkinson, & Tomasino, 2003). Although the physiological mechanisms underlying the therapeutic effects of HRV biofeedback continue to be explored, enhanced sensitivity of the baroreflex (i.e., the autonomic reflex that links dynamic cardiovascular processes to each other and to neural processing) has been implicated (Chernigovskaia, et al., 1990; Lehrer, Vaschillo, & Vaschillo, 2000). This theory suggests that the 0.1 Hz breathing element of HRV biofeedback “exercises” autonomic reflexes to increase cardiovascular variability and improve baroreflex functioning, much in the same way that exercising somatic muscles improves neuromuscular reflexes (i.e., coordination and balance). In a sample of healthy individuals, improvements in respiration (i.e., peak flow) and baroreflex gain were noted after several weeks of consistent HRV biofeedback training (Lehrer, Vaschillo, Vaschillo, Lu, Eckberg, Edelberg, Shih, Lin, Kuusela, Tahvanainen, & Hammer, 2003). This long-term improvement in baroreflex gain was not replicated (Lehrer et al., 2004) in a sample of asthma patients who showed no cumulative improvements in baroreflex gain, although their clinical symptoms improved and baroreflex gain was enhanced during HRV biofeedback suggesting that 0.1 Hz paced breathing, when properly performed, has in-the-moment effects on the baroreflex.
In this study, we sought to build on knowledge of how respiration influences cardiovascular variability by examining whether high-amplitude cardiovascular oscillations also could be elicited by using rhythmical sighing paced in the very low frequency (VLF) range (0.003 – 0.05 Hz). Prior studies (Vaschillo, Lehrer, Rishe, & Konstantinov, 2002; Hammer & Saul, 2005; van de Vooren, Gademan, Swenne, TenVoorde, Schalij, & Van der Wall, 2007; Vaschillo et al., 2011) suggest that the vascular tone branch of the baroreflex is active in the VLF range and, therefore, a vascular tone baroreflex-specific resonance frequency may be observed in this range. Theoretically, triggering resonance in this range could be beneficial for mental and physical health in much the same way as are HR baroreflex 0.1 Hz resonance responses in the low frequency range. However, a major challenge to demonstrating resonance properties in the VLF range is that while most individuals, regardless of health status, can be trained to breathe at 0.1 Hz to trigger HR baroreflex resonance, few individuals other than monks or yogis (Lehrer, Sasaki, & Saito, 1999) can sustain a breathing rate in the VLF range. We present results of a study that trained young healthy adults to rhythmically sigh at 0.02 Hz (1 sigh every 50 seconds, with normal breathing interspersed). This frequency is the approximate mid-point of the VLF range and served as a starting point for exploring the effects of rhythmical sighing and the identification of resonance in this frequency range.
A sigh is a deep breath with distinct neurobiological, physiological, and psychological properties. The specific details of what differentiates a sigh from a deep breath or large lung inflation remain uncertain, but it may be defined as an augmented breath that occurs during eupneic breathing and is followed by a respiratory pause called “post-sigh apnea” (Ramirez, 2014). Others define it as a quick deep inspiration with a tidal volume at least twice as large as the mean tidal volume, and a slower expiration (e.g., see Wilhelm, Trabert, & Roth, 2001), with little emphasis on the breath being augmented or followed by apnea. Nonetheless, sighing is a common respiratory phenomenon that can arise spontaneously or be actively produced (e.g., by following instructions). Although sighs are often regarded as a symptom of abnormal or dysregulated breathing [e.g., hyperventilation syndrome (Bass & Gardner, 1985; Berczeller, 1993; Brashear, 1983; Howell, 1990; Lum, 1981; Magarian, Middaugh, & Linz, 1983)], they can prevent reductions in lung compliance and gas exchange caused by breathing with a constant volume (Mcllroy, Butler, & Finley, 1962; Ferris & Pollard, 1960; Davis & Moscato, 1994). Vlemincx et al. (2010a, 2010b, 2011b, 2012a, 2012b, 2013) further showed that both spontaneous and instructed sighs restored structured respiratory variability when it was disturbed, such as by stress, emotions, or sustained attention. These latter authors thus defined sighing as a “resetter of the respiratory system”.
We hypothesized that a single sigh would produce immediate, strong oscillations across the cardiovascular system that would fade quickly over time. Further, it was predicted that rhythmical sighing at 0.02 Hz would acutely impose high-amplitude oscillations on multiple cardiovascular parameters in the VLF range, with a spectral peak at 0.02 Hz, that would not be observed in a normal respiration baseline task. The influence of paced sighing on baroreflex sensitivity was exploratory. We also compared the cardiovascular effects of paced sighing to those of 0.1 Hz paced breathing. This paced breathing comparison was included because it is the active element of HRV biofeedback, which has well-characterized physiological and therapeutic effects. These comparisons were exploratory and there were no a priori hypotheses.
2. Method
2.1. Participants
Twenty four young healthy participants (12 women) (mean ± standard deviation: 20.17 ± 1.32 years, range 18–24) who did not drink alcohol were enrolled in the study. Participants were recruited through university and community bulletin boards, electronic postings, and flyers as part of a larger study that aimed at understanding how chronic alcohol use behaviors affect the vasculature. The racial composition was: White (58.3%), Asian (20.8%), Black or African American (12.5%), and mixed or other (8.3%); 16.7% self-reported being Hispanic or Latino. All participants reported being in a good health without respiratory or cardiac disease, or any history of psychiatric disorders; they were not alcohol, nicotine, or recreational drug users. The experiment was approved by the Rutgers University Institutional Review Board for the Protection of Human Subjects Involved in Research. Written informed consent was obtained from all participants and they were compensated for their time spent in the experiment.
2.2. Physiological Assessment
A PowerLab Acquisition System (ADInstruments, Colorado Springs, CO) and Finometer MIDI (Finapres, Amsterdam) were used to collect electrocardiogram (ECG), respiration, beat-to-beat blood pressure, and skin conductance. The sampling rate for all data collection was 2000 Hz. A standard lead II was used for ECG measurement. A cuff-sensor for blood pressure measurement was attached to the second phalange of the right middle finger. Skin conductance metal electrodes were attached to the edges of the right palm, one below the thumb and the other below the little finger. Respiratory signals were collected from a single strain belt containing piezo-electric transducer that was set around the upper part of the chest, just below the underarms. This provided a measure of thoracic breathing.
Respiration was calibrated before the start of physiological records using a standard 800 ml bag. Clips were comfortably set on the nose to ensure that the participants breathed out of their mouths only. They were told to wrap their lips tightly around the edges of the calibration tube to create a closed-loop system with the lungs. They were then asked to completely fill the calibration bag with the air from their lungs and then fully empty the bag. This process was repeated five times. The calibration tube was held in the same position throughout this procedure.
2.3. Procedure
Each participant completed one laboratory session that started between 10 a.m. and 2 p.m. to minimize biological circadian variations. In preparation for the session, participants were asked to get a good night sleep and to eat a light meal prior to, but not within 1 hour of the session. Physiological assessment was completed in a sound-attenuated, dimly lit testing room. Participants were seated in a comfortable chair located 1 m in front of a LCD TV screen, and physiological sensors and electrodes were attached. ECG, blood pressure, skin conductance, and respiration were continuously collected while participants completed three tasks. The sequence of tasks in the study included a baseline task, rhythmical 0.02 Hz sighing task, and 0.1 Hz paced breathing task.
The baseline task (B1) was a 5-minute low-demand cognitive task (plain ‘Vanilla’ task; Jennings, 1992) that provides a more standardized baseline than an uncontrolled resting state. A rectangle presented in the center of the TV screen changed color every 10 sec for 5-min, and participants were asked to silently count the number of rectangles that were blue in color.
The 0.02 Hz paced sighing task (0.02Hz-PS) lasted 8.5 minutes during which participants performed 10 sighs. Participants were instructed to breathe normally until a red screen appeared on the TV, at which point they were asked to start a sigh. The time interval between red screens was every 50 s; the screen remained red for 2 s. The red screen presentation was programmed using E-Prime software (Psychology Software Tools, Inc., Pittsburgh, PA) with an accuracy of ±1 ms. Before starting the recording task, participants were asked to voluntarily perform a few sighs (to determine what was natural for them). They then underwent a short training to become familiarized with the task of sighing at paced intervals. They were informed that during the task, they should sigh naturally. It was emphasized that they should inhale and exhale through their mouth, and to avoid breathing too deeply, which could result in hyperventilation (i.e., feeling dizzy or lightheaded). Respiratory volume of the participants’ sighs was measured from a thoracic respiratory belt and monitored to ensure it was at least twice the volume of breaths taken during the baseline (normal respiration) task. Participants were asked to breathe normally between sighs, remain still other than to sigh along with presentation of the red screen, and avoid speaking during the task.
The 0.1 Hz paced breathing task (0.1Hz-PBr) lasted 5 minutes. Participants were instructed to breathe at a rate of approximately six complete breathing cycles per minute by following a visual pacer (Easy Air, Biofeedback Foundation of Europe, Montreal, Canada) presented on the TV screen. The pacer was set to allow for a 5.5 sec inhalation and a 5.5 exhalation. Participants inhaled as the visual pacer moved vertically up, and exhaled as it moved down. There was no pause between breaths except in the case when a participant completed their exhale prior to the pacer reaching the bottom. In this case, they were told to pause their breathing and begin again exactly when the pacer reached its bottom. If they completed the inhale prior to the pacer reaching its maximum, they were allowed to start exhaling early. It was emphasized that the key respiratory element was that each inhalation should synchronize with the bottom of the pacer. A short training and practice period was used to show participants how to avoid hyperventilating and to reduce effortful breathing.
2.4. Analysis of Physiological Data
Physiological data were analyzed using WinCPRS software (Absolute Alien Oy, Finland). Artifacts and missed or irregular beats were manually modified by interpolation prior to the analysis. Analysis was performed separately for each task (B1, 0.02Hz-PS, and 0.1Hz-PBr). The software was used to measure the time intervals between R-to-R waves of ECG (RRI), beat-to-beat systolic and diastolic arterial pressure (SAP and DAP) from finger pulse waves recorded by the Finometer, beat-to-beat pulse transit time as the time interval between the R-wave of the ECG and the apex of the finger pulse wave to evaluate the dynamics of vascular tone; higher pulse transit time corresponds to lower vascular tone (Naschitz, Bezobchuk, Mussafia-Priselac, Sundick, Dreyfuss, Khorshidi, Karidis, Manor, Nagar, Peck, Peck, Storch, Rosner, & Gaitini, 2004; Naka, Tweddel, Doshi, Goodfellow, & Henderson, 2006). The ModelFlow methodology for cardiac output measurement (Bogert & van Lieshout, 2005) was used to assess beat-to-beat stroke volume. Cubic interpolation of the non-equidistant waveforms of the beat-to-beat sequences was completed, and measurements were resampled at 4 Hz. The same software was used to measure thoracic respiration tidal volume.
Data were analyzed in two ways. First, the average physiological reactions across the 5-minute tasks were calculated for each individual and averaged across all 24 participants. Second, Fourier spectra for each task and each participant were calculated to allow calculation of peak power at 0.02 Hz and 0.1 Hz. The latter analysis allowed us to assess changes in variability caused by the 0.02Hz-PS and 0.1Hz-PBr tasks.
Activity of the HR, stroke volume, and vascular tone arcs of the baroreflex were evaluated as HR, stroke volume, and vascular tone baroreflex gains calculated using cross-spectral analysis (Cooke, Hoag, Crossman, Kuusela, Tahvanainen, & Eckberg, 1999) via the transfer functions, where SAP was the input, and HR, stroke volume, and vascular tone, respectively, were the output. The gains were calculated as the mean values of the corresponding transfer functions in the low frequency range (0.05 – 0.15 Hz) where coherence between the input and output functions was > 0.5 (Vaschillo, Vaschillo, Buckman, Pandina, & Bates, 2012).
In addition, the reaction of HR and SAP to sigh volume was measured. The peaks of the first positive phase in HR and SAP were used to estimate the cardiovascular response magnitude and the thoracic volume of each sigh was used to estimate sigh depth. The first positive phase peak was used but we note that cardiovascular reactions are complex and included several distinct phases (e.g., an acute increase, an abrupt decrease, and a gradual return to baseline).
2.5. Statistical Analysis
First, the association of cardiovascular reactivity to sigh inhalation depth was calculated using Pearson’s correlation coefficients. Within-person analyses correlated the thoracic volumes of each of 10 sighs with the corresponding magnitude of HR and SAP reactions. Across participants, the average thoracic volume was computed for each participant and correlated with average HR and SAP reaction.
Second, linear mixed effects models (SAS Proc Mixed) with one-way repeated measures was used to assess differences in physiological reactivity. The linear mixed effects model is a statistical test that parallels ANOVA, but is more flexible for testing random effects. Three tasks (B1, 0.02Hz-PS, 0.1Hz-PBr) were compared. For each task, average physiological responses and spectral power peaks at 0.02 and 0.1 Hz were analyzed. All physiological parameters were log transformed prior to analyses. We had a priori hypotheses regarding the differences in cardiovascular reactivity between paced sighing and baseline (B1) breathing, and also planned to explore differences between paced sighing and paced breathing. Thus, planned pair-wise comparisons included (a) a comparison of the 0.1Hz spectral power peak during baseline vs. the paced breathing task, (b) a comparison of 0.2Hz spectral power peak during baseline vs. the paced sighing task, and (c) a comparison of 0.1Hz spectral power peak during paced breathing vs. the 0.2Hz spectral power peak during the paced sighing task. The Tukey-Kramer adjustment was used for post-hoc pairwise comparisons. Analyses were performed using SAS 9.3. Effect size calculations were performed using Cohen’s drm (for repeated measures designs) as suggested by Lakens (2013). As a standard of reference, drm .20–.49 = small, drm .50–.79 = medium, and drm ≥ .80 = large effect sizes (Cohen, 1988).
3. Results
The mean thoracic respiratory volume across all sighs in all participants was 1590 ± 1188 ml. Individual participant’s average sigh volume (estimated from the thoracic belt) varied from 715 ml to 4823 ml, but the within-person variability in sigh volume typically remained within 15 % of their mean. Only 3 of the 24 participants showed a significant correlation between the volume of their 10 individual sighs and the magnitude of the corresponding HR or SAP responses. When average sigh volumes and cardiovascular responses were then correlated across individuals, there were no significant correlations between an individual’s average sigh volume and the magnitude of the HR or SAP responses (HR: r [24] = 0.06, p = 0.798; BP: r [24] = −0.11, p = 0.245.).
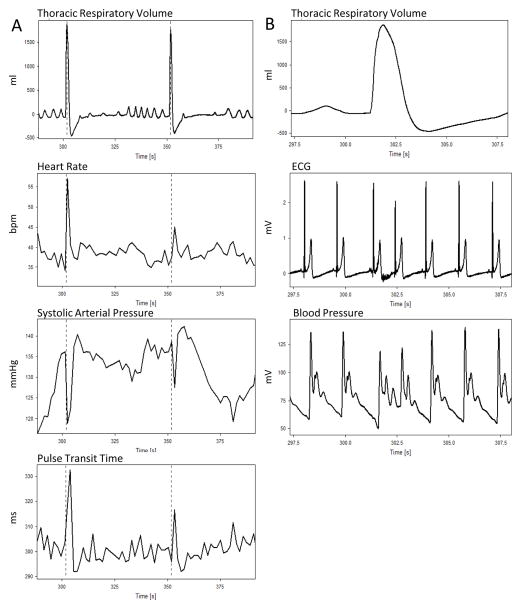
Figure 1 shows an example of the respiratory profile of an instructed sigh as well as the HR, SAP, and pulse transit time responses. Note that although the depth of both sighs shown in Figure 1A was equivalent, the magnitude of the HR, SAP, and pulse transit time reactions differed. In this example, eupneic breathing was observed immediately prior to the initiation of a deep inspiration, which in turn was followed by a brief apneic pause. Approximately 70% of all attempted sighs met this definition of a sigh; the other 30% happened in the absence of either normal breathing prior, broken inspiration patterns, or apnea after, but all attempted sighs presented as large lung inflations. Figure 1B shows that the raw respiratory signal (top panel) shortened the RR interval (middle panel) and decreased blood pressure (bottom panel).
Figure 1. Respiratory and cardiovascular reactions to a sigh.
(A) The top panel shows that the paced sighing task frequently elicited sighs with eupnea prior to the sigh, deep inspirations, and a post-sigh apneic pause. The cardiovascular reactions (middle and lower panels) were sizeable, but did not correlate with the depth of the inhalation.
(B) The raw respiratory (top panel) and cardiovascular signals observed during the paced sighing task. Note that the RR interval (middle panel) was shortened and systolic and diastolic blood pressure (bottom panel) was decreased.
Average respiration and cardiovascular responses during each task were calculated to test whether the paced sighing and breathing tasks led to overall increases in mean heart rate, blood pressure, or other cardiovascular parameters, compared to baseline. As shown in Table 1, 0.02Hz-PS had less effect on mean cardiovascular functioning than 0.1Hz-PBr. Specifically, 0.1Hz-PBr significantly increased mean thoracic respiratory tidal volume (drm = 1.8), SAP (drm = 0.8), and DAP (drm = 0.7) over B1 averages, whereas 0.02Hz-PS significantly increased only mean thoracic respiratory tidal volume (drm = 1.0) and SAP (drm = 0.4) over B1 averages. Moreover, 0.1Hz-PBr increased mean thoracic tidal volume (drm = 1.0) and SAP (drm = 0.4) to a significantly greater degree than 0.02Hz-PS (Table 1). The 0.1Hz-PBr task also significantly increased HR baroreflex (drm = 0.8), vascular tone baroreflex (drm = 0.7), and stroke volume (drm = 1.2) baroreflex gains over B1 averages, whereas 0.02Hz-PS did not.
Table 1.
Average physiological parameters during baseline, paced breathing, and paced sighing.
| Baseline | 0.1 Hz Paced Breathing | 0.02 Hz Paced Sighing | Overall Model F | |
|---|---|---|---|---|
| Thoracic Respiratory Vol [ml] | 209.4 ± 28.6 | 873.3 ± 181.0a | 380.4 ± 47.5a,b | F(2,46)=64.49* |
| Heart Rate [bpm] | 70.0 ± 2.2 | 71.2 ± 2.3 | 71.0 ± 2.3 | F(2,46)=0.55 |
| Systolic Arterial Pressure [mmHg] | 122.5 ± 2.8 | 134.5 ± 3.2a | 128.9 ± 3.3a,b | F(2,46)=11.78* |
| Diastolic Arterial Pressure [mmHg] | 65.0 ± 1.6 | 71.7 ± 2.4a | 67.9 ± 2.2b | F(2,46)=8.31* |
| Stroke Volume [ml] | 69.7 ± 1.9 | 73.1 ± 2.4 | 68.9 ± 1.6 | F(2,46)=1.74 |
| Pulse Transit Time [ms] | 291. 0 ± 4.1 | 287.5 ± 3.7 | 290.4 ± 4.0 | F(2,46)=1.81 |
| Heart Rate Baroreflex Gain [ms/mmHg] | 12.7 ± 1.2 | 17.8 ± 1.5a | 12.2 ± 1.1b | F(2,46)=10.05* |
| Vascular Tone Baroreflex Gain [ms/mmHg] | 0.7 ± 0.1 | 0.9 ± 0.1a | 0.8 ± 0.1b | F(2,46)=7.93* |
| Stroke Volume Baroreflex Gain [ml/mmHg] | 0.6 ± 0.1 | 1.0 ± 0.1a | 0.7 ± 0.1b | F(2,44)=7.88* |
Notes. Data are presented as mean ± standard error. Paced Sighing: Participants sighed at a rate of 1 sigh per 50 sec (0.02 Hz) with normal breathing interspersed for 8.5 minutes. Paced Breathing: Participants breathed at a rate of 6 breaths per min (0.1 Hz) for 5 minutes.
overall model was significant at p < .05;
p < .05 compared to baseline.
p < .05 compared to paced breathing. Statistical analyses were performed on log-transformed variables.
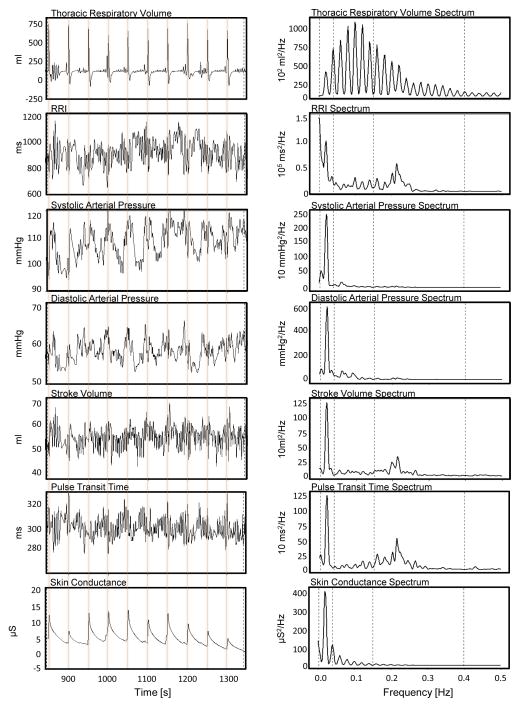
We next sought to examine how oscillations in the cardiovascular system changed in response to rhythmical sighing, and again compare these changes to those induced by 0.1 Hz paced breathing. Figure 2 shows data from one participant during the paced sighing task. The top left panel demonstrates the ability of this participant to rhythmically sigh at 0.02 Hz. The top right panel of Figure 2 shows the respiratory volume power spectrum from this participant, with clear harmonic features present. Time series data (left panels, Figure 2) show that each sigh produced powerful, but temporally constrained cardiovascular responses that gradually faded over approximately 50 s. Fourier transformed data (right panels, Figure 2) show that paced sighing at 0.02 Hz produced clear spectral peaks at 0.02 Hz. No habituation to paced sighing was observed in any cardiovascular process for this or any participant; in other words, each sigh generated a similar ~50s cardiovascular response that seemed unrelated to the thoracic volume of the sigh.
Figure 2. Reaction of respiration and cardiovascular processes to 0.02 Hz rhythmical sighing in one participant.
Each sigh elicited large, phasic shifts in all recorded cardiovascular functions as observed in the time series data (left panels). The corresponding frequency spectra (right panels) revealed a dominant peak at 0.02 Hz. RRI - time intervals between R-to-R waves of the electrocardiogram; PTT - pulse transit time as a time interval between R-wave of the ECG and the apex of the finger pulse that reflects the vascular tone. Note that lower PTT corresponds to higher vascular tone.
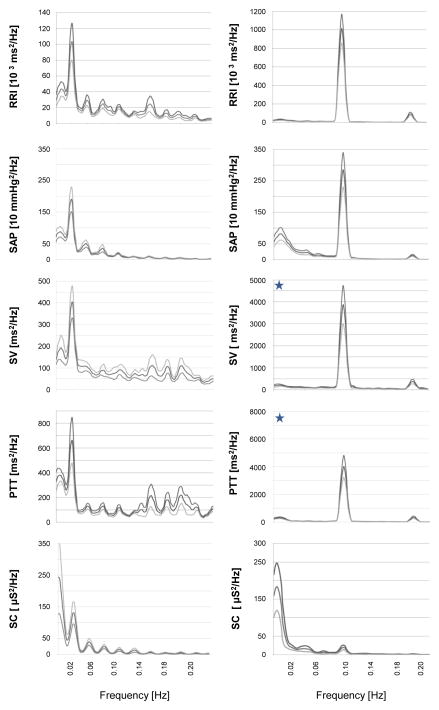
Figure 3 extends these observations by showing the averaged RRI, SAP, stroke volume, pulse transit time, and skin conductance spectra during 0.02 Hz paced sighing (left panels) and 0.1 Hz paced breathing (right panels). As expected, a power peak at 0.1 Hz was observed for all 24 participants in response to the paced breathing task. We also observed a power peak at 0.02Hz for all physiological processes in response to the paced sighing task. Note that the y-axis scale of the RRI, stroke volume, and pulse transit time panels differ (i.e., are ~10-fold larger for the paced breathing reactions [right] compared to paced sighing [left]). The peak spectral volumes at 0.02 and 0.1 Hz for each cardiovascular parameter are also shown in Table 2. The amplitude of the 0.02 Hz oscillation during the 0.02Hz-PS task was significantly higher than its amplitude during the baseline task for RRI (Cohen’s drm = 0.7), SAP (Cohen’s drm = 1.2), stroke volume (Cohen’s drm = 1.2), and pulse transit time (Cohen’s drm = 1.5). The amplitude of the 0.02Hz oscillation was also greater than the 0.1 Hz oscillation during the baseline task for RRI (Cohen’s drm = 1.0), stroke volume (Cohen’s drm = 1.1), pulse transit time (Cohen’s drm = 1.0). However, the amplitude of 0.02 Hz oscillation in all physiological processes elicited by the 0.02Hz-PS task, except for SAP, was significantly lower than the amplitude of 0.1 Hz oscillation elicited by the 0.1Hz-PBr task. The effect sizes for these comparisons are as follows: RRI (Cohen’s drm = 2.1), stroke volume (Cohen’s drm = 1.2), pulse transit time (Cohen’s drm = 1.8), and skin conductance (Cohen’s drm = 1.0).
Figure 3. Frequency spectra of cardiovascular functions averaged across 24 participants.
Spectra presented as mean power ± std error at each frequency. Left panels show spectra of cardiovascular processes for the 0.02 Hz paced sighing task. Right panels show spectra of cardiovascular processes for the 0.1 Hz paced breathing task. The blue stars denote that the y-axis scale of the RRI, stroke volume, and pulse transit time panels differ (i.e., are ~10-fold larger for the paced breathing [right] compared to paced sighing [left]). Peaks in the spectra at 0.02 Hz and 0.1 Hz demonstrate the presence of prominent oscillations at these frequencies in all cardiovascular processes measured. The 0.02 Hz oscillations elicited by paced sighing are considerably lower than corresponding 0.1 Hz oscillations elicited by paced breathing, except for systolic pressure and skin conductance spectra. Larger skin conductance oscillations at 0.02 Hz comparing to 0.1 Hz oscillations show that 0.02 Hz paced sighing more strongly activated the sympathetic system than 0.1 Hz breathing.
Table 2.
Comparison of average spectral power at 0.02 Hz and 0.1 Hz during the plain vanilla baseline task and in response to paced respiratory tasks.
| Power at 0.02 Hz | Power at 0.1 Hz | Overall Model F | |||
|---|---|---|---|---|---|
| Plain Vanilla | Paced Sighing | Plain Vanilla | Paced Breathing | ||
| RRI [10 3 ms2 /Hz] | 33.2 ± 7,8a | 103.4 ± 23.2 b,c | 14.5 ± 4.0 | 1013± 155a | F(3,96)=92.0* |
| SAP [mmHg2 /Hz] | 471 ± 92a | 1904 ± 388 b | 76 ± 15 | 2846 ± 545a | F(3,96)=62.6* |
| Stroke Volume [ml2 /Hz] | 100 ± 27 | 405 ± 74 b,c | 45 ± 18 | 3871 ± 862a | F(3,96)=43.4* |
| Pulse Transit Time [ms2 /Hz] | 153 ± 26 | 663 ± 185 b,c | 87 ± 35 | 4012 ± 793a | F(3,96)=90.5* |
| Skin Conductance [μS2 /Hz] | --- | 130 ± 35c | --- | 20 ± 6 | F(1,23)=29.7* |
Notes. Data are presented as mean ± standard error. Paced Sighing: Participants sighed at a rate of 1 sigh per 50 sec (0.02 Hz) with normal breathing interspersed for 8.5 minutes. Paced Breathing: Participants breathed at a rate of 6 breaths per min (0.1 Hz) for 5 minutes. RRI - R wave-to-R wave intervals of the electrocardiogram. SAP = systolic arterial pressure.
overall model was significant at p < .05;
p < .05 compared to plain vanilla at 0.1 Hz power,
p < .05 compared to plain vanilla at 0.02 Hz power,
p < .05 compared to paced breathing at 0.1 Hz power
4. Discussion
This paper represents an initial attempt to understand how rhythmical sighing influences the cardiovascular system. We characterized the effects of paced 0.02 Hz sighing on the cardiovascular system, and compared paced 0.02 Hz sighing to 0.1 Hz breathing. Both maneuvers produced high-amplitude oscillations in the cardiovascular system, with robust spectral peaks found at the respective task frequencies (Figure 3, Table 2). Paced sighing had less effect on average cardiovascular functioning compared to paced breathing (Table 1). We frame the results within the context of current theories on sighing, and focus on the potential mechanistic importance and clinical implications of being able to manipulate the cardiovascular system in the very low frequency range.
4.1. The cued sigh procedure
The present results come from an experiment in which young, healthy participants were asked to voluntarily produce sighs when cued. Recently, Ramirez (2014) defined the sigh as including three components: pre-sigh eupnea, a large lung inflation, and post-sigh apnea. Analysis of the cued sighs performed by our participants revealed that approximately 70% of cued sighs demonstrated all three of these sigh components. This provides support that the paced sighing task did not simply produce repeated, large lung inflations; rather most respiratory responses appeared to fit Ramirez’s (2014) operational definition of a sigh. Of the 30% that did not meet criteria, several were preceded by irregular, rather than normal breathing, and others showed no post-sigh apnea. This does not imply necessarily that these responses were not true sighs, as sighing in humans is less well understood than in the animal literature, but suggests that we cannot say definitively that they were. Although we visually observed no consistent differences in cardiovascular responses to sighs that met Ramirez’s criteria versus those that did not, it was not possible to explicitly test this. Future studies that differentiate the effects of the sigh elements on physiological and psychological reactivity are needed, as are studies specifically designed to test similarities and differences between cued and spontaneous sighs.
Nonetheless, the present study shows a link between respiratory manipulations in the very low frequency range and cardiovascular reactivity. The idea that a cued, large lung inflation, rather than fully defined sighs, was sufficient to elicit the noted cardiovascular reactions is compelling in itself. It is also noteworthy that we saw no correlation between the depth of these breaths and HR or BP reactivity, while during normal breathing, HR and BP are typically dependent on respiratory depth. For simplicity, and because the majority of cued respiratory responses to our paced sighing task did elicit fully defined sighs, we frame the rest of this discussion around “sighing”, although we acknowledge that more research is needed.
4.2. Rhythmical sighing and its cardiovascular effects
A major goal of our current research program is to identify new ways of stimulating the cardiovascular system in the very low frequency range to explore the vascular tone branch of the baroreflex and identify resonance properties that may be useful clinically. In this study, we asked participants to produce a sigh every 50 seconds (0.02 Hz, in the very low frequency range) and to otherwise breathe normally. We observed well-defined cardiovascular reactions in response to each individual cued sigh that lasted about 50 s and included multiple components such as, in the case of heart rate, a short-term large amplitude increase that lasted ~3 seconds, an abrupt decrease with overshot baseline HR, followed by a gradual return to baseline (and return to normal oscillations). Repeatedly sighing produced systematic cardiovascular reactions across the 8.5-min paced sighing task, with no evident cardiovascular habituation as sighing continued. The ability to pace a simple respiratory maneuver and see a consistent, well-defined cardiovascular reaction has potential clinical value, as described below. Moreover, the fact that cardiovascular reactions were observed to individual sighs suggests that it is plausible that high amplitude cardiovascular oscillations also could be elicited by rhythmical sighing at other frequencies and may be particularly useful for exploring cardioregulatory mechanisms at play in the very low frequency range. Mechanistically, it may be proposed that each sigh causes an acute decrease in vascular tone, which leads to an acute decrease in blood pressure. This, in turn, leads to an increase in HR, followed by a slow normalization of blood pressure and HR (e.g., see Fig. 1). Thus, sighing may modulate afferent outflow from the baroreceptors and consequently amplify neural inhibition.
Like paced breathing, paced sighing elicited high-amplitude oscillations in heart rate, blood pressure, stroke volume, pulse transit time, and skin conductance in young, healthy people (Figure 3, Table 2). These large oscillations occurred in the absence of correspondingly large shifts in average levels of cardiovascular functions (Table 1). In other words, repeated sighing at a given frequency successfully imposed oscillations in cardiovascular processes at that frequency without provoking major increases in HR, stroke volume, pulse transit time, or diastolic blood pressure; increases in systolic pressure were statistically significant but clinically negligible.
Paced sighing did not lead to an increase in HR-, vascular tone-, or stroke volume-baroreflex gain, whereas 0.1 Hz breathing did (Table 1). This may suggest that paced 0.1 Hz breathing is more powerful at activating the baroreflex mechanism than paced 0.02 Hz sighing. Conversely, this result may be a methodological artifact from using the common method of cross-spectrum analysis and measuring gain in the low frequency range where coherence was > 0.5. Importantly, 0.02 Hz sighing did not change coherence in the low frequency range whereas 0.1 Hz breathing did. It is possible that 0.02 Hz sighing would increase baroreflex gain in the very low frequency range, but to our knowledge, this has not been tested.
4.3. Clinical applications of rhythmical sighing through generation of cardiovascular oscillations
The rhythmical sighing manipulation used in this study was meant to emulate the rhythmical breathing procedures used as part of HRV biofeedback to affect the cardiovascular system and produce measureable clinical benefits (e.g., Lehrer et al., 2003, 2004). It has been suggested that the therapeutic effects of HRV biofeedback stem from the high-amplitude cardiovascular oscillations that are elicited by rhythmical 0.1 Hz breathing. Two mechanisms are known to underlie the resulting high-amplitude cardiovascular oscillations. The first mechanism is respiratory sinus arrhythmia, or the respiratory modulation of HR through the vagus (Porges, 2009). The second mechanism is resonance in the HR baroreflex system (Hammer & Saul, 2005; Vaschillo et al., 2011). These high-amplitude oscillations act by training autonomic reflexes, supporting the active state of these reflexes, and restoring sympathetic-vagal balance (Chernigovskaia, et al., 1990; Lehrer, Vaschillo, & Vaschillo, 2000; Lehrer, Vaschillo, Vaschillo, Lu, Eckberg, Edelberg, Shih, Lin, Kuusela, Tahvanainen, & Hammer, 2003). The same oscillations modulate the afferent firing from baroreceptors, which elicits direct central nervous system responding through the central autonomic network. In doing so, high-amplitude cardiovascular oscillations appear to promote neuroinhibitory processes and restore excitation-inhibition balance in the brain (Lacey, & Lacey, 1970; Dworkin, Elbert, Rau, Birbaumer, Pauli, Droste, & Brunia, 1994; Elbert, Roberts, Lutzenberger, & Birbaumer, 1992; Rau, Pauli, Brody, Elbert, & Birbaumer, 199; Nyklicek, Wijnen, & Rau, 2005; Reyes del Paso, Gonzalez, & Hernandez, 2004; Yasumasu, Reyes Del Paso, Takahara, & Nakashima, 2006; Duschek, Wörsching, & Reyes del Paso, 2013). Thus, HRV biofeedback has therapeutic value because its core component, rhythmical 0.1 Hz breathing, modulates neurocardiac signaling, which, in turn, promotes mental and physical health.
HRV biofeedback continues to gain popularity in the clinic and laboratory, but pacing one’s breathing at 0.1 Hz (6 breaths per minute) presents challenges to first-time practitioners and to individuals with health conditions that preclude their ability to properly slow their breathing without strain. This is evident in our study where all participants were naïve to the paced breathing task and thus showed modest elevations in average systolic and diastolic blood pressure during the task. The slow paced breathing techniques used in HRV biofeedback are also limited in their inability to activate the cardiovascular system in the very low frequency range (i.e., 3 or fewer breaths per minute). Thus, HRV biofeedback breathing is less capable of “exercising” vascular dynamics compared to its effects on cardiac dynamics.
Very little has been done to assess the clinical utility of alternative paced respiratory maneuvers that may allow insight into vascular dynamics and very low frequency mechanisms. In a study of children who had undergone a major surgery and received either pressure support ventilation alone or pressure support ventilation with rhythmical sighing paced at one per minute (Nacoti, Spagnolli, Bonanomi, Barbanti, Cereda, & Fumagalli, 2012), paced sighing was shown to improve gas exchange and decreased respiratory drive without major short-term complications. We know of no other studies that have assessed rhythmical sighing in the very low frequency range. In the present study, we showed that paced sighing (or, possibly, paced large lung inflations) produces similar types of cardiovascular reaction (albeit sometimes smaller) to paced breathing, and that paced sighing is simple to perform and easy to teach. Thus, sighing paced at very low frequencies potentially may be useful as a foundation for clinical interventions aimed at improving vascular tone regulation. More research is obviously needed to determine its clinical utility, but the present findings provide a framework upon which new studies could be designed.
4.4. Limitations
The results of this study should be considered in light of several limitations. First, a critical element of a sigh is its ability to generate subjective psychological relief. We did not, however, directly assess whether the paced sighing task promoted a state of psychological well-being. Second, we measured respiration using a single respiratory belt placed thoracically. This was due to other elements of the experimental design that impeded the use of a belt placed abdominally. Thus, although thoracic respiratory depth was unrelated to cardiovascular response in this study, it is possible that abdominal respiratory depth would have been. Moreover, it is possible that the inter-individual variability in sigh depth may have been reduced if abdominal respiration had also been assessed. Third, we did not measure end tidal CO2 and thus cannot address whether some of the observed cardiovascular effects were due to hypocapnia. Fourth, as discussed above, not all respiratory responses during the paced sighing technique met the full criteria (Ramirez, 2014) for a sigh. Finally, this study assessed rhythmical sighing paced at 0.02 Hz only.
As noted above, neither paced sighing nor paced breathing changed mean HR compared to baseline, but both significantly increased mean systolic arterial pressure compared to baseline. Paced breathing at 0.1 Hz also led to significant elevations in mean diastolic pressure, and the systolic pressure elevation was significantly larger compared to that observed during paced sighing. The respiratory muscle activation involved in pacing may have elevated metabolic demand and thus impacted average cardiovascular functioning. However, it is unlikely that the voluntary muscle activation in the two tasks was equal, since paced breathing requires constant voluntary activation whereas the paced sighing task required only intermittent activation. The lack of HR changes in response to paced breathing parallels prior studies (e.g., Lehrer et al., 2000, 2003). The increase in systolic and diastolic pressure in response to paced breathing was likely due to the lack of training time: often, individuals find slow breathing effortful at first, before learning to appropriately regulate breathing frequency without hyperventilating. HRV biofeedback includes multiple training sessions to promote effortlessness and, over time, 0.1 breathing is subjectively experienced by most practitioners as relaxing. The fact that paced sighing did not change HR or diastolic pressure and resulted in a significantly smaller increase in systolic pressure suggests that this rhythmical respiratory manipulation may be easier and quicker to learn, be experienced as more natural (or relaxing) to the participant, and/or acts more passively.
4.5. Conclusions
Prior studies showed that a sigh is a respiratory maneuver associated with stressful events, negative emotions, and unpleasant thoughts as well as cognitive loading and sustained attention (Vlemincx, Van Diest, De Peuter, Bresseleers, Bogaerts, Fannes, Li, & Van Den Bergh, 2009; Blechert, Michael, Grossman, Lajtman, Wilhelm, 2007; Wilhelm, Trabert, & Roth, 2001). Vlemincx et al. (2013) proposed a “sigh-resetter” model wherein a sigh was conceptualized as a physiological and psychological phenomenon that restores respiratory control and normalizes respiration variability, thereby mitigating stress and emotional arousal. The model further implied that sighing is part of a homeostatic system and, as such, serves as an acute response to stress. The present study extends this model by suggesting that a sigh also can be used to activate the cardiovascular system.
We speculate that a sigh triggers cardiovascular oscillations that activate afferent neural streams from the baroreceptors, which reduce cortical excitation and produce subjective reductions in stress. Under normal circumstances, this baroreflex protective mechanism adaptively adjusts to changes in the internal milieu and from the external environment. We hypothesize that a sigh arises when this protective mechanism becomes compromised by stress or strong emotions. According to this hypothesis, the sigh “jump starts” the baroreflex protective mechanism by triggering large-scale cardiovascular changes. In doing so, the sigh mitigates stress and emotional arousal, and this reduction in stress and emotional arousal leads to restoration of respiratory variability.
The present study demonstrates that paced sighing is a reliable method for imposing stable very low frequency oscillations in the cardiovascular system. This method may be a valuable research tool for evaluating amplitude and phase interrelations between cardiovascular parameters in the very low frequency range to investigate mechanisms that regulate processes in this range (e.g., identifying resonance frequencies of the cardiovascular system). Moreover, this method may have clinical implications that should be explored in future studies.
Highlights.
A single sigh strongly affects a number of cardiovascular functions.
Rhythmical sighing at 0.02 Hz imposes high-amplitude oscillations in functions.
Imposed oscillations can be used to study the mechanisms that regulate functions.
A sigh may be an acute respiratory maneuver to mitigate stress via baroreflex mechanism.
High-amplitude oscillations imposed by rhythmical sighing may be clinically useful.
Acknowledgments
Funding was provided by R21AA020367, K01AA017473, R01AA015248, HHSN275201000003C, K02AA00325, and K24AA021778 (NIH-NIAAA). These funding sources were not involved in the study design, analysis, or interpretation of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bass C, Gardner WN. Respiratory and psychiatric abnormalities in chronic symptomatic hyperventilation. British Medical Journal. 1985;290(6479):1387–90. doi: 10.1136/bmj.290.6479.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berczeller PH. Hyperventilation and sighing. Hospital Practice. 1993;28(7):37–41. doi: 10.1080/21548331.1993.11442818. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respiratory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30(2):183–96. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Grossman P, Lajtman M, Wilhelm FH. Autonomic and respiratory characteristics of posttraumatic stress disorder and panic disorder. Psychosomatic Medicine. 2007;69(9):935–43. doi: 10.1097/PSY.0b013e31815a8f6b. [DOI] [PubMed] [Google Scholar]
- Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Experimental Physiology. 2005;90(4):437–46. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- Brashear RE. Hyperventilation syndrome. Lung. 1983;161(5):257–73. doi: 10.1007/BF02713872. [DOI] [PubMed] [Google Scholar]
- Chernigovskaia NV, Vashchillo EG, Petrash VV, Rusanovski VV. Voluntary regulation of the heart contraction rate as a method for correcting the functional status of neurosis patients. Fiziologiia Cheloveka. 1990;16(2):58–64. [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Cooke WH, Hoag JB, Crossman AA, Kuusela TA, Tahvanainen KU, Eckberg DL. Human responses to upright tilt: a window on central autonomic integration. The Journal of Physiology. 1999;517(2):617–28. doi: 10.1111/j.1469-7793.1999.0617t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GM, Moscato J. Changes in lung mechanics following sighs in premature newborns without lung disease. Pediatric Pulmonology. 1994;17(1):26–30. doi: 10.1002/ppul.1950170106. [DOI] [PubMed] [Google Scholar]
- Del Paso GA, Gonzalez MI, Hernandez JA, Duschek S, Gutierrez N. Tonic blood pressure modulates the relationship between baroreceptor cardiac reflex sensitivity and cognitive performance. Psychophysiology. 2009;46(5):932–8. doi: 10.1111/j.1469-8986.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- Delgado LC, Vila J, Reyes Del Paso GA. Proneness to worry is negatively associated with blood pressure and baroreflex sensitivity: Further evidence of the blood pressure emotional dampening hypothesis. Biological Psychology. 2014;96:20–7. doi: 10.1016/j.biopsycho.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Duschek S, Wörsching J, Reyes del Paso GA. Interactions between autonomic cardiovascular regulation and cortical activity: a CNV study. Psychophysiology. 2013;50(4):388–97. doi: 10.1111/psyp.12026. [DOI] [PubMed] [Google Scholar]
- Duschek S, Muckenthaler M, Werner NS, Reyes del Paso GA. Relationships between features of cardiovascular control and cognitive performance. Biological Psychology. 2009;81(2):110–117. doi: 10.1016/j.biopsycho.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Dworkin BR, Elbert T, Rau H, Birbaumer N, Pauli P, Droste C, Brunia CH. Central effects of baroreceptor activation in humans: attenuation of skeletal reflexes and pain perception. Proceedings of the National Academy of Sciences of the U S A. 1994;91(14):6329–33. doi: 10.1073/pnas.91.14.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Roberts LE, Lutzenberger W, Birbaumer N. Modulation of slow cortical potentials by instrumentally learned blood pressure responses. Psychophysiology. 1992;29(2):154–164. doi: 10.1111/j.1469-8986.1992.tb01678.x. [DOI] [PubMed] [Google Scholar]
- Ferris BG, Pollard DS. Effect of deep and quiet breathing on pulmonary compliance in man. The Journal of Clinical Investigation. 1960;39:143–9. doi: 10.1172/JCI104012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Barrett AB, Minati L, Dolan RJ, Seth AK, Critchley HD. What the heart forgets: Cardiac timing influences memory for words and is modulated by metacognition and interoceptive sensitivity. Psychophysiology. 2013;50(6):505–12. doi: 10.1111/psyp.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74(2):263–85. doi: 10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Hammer PE, Saul JP. Resonance in a mathematical model of baroreflex control: arterial blood pressure waves accompanying postural stress. American Journal of Physiology (Regulatory, Integrative and Comparative Physiology) 2005;288(6):R1637–48. doi: 10.1152/ajpregu.00050.2004. [DOI] [PubMed] [Google Scholar]
- Hassett AL, Radvanski DC, Vaschillo EG, Vaschillo B, Sigal LH, Karavidas MK, Buyske S, Lehrer PM. A pilot study of the efficacy of heart rate variability (HRV) biofeedback in patients with fibromyalgia. Applied Psychophysiology and Biofeedback. 2007;32(1):1–10. doi: 10.1007/s10484-006-9028-0. [DOI] [PubMed] [Google Scholar]
- Hirose S. Restlessness of respiration as a manifestation of akathisia: five case reports of respiratory akathisia. The Journal of Clinical Psychiatry. 2000;61(10):737–41. doi: 10.4088/JCP.v61n1005. [DOI] [PubMed] [Google Scholar]
- Howell JB. Behavioural breathlessness. Thorax. 1990;45(4):287–92. doi: 10.1016/0022-3999(81)90065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingjaldsson JT, Laberg JC, Thayer JF. Reduced heart rate variability in chronic alcohol abuse: relationship with negative mood, chronic thought suppression, and compulsive drinking. Biological Psychiatry. 2003;54(12):1427–36. doi: 10.1016/S0006-3223(02)01926-1. [DOI] [PubMed] [Google Scholar]
- Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29(6):742–50. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- Karavidas MK, Lehrer PM, Vaschillo E, Vaschillo B, Marin H, Buyske S, Malinovsky I, Radvanski D, Hasset A. Preliminary results of an open label study of heart rate variability biofeedback for the treatment of major depression. Applied Psychophysiology and Biofeedback. 2007;32(1):19–30. doi: 10.1007/s10484-006-9029-z. [DOI] [PubMed] [Google Scholar]
- Lacey BC, Lacey JI. Some autonomic-central nervous system interrelationships. In: Blank P, editor. Physiological Correlations of Emotion. New York, NY: Academic Press; 1970. pp. 205–227. [DOI] [Google Scholar]
- Lacey BC, Lacey JI. Two-way communication between the heart and the brain. Significance of time within the cardiac cycle. The American Psychologist. 1978;33(2):99–113. doi: 10.1037/0003-066X.33.2.99. [DOI] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Frontiers in Psychology. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Scardella A, Siddique M, Habib RH. Biofeedback treatment for asthma. Chest. 2004;126(2):352–61. doi: 10.1378/chest.126.2.352. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B, Lu SE, Eckberg DL, Edelberg R, Shih WJ, Lin Y, Kuusela TA, Tahvanainen KU, Hammer RM. Heart rate variability biofeedback increases baroreflex gain and peak expiratory flow. Psychosomatic Medicine. 2003;65(5):796–805. doi: 10.1097/01.PSY.0000089200.81962.19. [DOI] [PubMed] [Google Scholar]
- Lehrer PM, Vaschillo E, Vaschillo B. Resonant frequency biofeedback training to increase cardiac variability: rationale and manual for training. Applied Psychophysiology and Biofeedback. 2000;25(3):177–91. doi: 10.1023/A:1009554825745. [DOI] [PubMed] [Google Scholar]
- Lehrer P, Sasaki Y, Saito Y. Zazen and cardiac variability. Psychosomatic Medicine. 1999;61(6):812–21. doi: 10.1097/00006842-199911000-00014. [DOI] [PubMed] [Google Scholar]
- Leiner GC, Abramowitz S. The symptom of sighing: physiologic and pathologic observations. Diseases of the Chest. 1958;34(1): 60–72. doi: 10.1378/chest.34.1.60. [DOI] [PubMed] [Google Scholar]
- Lum LC. Hyperventilation and anxiety state. Journal of the Royal Society of Medicine. 1981;74(1):1–4. doi: 10.1177/014107688107400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naka KK, Tweddel AC, Doshi SN, Goodfellow J, Henderson AH. Flow-mediated changes in pulse wave velocity: a new clinical measure of endothelial function. European Heart Journal. 2006;27(3):302–9. doi: 10.1093/eurheartj/ehi619. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, van de Borne P, Montano N, Hering D, Kara T, Somers VK. Sympathetic neural outflow and chemoreflex sensitivity are related to spontaneous breathing rate in normal men. Hypertension. 2006;47(1):51–5. doi: 10.1161/01.HYP.0000197613.47649.02. [DOI] [PubMed] [Google Scholar]
- Naschitz JE, Bezobchuk S, Mussafia-Priselac R, Sundick S, Dreyfuss D, Khorshidi I, Karidis A, Manor H, Nagar M, Peck ER, Peck S, Storch S, Rosner I, Gaitini L. Pulse transit time by R-wave-gated infrared photoplethysmography: review of the literature and personal experience. Journal of Clinical Monitoring and Computing. 2004;18(5–6):333–42. doi: 10.1007/s10877-005-4300-z. [DOI] [PubMed] [Google Scholar]
- Magarian GJ, Middaugh DA, Linz DH. Hyperventilation syndrome: a diagnosis begging for recognition. The Western Journal of Medicine. 1983;138(5):733–6. [PMC free article] [PubMed] [Google Scholar]
- McCraty R, Atkinson M, Tomasino D. Impact of a workplace stress reduction program on blood pressure and emotional health in hypertensive employees. Journal of Alternative and Complementary medicine. 2003;9(3): 355–69. doi: 10.1089/107555303765551589. [DOI] [PubMed] [Google Scholar]
- Mcllroy MB, Butler J, Finley TN. Effects of chest compression on reflex ventilator drive and pulmonary function. Journal of Applied Physiology. 1962;17:701–5. [Google Scholar]
- Nacoti M, Spagnolli E, Bonanomi E, Barbanti C, Cereda M, Fumagalli R. Sigh improves gas exchange and respiratory mechanics in children undergoing pressure support after major surgery. Minerva Anestesiologica. 2012;78(8):920–9. [PubMed] [Google Scholar]
- Nyklicek I, Wijnen V, Rau H. Effects of baroreceptor stimulation and opioids on the auditory startle reflex. Psychophysiology. 2005;42(2):213–22. doi: 10.1111/j.1469-8986.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: new insights into adaptive reactions of the autonomic nervous system. Cleveland Clinic Journal of Medicine. 2009;76(2):86–90. doi: 10.3949/ccjm.76.s2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM. The integrative role of the sigh in psychology, physiology, pathology, and neurobiology. Progress in Brain Research. 2014;209:91–129. doi: 10.1016/B978-0-444-63274-6.00006-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau H, Pauli P, Brody S, Elbert T, Birbaumer N. Baroreceptor stimulation alters cortical activity. Psychophysiology. 1993;30(3):322–325. doi: 10.1111/j.1469-8986.1993.tb03359.x. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Gonzalez I, Hernandez JA. Baroreceptor sensitivity and effectiveness varies differentially as a function of cognitive-attentional demands. Biological Psychology. 2004;67(3):385–95. doi: 10.1016/j.biopsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, González MI, Hernández JA, Duschek S, Gutiérrez N. Tonic blood pressure modulated the relationship between baroreceptor cardiac reflex sensitivity and cognitive performance. Psychophysiology. 2009;46(5):932–938. doi: 10.1111/j.1469-8986.2009.00832.x. [DOI] [PubMed] [Google Scholar]
- Reyes del Paso GA, Mata JL, Martín-Vázquez M. Relationships between baroreceptor cardiac reflex sensitivity and cognitive performance: Modulations by gender and blood pressure. Psychophysiology. 2012;49(1):138–144. doi: 10.1111/j.1469-8986.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- Ritz T. Studying noninvasive indices of vagal control: the need for respiratory control and the problem of target specificity. Biological Psychology. 2009;80(2):158–168. doi: 10.1016/j.biopsycho.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61(3):201–16. doi: 10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hall M, Sollers JJ, 3rd, Fischer JE. Alcohol use, urinary cortisol, and heart rate variability in apparently healthy men: Evidence for impaired inhibitory control of the HPA axis in heavy drinkers. International Journal of Psychophysiology. 2006;59(3):244–50. doi: 10.1016/j.ijpsycho.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Hansen AL, Saus-Rose E, Johnsen BH. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Annals of Behavioral Medicine. 2009;37(2):141–53. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- van de Vooren H, Gademan MG, Swenne CA, TenVoorde BJ, Schalij MJ, Van der Wall EE. Baroreflex sensitivity, blood pressure buffering, and resonance: what are the links? Computer simulation of healthy subjects and heart failure patients. Journal of Applied Physiology. 2007;102(4):1348–1356. doi: 10.1152/japplphysiol.00158.2006. [DOI] [PubMed] [Google Scholar]
- Vaschillo E, Lehrer P, Rishe N, Konstantinov M. Heart rate variability biofeedback as a method for assessing baroreflex function: a preliminary study of resonance in the cardiovascular system. Applied Psychophysiology and Biofeedback. 2002;27(1):1–27. doi: 10.1023/A:1014587304314. [DOI] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, Bates ME. The investigation and clinical significance of resonance in the heart rate and vascular tone baroreflexes. In: Fred A, Filipe J, Gamboa H, editors. Biomedical Engineering Systems and Technologies: Communications in Computer and Information Science. Heidelberg, Germany: Springer; 2011. pp. 224–237. [DOI] [Google Scholar]
- Vaschillo EG, Bates ME, Vaschillo B, Lehrer P, Udo T, Mun EY, Ray S. Heart rate variability response to alcohol, placebo, and emotional picture cue challenges: effects of 0.1-Hz stimulation. Psychophysiology. 2008;45(5):847–858. doi: 10.1111/j.1469-8986.2008.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaschillo EG, Vaschillo B, Buckman JF, Pandina RJ, Bates ME. Measurement of vascular tone and stroke volume baroreflex gain. Psychophysiology. 2012;49(2):193–7. doi: 10.1111/j.1469-8986.2011.01305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashchillo EG, Zingerman AM, Konstantinov MA, Menitsky DN. Research of the resonance characteristics for cardiovascular system. Fiziologiia Cheloveka. 1983;9:257–265. [Google Scholar]
- Vlemincx E, Abelson JL, Lehrer PM, Davenport PW, Van Diest I, Van den Bergh O. Respiratory variability and sighing: a psychophysiological reset model. Biological Psychology. 2013;93(1):24–32. doi: 10.1016/j.biopsycho.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, Van den Bergh O. A sigh following sustained attention and mental stress: effects on respiratory variability. Physiology & Behavior. 2012a;107(1):1–6. doi: 10.1016/j.physbeh.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, Van den Bergh O. Imposing respiratory variability patterns. Applied Psychophysiology & Biofeedback. 2012b;37(3):153–60. doi: 10.1007/s10484-012-9187-0. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Taelman J, De Peuter S, Van Diest I, Van den Bergh O. Sigh rate and respiratory variability during mental load and sustained attention. Psychophysiology. 2011;48(1):117–20. doi: 10.1007/s10484-012-9187-0. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, Lehrer PM, Aubert AE, Van den Bergh O. Respiratory variability preceding and following sighs: a resetter hypothesis. Biological Psychology. 2010a;84(1):82–7. doi: 10.1016/j.biopsycho.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Taelman J, Van Diest I, Van den Bergh O. Take a deep breath: the relief effect of spontaneous and instructed sighs. Physiology & Behavior. 2010b;101(1):67–73. doi: 10.1016/j.physbeh.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Vlemincx E, Van Diest I, De Peuter S, Bresseleers J, Bogaerts K, Fannes S, Li W, Van Den Bergh O. Why do you sigh? Sigh rate during induced stress and relief. Psychophysiology. 2009;46(5):1005–13. doi: 10.1111/j.1469-8986.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Trabert W, Roth WT. Characteristics of sighing in panic disorder. Biological Psychiatry. 2001;49(7):606–14. doi: 10.1016/S0006-3223(00)01014-3. [DOI] [PubMed] [Google Scholar]
- Yasumasu T, Reyes Del Paso GA, Takahara K, Nakashima Y. Reduced baroreflex cardiac sensitivity predicts increased cognitive performance. Psychophysiology. 2006;43(1):41–45. doi: 10.1111/j.1469-8986.2006.00377.x. [DOI] [PubMed] [Google Scholar]