Abstract
Objective
The objective of this prospective, longitudinal study of patients with normal-tension glaucoma (NTG) was to determine whether patients with nocturnal hypotension are at greater risk for visual field (VF) loss over 12 months than those without nocturnal hypotension.
Design
Prospective, longitudinal study.
Participants
Consecutive patients with NTG with at least 5 prior VF tests were screened for eligibility.
Methods
The baseline evaluation assessed demographic and clinical characteristics, covering systemic comorbid conditions, including systemic hypertension. All oral and ophthalmologic medications were recorded. A complete ophthalmological examination was performed at baseline and follow-up. Patients had their blood pressure (BP) monitored every 30 minutes for 48 hours with an ambulatory recording device at baseline and 6 and 12 months.
Main Outcome Measures
The primary outcome was based on the global rates of VF progression by linear regression of the mean VF threshold sensitivity over time (decibels/year).
Results
Eighty-five patients with NTG (166 eyes; mean age, 65 years; 67% were women) were included. Of the 85 patients, 29% had progressed in the 5 VFs collected before study enrollment. The nocturnal mean arterial pressure (MAP) was compared with the daytime MAP. Multivariate analysis showed that the total time that sleep MAP was 10 mmHg below the daytime MAP was a significant predictor of subsequent VF progression (P<0.02).
Conclusions
Cumulative nocturnal hypotension predicted VF loss in this cohort. Our data suggest that the duration and magnitude of decrease in nocturnal blood pressure below the daytime MAP, especially pressures that are 10 mmHg lower than daytime MAP, predict progression of NTG. Low nocturnal blood pressure, whether occurring spontaneously or as a result of medications, may lead to worsening of VF defects.
Glaucoma is an optic neuropathy characterized by progressive loss of retinal ganglion cells and their axons, which leads to structural and functional damage to the optic nerve. Despite being an arbitrary definition, primary open-angle glaucoma (POAG) with intraocular pressure (IOP) within the statistically normal range (i.e., <21 mmHg) is commonly defined in practice and research as normal-tension glaucoma (NTG), when in fact POAG and NTG share similar pathogenesis. Presently, IOP reduction, the only treatable risk factor in glaucoma, tends to slow, not halt, disease progression, suggesting that mechanisms other than IOP may contribute to visual field (VF) loss, particularly in the lower ranges of IOP in patients with glaucoma.1–4 Epidemiologic studies, cross-sectional studies, and randomized clinical trials (RCTs) have suggested that systemic blood pressure (BP) plays a role in pathogenesis of glaucoma. Low BP and lower diastolic perfusion pressure were among the first risk factors associated with incident glaucoma in population-based studies.3,5–11 Thus, many studies suggest that nocturnal hypotension may identify patients at higher risk for VF progression.9,12–17 Presumably, it is the difference between arterial and venous pressure that determines blood flow after vessels are maximally dilated by autoregulation that matters.18 When systemic BP decreases 20 mmHg below the patients’ usual BP, vessels dilate to preserve constant perfusion in vital organs, if the BP decreases below the lower limit, ischemia occurs.
In this first longitudinal study of NTG using 48-hour BP monitoring at 6-month intervals, we tested the hypotheses that nocturnal systemic hypotension is associated with progressive VF loss and that the extent and duration of the nocturnal decrease in mean arterial pressure (MAP) predict progression of VF loss. The objective was to determine whether patients with nocturnal hypotension are at greater risk for VF loss over 12 months than those without nocturnal hypotension.
Methods
This longitudinal study was approved by the New York Eye and Ear Infirmary and Weill Cornell Medical College Institutional Review Boards for Human Research. The study was performed in compliance with the Health Insurance Portability and Accountability Act and the tenets set forth in the Declaration of Helsinki. Written informed consent was obtained from all eligible patients. A total of 456 patients who were followed at the New York Eye and Ear Infirmary with a diagnosis of NTG were screened for potential eligibility. At baseline examination, the diagnosis of NTG was based on the presence of glaucomatous optic neuropathy, abnormal 24-2 Swedish Interactive Thresholding Algorithm (SITA) standard automated perimetry (SAP) examinations (SITA-SAP, Humphrey Visual Field Analyzer; Carl Zeiss Meditec, Inc., Dublin, CA) in 1 or both eyes, open anterior chamber angles, no identifiable secondary cause of glaucoma, and all known untreated IOPs ≤21 mmHg by Goldmann applanation tonometry. Glaucomatous optic neuropathy was defined on the basis of stereophotographic evaluation by glaucoma specialists using the following criteria: focal or diffuse neuroretinal rim thinning, focal or diffuse retinal nerve fiber layer loss, or an inter-eye vertical cup-to-disc ratio asymmetry >0.2 not explained by differences in disc size. The 24-2 VF tests were considered abnormal if the glaucoma hemifield test result was outside normal limits or the pattern standard deviation (PSD) had a P value <5%. At least 2 consecutive VF results had to be reliable on the basis of false-positive rates ≤25%, false-negative rates, and fixation losses ≤33%. Patients with significant lens opacity, ocular conditions that would affect VF results, fewer than five 24-2 SITA-SAP tests, IOPs ≥21 mmHg, or severe glaucoma VF loss (mean deviation19 <−20 decibels [dB]) were excluded. A total of 216 patients were eligible for the study. As shown in Figure 1, 97 patients consented to participate.
Figure 1.
Consolidated standards of reporting trials diagram. BP = blood pressure; IOP = intraocular pressure; NTG = normal-tension glaucoma.
Demographic and clinical history, including the presence of atherosclerotic disease, diabetes, hypertension, migraine, and Raynaud’s disease, were assessed. Comorbidity was assessed using the Charlson index.20 All oral medications were recorded. A complete ophthalmic history and searching for evidence of prior disc hemorrhage, laser and incisional surgical interventions, was obtained.
At follow-up examinations, complete ophthalmic examination was performed, including best-corrected visual acuity, slit-lamp biomicroscopy, gonioscopy, dilated fundoscopy, stereodisc photography, automated perimetry, and Goldmann applanation tonometry. Central corneal thickness was measured using ultrasonic pachymetry (DGH-550; DGH Technology Inc., Exton, PA). Visual function was assessed using 24-2 SITA-SAP tests at all follow-up visits. Stereoscopic optic disc photographs were reviewed by 2 masked experts (C.G.dM., J.M.L.) searching for disc hemorrhages (i.e., a splinter-like or flame-shaped hemorrhage on or within the retinal nerve fiber layer or neuroretinal rim).21 If the 2 investigators disagreed, a third investigator adjudicated.
Systemic BP was monitored every 30 minutes for 48 hours with an ambulatory recording device. Spacelabs ambulatory BP monitoring equipment (Spacelabs Healthcare, Issaquah, WA) was used.22–24 Patients used a diary to record times of going to bed and rising, taking medications, and any systemic symptoms. Of those who consented to participate, 85 patients completed the baseline 48-hour BP recording; overall, 67 patients had 3 BP sessions, 5 patients had 2 BP sessions, and 13 patients had 1 BP session (Fig 1). Follow-up evaluations were performed at 6 and 12 months, at which time the medical and ophthalmic histories were reviewed for change, 48-hour ambulatory BP measurement was obtained, and the eye examination, including tonometry and perimetry, was repeated. The most recent 5 VFs before enrollment and the 3 VFs obtained during the study period were used for outcome analyses. Automated pointwise linear regression analysis was performed using PROGRESSOR software (version 3.3; Medisoft, Ltd., Leeds, UK) providing slopes (dB/year) of progression both globally and locally for each point based on threshold sensitivity maps, as well as its level of significance (P values). Details of the software have been described elsewhere.25 Global rates of sensitivity change are provided automatically by PROGRESSOR and represent the slopes of the average threshold sensitivity of each field of the sequence of VF tests analyzed using least-squares linear regression. Therefore, the main outcome measure was the numeric value of the rate of VF change for each eye over the study period. The mean deviation and PSD values of the baseline VF tests were recorded. For a binary classification of progression (i.e., “progressing” vs. “stable” eyes), pointwise linear regression progression criteria were applied to the sequences of 8 SITA 24-2 VF tests. A VF test point was deemed “progressing” if it presented a slope of sensitivity change over time more negative than −1.0 dB loss/year at P<0.01. An eye was deemed “progressing” if the sequence of VF tests presented at least 2 adjacent VF test points in the same hemifield meeting the aforementioned criterion. These pointwise linear regression progression criteria have been used and validated in previous studies.26,27
Sample Size and Data Analysis
We projected 22% progression over 1 year.9 To increase the power of the study, we preferentially enrolled patients who had previously progressed based on clinical judgement, because they are thought to be at higher risk for subsequent progression.28,29 With a power of 80% and an α level of 0.05, we detected a difference of proportions of 0.22 with 72 eyes per group, or 144 eyes overall. With a loss to follow-up of 15%, a total sample of 170 eyes was required.
By using the data from the patient’s diary card for each 48-hour period, we calculated (1) the 48-hour daytime average MAP while the patient was awake; (2) the 48-hour nocturnal MAP while the patient was asleep; and (3) the variability of nocturnal MAP. We characterized BP in several ways: (1) the daytime average MAP at each follow-up, which we calculated using only the data from when the patient is awake, according to his/her diary card; (2) measures of nighttime mean MAP; and (3) variability of BP, particularly its departure from its usual daytime MAP. The first measure of variability was simply the time (averaged across the 48 hours) that the nighttime BP was less than the daytime average. The second measure of variability is similar: the time spent at least some level c below the daytime average, where c=10 and 20. The third measure of variability was the area under curve, representing total nighttime time*magnitude spent below the daytime average; the fourth was the area below the defined constant c.
The main analysis used the rate of VF change for each eye over the study period. A seemingly unrelated regression equations approach was used to assess the relationship of predictors to outcome.30 The multivariate approach is appropriate because the outcome equation for each eye has eye-specific (right eye/left eye) explanatory variables. In addition, because the 2 eye outcomes are correlated (Breusch–Pagan test of independence; P = 0.04), the statistical efficiency of the estimators is gained by estimating the 2 equations simultaneously. The regression equations control for age, sex, prior progression, IOP, presence of cataract, central corneal thickness, use of topical pilocarpine, α-agonists, β-blockers, oral statins, and hypertension medications. Hypothesis tests use a Wald test to assess the significance of effects in the right and left eye regression equations simultaneously rather than separate tests using each eye’s equation. To avoid the undesired effect of collinearity between predictors (“time” and “area”), 2 distinct models were built using each of these predictors separately, but also adjusting for the same confounders.
Results
Baseline Characteristics
Overall, 29% of patients had progression of VF loss during the 36 months before enrollment. Table 1 contrasts the 29% (25/85) of patients with VF progression in the prior 36 months versus those without progression. Most patients were female, the average age was 65 years, and 32% had systemic hypertension, of whom 77% were taking oral medications to reduce systemic BP. Most patients were receiving prostaglandin analog eye drops, and half of patients were receiving β-adrenergic antagonist drops. There were no significant differences in any of the characteristics listed in Table 1 for those who did and did not have progression during the 36 months before the first 48 hour BP monitoring.
Table 1.
Clinical and Demographic Characteristics of Patients with Progression Before Enrollment versus Patients without Prior Progression
| Prior Progression (n=25) | % | No Prior Progression (n=60) | % | P Value | |
|---|---|---|---|---|---|
| Age, yrs | 65.3±13.5 | 65.9±10.3 | 0.84 | ||
| Female sex | 19 | 76.0 | 38 | 63.3 | 0.26 |
| Angina | 0 | 0.0 | 3 | 5.0 | 0.55 |
| Myocardial infarction | 2 | 8.0 | 1 | 1.7 | 0.21 |
| Cerebrovascular accident | 0 | 0.0 | 4 | 6.7 | 0.32 |
| Hypertension | 6 | 24.0 | 21 | 35.0 | 0.32 |
| Antihypertensive medication | |||||
| Angiotensin converter enzyme inhibitor | 2 | 33.3 | 3 | 14.3 | 0.30 |
| Angiotensin II receptor blockers | 1 | 16.7 | 5 | 23.8 | 1.00 |
| β-Blocker | 4 | 66.7 | 5 | 23.8 | 0.14 |
| Calcium channel blocker | 3 | 50.0 | 5 | 23.8 | 0.32 |
| Diuretics | 1 | 16.7 | 4 | 19.1 | 1.00 |
| Baseline blood pressure | |||||
| Nonhypertensives | |||||
| Systolic | 132.9±15.5 | 133.5±17.9 | 0.92 | ||
| Diastolic | 76.8±8.5 | 79.6±10.6 | 0.32 | ||
| Mean arterial pressure | 96.5±9.1 | 97.7±12.2 | 0.71 | ||
| Hypertensives | |||||
| Systolic | 135.3±10.3 | 142.8±10.9 | 0.15 | ||
| Diastolic | 82.3±12.9 | 84.5±7.1 | 0.59 | ||
| Mean arterial pressure | 101.2±13.7 | 104.6±7.5 | 0.58 | ||
| Diabetes | 1 | 4.0 | 3 | 5.0 | 1.00 |
| Renal disease | 3 | 12.0 | 1 | 1.7 | 0.074 |
| Migraine | 5 | 20.0 | 12 | 20.0 | 1.00 |
| Raynaud’s disease | 5 | 20.0 | 10 | 16.7 | 0.76 |
| Thyroid condition | 6 | 24.0 | 16 | 26.7 | 0.80 |
| Comorbidity | |||||
| 0 | 11 | 44.0 | 31 | 51.7 | 0.70 |
| 1 | 5 | 20.0 | 13 | 21.7 | |
| 2 | 6 | 24.0 | 11 | 18.3 | |
| ≥3 | 3 | 12.0 | 5 | 8.4 | |
| Age at diagnosis | 53±12 | 55±12 | 0.53 | ||
| Family history of glaucoma | 10 | 40.0 | 28 | 46.7 | 0.65 |
| Glaucoma medication | |||||
| α-Agonists | 7 | 28.0 | 12 | 20.0 | 0.41 |
| β-Blockers | 13 | 52.0 | 28 | 46.7 | 0.65 |
| Pilocarpine | 2 | 8.0 | 4 | 6.7 | 1 |
| Carbonic anhydrase inhibitors | 8 | 32.0 | 23 | 38.3 | 0.58 |
| Prostaglandins | 21 | 84.0 | 54 | 90.0 | 0.47 |
Data are n (%) or mean ± standard deviation.
Table 2 displays the baseline ophthalmologic characteristics according to whether the specific eye (right or left eye) had prior progression. The mean follow-up IOP was 13 mmHg. Some 36% of eyes had a threat to fixation, whereas 74% of eyes had a prior disc hemorrhage. The mean corneal thickness was 535 μm for both groups. As expected change in mean deviation and PSD was significantly greater in patients with progression in the prior 36 months. Otherwise, there were no other significant differences in eyes according to recent progression.
Table 2.
Ophthalmologic Characteristics by Individual Eye for Eyes that Showed Prior Progression versus No Prior Progression (n=166)
| Prior Progression (n=28) | % | No Prior Progression (n=138) | % | P Value | |
|---|---|---|---|---|---|
| Optic | |||||
| Mean IOP over 3 yrs (±SD; mmHg) | 12.9±2.5 | 13.2±2.4 | 0.62 | ||
| Myopia | 8 | 28.6 | 44 | 31.9 | 0.73 |
| Visual acuity (logMAR) | 0.19±0.42 | 0.16±0.27 | 0.69 | ||
| Disk size (microns) | 1.84±0.35 | 2.01±0.35 | 0.16 | ||
| Rim area (microns) | 0.91±0.28 | 1.02±0.48 | 0.25 | ||
| Spheric equivalent (diopters) | −2.4±3.53 | −1.87±7.49 | 0.69 | ||
| Vertical cup-to-disc ratio | 0.80±0.11 | 0.80±0.13 | 0.78 | ||
| CCT (microns) | 535±43 | 539±34 | 0.64 | ||
| MD (dB) | −10.53±5.07 | −7.48±6.15 | 0.02 | ||
| PSD (dB) | 10.75±3.10 | 7.98±4.39 | 0.00 | ||
| Prior disc hemorrhage | 13 | 46.4 | 46 | 33.3 | 0.19 |
| Threat to fixation | 21 | 75.0 | 103 | 74.6 | 0.97 |
| Cataracts | 17 | 60.7 | 62 | 44.9 | 0.13 |
| Previous surgery | |||||
| ALT | 4 | 14.3 | 10 | 7.2 | 0.26 |
| SLT | 4 | 14.3 | 28 | 20.3 | 0.60 |
| Filtering surgery | 2 | 7.1 | 8 | 5.8 | 0.68 |
| Lasik | 0 | 0.0 | 4 | 2.9 | 1.00 |
| Cataract surgery | 9 | 32.1 | 26 | 18.8 | 0.12 |
ALT = argon-laser trabeculoplasty; CCT = central corneal thickness; dB = decibels; IOP = intraocular pressure; logMAR = logarithm of the minimum angle of resolution; MD = mean deviation; PSD = pattern standard deviation; SD = standard deviation; SLT = selective-laser trabeculoplasty.
Progression was defined on the basis of pointwise linear regression criteria, as described in the text.
Statistical significance at 5% level is shown in boldface.
Outcomes
Overall, 26 of the 85 patients (29%) progressed after 1 year of follow-up on the basis of the pointwise linear regression progression criteria. Of the patients who had progression before study entry, 52% (13 of 25) continued to progress after 1 year of follow-up versus 21% of those without prior progression. Thus, prior progression was a significant predictor of subsequent progression (P<0.01).
Forty-Eight-Hour Nocturnal Blood Pressures
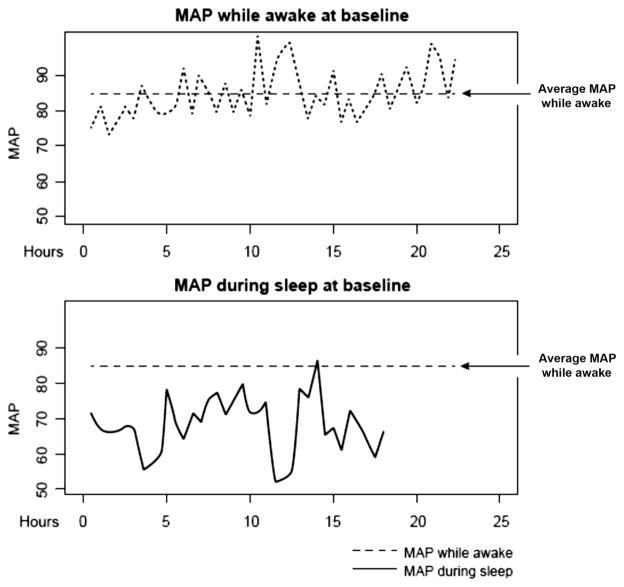
Figure 2 shows the MAP when awake versus the MAP at night at baseline for a patient who had progression over 12 months. The total time that patients spent during sleep at MAPs below their daytime MAP and the total area below their daytime MAP were both evaluated. At any given time point, total time and total area during sleep below daytime MAP were highly correlated (Pearson’s r = 0.78 at baseline, r = 0.73 at 6 months, and r = 0.77 at 12 months). The total time that patients spent during sleep below their daytime BP was correlated across baseline and 6 and 12 months (intraclass correlation coefficient = 0.37). The total area during sleep below their daytime BP was also moderately correlated at the 3 time points (intraclass correlation coefficient = 0.53).
Figure 2.
Example of how mean arterial pressures (MAPs) while awake versus during sleep were calculated. This example depicts a patient who progressed on the basis of our visual field (VF) criteria (see text). The time range (x-axis) corresponds to the time period awake or asleep during a 48-hour of blood pressure (BP) monitoring. The top graph depicts the MAP variation while awake (dotted line) and the corresponding average (vertical dashed line). In the bottom graph, the dashed line depicts the average MAP (calculated in the previous plot) while the patient was awake and the solid line depicts the MAP variation during sleep.
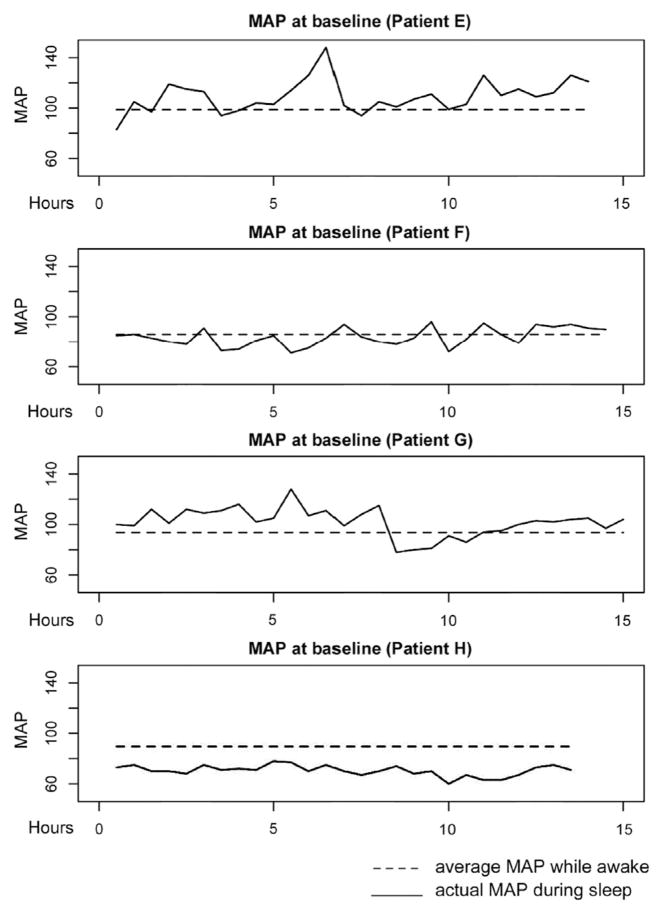
As an example, Figure 3 shows the MAP during sleep at baseline for patients who had VF progression, and Figure 4 shows patients who did not have VF progression. The analysis considers progression over 12 months in relation to cumulative nocturnal BP over three 48-hour periods of monitoring: at baseline and 6 and 12 months.
Figure 3.
Each graph depicts the mean arterial pressures (MAPs) during sleep of progressing patients. The time range (x-axis) corresponds to the time asleep during the baseline 48-hour of blood pressure monitoring. The dashed line depicts the average MAP while the patient was awake, and the continuous line depicts the MAP variation during sleep.
Figure 4.
Each graph depicts the mean arterial pressure (MAPs) during sleep of nonprogressing patients. The time range (x-axis) corresponds to the time asleep during the baseline 48-hour of blood pressure monitoring. The dashed line depicts the average MAP while the patient was awake, and the continuous line depicts the MAP variation during sleep.
Predictors of Visual Field Outcomes
To identify predictors of progression, eye-specific multivariate analyses controlling for age, sex, and other variables, as well as eye-specific (right eye/left eye) variables, were used to assess the various predictors of progression. Taken together, the three 48-hour measures of BP contrasting daytime mean BP with BP during sleep shows that the total time that the MAP during sleep is more than 10 mmHg below the daytime mean is a significant predictor of global progression (P = 0.02). The area that represents the time multiplied by the magnitude of nocturnal BP that was >10 mmHg below daytime MAP also predicted progression (P = 0.03). Progression also was associated with prior progression (P = 0.01) and corneal thickness (P = 0.02) and was weakly associated with age (P = 0.1). Progression was not associated with previous cataract surgery (P = 0.78) or the use of ophthalmic β-blockers (P = 0.53), α-2 adrenergic agonists (P = 0.50), or other medications such as statins (P = 0.22). There were no significant differences in progression between systemic hypertensive and normotensive individuals (P = 0.59). Over the follow-up year, multivariate analysis showed that IOP in either eye was not a predictor of subsequent VF progression (P = 0.37).
Alternate Definitions of Hypotension
Time
We examined the associations of the progression outcome with the other definitions of time, specifically the time a patient spent during sleep when his or her MAP was below the daytime MAP and the time during sleep more than 20 mmHg under the daytime MAP. Figure 5 shows the distribution of patients according to the time spent under the daytime MAP. Many patients had time under the daytime MAP, but only a few had time <20 mmHg. A multivariate analysis showed that the total time that a patient’s MAP during sleep was >20 mmHg below daytime MAP was associated with global progression (P = 0.01), but total time under daytime MAP was not (P = 0.35). Thus, the total time <10 mmHg predicted progression, as did time <20 mmHg; however, not many patients had long times <20 mmHg.
Figure 5.
The percentage distribution of patients according to the time spent below the daytime mean arterial pressure (MAP).
Area
We examined the associations of the progression outcome with the other areas measures, that is, area during sleep under the daytime MAP and area during sleep >20 mmHg under the daytime MAP. Figure 6 shows the distribution of patients according to the total area under the daytime MAP. A multivariate analysis showed that the area under daytime MAP (P = 0.03) and the area >10 mmHg below daytime MAP (P = 0.03) did predict progression, whereas the area >20 mmHg below daytime MAP (P = 0.11) was a weaker predictor. Thus, cumulative nocturnal hypotension predicts VF loss.
Figure 6.
The percentage distribution of patients according to the total area below the daytime mean arterial pressure (MAP).
Discussion
In a population with treated NTG followed longitudinally with 48-hour BP monitoring, this study demonstrated that the duration and magnitude of nocturnal systemic hypotension, that is, MAP during sleep below the daytime MAP, are associated with progressive VF loss. These data have important implications for our understanding of glaucoma pathophysiology and future treatment strategies. Our study defined decreases in nocturnal MAP consonant with autoregulatory theory. The range in which autoregulation can preserve constant perfusion is approximately 20 mmHg surrounding the patients’ usual MAP.31 Once the BP decreases below the lower limit, ischemia occurs. Ambulatory BP monitoring has long established that BP decreases during the night in the majority of individuals, but the issue is how much and for how long.32 Nocturnal hypotension poses ischemic risk for other regional circulations, specifically stroke and myocardial infarction. The concept of autoregulation provided the framework for our earlier RCT of patients undergoing coronary artery bypass that showed maintaining higher MAPs during cardiopulmonary bypass significantly reduced major ischemic cardiac and neurologic morbidity and mortality.17
Epidemiologic studies, cross-sectional studies, and RCTs have suggested that systemic BP plays a role in the pathogenesis of glaucoma. Low BP and lower diastolic perfusion pressure were among the first risk factors associated with incident glaucoma in population-based studies.3,5–11 Numerous cross-sectional studies, most with a single 24-hour BP recording, have evaluated VF loss in relation to BP using many different criteria.13,33,34 These cross-sectional studies have suggested that patients with VF progression had more nocturnal hypotension.12–14,35 In those with VF progression, greater nocturnal hypotension was found in both normotensive and hypertensive patients.13,14 Randomized clinical trials also supported that systemic BP may be an important determinant of VF progression.26,36,37 In the Low-pressure Glaucoma Study, the use of oral anti-hypertensive medication and lower MAP both increased the risk VF progression.26 Of note, our study and the others cited measured only brachial BP and not actual ocular perfusion pressure.
Glaucoma is a progressive optic neuropathy with a variety of phenotypes, including NTG and high-tension glaucoma. Although they are both the same clinical entity (POAG), patients with NTG have features that suggest IOP-independent mechanisms may play a significant role in its progression.38–41 For instance, in NTG, optic disc phenotypes are more often described as senile-sclerotic or focal-ischemic.42,43
To date, IOP reduction is the only proven treatment for all types of open-angle glaucoma, including NTG. The Early Manifest Glaucoma Trial, which compared treatment with observation alone among patients with open-angle glaucoma (high-tension glaucoma or NTG) showed that lowering IOP decreases the risk of VF progression.44 Focused only on NTG, the Collaborative Normal-tension Glaucoma Study compared treated with untreated patients; the intent-to-treat analysis of the Collaborative Normal-tension Glaucoma Study, which includes all patients from randomization, revealed that IOP lowering did not slow rates of progression.1 Nonetheless, the pragmatic analysis showed that IOP lowering was beneficial, as also proven in other clinical trials, but seemingly at the expense of treatment-induced cataract.36 In the Low-pressure Glaucoma Study, an RCT in which both arms were treated with IOP-lowering medications, none of the IOP parameters were associated with progression.26 It is important to realize that our study was not designed or powered to disprove the importance of lowering the IOP in NTG. In fact, one should be reminded that IOP lowering has been reported to improve ocular perfusion in NTG.19
Current guidelines for treatment of hypertensive patients urge aggressive targets for reducing systemic BP, especially in those with diabetes or renal disease.45 If such aggressive BP goals are accompanied by nocturnal hypotension in patients with glaucoma, new, less-aggressive targets might have to be set to prevent further VF loss. Despite the unquestionable benefits of treating systemic hypertension, our data suggest that lowering BP too aggressively—particularly at night—may be detrimental to peripheral tissues such as the optic nerve, a watershed zone.46
In conclusion, the magnitude and duration of nocturnal hypotension identify patients with NTG who have VF progression. Ambulatory monitoring of systemic BP should become part of routine assessment of patients with NTG, particularly among those who continue to progress despite IOP lowering. Nocturnal BP should be considered a modifiable risk factor in NTG. Randomized trials will be required to assess the efficacy of different interventions designed to avoid nocturnal hypotension to prevent VF loss in patients with NTG, as well as to test the effect of more aggressive IOP-lowering therapy in these cases.
Abbreviations and Acronyms
- BP
blood pressure
- IOP
intraocular pressure
- MAP
mean arterial pressure
- NTG
normal-tension glaucoma
- POAG
primary open-angle glaucoma
- PSD
pattern standard deviation
- RCT
randomized clinical trial
- SAP
standard automated perimetry
- SITA
Swedish Interactive Thresholding Algorithm
- VF
visual field
Footnotes
Financial Disclosure(s):
The author has no proprietary or commercial interest in any materials discussed in this article.
References
- 1.Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. doi: 10.1016/s0002-9394(98)00272-4. [DOI] [PubMed] [Google Scholar]
- 2.Musch DC, Gillespie BW, Niziol LM, et al. CIGTS Study Group. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2000;118:1766–73. doi: 10.1016/j.ophtha.2011.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leske MC, Heijl A, Hyman L, et al. EMGT Group. Predictors of long-term progression in the Early Manifest Glaucoma Trial. Ophthalmology. 2007;114:1965–72. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. discussion 829–30. [DOI] [PubMed] [Google Scholar]
- 5.Francois J, Neetens A. The deterioration of the visual field in glaucoma and the blood pressure. Doc Ophthalmol. 1970;28:70–132. doi: 10.1007/BF00153874. [DOI] [PubMed] [Google Scholar]
- 6.Drance SM. Some factors in the production of low tension glaucoma. Br J Ophthalmol. 1972;56:229–42. doi: 10.1136/bjo.56.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer JH, Brandi-Dohrn J, Funk J. Twenty four hour blood pressure monitoring in normal tension glaucoma. Br J Ophthalmol. 1996;80:864–7. doi: 10.1136/bjo.80.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 9.Graham SL, Drance SM, Wijsman K, et al. Ambulatory blood pressure monitoring in glaucoma. The nocturnal dip. Ophthalmology. 1995;102:61–9. doi: 10.1016/s0161-6420(95)31053-6. [DOI] [PubMed] [Google Scholar]
- 10.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma. A population-based assessment. Arch Ophthalmol. 1995;113:216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 11.Leske MC, Wu SY, Hennis A, et al. BESs Study Group. Risk factors for incident open-angle glaucoma: the Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser HJ, Flammer J, Graf T, Stumpfig D. Systemic blood pressure in glaucoma patients. Graefes Arch Clin Exp Ophthalmol. 1993;231:677–80. doi: 10.1007/BF00919280. [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–24. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, Podhajsky P, Zimmerman MB. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96. doi: 10.1159/000027399. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, MacKenzie CR, Gold JP, et al. The preoperative and intraoperative hemodynamic predictors of postoperative myocardial infarction or ischemia in patients undergoing noncardiac surgery. Ann Surg. 1989;210:637–48. doi: 10.1097/00000658-198911000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlson ME, MacKenzie CR, Gold JP, et al. Intra-operative blood pressure. What patterns identify patients at risk for postoperative complications? Ann Surg. 1990;212:567–80. doi: 10.1097/00000658-199011000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gold JP, Charlson ME, Williams-Russo P, et al. Improvement of outcomes after coronary artery bypass. A randomized trial comparing intraoperative high versus low mean arterial pressure. J Thorac Cardiovasc Surg. 1995;110:1302–14. doi: 10.1016/S0022-5223(95)70053-6. [DOI] [PubMed] [Google Scholar]
- 18.Costa VP, Harris A, Anderson D, et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014;92:e252–66. doi: 10.1111/aos.12298. [DOI] [PubMed] [Google Scholar]
- 19.Ramdas WD, Wolfs RC, Hofman A, et al. Ocular perfusion pressure and the incidence of glaucoma: real effect or artifact? The Rotterdam Study Invest. Ophthalmol Vis Sci. 2011;52:6875–81. doi: 10.1167/iovs.11-7376. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Jonas JB, Iester M. Disc hemorrhage and glaucoma [letter] Ophthalmology. 1995;102:365–6. doi: 10.1016/s0161-6420(13)30831-8. [DOI] [PubMed] [Google Scholar]
- 22.Groppelli A, Omboni S, Parati G, Mancia G. Evaluation of noninvasive blood pressure monitoring devices Spacelabs 90202 and 90207 versus resting and ambulatory 24-hour intra-arterial blood pressure. Hypertension. 1992;20:227–32. doi: 10.1161/01.hyp.20.2.227. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien E, Atkins N, Mee F, O’Malley K. Comparative accuracy of six ambulatory devices according to blood pressure levels. J Hypertens. 1993;11:673–5. doi: 10.1097/00004872-199306000-00012. [DOI] [PubMed] [Google Scholar]
- 24.O’Brien E, O’Malley K, Mee F, et al. Ambulatory blood pressure measurement in the diagnosis and management of hypertension. J Hum Hypertens. 1991;5(Suppl):23–30. [PubMed] [Google Scholar]
- 25.Fitzke FW, Hitchings RA, Poinoosawmy D, et al. Analysis of visual field progression in glaucoma. Br J Ophthalmol. 1996;80:40–8. doi: 10.1136/bjo.80.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Moraes CG, Liebmann JM, Greenfield DS, et al. Low-pressure Glaucoma Treatment Study Group. Risk factors for visual field progression in the Low-pressure Glaucoma Treatment Study. Am J Ophthalmol. 2012;154:702–11. doi: 10.1016/j.ajo.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 27.De Moraes CG, Liebmann CA, Susanna R, et al. Examination of the performance of pointwise linear regression progression criteria to detect glaucomatous visual field change [report online] Clin Experiment Ophthalmol. 2012;40:e190–6. doi: 10.1111/j.1442-9071.2011.02680.x. [DOI] [PubMed] [Google Scholar]
- 28.Nouri-Mahdavi K, Hoffman D, Gaasterland D, Caprioli J. Prediction of visual field progression in glaucoma. Invest Ophthalmol Vis Sci. 2004;45:4346–51. doi: 10.1167/iovs.04-0204. [DOI] [PubMed] [Google Scholar]
- 29.Bengtsson B, Patella VM, Heijl A. Prediction of glaucomatous visual field loss by extrapolation of linear trends. Arch Ophthalmol. 2009;127:1610–5. doi: 10.1001/archophthalmol.2009.297. [DOI] [PubMed] [Google Scholar]
- 30.Zellner A. An efficient method of estimating seemingly unrelated regressions and tests for aggregation bias. J Am Stat Assoc. 1962;57:348–68. [Google Scholar]
- 31.Skinhoj E, Strandgaard S. Pathogenesis of hypertensive encephalopathy. Lancet. 1973;1:461–2. doi: 10.1016/s0140-6736(73)91884-9. [DOI] [PubMed] [Google Scholar]
- 32.Mancia G, Parati G, Omboni S, et al. Ambulatory blood pressure monitoring. Clin Exp Hypertens. 1999;21:703–15. doi: 10.3109/10641969909061001. [DOI] [PubMed] [Google Scholar]
- 33.Graham SL, Drance SM. Nocturnal hypotension: role in glaucoma progression. Surv Ophthalmol. 1999;43(Suppl):S10–6. doi: 10.1016/s0039-6257(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 34.Tokunaga T, Kashiwagi K, Tsumura T, et al. Association between nocturnal blood pressure reduction and progression of visual field defect in patients with primary open-angle glaucoma or normal-tension glaucoma. Jpn J Ophthalmol. 2004;48:380–5. doi: 10.1007/s10384-003-0071-6. [DOI] [PubMed] [Google Scholar]
- 35.Collignon N, Dewe W, Guillaume S, Collignon-Brach J. Ambulatory blood pressure monitoring in glaucoma patients. The nocturnal systolic dip and its relationship with disease progression. Int Ophthalmol. 1998;22:19–25. doi: 10.1023/a:1006113109864. [DOI] [PubMed] [Google Scholar]
- 36.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol. 1998;126:487–97. doi: 10.1016/s0002-9394(98)00223-2. [DOI] [PubMed] [Google Scholar]
- 37.Greenfield DS, Liebmann JM, Ritch R, Krupin T Low-Pressure Glaucoma Study Group. Visual field and intraocular pressure asymmetry in the Low-pressure Glaucoma Treatment Study. Ophthalmology. 2007;114:460–5. doi: 10.1016/j.ophtha.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 38.King D, Drance SM, Douglas G, et al. Comparison of visual field defects in normal-tension glaucoma and high-tension glaucoma. Am J Ophthalmol. 1986;101:204–7. doi: 10.1016/0002-9394(86)90596-9. [DOI] [PubMed] [Google Scholar]
- 39.Chauhan BC, Drance SM, Douglas GR, Johnson CA. Visual field damage in normal-tension and high-tension glaucoma. Am J Ophthalmol. 1989;108:636–42. doi: 10.1016/0002-9394(89)90854-4. [DOI] [PubMed] [Google Scholar]
- 40.Araie M, Yamagami J, Suziki Y. Visual field defects in normal-tension and high-tension glaucoma. Ophthalmology. 1993;100:1808–14. doi: 10.1016/s0161-6420(93)31394-1. [DOI] [PubMed] [Google Scholar]
- 41.Caprioli J, Spaeth GL. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol. 1984;97:730–7. doi: 10.1016/0002-9394(84)90505-1. [DOI] [PubMed] [Google Scholar]
- 42.Yamazaki Y, Hayamizu F, Miyamoto S, et al. Optic disc findings in normal tension glaucoma. Jpn J Ophthalmol. 1997;41:260–7. doi: 10.1016/s0021-5155(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 43.Ahrlich KG, De Moraes CG, Teng CC, et al. Visual field progression differences between normal-tension and exfoliative high-tension glaucoma. Invest Ophthalmol Vis Sci. 2010;51:1458–63. doi: 10.1167/iovs.09-3806. [DOI] [PubMed] [Google Scholar]
- 44.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 45.Lenfant C, Chobanian AV, Jones DW, Roccella EJ. Seventh report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–9. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 46.Kario K, Shimada K. Risers and extreme-dippers of nocturnal blood pressure in hypertension: antihypertensive strategy for nocturnal blood pressure. Clin Exp Hypertens. 2004;26:177–89. doi: 10.1081/ceh-120028556. [DOI] [PubMed] [Google Scholar]