Abstract
OBJECTIVE
The primary cervical cancer screening strategy for women over age 30 is high-risk human papillomavirus (HPV) testing combined with Papanicolaou (Pap) testing (cotesting) every 5 years. This combination strategy is a preventive service that is required by the Affordable Care Act to be covered with no cost-sharing by most health insurance plans. The cotesting recommendation was made based entirely on prospective data from an insured population that may have a lower proportion of women with HPV positive and Pap negative results (ie, discordant results). The discordant group represents a very difficult group to manage. If the frequency of discordant results among underserved women is higher, health care providers may perceive the cotesting strategy to be a less favorable screening strategy than traditional Pap testing every 3 years.
STUDY DESIGN
The Centers for Disease Control and Prevention’s Cervical Cancer Study was conducted at 15 clinics in 6 federally qualified health centers across Illinois. Providers at these clinics were given the option of cotesting for routine cervical cancer screening. Type-specific HPV detection was performed on residual extracts using linear array.
RESULTS
Pap test results were abnormal in 6.0% and HPV was positive in 7.2% of the underserved women screened in this study (mean age, 45.1 years). HPV prevalence decreased with age, from 10.3% among 30- to 39-year-olds to 4.5% among 50- to 60-year-olds. About 5% of the women had a combination of a positive HPV test and normal Pap test results; HPV 16/18 was identified in 14% of discordant women.
CONCLUSION
The rate of discordant results among underserved women was similar to those reported throughout the US in a variety of populations. Typing for HPV 16/18 appears to assist in the management in a small proportion of women with discordant results.
Keywords: cotesting, genotying, HPV testing, Pap test, underserved populations
Since 2003, in the United States, cervical cancer screening with human papillomavirus (HPV) and Papanicolaou (Pap) tests (“cotesting”) has been recommended by the American College of Obstetricians and Gynecologists and the American Cancer Society.1,2 In 2012, the United States Preventive Services Task Force3 endorsed cotesting every 5 years as an alternative screening strategy to Pap-testing alone every 3 years among women 30 to 65 years of age. The American Cancer Society and American College of Obstetricians and Gynecologists made similar recommendations, although they deemed cotesting as the preferred strategy.4,5 However, provider and patient surveys have indicated that cotesting and the increase in screening intervals have not been widely adopted; in fact, lengthening screening intervals beyond annual screening is uncommon.6-9
In addition to potentially improving acceptability and compliance with appropriate lengthened screening intervals, an important need has been to define the optimal follow-up of women who have HPV-positive and Pap-negative results (from here on, this will be called discordant results). Women with discordant results have a low prevalence of cervical intraepithelial neoplasia of grade 2 or worse (CIN 2+).10 In 2006, the American Society for Colposcopy and Cervical Pathology (ASCCP) published guidelines recommending that these women either be retested with both HPV and Pap tests in a year, or tested for HPV 16 and 18 (using a test approved by the US Food and Drug Administration) and directed to colposcopy if results are positive.11,12 According to data from a large managed care organization that includes over half a million women, followed prospectively, approximately 4% of women 30 and older can be expected to have discordant results.13 However, there is concern that data from a managed care population may not be generalizable to low-income, underinsured women. Moreover, if the frequency of discordant results among underserved women is higher, health care providers may perceive the cotesting strategy to be less favorable than traditional Pap testing every 3 years.
Our Centers for Disease Control and Prevention’s (CDC) Cervical Cancer Study (referred to as the Cx3 Study) offered a unique opportunity to examine the performance of cotesting in a cohort of underserved women presenting to federally qualified health centers (FQHCs) for routine cervical cancer screening. We also sought to compare the results of HPV type-specific testing in this population of women with that of other populations, to estimate the effect of type-specific HPV testing on referral to colposcopy. This population of women is of increased concern when determining optimal management strategies to ensure proper follow-up for those at highest risk of cervical cancer.
Materials and Methods
Participants
The data for this study were obtained from women, recruited between Sept. 2009 and May 2011 as part of CDC’s Cx3 Study. The study was conducted in 15 clinics associated with 6 FQHCs serving low-income women in Illinois. FQHCs provide comprehensive primary health care services to medically underserved communities and vulnerable populations in high-need areas across the United States.14 Women between the ages of 30 and 60 who were being seen in one of the FQHCs for a routine screening Pap test were identified through medical chart review by clinic staff. This group had no abnormal Pap test results in the preceding year survey, no history of cervical cancer, no record of being HIV positive, and no hysterectomy. They were invited to participate in our study when they arrived at the clinic for their routine visit. Further details on study design can be found in Benard et al.15 This study was approved by CDC’s Institutional Review Board. Informed consent was obtained from all study participants.
Two samples of exfoliated cervical cells were collected during the pelvic examination after visualization of the cervix. The first sample was collected per clinic protocol for routine Pap tests (either liquid or conventional) using the Bethesda system for reporting.16 The second sample was collected with the Digene Cervical Sampler (Qiagen Inc., Valencia, CA) and placed in specimen transport media (STM; Qiagen) for high-risk HPV testing (Hybrid Capture 2, HC2; Digene, Gaithersburg, MD). The STM specimens were stored and shipped to CDC at ambient temperature within 1 week of collection.
Laboratory
Specimens received at CDC were stored at 4°C and processed within 1 week of receipt. High-risk HC2 testing was performed on 250 μL aliquot, according to the manufacturer’s specification. A positive result indicates the presence of 1 or more of the 13 high-risk types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68. A second 200 μL aliquot was treated with Proteinase K at 65°C to lyse cells and the DNA from lysate was purified using the automated Chemagic Magnetic Separation Module 1 (PerkinElmer chemagen Technologie GmbH, Baesweiler, Germany) with the ViralNA/gDNA kit (Chemagen). The resulting extract (100 μL total volume) was tested immediately or stored at −20°C. Water blanks were processed through all laboratory steps as contamination control.
All DNA extracts were tested with the Research Use Only Linear Array genotyping assay (Roche Diagnostics, Indianapolis, IN). This assay uses HPV L1 consensus polymerase chain reaction (PCR) with PGMY09/11 primers and consensus PCR with β-globin primers as an internal control for amplification and cellular DNA. The typing strips include probes for 37 different HPV types (6, 11, 16, 18, 26, 31, 33, 35, 39, 40, 42, 45, 51, XR(52), 53, 54, 55, 56, 58, 59, 61, 62, 64, 66, 67, 68, 69, 70, 71, 72, 73, 81, 82, 83, 84, 89, IS39). There are 14 specific high-risk types examined in this assay: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68. The manufacturer’s protocol was modified to use 10 μL extract in the 100 μL PCR reaction and automated hybridization and wash steps with Bee Blot instrument (Bee Robotics, Caernafon, UK). Because HPV52 is detected with XR probe that cross-hybridizes with HPV 33, 35, and 58, all XR- high-risk positive samples with 1 or more cross-hybridizing types present were tested using a type-specific HPV 52 quantitative PCR.17 We had 15 samples that were considered negative for high-risk HC2, but these were positive for high risk by linear array. We excluded these in the analysis that was based on the assumption that HC2 would be the first test used. Samples negative for all HPV types and β-globin were considered inadequate and were omitted from further analysis.
Analysis
We analyzed baseline data from the 2246 women enrolled in the study who had both HPV and Pap testing. Pap test results defined as positive included atypical squamous cells of undetermined significance. Atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion, low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion, squamous cell carcinoma, and atypical glandular cells (atypical glandular cells of undetermined significance, adenocarcinoma). Results for cotesting included negative (HPV negative and Pap normal), discordant (HPV positive and Pap normal) or positive (HPV positive and Pap positive; HPV negative and Pap positive). Binomial exact 95% confidence intervals (CIs) were calculated for Pap and HPV results prevalence. Typing results are reported only for those samples that were HC2 HPV-positive. Other factors that were collected included the woman’s age and clinic location, including Chicago area and nonChicago area (ie, southern Illinois and mid-Illinois). Prevalence ratios (HPV/Pap) with 95% CIs were calculated to show the relative contribution of HPV to Pap for screening positive by cotesting. McNemar’s χ2 test was used to test for statistical differences (P < .05) for testing HPV-positive vs testing Pap-positive for all women and within 10-year age groups. Logistic regression was used to test the statistical differences of overall HPV positivity (HPV positive vs HPV negative) and discordant result (HPV negative and Pap negative vs all other categories combined) by 10-year age groups. The 50- to 60-year-old age group was included as the reference category. We used Stata version 12.1 (StataCorp, College Station, TX) for statistical analyses.18
Results
For the 2246 women enrolled in the study, the mean age was 45.1 years (Table 1). Two-thirds of the women were from Chicago. Two-thirds of the samples used liquid-based cytology. Overall, the HPV test result was positive in 7.2% (95% CI, 6.2–8.4%; n = 162) of the women; while 6.0% (95% CI, 5.0–7.0%; n = 134) had a positive Pap test result. Most (89.1%, 95% CI, 87.8–90.4%; n = 2002) were cotest negative and 4.9% (95% CI, 4.0–5.9%; n = 110) had discordant results (HPV positive and Pap negative results).
TABLE 1.
Demographics and laboratory test results, CDC Cervical Cancer Study 2009-2011
| Demographic | Category | n | % |
|---|---|---|---|
| Age at enrollment, by 5-y age category |
30-34 | 149 | 6.6 |
| 35-39 | 471 | 21.0 | |
| 40-44 | 473 | 21.1 | |
| 45-49 | 468 | 20.8 | |
| 50-54 | 385 | 17.1 | |
| 55-60 | 300 | 13.4 | |
| Total | 2246 | 100.0 | |
| Age at enrollment, mean (SD) | 45.1 (7.6) | ||
| Clinic is located in Chicago | No | 772 | 34.4 |
| Yes | 1474 | 65.6 | |
| Total | 2246 | 100.0 | |
| Laboratory tests | |||
| Pap test type | Liquid | 1466 | 65.3 |
| Conventional | 768 | 34.2 | |
| Not reported | 12 | 0.5 | |
| Total | 2246 | 100 | |
| Pap results | Negative | 2112 | 94.0 |
| ASC-US | 95 | 4.2 | |
| LSIL | 25 | 1.1 | |
| ASC-H | 4 | 0.2 | |
| HSIL | 5 | 0.2 | |
| AGUS | 3 | 0.1 | |
| AGC | 1 | 0.04 | |
| Adenocarcinoma | 1 | 0.04 | |
| Total | 2246 | 100.0 | |
| Pap resultsa | Negative | 2112 | 94.0 |
| Positive | 134 | 6.0 | |
| Total | 2246 | 100 | |
| HPV resultsb | Negative | 2084 | 92.8 |
| Positive | 162 | 7.2 | |
| Total | 2246 | 100 | |
| Pap and HPV combinations | Pap− / HPV− | 2002 | 89.1 |
| Pap+ / HPV − | 82 | 3.7 | |
| Pap− / HPV+ | 110 | 4.9 | |
| Pap+ / HPV+ | 52 | 2.3 | |
| 2246 | 100.0 |
AGC, atypical glandular cells; AGUS, atypical glandular cells of undetermined significance; ASC-H, atypical squamous cells cannot exclude high-grade; ASC-US, atypical squamous cells of undetermined significance; HPV, human papillomavirus test; HSIL, high grade squamous intraepithelial lesion; LSIL, low grade squamous intraepithelial lesion; Pap, Papanicolaou test; SD, standard deviation.
Negative includes within normal limits and negative for intraepithelial lesion or malignancy; Positive includes ASC-US, LSIL, HSIL, AGUS, AGC, adenocarcinoma;
Negative includes HPV negative; positive includes HPV positive.
The various combinations of HPVand Pap test results are presented in Table 2. In general, the percentage of Pap-test results considered abnormal remained relatively constant across age categories. However, the percentage of HPV-positive tests decreased from 10.3% among women aged 30 to 39 years to 4.5% among those aged 50 to 60 years (odds ratio, 2.43; P < .001). The prevalence of discordant cotest results decreased by age group with a higher rate for women aged 30 to 39 (6.5%) than among women aged 50 to 60 years (2.9%) (odds ratio, 2.2; P = .003).
TABLE 2.
Summary of results from cotesting with Pap and HPV by 10-year age groups
| Total |
Pap + |
HPV+ |
Pap− / HPV− |
Pap+/ HPV− |
Pap−/HPV+ |
Pap+/ HPV+ |
Prevalence ratio (HPV/Pap) (95% CI) |
P valuea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age group | n | % | n | % | n | % | n | % | n | % | n | % | n | % | ||
| 30-39 | 620 | 27.6% | 38 | 6.1% | 64 | 10.3% | 542 | 87.4% | 14 | 2.3% | 40 | 6.5% | 24 | 3.9% | 1.68 (1.26–2.26) | < .001b |
|
| ||||||||||||||||
| 40-49 | 941 | 41.9% | 57 | 6.1% | 67 | 7.1% | 834 | 88.6% | 40 | 4.3% | 50 | 5.3% | 17 | 1.8% | 1.18 (0.87–1.59) | .292 |
|
| ||||||||||||||||
| 50-60 | 685 | 30.5% | 39 | 5.7% | 31 | 4.5% | 626 | 91.4% | 28 | 4.1% | 20 | 2.9% | 11 | 1.6% | 0.79 (0.54–1.17) | .248 |
|
| ||||||||||||||||
| Total | 2246 | 100% | 134 | 6.0% | 162 | 7.2% | 2002 | 89.1% | 82 | 3.7% | 110 | 4.9% | 52 | 2.32% | 1.21 (1.01–1.45) | .043a,b |
Prevalence ratio (HPV/Pap), ratio of the prevalence of a positive HPV test to the prevalence of a positive cytologic interpretation. Pap + includes atypical squamous cells of undetermined significance, low grade squamous intraepithelial lesion, high grade squamous intraepithelial lesion, atypical glandular cells of undetermined significance, atypical glandular cells, adenocarcinoma; HPV + includes HPV positive.
CI, confidence interval; HPV, human papillomavirus test; Pap, Papanicolaou test.
McNemar’s χ2 test;
Statistically significantly (P < .05) greater number of HPV positives vs Pap positives.
Figure 1 shows the HPV genotype distribution among HPV-positive (HC2) women with Pap positive and negative results. Among women with positive Pap results (n = 52), the most common genotypes were HPV 16 (23%), HPV 31 (17%), HPV 52 (17%), HPV 18 (12%), and HPV 58 (12%). Among women with negative Pap results (n = 110), the most frequent HPV genotypes were HPV 31 (11%), HPV 59 (9%), HPV 51 (8%), and HPV 52 (8%). HPV 16 and 18 represented 7% and 6% respectively.
FIGURE 1. Percent distribution of high risk genotypesa by Pap test results (Cx3 Study, n [ 162).
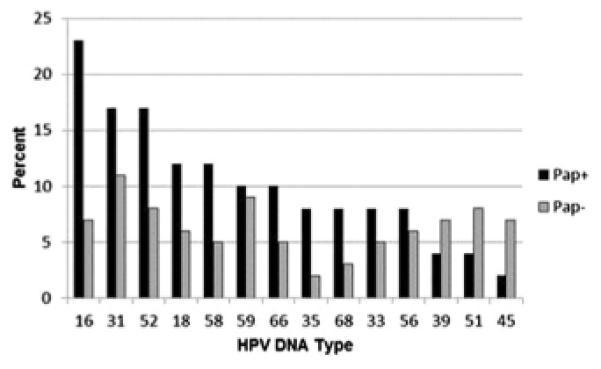
Pap+ includes atypical squamous cells of undetermined significance, low grade squamous intraepithelial lesion, high grade squamous intraepithelial lesion, atypical glandular cells of undetermined significance, atypical glandular cells, adenocarcinoma; Pap− includes Pap negative/normal.
Cx3 Study, Centers for Disease Control and Prevention’s Cervical Cancer Study; HPV, human papillomavirus test; Pap, Papanicolaou test.
aHigh risk genotypes were assessed for 14 high-risk types using Roche Linear Array (Roche Diagnostics, Indianapolis, IN) among all samples that initially tested positive for pooled HPV (Hybrid Capture 2 [HC2; Digene, Gaithersburg, MD] HPV positive).
We used the information from this study to project the percentage of women who would require more vigilant surveillance if current management guidelines from the ASCCP were followed (Figure 2). According to our data, approximately 5% of women aged 30 to 60 years would have discordant results and would require further workup. If genotyping were conducted for all of these women, most (86%) would not be triaged for immediate colposcopy. HPV results in the cotest discordant group were 5.5% HPV 16 only, 1.8% HPV 16 with at least 1 other high-risk HPV type, 5.5% HPV 18 only, 0.9% HPV 18 with at least 1 other high-risk HPV type.
FIGURE 2. Cx3 Study data with current national algorithmsa.
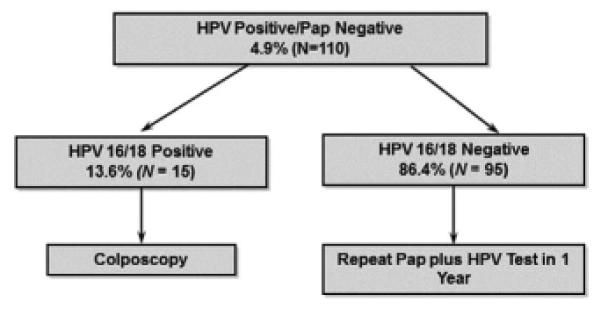
This management algorithm for discordant results shows the percentage of women who would have various test results and require further workup using data from the study and may inform the CDC program.
Cx3 Study, Centers for Disease Control and Prevention’s Cervical Cancer Study; HPV, human papillomavirus test; Pap, Papanicolaou test; s.d., standard deviation.
aAmerican Society of Colposcopy and Cervical Pathology (ASCCP) National Algorithms.
Discussion
In developing both cervical cancer screening and management guidelines, disease risk determinations are often based on longitudinal studies of women in settings such as managed care organizations, where women have easy access to care. There is general concern that data derived from such populations may be different from data on underserved women. We found that the discordance rates were consistent with a recent large-scale study in Kaiser Permanente Northern California (Berkeley, CA), a health maintenance organization,13 with 6.3% (compared with 7.2% in our study) HPV positive and 4.0% (compared with 4.9% in our study) discordant rate (HPV positive/Pap negative). These findings suggest that the recommended guidelines for cervical cancer screening and management can be followed, even in this vulnerable population.
Higher confidence and use of cotesting in cervical screening is supported primarily by the low risk of CIN3+ among women whose results are negative for both tests and in whom screening intervals may be substantially lengthened.10 A major limitation of HPV testing is that benign infections likely to clear up are common enough to lead to a low predictive value for a single positive test. In other words, most discordant women have a low risk of developing cancer.10 Immediate referral of all of these women for colposcopy could easily triple referral rates to colposcopy and may represent excessive intervention, given the low risk for imminent cancer in these women. However, the 5-year cumulative risk of CIN 3+ among women with discordant results is considerably higher than after a Pap-negative result alone.19 Therefore, the new ASCCP guidelines recommend following women who are HPV-positive/Pap-negative with a repeat cotest at 1 year.12
An alternative management strategy for discordant women would be to perform HPV 16/18 typing with immediate colposcopy referral for women with positive results and 1-year follow-up cotesting for women with negative results. We found approximately 14% of HPV-positive/Pap-negative women were positive for HPV 16/18 and would have required immediate colposcopy. This would mean that in a group of 2000 cotested women, 100 would have discordant results. A clinic would incur the cost of the HPV 16/18 test for all 100 women to identify 14 for colposcopy; based on results from the ATHENA trial,20 1 to 2 women among these 14 could be expected to be identified as having CIN 3+. The remaining 86 who are HPV 16/18 negative would have a lower risk of CIN 3+, though apparently not low enough to return to routine screening; recommendations are for these women to return the following year for repeat cotesting. Another concern is short-term anxiety of having a positive HPV test with recommendations to wait a year before intervening.21
These findings help inform the larger Cx3 Study designed to understand the barriers associated with cotest use and the extension of the interval between screenings in the underserved population8,9 by providing information on the cotest results and the number that may require additional management. To improve cervical cancer screening among medically underserved women, Congress authorized the CDC to develop the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) in 1990.22 The NBCCEDP is a comprehensive public health program that helps low-income, under-, and uninsured women gain access to breast and cervical screening services. Before the 2012 revision to the United States Preventive Services Task Force screening guidelines, the NBCCEDP reimbursed providers for the HPV test for management of abnormal Pap results, but not as a cotest. In July 2012, CDC adopted these new cotesting guidelines, but the implications of the extended screening interval and the management strategies have not been fully explored. Currently, the programs are reimbursing for repeating the cotest in a year, but not for genotyping. The ASCCP guidelines state that the additional genotyping is an alternate strategy, but do not report a preference.12 More modeling and cost-effectiveness studies may be done on the strategy of HPV 16/18 testing, as this would be very useful information for the NBCCEDP and other programs that treat low-income women.
This study has several strengths, including enrollment of over 2000 low-income, un-, and underinsured patients in the setting of FQHCs across a diverse population of urban and rural clinics in Illinois. As a demonstration study in 1 state, our results may not be generalizable to other settings. In addition, the number of women with abnormal results is small; extending the study to more sites would improve the estimate of the discordant cotests in this population. However, when we examined the cytological outcomes of the study population in Illinois, it was similar to the NBCCEDP cytologic distribution (the HPV data were not available for comparison). An additional limitation was that the baseline data provides only cross-sectional HPV and Pap test results with no histologic outcomes. However, this study is collecting medical chart data as the final end point to be able to link the testing results to the clinical outcomes.
Comment
The rate of discordant cotesting results in this study of underserved women was similar to rates reported throughout the US in various populations, including those who are insured. This is important reassurance for providers who might have been unsure if they should follow national guidelines in this vulnerable population, because most of the data generated for the recommendations were based solely on managed-care populations.
Acknowledgments
This manuscript was written in the course of employment by the United States Government with support from services provided by a contract with Battelle (200-2002-00573, Task Order no. 0006) and is not subject to copyright in the United States. Qiagen and Roche Molecular Diagnostics provided in-kind support for testing reagents through the CDC Foundation. Diane Manninen, PhD was the project manager from Battelle.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC).
The authors report no conflict of interest.
REFERENCES
- 1.American College of Obstetricians and Gynecologists Cervical cytology screening. ACOG practice bulletin no. 45, August 2003. Clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2003;102:417–27. doi: 10.1016/s0029-7844(03)00745-2. [DOI] [PubMed] [Google Scholar]
- 2.Saslow D, Runowicz CD, Solomon D, et al. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin. 2002;52:342–62. doi: 10.3322/canjclin.52.6.342. [DOI] [PubMed] [Google Scholar]
- 3.Moyer VA. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. doi: 10.7326/0003-4819-156-12-201206190-00424. W312. [DOI] [PubMed] [Google Scholar]
- 4.American College of Obstetricians and Gynecologists Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol. 2009;114:1409–20. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 5.Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology Screening Guidelines for the prevention and early detection of cervical cancer. J Low Genit Tract Dis. 2012;16:175–204. doi: 10.1097/LGT.0b013e31824ca9d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roland KB, Soman A, Benard VB, Saraiya M. Human papillomavirus and Papanicolaou tests screening interval recommendations in the United States. Am J Obstet Gyn. 2011;205:441–8. doi: 10.1016/j.ajog.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 7.Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: a national survey, 2006 to 2007. Ann IntMed. 2009;151:602–11. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins NA, Benard VB, Greek A, Roland KB, Manninen D, Saraiya M. Patient knowledge and beliefs as barriers to extending cervical cancer screening intervals in Federally Qualified Health Centers. Prev Med. 2013;57:641–5. doi: 10.1016/j.ypmed.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roland KB, Benard VB, Greek A, Hawkins NA, Manninen D, Saraiya M. Primary care provider practices and beliefs related to cervical cancer screening with the HPV test in Federally Qualified Health Centers. Prev Med. 2013;57:419–25. doi: 10.1016/j.ypmed.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol. 2011;12:663–72. doi: 10.1016/S1470-2045(11)70145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright TC, Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol. 2007;197:346–55. doi: 10.1016/j.ajog.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 12.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829–46. doi: 10.1097/AOG.0b013e3182883a34. [DOI] [PubMed] [Google Scholar]
- 13.Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol. 2009;113:595–600. doi: 10.1097/AOG.0b013e3181996ffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.What are federally qualified health centers (FQHCs)? HRSA; Washington, DC: US Department of Health and Human Services. Health Information Technology. Available at: http://www.hrsa.gov/healthit/toolbox/ruralhealthittool box/introduction/qualified.html. Accessed June 10, 2013. [Google Scholar]
- 15.Benard VB, Saraiya M, Greek A, et al. Overview of the CDC Cervical Cancer (Cx3) Study: an educational intervention of HPV testing for cervical cancer screening. J Women’s Health. 2014;23:197–203. doi: 10.1089/jwh.2013.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA. 2002;287:2114–9. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.Onyekwuluje JM, Steinau M, Swan DC, Unger ER. A real-time PCR assay for HPV52 detection and viral load quantification. Clin Lab. 2012;58:61–6. [PubMed] [Google Scholar]
- 18.StataCorp . College Station, TX: Available at: http://www.stata.com. Accessed June 10, 2013. [Google Scholar]
- 19.Katki HA, Schiffman M, Castle PE, et al. Benchmarking CIN 3+ risk as the basis for incorporating HPV and Pap cotesting into cervical screening and management guidelines. J Low Genit Tract Dis. 2013;17(Suppl 1):S28–35. doi: 10.1097/LGT.0b013e318285423c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright TC, Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206:46.e1–11. doi: 10.1016/j.ajog.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 21.Vesco KK, Whitlock EPE, Eder M, et al. A Systematic Evidence Review for the US Preventive Services Task Force. Screen Cerv Cancer. 2011 Evidence Syntheses, no. 86. Available at: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0016542/. Accessed June 10, 2013.
- 22.Ryerson AB, Benard VB, Majors A. National Breast and Cervical Cancer Early Detection Program: Summarizing the first 12 years of parnterships and progress against breast and cervical cancer. 2003 Available at: http://www.cdc.gov/cancer/nbccedp/data/. Accessed June 10, 2013.


