Abstract
The purpose of the study was to examine the impact of race/ethnicity on second breast tumors among women with ductal carcinoma in situ (DCIS). We identified 102,489 women diagnosed with primary DCIS between 1988 and 2009 from the 18 NCI-SEER Registries. Cox proportional hazard regression was used to estimate race/ethnicity-associated relative risks (RRs) and their 95 % confidence intervals (CI) of ipsilateral breast tumors (IBT; defined as DCIS or invasive carcinoma in the ipsilateral breast) and contralateral breast tumors (CBT; defined as DCIS or invasive carcinoma in the contralateral breast). Overall, 2,925 women had IBT and 3,723 had CBT. Compared with white women, black (RR 1.46; 95 % CI 1.29–1.65), and Hispanic (RR 1.18; 95 % CI 1.03–1.36) women had higher IBT risk, which was similar for invasive IBT and ipsilateral DCIS. A significant increase in IBT risk among black women persisted, regardless of age at diagnosis, treatment, tumor grade, tumor size, and histology. The CBT risk was significantly increased among black (RR 1.21; 95 % CI 1.08–1.36) and Asian/PI (RR 1.16; 95 % CI 1.02–1.31) women compared with white women. The association was stronger for invasive CBT among black women and for contralateral DCIS among Asian/PI women (Pheterogeneity <0.0001). The black race-associated CBT risk was more pronounced among women ≥50 years at diagnosis and those with comedo DCIS; in contrast, a significant increase in risk among Asian/PI women was restricted to those <50 years and those with noncomedo DCIS. Racial/ethnic differences in risks of second breast tumors after DCIS could not be explained by pathologic features and treatment.
Keywords: Ductal carcinoma in situ, Breast cancer, Race, Second breast tumors
The diagnosis of ductal carcinoma in situ (DCIS) has increased considerably since the 1980s, largely due to widespread use of screening mammography [1]. Approximately, 45,900 new DCIS cases were diagnosed in the United States in 2010 [2]; the age-adjusted incidence was 32 per 100,000 persons in white women and 21–30 per 100,000 persons in nonwhite women [3]. Although 10-year breast cancer mortality is less than 2 % [4], 10–24 % of DCIS patients experience second breast tumors 10 or more years after treatment [5]. Black women with invasive breast cancer are more likely than their white counterparts to present with more aggressive pathologic features and have higher breast cancer-specific mortality [6–8]. However, the impact of race/ethnicity on DCIS outcomes has not been well defined. Only a few population-based [9–12] and small institution-based studies [13–17] have compared DCIS outcomes between black and white women. The information on DCIS outcomes in women of other races is sparser [9–11, 13, 15].
Prior analyses of the Surveillance, Epidemiology, and End Results (SEER) data through 2001 that were adjusted for demographic factors and treatment reported statistically significant differences in ipsilateral and contralateral invasive breast cancers in racial/ethnic minority groups [9, 11]. There was no significant difference in risk of second breast tumors after adjustment for histopathologic factors by race/ethnicity [13, 16, 18]. However, these studies had limited power to detect racial/ethnic differences in outcomes due to small numbers of minority patients and few second breast events.
Therefore, we examined racial disparities in second breast tumors in a large cohort of women with DCIS diagnosed between 1988 and 2009 in the 18 SEER registries, controlling for age at diagnosis, treatment patterns, and histopathologic features. Further, we assessed effect modification of patient and tumor characteristics on this relationship.
Methods
Patient population
From the SEER 18 Registries Database (November 2011 submission), we identified women diagnosed with primary unilateral DCIS [11] between January 1988 and June 2009 with no cancer history who were followed through December 31, 2009 (n = 109,938); these registries cover approximately 28 % of the population. De-identified SEER data were used, exempting the study from review by our Institutional Review Board. We excluded patients younger than 20 years or older than 84 years (n = 1,949) and those with bilateral mastectomy (n = 4,263). White, black, and Asian/Pacific Islander women comprised 98.7 % of eligible cases, therefore, women of other races or unknown race (n = 1,237) were excluded if they were non-Hispanic. Thus, 102,489 women with DCIS were included in the analysis. Since women undergoing mastectomy experience extremely low risk of ipsilateral breast tumors (IBTs) [5], we excluded 27,680 women treated with mastectomy for their first DCIS and thus included 74,809 in the analysis of IBTs.
Race/ethnicity was classified into mutually exclusive categories of non-Hispanic white (hereafter referred to as white), non-Hispanic black (black), non-Hispanic Asian/Pacific Islander (Asian/PI), and Hispanic. Whites accounted for 95.8 % of Hispanics. Since the exclusion of Hispanic nonwhites and Pacific Islanders did not significantly influence the result, we combined all Hispanics as a single group and non-Hispanic Asians and Pacific Islanders as a single group.
Outcomes
Second primary breast tumor was defined as an invasive breast cancer (including all histologic types) or DCIS diagnosed at least 6 months after the first DCIS. The outcomes included IBTs (defined as local recurrence of DCIS or invasive carcinoma in the ipsilateral breast), invasive IBTs, ipsilateral DCIS, contralateral breast tumors (CBTs; defined as DCIS or invasive carcinoma in the contralateral breast), invasive CBTs, and contralateral DCIS. Person-years were calculated from 6 months after the first DCIS until the date of second primary breast tumors, death, or December 31, 2009, whichever occurred first.
Statistical analysis
The x2 test and analysis of variance were used, respectively, to compare baseline categorical and continuous variables across racial/ethnic groups. Kaplan–Meier estimates of 5-year probabilities of IBTs and CBTs were calculated for each group, with P values given by the log-rank test. We used Cox proportional hazards models to compute race-associated relative risk (RR) and 95 % confidence interval (CI). Assumption of proportionality for Cox models were confirmed using scaled Schoenfeld residuals. The models were controlled for age (20–39, 40–49, 50–59, 60–69, or 70–84 years) and year of the first DCIS diagnosis (1988–1989, 1990–1994, 1995–1999, 2000–2004, or 2005–2009), registry, treatment for the first DCIS (no surgical treatment, breast-conserving surgery(BCS) alone, BCS plus radiotherapy, mastectomy, or unknown) and histopathological features including tumor size (<2 cm, 2–5 cm, ≥5 cm, or unknown), grade (well differentiated, moderately differentiated, poorly differentiated, or unknown), and histology (comedo, papillary, cribriform, solid, or NOS). The analyses were stratified by age at diagnosis of the first DCIS, treatment, and histopathologic features. Interactions between race/ethnicity and characteristics of DCIS were assessed by entering cross-product terms in multivariable-adjusted models. The statistical significance of an interaction term was evaluated by the likelihood ratio test. To determine whether race/ethnicity is differentially associated with types of second breast tumors (invasive cancer vs DCIS), the Cox proportional hazards model was used with types of second breast tumors treated as competing risks [19]. Specifically, we estimated RRs of different types of second breast tumors using the approach described by Lunn and McNeil [20, 21] and used the likelihood ratio test for heterogeneity. All statistical analyses were performed by SAS (version 9.3; SAS Institute, Cary, NC). Statistical significance was assessed as two-sided P < 0.05.
Results
Among 102,489 women with DCIS, 75,431 (73.6 %) were white, 9,921 (9.7 %) were black, 9,246 (9.0 %) were Asian/PI, and 7,891 (7.7 %) were Hispanic. Mean age was 58.5 years (range 20–84). Most (88.7 %) were diagnosed after 1995. The patient population was characterized pathologically by 45.5 % with poorly differentiated tumors, 22.9 % with tumors ≥2 cm, and 14.7 % with comedocarcinoma. Information on estrogen receptor (ER) status was available for 41.6 % patients, of which 77.8 % were reported after 2003 and 82.0 % were positive. Overall, 2.2 % of patients received no surgical treatment for their first DCIS, 27.5 % were treated with mastectomy, 26.5 % received BCS alone, and 43.8 % received BCS and radiotherapy.
Compared with white women, racial/ethnic minority women were significantly younger at the diagnosis of first DCIS and were more likely to have large, well or moderately differentiated, and noncomedo lesions (each P < 0.0001) (Table 1). ER positivity was reported in 85.0 % of black women with available ER data, which was significantly higher than the other racial/ethnic groups (white 81.6 %, Asian/PI 81.7 %, and Hispanic 81.9 %; P < 0.0001). A larger proportion of black and Asian/PI patients underwent mastectomy for their first DCIS (P < 0.0001). Among 72,232 patients treated with BCS, radiotherapy was received more often in Asian/PI patients (64.8 %) than other racial/ethnic groups (white 62.3 %, black 61.9 %, and Hispanic 60.4 %; P < 0.0001).
Table 1.
Characteristics of women with unilateral ductal carcinoma in situ (DCIS) in the SEER 18 Cancer Registries, 1988–2009, stratified by race and ethnicitya (n = 102,489)
| No. of patients (%) | |||||
|---|---|---|---|---|---|
| White | Black | Asian/PI | Hispanic | Pb | |
| Total | 75,431 (73.6) | 9,921 (9.7) | 9,246 (9.0) | 7,891 (7.7) | – |
| Age at diagnosis, y | |||||
| Mean (SD) | 59.1 (12.1) | 57.6 (12.0) | 56.0 (11.6) | 56.0 (11.7) | <0.0001 |
| 20–39 | 2,505 (3.3) | 512 (5.2) | 457 (4.9) | 378 (4.8) | – |
| 40–49 | 16,182 (21.5) | 2,267 (22.9) | 2,598 (28.1) | 2,262 (28.7) | – |
| 50–59 | 21,165 (28.1) | 2,881 (29.0) | 2,787 (30.1) | 2,320 (29.4) | – |
| 60–69 | 18,181 (24.1) | 2,384 (24.0) | 2,001 (21.6) | 1,733 (22.0) | – |
| ≥70 | 17,398 (23.1) | 1,877 (18.9) | 1,403 (15.2) | 1,198 (15.2) | <0.0001 |
| Length of follow-up, months | |||||
| Mean (range) | 82.6 (6,263) | 72.2 (6,263) | 77.4 (6,263) | 69.8 (6,263) | <0.0001 |
| 6–11 | 3,743 (5.0) | 625 (6.3) | 560 (6.1) | 556 (7.1) | – |
| 12–59 | 27,131 (36.0) | 4,174 (42.1) | 3,663 (39.6) | 3,437 (43.6) | – |
| 60–119 | 28,301 (37.5) | 3,534 (35.6) | 3,130 (33.9) | 2,731 (34.6) | – |
| ≥120 | 16,256 (21.6) | 1,588 (16.0) | 1,893 (20.5) | 1,167 (14.8) | <0.0001 |
| Year of the first DCIS diagnosisc | |||||
| 1988–1989 | 1,915 (2.5) | 179 (1.8) | 157 (1.7) | 75 (1.0) | – |
| 1990–1994 | 7,374 (9.8) | 720 (7.3) | 721 (7.8) | 488 (6.2) | – |
| 1995–1999 | 11,946 (15.8) | 1,411 (14.2) | 1,545 (16.7) | 1,004 (12.7) | – |
| 2000–2004 | 28,284 (37.5) | 3,589 (36.2) | 3,173 (34.2) | 2,847 (36.1) | – |
| 2005–2009 | 25,912 (34.4) | 4,022 (40.5) | 3,650 (39.5) | 3,477 (44.1) | <0.0001 |
| Histological subtype | |||||
| Not otherwise specified | 50,510 (67.0) | 6,795 (68.5) | 6,319 (68.3) | 5,394 (68.4) | – |
| Comedo | 11,523 (15.3) | 1,293 (13.0) | 1,174 (12.7) | 1,025 (13.0) | – |
| Papillary | 4,397 (5.8) | 820 (8.3) | 545 (5.9) | 509 (6.5) | – |
| Cribiform | 5,616 (7.5) | 662 (6.7) | 814 (8.8) | 657 (8.3) | – |
| Solid | 3,385 (4.5) | 351 (3.5) | 394 (4.3) | 306 (3.9) | <0.0001 |
| Grade | |||||
| Well differentiated | 7,432 (14.2) | 1,185 (17.2) | 990 (13.9) | 785 (13.1) | – |
| Moderately differentiated | 20,688 (39.4) | 2,866 (41.6) | 2,978 (41.7) | 2,553 (42.7) | – |
| Poorly differentiated | 24,356 (46.4) | 2,847 (41.3) | 3,173 (44.4) | 2,636 (44.1) | <0.0001 |
| Missing | 22,955 | 3,023 | 2,105 | 1,917 | – |
| Tumor size, cm | |||||
| <2.0 | 39,429 (78.9) | 4,519 (72.8) | 5,052 (72.2) | 3,838 (72.0) | – |
| 2.0–4.9 | 8,326 (16.7) | 1,233 (19.9) | 1,592 (22.8) | 1,160 (21.8) | – |
| ≥5.0 | 2,235 (4.5) | 456 (7.4) | 350 (5.0) | 335 (6.3) | <0.0001 |
| Missing | 25,441 | 3,713 | 2,252 | 2558 | – |
| Estrogen receptor | |||||
| Negative | 5,624 (18.4) | 684 (15.0) | 728 (18.3) | 631 (18.1) | – |
| Positive | 25,013 (81.6) | 3,882 (85.0) | 3251 (81.7) | 2855 (81.9) | <0.0001 |
| Missing | 44,794 | 5,355 | 5267 | 4405 | – |
| Surgery for first DCIS | |||||
| None | 1,599 (2.1) | 277 (2.8) | 121 (1.3) | 212 (2.7) | – |
| BCSd | 53,644 (71.4) | 6,685 (67.7) | 6,265 (68.0) | 5,638 (71.7) | – |
| Mastectomye | 19,927 (26.5) | 2,915 (29.5) | 2,828 (30.7) | 2,010 (25.6) | <0.0001 |
| Missing | 261 | 44 | 32 | 31 | – |
| Radiation therapy for first DCIS | |||||
| No | 40,506 (54.7) | 5,546 (56.9) | 5,053 (55.3) | 4,308 (55.7) | – |
| Yes | 33,556 (45.3) | 4,200 (43.1) | 4,081 (44.7) | 3,425 (44.3) | 0.0003 |
| Missing | 1,369 | 175 | 112 | 158 | – |
| Surgery and radiation therapy for first DCIS | |||||
| No surgery | 1,599 (2.2) | 277 (2.8) | 121 (1.3) | 212 (2.7) | – |
| BCSd alone | 19,852 (26.8) | 2,506 (25.7) | 2,176 (23.9) | 2,183 (28.2) | – |
| BCSd and radiation | 32,787 (44.2) | 4,064 (41.6) | 3,998 (43.8) | 3,324 (43.0) | – |
| Mastectomye | 19,927 (26.9) | 2,915 (29.9) | 2,828 (31.0) | 2,010 (26.0) | <0.0001 |
| Missing | 1,266 | 159 | 123 | 162 | – |
SD standard deviation, BCS breast-conserving surgery
Race and ethnicity were classified into mutually exclusive categories of non-Hispanic white (hereafter referred to as white), non-Hispanic black (black), non-Hispanic Asian or Pacific Islander (Asian/PI), and Hispanic (Hispanic)
P values were calculated from a comparison across all groups except the groups with missing values
Cases diagnosed between 1988 and 1989 were from nine registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah). Cases diagnosed between 1990 and 1999 were from 12 registries (the above nine registries, plus Los Angeles, San Jose-Monterey, and Rural Georgia). Cases diagnosed since 2000 were from 17 registries (the above 12 registries, plus Greater California, Kentucky, Louisiana, New Jersey, and Greater Georgia). Cases from the Alaska Native Tumor Registry were excluded due to the small number of Alaskan Native patients
Breast-conserving surgery consisted of excisional biopsy, lumpectomy, nipple resection, wedge resection, quadrantectomy, segmental mastectomy, tylectomy, and partial mastectomy, NOS
Mastectomy included total mastectomy, modified radical mastectomy, radical mastectomy, subcutaneous mastectomy and mastectomy, not otherwise specified
IBTs
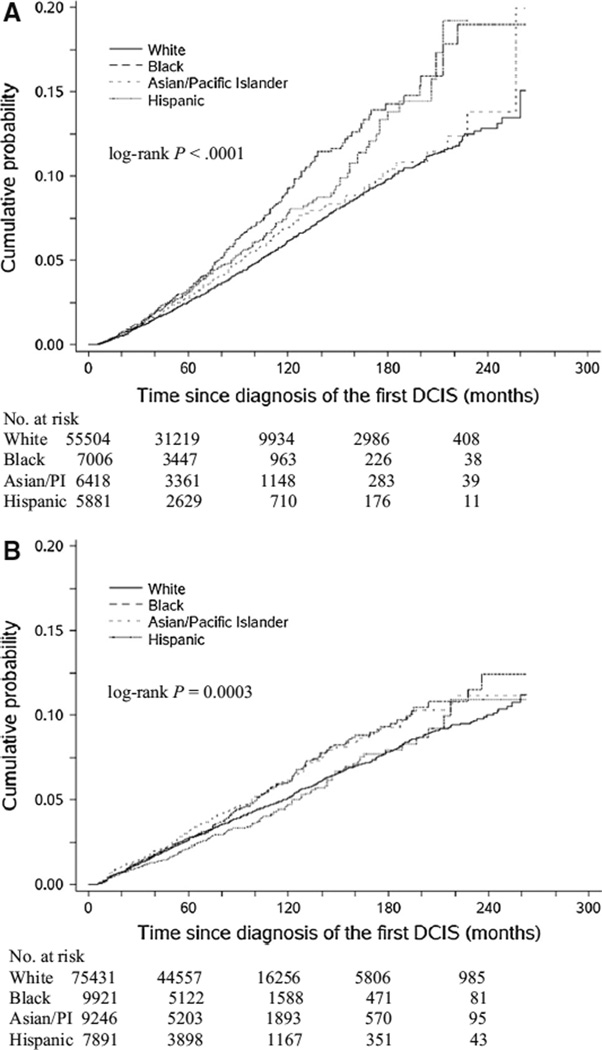
Among 74,809 women treated with BCS or with no surgical treatment, 2,925 (3.9 %) experienced IBTs during a median follow-up of 66 months (range 6–263). Of these IBTs, 824 (28.2 %) were DCIS and 2,101 (71.8 %) were invasive cancer. There was a statistically significant difference in the cumulative incidence of IBTs by race/ethnicity; the 5-year rate was 3.3 % in blacks and 3.1 % in Hispanics compared to 2.5 % in whites and 2.8 % in Asians/PIs (Fig. 1a, P < 0.0001).
Fig. 1.
Cumulative incidences of second breast tumors in the ipsilateral breast (a) and contralateral breast (b) among four racial/ethnic groups of women with DCIS
The multivariable-adjusted analysis showed that black (RR 1.46; 95 % CI 1.29–1.65) and Hispanic women (RR 1.18; 95 % CI 1.03–1.36) had significantly higher IBT risk compared with white women. There was no significant difference in risk between white and Asian/PI (Table 2) or individual Asian groups (Supplement Table 1). We restricted the analysis to 32,016 women (white 24,587, black 2,841, Asian/PI 2,373, and Hispanic 2,215) with available ER data. The RR was 1.29 (95 % CI 1.00–1.68; P = 0.06) for blacks, 1.27 (95 % CI 0.93–1.74) for Asians/PIs, and 1.25 (95 % CI 0.94–1.65) for Hispanics.
Table 2.
Risk of ipsilateral breast tumorsa associated with race and ethnicity among women with unilateral ductal carcinoma in situ (DCIS) diagnosed between 1988 and 2009b (n = 74,809)
| Person-years | Ipsilateral breast tumors | Ipsilateral DCIS | Ipsilateral invasive cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | RRc | 95 % CIc | Cases | RRc | 95 % CIc | Cases | RRc | 95 % CIc | ||
| White | 355,850 | 2,104 | 1.00 | Referent | 595 | 1.00 | Referent | 1,509 | 1.00 | Referent |
| Black | 39,822 | 3,30 | 1.46 | 1.29–1.65 | 98 | 1.48 | 1.18–1.86 | 232 | 1.45 | 1.25–1.67 |
| Asian/PI | 39,132 | 255 | 1.11 | 0.96–1.29 | 62 | 0.94 | 0.70–1.27 | 193 | 1.18 | 0.99–1.39 |
| Hispanic | 32,118 | 236 | 1.18 | 1.03–1.36 | 69 | 1.33 | 1.02–1.72 | 167 | 1.13 | 0.96–1.34 |
| Pheterogeneity = 0.25 | ||||||||||
RR relative risk, 95 % CI 95 % confidence interval
Ipsilateral breast tumors were defined as local recurrence of DCIS or invasive carcinoma in the ipsilateral breast that was diagnosed at least 6 months after the first DCIS
Patients who had been treated with mastectomy for their first DCIS (n = 27,680) were excluded
Relative risks were adjusted for age (20–39, 40–49, 50–59, 60–69, or 70–84 years) and year of the first DCIS diagnosis (1988–1989, 1990–1994, 1995–1999, 2000–2004, or 2005–2009), registry, treatment for the first DCIS (no surgical treatment, breast-conserving surgery alone, or breast-conserving surgery plus radiation therapy) and histopathological features including tumor size (<2 cm, 2–5 cm, ≥5 cm, or unknown), grade (well differentiated, moderately differentiated, poorly differentiated, or unknown), and histology (comedo, papillary, cribriform, solid, or NOS)
We determined whether race/ethnicity is differentially associated with types of IBTs (Table 2). Among black women, the RR was 1.48 (95 % CI 1.18–1.86) for ipsilateral DCIS and 1.45 (95 % CI 1.25–1.67) for invasive IBT. Hispanic ethnicity was associated with ipsilateral DCIS (RR 1.33; 95 % CI 1.02–1.72), but not with invasive IBT (RR 1.13; 95 % CI 0.96–1.34). However, this difference was not statistically significant.
We further analyzed the impact of race/ethnicity on IBTs according to pathologic features and treatment for the first DCIS (Table 3). The risk was significantly and consistently increased among black compared to white women treated with BCS, regardless of age at diagnosis, receipt of radiotherapy, tumor grade, size, and architectural patterns. There was no statistically significant difference in the risk associated with Hispanic ethnicity according to tumor characteristics and treatment.
Table 3.
Stratified analysis of racial/ethnic differences in risk of ipsilateral breast tumors by characteristics of the first ductal carcinoma in situ (DCIS)
| Cases | Person-years | RRa | 95 % CIa | |
|---|---|---|---|---|
| Age at diagnosis <50 years | ||||
| White | 696 | 90,678 | 1.00 | Referent |
| Black | 122 | 11,431 | 1.49 | 1.22–1.83 |
| Asian/PI | 114 | 13,137 | 1.13 | 0.91–1.41 |
| Hispanic | 94 | 10,707 | 1.10 | 0.88–1.38 |
| Age at diagnosis ≥50 years | ||||
| White | 1,408 | 265,172 | 1.00 | Referent |
| Black | 208 | 28,391 | 1.45 | 1.25–1.69 |
| Asian/PI | 141 | 25,995 | 1.09 | 0.90–1.33 |
| Hispanic | 142 | 21,411 | 1.24 | 1.03–1.48 |
| Pinteraction = 0.45 | ||||
| Breast-conserving surgery alone | ||||
| White | 1,103 | 135,774 | 1.00 | Referent |
| Black | 181 | 15,372 | 1.51 | 1.28–1.78 |
| Asian/PI | 126 | 14,063 | 1.03 | 0.85–1.26 |
| Hispanic | 132 | 12,587 | 1.27 | 1.05–1.54 |
| Breast-conserving surgery and radiation therapy | ||||
| White | 848 | 202,215 | 1.00 | Referent |
| Black | 117 | 22,223 | 1.33 | 1.09–1.63 |
| Asian/PI | 108 | 23,808 | 1.21 | 0.96–1.53 |
| Hispanic | 86 | 17,502 | 1.19 | 0.95–1.51 |
| Pinteraction = 0.40 | ||||
| Well or moderately differentiated DCIS | ||||
| White | 610 | 125,031 | 1.00 | Referent |
| Black | 103 | 15,280 | 1.63 | 1.31–2.03 |
| Asian/PI | 82 | 16,142 | 0.97 | 0.75–1.26 |
| Hispanic | 77 | 12,913 | 1.08 | 0.85–1.39 |
| Poorly differentiated DCIS | ||||
| White | 522 | 91,332 | 1.00 | Referent |
| Black | 83 | 9,121 | 1.39 | 1.09–1.78 |
| Asian/PI | 67 | 10,946 | 1.12 | 0.85–1.47 |
| Hispanic | 63 | 8,795 | 1.19 | 0.91–1.57 |
| Pinteraction = 0.47 | ||||
| DCIS <2 cm | ||||
| White | 1,212 | 208,235 | 1.00 | Referent |
| Black | 150 | 20,843 | 1.32 | 1.11–1.58 |
| Asian/PI | 152 | 24,548 | 1.13 | 0.94–1.37 |
| Hispanic | 127 | 17,749 | 1.21 | 1.00–1.46 |
| DCIS ≥2 cm | ||||
| White | 189 | 32,937 | 1.00 | Referent |
| Black | 52 | 4,688 | 1.86 | 1.34–2.58 |
| Asian/PI | 35 | 4,970 | 1.20 | 0.80–1.78 |
| Hispanic | 36 | 4,146 | 1.55 | 1.07– 2.26 |
| Pinteraction = 0.07 | ||||
| Comedo type | ||||
| White | 440 | 59,707 | 1.00 | Referent |
| Black | 60 | 5,360 | 1.44 | 1.08–1.91 |
| Asian/PI | 39 | 5,295 | 0.97 | 0.68–1.38 |
| Hispanic | 45 | 4,868 | 1.14 | 0.83–1.58 |
| Noncomedo type | ||||
| White | 1,664 | 296,144 | 1.00 | Referent |
| Black | 270 | 34,462 | 1.47 | 1.28–1.68 |
| Asian/PI | 216 | 33,837 | 1.14 | 0.97–1.34 |
| Hispanic | 191 | 27,250 | 1.19 | 1.01–1.39 |
| Pinteraction = 0.40 | ||||
RR relative risk, 95 % CI 95 % confidence interval
Relative risks were adjusted for the covariates listed in the footnote of Table 2
CBTs
A total of 3,723 (3.6 %) patients developed CBTs among 102,489 patients with a median follow-up of 70 months (range 6–263), of which 1,145 (30.8 %) were DCIS and 2,578 (69.2 %) were invasive cancer. A statistically significant difference in the incidence of CBTs was observed across races/ethnicities, with a 5-year rate of 2.7 % in whites and blacks, 3.2 % in Asians/PIs, and 2.1 % in Hispanics (Fig. 1b, P < 0.001).
The multivariable-adjusted CBT risk was significantly increased among black (RR 1.21; 95 % CI 1.08–1.36) and Asian/PI, especially Filipino (Supplement Table 2), women (RR 1.16; 95 % CI 1.02–1.31) compared to their white counterparts. This association depended on types of CBTs (Pheterogeneity <0.0001). The elevated risk among blacks was stronger for invasive CBT (RR 1.25; 95 % CI 1.09–1.43) than contralateral DCIS (RR 1.12; 95 % CI 0.91–1.39). In contrast, Asian/PI patients had significantly higher risk for contralateral DCIS (RR 1.59; 95 % CI 1.30–1.95) but not for invasive CBT (RR 0.96; 95 % CI 0.82–1.13) (Table 4).
Table 4.
Risk of second breast tumors in the contralateral breast associated with race and ethnicity among women with unilateral ductal carcinoma in situ (DCIS) diagnosed between 1988 and 2009 (n = 102,489)
| Person-years | Contralateral breast tumorsa | Contralateral DCIS | Contralateral invasive cancer | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cases | RRb | 95 % CIb | Cases | RRb | 95 % CIb | Cases | RRb | 95 % CIb | ||
| White | 519,306 | 2,765 | 1.00 | Referent | 816 | 1.00 | Referent | 1,949 | 1.00 | Referent |
| Black | 59,670 | 362 | 1.21 | 1.08–1.36 | 102 | 1.12 | 0.91–1.39 | 260 | 1.25 | 1.09–1.43 |
| Asian/PI | 59,638 | 378 | 1.16 | 1.02–1.31 | 160 | 1.59 | 1.30–1.95 | 218 | 0.96 | 0.82–1.13 |
| Hispanic | 46,067 | 218 | 0.95 | 0.82–1.09 | 67 | 0.99 | 0.77–1.29 | 151 | 0.93 | 0.79–1.11 |
| Pheterogeneity < 0.0001 | ||||||||||
RR relative risk, 95 % CI 95 % confidence interval
Contralateral breast tumors were defined as DCIS or invasive carcinoma developed in the contralateral breast at least 6 months after first DCIS
Relative risks were adjusted for age (20–39, 40–49, 50–59, 60–69, or 70–84 years) and year of the first DCIS diagnosis (1988–1989, 1990–1994, 1995–1999, 2000–2004, or 2005–2009), registry, treatment for the first DCIS (no surgical treatment, breast-conserving surgery(BCS) alone, BCS plus radiation therapy, mastectomy, or unknown) and histopathological features including tumor size (<2 cm, 2–5 cm, ≥5 cm, or unknown), grade (well differentiated, moderately differentiated, poorly differentiated, or unknown), and histology (comedo, papillary, cribriform, solid, or NOS)
Racial differences in CBTs varied by age (Pinteraction = 0.01) and architectural patterns of first DCIS (Pinteraction = 0.049) (Table 5). The black race-associated risk was much higher among women ≥50 years at diagnosis of first DCIS and those with comedo DCIS. Among Asian/PI women, a statistically significant increase in risk was found among those <50 years at diagnosis and those with noncomedo DCIS.
Table 5.
Stratified analysis of racial/ethnic differences in risk of contralateral breast tumors by characteristics of the first ductal carcinoma in situ (DCIS)
| Cases | Person-years | RRa | 95 % CIa | |
|---|---|---|---|---|
| Age at diagnosis <50 years | ||||
| White | 658 | 141,171 | 1.00 | Referent |
| Black | 79 | 18,247 | 1.03 | 0.81–1.31 |
| Asian/PI | 134 | 20,566 | 1.39 | 1.12–1.71 |
| Hispanic | 61 | 15,870 | 0.87 | 0.67–1.15 |
| Age at diagnosis ≥50 years | ||||
| White | 2,107 | 378,135 | 1.00 | Referent |
| Black | 283 | 41,423 | 1.34 | 1.15–1.56 |
| Asian/PI | 244 | 39,073 | 0.91 | 0.75–1.11 |
| Hispanic | 157 | 30,031 | 0.96 | 0.78–1.17 |
| Pinteraction = 0.01 | ||||
| Breast-conserving surgery alone | ||||
| White | 708 | 135,774 | 1.00 | Referent |
| Black | 82 | 15,372 | 1.01 | 0.80–1.28 |
| Asian/PI | 85 | 14,063 | 1.06 | 0.83–1.35 |
| Hispanic | 66 | 12,587 | 1.03 | 0.79–1.34 |
| Breast-conserving surgery and radiation therapy | ||||
| White | 1,079 | 202,215 | 1.00 | Referent |
| Black | 143 | 22,223 | 1.34 | 1.12–1.61 |
| Asian/PI | 144 | 23,808 | 1.18 | 0.96–1.46 |
| Hispanic | 79 | 17,502 | 0.91 | 0.72–1.16 |
| Mastectomy | ||||
| White | 906 | 163,455 | 1.00 | Referent |
| Black | 121 | 19,848 | 1.16 | 0.95–1.41 |
| Asian/PI | 144 | 20,507 | 1.20 | 0.98–1.48 |
| Hispanic | 71 | 13,783 | 1.00 | 0.78–1.28 |
| Pinteraction = 0.13 | ||||
| Well or moderately differentiated DCIS | ||||
| White | 817 | 159,784 | 1.00 | Referent |
| Black | 112 | 20,476 | 1.14 | 0.93–1.40 |
| Asian/PI | 122 | 21,842 | 1.07 | 0.86–1.33 |
| Hispanic | 82 | 16,882 | 0.98 | 0.77–1.24 |
| Poorly differentiated DCIS | ||||
| White | 691 | 134,319 | 1.00 | Referent |
| Black | 98 | 14,275 | 1.45 | 1.16–1.81 |
| Asian/PI | 103 | 16,957 | 1.11 | 0.88–1.39 |
| Hispanic | 51 | 13,218 | 0.75 | 0.56–1.01 |
| Pinteraction = 0.12 | ||||
| DCIS <2 cm | ||||
| White | 1,492 | 278,952 | 1.00 | Referent |
| Black | 176 | 27,547 | 1.28 | 1.09–1.51 |
| Asian/PI | 204 | 32,930 | 1.10 | 0.92–1.31 |
| Hispanic | 116 | 22,690 | 1.03 | 0.85–1.26 |
| DCIS ≥2 cm | ||||
| White | 366 | 64,236 | 1.00 | Referent |
| Black | 56 | 9,606 | 1.09 | 0.81–1.46 |
| Asian/PI | 80 | 10,988 | 1.28 | 0.97–1.68 |
| Hispanic | 38 | 8,017 | 0.85 | 0.60–1.20 |
| Pinteraction = 0.23 | ||||
| Comedo type | ||||
| White | 512 | 98,174 | 1.00 | Referent |
| Black | 67 | 9,302 | 1.47 | 1.13–1.92 |
| Asian/PI | 58 | 9,821 | 1.07 | 0.78–1.45 |
| Hispanic | 28 | 7,862 | 0.68 | 0.46–1.01 |
| Non-comedo type | ||||
| White | 2,253 | 421,132 | 1.00 | Referent |
| Black | 295 | 50,368 | 1.16 | 1.02–1.32 |
| Asian/PI | 320 | 49,817 | 1.18 | 1.02–1.35 |
| Hispanic | 190 | 38,038 | 1.01 | 0.87–1.18 |
| Pinteraction = 0.049 | ||||
RR relative risk, 95 % CI 95 % confidence interval
Relative risks were adjusted for the covariates listed in the footnote of Table 4
Discussion
This large, multiethnic cohort of women with DCIS allowed adequately powered detection of racial/ethnic disparities in second breast tumors according to types of second breast events and characteristics of the first DCIS. The risk for second breast tumors was significantly and consistently increased in black compared to white women, regardless of whether the outcome was ipsilateral DCIS or subsequent invasive cancer in the ipsilateral or contralateral breast. More importantly, the significantly elevated IBT risk in blacks persisted despite the presence of established prognostic factors (e.g., younger age at diagnosis, aggressive pathology). The black race-associated increase in CBT risk was more obvious among women ≥50 years at diagnosis and those with comedo DCIS. Compared with white women, Hispanic women were more likely to experience IBTs and Asian/PI women were more likely to develop CBT, particularly presented as DCIS. The positive association between Asian/PI race and CBT risk was stronger among those <50 years at diagnosis and those with noncomedo DCIS.
Invasive breast cancer is pathologically and biologically more aggressive in black compared to white women [22, 23], largely contributing to worse prognosis for blacks [7]. However, the impact of black race on pathologic features and clinical outcomes of DCIS remains unclear. In this analysis, black women were younger at diagnosis of DCIS and had larger lesions than white women. However, DCIS lesions from blacks were more likely than those from whites to be well differentiated and noncomedo subtypes, both related to favorable prognosis [24]. Institution-based studies reported that black DCIS patients presented large-size lesions more frequently than white patients despite no difference in tumor grade or architectural patterns [13, 17].
Prior analyses of SEER data through 2001 showed significantly higher incidences of invasive IBTs and invasive CBTs among black compared to white women with DCIS after adjusting for age, year, registry, and treatment, but not pathologic factors [9, 11]. This difference was no longer statistically significant after adjustment for pathologic variables [13, 16, 18]. Thus, the worse outcome among black DCIS patients may be attributable to more aggressive tumor pathology [24]. However, these studies included small numbers of black patients and thus had limited power to detect a moderate increase in risk of second breast tumors. In the current study, black race was associated with 46 % increased risk for IBTs and 21 % increased risk for CBTs after adjustment for pathologic factors, age and year of diagnosis of first DCIS, registry, and treatment. The elevated IBT risk in blacks was maintained across tumor size, grade, and architectural patterns. Future research should focus on identifying molecular markers of DCIS that may explain outcome disparities.
Notably, DCIS lesions from black women were more likely than white women to be ER+, and controlling for ER slightly reduced racial differences in IBTs. In a multiethnic cohort of 1,902 women with DCIS, blacks accounted for 11.3 % and exhibited the highest rate of ER+ lesions among all racial groups [13]. Using immunohistochemical markers (ER, PR, HER2, and Ki67), Sharaf Aldeen et al. [25] classified 94 DCIS cases into five subtypes and found a similar distribution of molecular subtypes between black and white women. In addition, the basal-like DCIS defined by ER, PR, HER2, CK5/6, and EGFR displayed a comparable risk of IBTs to the other molecular subtypes [26]. Future efforts should focus on the clinical relevance of these findings and novel molecular markers that mediate poorer DCIS outcomes in black women.
Also, studies have suggested that socioeconomic disadvantages account for the disproportionately elevated mortality risk in black women with invasive breast cancer [27–30]. However, a meta-analysis of 20 studies evaluating survival of black and white breast cancer patients demonstrated that blacks maintained statistically significant excess risk of mortality after adjusting for socioeconomic status [6]. Future studies to disentangle the impacts of race and socioeconomic status on DCIS outcomes are warranted.
Two prior studies of DCIS outcomes reported that black race was differentially associated with types of IBTs. Collins et al. [15] evaluated IBTs among 2,995 DCIS patients treated with BCS, of which 9.6 % were black, and reported an 80 % increased risk of ipsilateral DCIS and a 30 % increased risk of invasive IBTs among black compared with white women. In contrast, the analysis of California Cancer Registry data showed that the risk for invasive IBTs was significantly increased among black DCIS patients but not for ipsilateral DCIS [9]. We instead found that black race was similarly associated with risks for ipsilateral DCIS and invasive IBTs after DCIS. Therefore, racial differences in local DCIS recurrence and the progression to invasive cancer in the ipsilateral breast may be mediated by common biological pathways.
Few studies have compared DCIS outcomes between Asian/PI, Hispanic, and white women [9–11, 13], with the majority reporting no difference [9, 10, 13]. However, risk of advanced invasive IBT was 130 % higher in Hispanic than white women with DCIS [11]. The current study with more racial minority women found that Hispanic ethnicity was associated with significantly increased risk for IBTs but not for CBTs. Yet, Asian/PI women had increased risk for CBTs, largely driven by a significant increase in contralateral DCIS, but not for IBTs. Whether the elevated risk of contralateral DCIS among Asian/PI women resulted from their greater use of surveillance mammography is unknown. Among breast cancer patients with equal access to health care, Asians/PIs were more likely than whites to undergo surveillance mammography [31]. However, lower use of mammography was found among older Asians/PIs compared to their white counterparts [32].
We found that black and Hispanic women treated with BCS alone had a higher risk of IBTs than white women. Given that radiotherapy following BCS reduces IBTs by 50 % after DCIS [33, 34], adding radiation after surgical excision may be appropriate for black and Hispanic patients who are at high IBT risk. The increased IBT risk persisted in blacks treated with BCS and radiotherapy, indicating the need for more intensive follow-up to improve outcomes. Many DCIS cases are over-diagnosed because more than half of DCIS patients would not develop invasive breast cancer if left untreated [35–37]. A challenge in DCIS management is the identification of DCIS patients who are most likely to go on to caner, which should integrate routine clinicopathological factors and novel molecular markers and take into account race/ethnicity and other patient-related factors.
Our study has limitations. Variables that could influence DCIS outcomes were unavailable in the SEER database. Positive surgical margins are consistently associated with increased IBT risk after DCIS [24]. Five-year tamoxifen use could reduce the risk of ipsilateral and contralateral breast tumors among DCIS patients treated with BCS and radiation by 35 % and 41 %, respectively [38]. Prompt initiation and completion of adjuvant therapy are also important for breast cancer patients with BCS to lower their risks of local recurrence and mortality [39–41]. Studies reported no racial difference in surgical margins or receipt of hormone therapy in DCIS patients [13, 14, 16, 42, 43]. However, black race was associated with longer waiting time for and lower probabilities of completing radiotherapy following BCS [40, 41]. Hispanics and Chinese with hormone receptor-positive breast cancer were less likely than other groups to initiate adjuvant hormone therapy within a year after diagnosis [44]. Of those who initiated adjuvant hormone therapy, blacks were more likely to be nonadherent to therapy and Asians/PIs were more likely to continue therapy [45].
Evidence suggests the associations of obesity and alcohol consumption with increased risk of second breast tumors in DCIS survivors [10, 46, 47]. Compared with white patients, the odds of being overweight or obese were higher in black and Hispanic DCIS patients and lower in Asian/PI DCIS patients [48]. Alcohol intake was less prevalent in black and Asian/PI than white and Hispanic women with breast cancer [49]. Due to the lack of lifestyle information, we could not assess the contribution of lifestyles to racial disparities in DCIS prognosis.
Additionally, data on tumor size and grade were unavailable for approximately 30 % of DCIS cases. Missing indicators were created for the current analysis. However, the associations between black race and risks of IBTs (RR 1.54; 95 % CI 1.27–1.88) and CBTs (RR 1.33; 95 % CI 1.11–1.59) remained statistically significant in the sensitivity analysis of complete cases.
Our study provides a more comprehensive examination of racial/ethnic differences in DCIS outcomes than previous studies. Black women with DCIS experienced disproportionately higher risks for second breast tumors, even when uniformly treated with BCS and radiotherapy. They may then need more intensive post-treatment followup. Hispanic DCIS patients had a greater IBT risk if treated with BCS alone and might be appropriate candidates for additional treatment. Differences in tumor pathology and treatment could not account for elevated risk for second breast tumors in racial minority women. Further studies are needed to determine whether biological and nonbiological (e.g., socioeconomic status) characteristics of DCIS are different across racial groups and whether these contribute to outcome disparities. Detailed treatment and followup information is needed to understand the contributions of healthcare quality and surveillance mammography use to racial disparities in DCIS outcomes.
Supplementary Material
Acknowledgments
Y. L. was supported by the Barnes-Jewish Hospital Foundation, St. Louis, Missouri. G. A. C. was also supported by the Breast Cancer Research Foundation. M. S. G was supported by the Barnes-Jewish Hospital Foundation, Siteman Cancer Center, National Institutes of Health, National Cancer Institute Grant U54CA153460, and Washington University School of Medicine Faculty Diversity Scholars Program. The funding agency had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-014-3151-z) contains supplementary material, which is available to authorized users.
Conflict of interest
None.
Contributor Information
Ying Liu, Email: liuyi@wudosis.wustl.edu, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8100, St. Louis, MO 63110, USA.
Graham A. Colditz, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8100, St. Louis, MO 63110, USA Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA.
Sarah Gehlert, Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA; The Brown School, Washington University, St. Louis, MO, USA.
Melody Goodman, Division of Public Health Sciences, Department of Surgery, Washington University School of Medicine, 660 South Euclid Ave, Campus Box 8100, St. Louis, MO 63110, USA; Alvin J. Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine, St. Louis, MO, USA.
References
- 1.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, Yankaskas BC, Rosenberg R, Carney PA, Kerlikowske K, Taplin SH, Urban N, Geller BM. Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst. 2002;94:1546–1554. doi: 10.1093/jnci/94.20.1546. [DOI] [PubMed] [Google Scholar]
- 2.US American Cancer Society. Cancer Facts and Figures for 2010. Atlanta: American Cancer Society; 2010. [Google Scholar]
- 3.Bethesda, MD: National Cancer Institute; SEER Cancer Statistics Review, 1975–2010. based on November 2012 SEER data submission, posted to the SEER web site, April. Available from: http://seer.cancer.gov/csr/1975_2010/ [Google Scholar]
- 4.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R. Mortality among women with ductal carcinoma in situ of the breast in the population-based surveillance, epidemiology and end results program. Arch Intern Med. 2000;160:953–958. doi: 10.1001/archinte.160.7.953. [DOI] [PubMed] [Google Scholar]
- 5.Virnig BA, Tuttle TM, Shamliyan T, Kane RL. Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. J Natl Cancer Inst. 2010;102:170–178. doi: 10.1093/jnci/djp482. [DOI] [PubMed] [Google Scholar]
- 6.Newman LA, Griffith KA, Jatoi I, Simon MS, Crowe JP, Colditz GA. Meta-analysis of survival in African American and white American patients with breast cancer: ethnicity compared with socioeconomic status. J Clin Oncol. 2006;24:1342–1349. doi: 10.1200/JCO.2005.03.3472. [DOI] [PubMed] [Google Scholar]
- 7.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR. Characteristics associated with differences in survival among black and white women with breast cancer. JAMA. 2013;310:389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 8.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 9.Innos K, Horn-Ross PL. Risk of second primary breast cancers among women with ductal carcinoma in situ of the breast. Breast Cancer Res Treat. 2008;111:531–540. doi: 10.1007/s10549-007-9807-1. [DOI] [PubMed] [Google Scholar]
- 10.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, Chew K, Moore DH, II, Waldman F. Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst. 2003;95:1692–1702. doi: 10.1093/jnci/djg097. [DOI] [PubMed] [Google Scholar]
- 11.Li CI, Malone KE, Saltzman BS, Daling JR. Risk of invasive breast carcinoma among women diagnosed with ductal carcinoma in situ and lobular carcinoma in situ, 2006–1988. Cancer. 2001;106:2104–2112. doi: 10.1002/cncr.21864. [DOI] [PubMed] [Google Scholar]
- 12.Smith BD, Haffty BG, Buchholz TA, Smith GL, Galusha DH, Bekelman JE, Gross CP. Effectiveness of radiation therapy in older women with ductal carcinoma in situ. J Natl Cancer Inst. 2006;98:1302–1310. doi: 10.1093/jnci/djj359. [DOI] [PubMed] [Google Scholar]
- 13.Bailes AA, Kuerer HM, Lari SA, Jones LA, Brewster AM. Impact of race and ethnicity on features and outcome of ductal carcinoma in situ of the breast. Cancer. 2013;119:150–157. doi: 10.1002/cncr.27707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stark A, Stapp R, Raghunathan A, Yan X, Kirchner HL, Griggs J, Newman L, Chitale D, Dick A. Disease-free probability after the first primary ductal carcinoma in situ of the breast: a comparison between African–American and White-American women. Breast Cancer Res Treat. 2012;131:561–570. doi: 10.1007/s10549-011-1742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins LC, Achacoso N, Haque R, Nekhlyudov L, Fletcher SW, Quesenberry CP, Jr, Schnitt SJ, Habel LA. Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat. 2013;139:453–460. doi: 10.1007/s10549-013-2539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nelson C, Bai H, Neboori H, Takita C, Motwani S, Wright JL, Hobeika G, Haffty BG, Jones T, Goyal S, Moran MS. Multi-institutional experience of ductal carcinoma in situ in black vs white patients treated with breast-conserving surgery and whole breast radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:e279–e283. doi: 10.1016/j.ijrobp.2012.03.068. [DOI] [PubMed] [Google Scholar]
- 17.Nassar H, Sharafaldeen B, Visvanathan K, Visscher D. Ductal carcinoma in situ in African American versus Caucasian American women: analysis of clinicopathologic features and outcome. Cancer. 2009;115:3181–3188. doi: 10.1002/cncr.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JL, Weaver DL, Bocklage T, Key CR, Platz CE, Cronin KA, Ballard-Barbash R, Willey SC, Harlan LC. The frequency of ipsilateral second tumors after breast-conserving surgery for DCIS: a population based analysis. Cancer. 2005;104:1840–1848. doi: 10.1002/cncr.21406. [DOI] [PubMed] [Google Scholar]
- 19.Glynn RJ, Rosner B. Methods to evaluate risks for composite end points and their individual components. J Clin Epidemiol. 2004;57:113–122. doi: 10.1016/j.jclinepi.2003.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Baer HJ, Glynn RJ, Hu FB, Hankinson SE, Willett WC, Colditz GA, Stampfer M, Rosner B. Risk factors for mortality in the nurses’ health study: a competing risks analysis. Am J Epidemiol. 2011;173:319–329. doi: 10.1093/aje/kwq368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 22.Danforth DN., Jr Disparities in breast cancer outcomes between Caucasian and African American women: a model for describing the relationship of biological and nonbiological factors. Breast Cancer Res. 2013;15:208. doi: 10.1186/bcr3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris GJ, Mitchell EP. Higher incidence of aggressive breast cancers in African–American women: a review. J Natl Med Assoc. 2008;100:698–702. doi: 10.1016/s0027-9684(15)31344-4. [DOI] [PubMed] [Google Scholar]
- 24.Shamliyan T, Wang SY, Virnig BA, Tuttle TM, Kane RL. Association between patient and tumor characteristics with clinical outcomes in women with ductal carcinoma in situ. J Natl Cancer Inst Monogr. 2010;2010:121–129. doi: 10.1093/jncimonographs/lgq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sharaf Aldeen B, Feng J, Wu Y, Nassar Warzecha H. Molecular subtypes of ductal carcinoma in situ in African American and Caucasian American women: distribution and correlation with pathological features and outcome. Cancer Epidemiol. 2013;37:474–478. doi: 10.1016/j.canep.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Jirstrom K, Johansson C, Amini RM, Blomqvist C, Agbaje O, Warnberg F. Long-term survival of women with basal-like ductal carcinoma in situ of the breast: a population-based cohort study. BMC Cancer. 2010;10:653. doi: 10.1186/1471-2407-10-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000;88:1256–1264. doi: 10.1002/(sici)1097-0142(20000301)88:5+<1256::aid-cncr13>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94:490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 29.Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95:1988–1999. doi: 10.1002/cncr.10830. [DOI] [PubMed] [Google Scholar]
- 30.Du W, Simon MS. Racial disparities in treatment and survival of women with stage I–III breast cancer at a large academic medical center in metropolitan Detroit. Breast Cancer Res Treat. 2005;91:243–248. doi: 10.1007/s10549-005-0324-9. [DOI] [PubMed] [Google Scholar]
- 31.Enewold L, McGlynn KA, Zahm SH, Jatoi I, Anderson WF, Gill AA, Shriver CD, Zhu K. Surveillance mammography among female Department of Defense beneficiaries: a study by race and ethnicity. Cancer. 2013;119:3531–3538. doi: 10.1002/cncr.28242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Field TS, Doubeni C, Fox MP, Buist DS, Wei F, Geiger AM, Quinn VP, Lash TL, Prout MN, Yood MU, Frost FJ, Silliman RA. Under utilization of surveillance mammography among older breast cancer survivors. J Gen Intern Med. 2008;23:158–163. doi: 10.1007/s11606-007-0471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisher B, Costantino J, Redmond C, Fisher E, Margolese R, Dimitrov N, Wolmark N, Wickerham DL, Deutsch M, Ore L, et al. Lumpectomy compared with lumpectomy and radiation therapy for the treatment of intraductal breast cancer. N Engl J Med. 1993;328:1581–1586. doi: 10.1056/NEJM199306033282201. [DOI] [PubMed] [Google Scholar]
- 34.Julien JP, Bijker N, Fentiman IS, Peterse JL, Delledonne V, Rouanet P, Avril A, Sylvester R, Mignolet F, Bartelink H, Van Dongen JA. Radiotherapy in breast-conserving treatment for ductal carcinoma in situ: first results of the EORTC randomised phase III trial 10853. EORTC Breast Cancer Cooperative Group and EORTC Radiotherapy Group. Lancet. 2000;355:528–533. doi: 10.1016/s0140-6736(99)06341-2. [DOI] [PubMed] [Google Scholar]
- 35.Jenks S. Downgrading cancer definitions: overdiagnosis fuels the discussion. J Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju070. dju070. [DOI] [PubMed] [Google Scholar]
- 36.Benson JR, Wishart GC. Predictors of recurrence for ductal carcinoma in situ after breast-conserving surgery. Lancet Oncol. 2013;14:e348–e357. doi: 10.1016/S1470-2045(13)70135-9. [DOI] [PubMed] [Google Scholar]
- 37.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, Pike MC, Reed SD, Saftlas AF, Scarvalone SA, Schwartz AM, Slomski C, Yothers G, Zon R. National Institutes of Health State-of-the-Science Conference statement: Diagnosis and Management of Ductal Carcinoma In Situ September 22–25, 2010. J Natl Cancer Inst. 2009;102:161–169. doi: 10.1093/jnci/djp485. [DOI] [PubMed] [Google Scholar]
- 38.Fisher B, Dignam J, Wolmark N, Wickerham DL, Fisher ER, Mamounas E, Smith R, Begovic M, Dimitrov NV, Margolese RG, Kardinal CG, Kavanah MT, Fehrenbacher L, Oishi RH. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 39.Punglia RS, Saito AM, Neville BA, Earle CC, Weeks JC. Impact of interval from breast conserving surgery to radiotherapy on local recurrence in older women with breast cancer: retrospective cohort analysis. BMJ. 2010;340:c845. doi: 10.1136/bmj.c845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut AI. Delay in initiating adjuvant radiotherapy following breast conservation surgery and its impact on survival. Int J Radiat Oncol Biol Phys. 2006;65:1353–1360. doi: 10.1016/j.ijrobp.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Srokowski TP, Fang S, Duan Z, Buchholz TA, Hortobagyi GN, Goodwin JS, Giordano SH. Completion of adjuvant radiation therapy among women with breast cancer. Cancer. 2008;113:22–29. doi: 10.1002/cncr.23513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haque R, Achacoso NS, Fletcher SW, Nekhlyudov L, Collins LC, Schnitt SJ, Quesenberry CP, Jr, Habel LA. Treatment of ductal carcinoma in situ among patients cared for in large integrated health plans. Am J Manag Care. 2010;16:351–360. [PMC free article] [PubMed] [Google Scholar]
- 43.Nakhlis F, Lazarus L, Hou N, Acharya S, Khan SA, Staradub VL, Rademaker AW, Morrow M. Tamoxifen use in patients with ductal carcinoma in situ and T1a/b N0 invasive carcinoma. J Am Coll Surg. 2005;201:688–694. doi: 10.1016/j.jamcollsurg.2005.06.195. [DOI] [PubMed] [Google Scholar]
- 44.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, Neugut AI, Fehrenbacher L, Thompson B, Coronado GD. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131:607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, Fehrenbacher L, Gomez SL, Miles S, Neugut AI. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Habel LA, Daling JR, Newcomb PA, Self SG, Porter PL, Stanford JL, Seidel K, Weiss NS. Risk of recurrence after ductal carcinoma in situ of the breast. Cancer Epidemiol Biomarkers Prev. 1998;7:689–696. [PubMed] [Google Scholar]
- 47.McLaughlin VH, Trentham-Dietz A, Hampton JM, Newcomb PA, Sprague BL. Lifestyle factors and the risk of a second breast cancer after ductal carcinoma in situ. Cancer Epidemiol Biomarkers Prev. 2014;23:450–460. doi: 10.1158/1055-9965.EPI-13-0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuerer HM, Lari SA, Arun BK, Hu CY, Brewster A, Mittendorf EA, Albarracin CT, Babiera GV, Caudle AS, Wagner JL, Litton JK, Bedrosian I, Meric-Bernstam F, Lucci A, Hunt KK. Biologic features and prognosis of ductal carcinoma in situ are not adversely impacted by initial large body mass. Breast Cancer Res Treat. 2012;133:1131–1141. doi: 10.1007/s10549-012-1999-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwan ML, Kushi LH, Weltzien E, Tam EK, Castillo A, Sweeney C, Caan BJ. Alcohol consumption and breast cancer recurrence and survival among women with early-stage breast cancer: the life after cancer epidemiology study. J Clin Oncol. 2010;28:4410–4416. doi: 10.1200/JCO.2010.29.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.