Abstract
Matrix metalloproteinases (MMPs) play an important role in modeling of the extracellular matrix. There is increasing evidence that these proteases are important in neurite elongation and axonal guidance during development in the central nervous system and retina. Moreover, they are also expressed after acute injury and can be the key mediators of pathogenesis. However, the role of MMPs in the inner ear is largely unknown. Our group recently demonstrated that general inhibition of MMPs resulted in auditory hair cell loss in vitro. In the present study, we investigated the role of MMPs in inner ear spiral ganglion neuron (SGN) survival, neuritogenesis and neurite extension by blocking MMPs known to be involved in axonal guidance, neurite elongation, and apoptosis in other neuronal systems. Spiral ganglion (SG) explants from 5-day-old Wistar rats were treated with different concentrations of the general MMP inhibitor GM6001, a specific MMP-2 inhibitor, and a specific MMP-9 inhibitor, in vitro. The general inhibitor of MMPs and the specific inhibition of MMP-2 significantly reduced both the number of neurites that extended from SG explants, as well as the length of individual neurites. However, neither the general inhibitor of MMPs nor the specific inhibition of MMP-2 influenced SGN survival. Inhibition of MMP-9 had no influence on SGNs. The data suggest that MMPs, and more specifically MMP-2, influence the growth of developing afferent neurites in the mammalian inner ear in vivo.
Keywords: Extracellular matrix, Inner ear, Matrix metalloproteinase, Spiral ganglion neurons
Introduction
Matrix metalloproteinases (MMPs) are zinc-dependent proteases that play an important role in modeling the extracellular matrix (ECM), which proves structural and biochemical support to the cells that they surround. In the brain, the ECM occupies the space between neural and glial cells and mediates various structural and functional interactions between the cells (Sorokin 2010; Faissner et al. 2010). During development, the ECM plays a crucial role in proliferation, migration, and differentiation of neural cells. The ECM of the nervous system is modulated by various proteinases secreted by neurons and glia (Page-McCaw et al. 2007; Yong 2005). Among these are the MMPs, which can be divided into four main subgroups based on their targets and structural domains: collagenases, gelatinases, stromelysins, and membrane-type MMPs (Rosenberg 2009). Currently, the two secreted gelatinases, MMP-2 and MMP-9, are the most frequently investigated MMPs in the central nervous system because they are detected relatively easily (Fujioka et al. 2012).
There is increasing evidence that MMPs are important in neurite elongation and axonal guidance during development, since the ECM is typically remodeled as axons elongate between cells. In vivo experiments on Xenopus using the general MMP inhibitor GM6001 demonstrated the importance of MMPs for axon guidance and extension in the developing visual system (Webber et al. 2002). Moreover, MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing murine cerebellum (Vaillant et al. 2003). MMPs not only play a crucial role in development, but are also expressed acutely after injury and are the key mediators of pathogenesis (Zhang et al. 2011; Agrawal et al. 2008). Studies in cerebral hypoxia/ischemia in rodents and non-human primates showed an elevation in MMP-2 expression 2 h after reperfusion (Chang et al. 2003; Yang et al. 2007). A marked increase in MMP-9 expression between 24 and 48 h after reperfusion provides a molecular basis for both the transient and long-term alterations that occur in the blood–brain barrier due to MMPs in reperfusion injury (Yang et al. 2007). In human ischemic strokes, active MMP-2 is increased on days 2–5 compared with active MMP-9, which can remain elevated for months after the ischemic episode (Clark et al. 1997). Furthermore, studies have shown that ischemic-induced retinal ganglion cell loss in mice correlates with an up-regulation of MMP-9. In addition, MMP-9 knockout mice, and mice treated with MMP synthetic inhibitor, are resistant to ischemic-induced retinal ganglion cell loss, indicating that MMP-9 plays a causative role (Chintala et al. 2002; Zhang and Chintala 2004).
As in the brain, the cells of the mammalian cochlea are embedded in a highly organized ECM. Several ECM molecules are positioned along the path of the developing afferent dendrites that link the cell bodies of spiral ganglion neurons (SGNs) to their target cells in the sensory epithelium (Woolf et al. 1992; Whitlon et al. 1999a, b). Moreover, the neurites of SGNs have been shown to respond strongly to ECM molecules in vitro. For example, both laminin and fibronectin strongly stimulate the growth of neurites when presented as a uniform surface (Aletsee et al. 2002; Evans et al. 2007). Interestingly, neurites avoid terminating on stripes of fibronectin when given a choice with a neutral molecule (poly-l-lysine), and either avoid or are attracted to laminin stripes depending upon concentration (Evans et al. 2007). It, therefore, seems reasonable that MMPs would be capable of modulating the responses of spiral ganglion (SG) neurites.
In contrast to the central nervous system and the retina, the role of MMPs in the inner ear is largely unknown. MMP-2 is strongly expressed in the embryonic inner ear, along the pathway between the SG and the hair cells (HCs) (Genepaint 2013). Inactivating the Mpv17 gene in mice, which encodes for a peroxisomal protein, induced a strong increase in MMP-2 expression in the inner ear (Reuter et al. 1998). These mice developed a degeneration of various inner ear structures including the loss of SGNs and degeneration of the organ of Corti as well as hearing loss. Cochlear ablation experiments in rats indicate that MMP-9 tends to be associated with neuropil reorganization related to fiber and terminal degeneration, whereas MMP-2 is predominantly involved in aiding reinnervation and synaptogenesis (Fredrich and Illing 2011). Interestingly, mice with hyperhomocysteinemia display an increase in cochlear expression of MMP-2 and MMP-9 (Kundu et al. 2009). However, hearing tests in these mice have not been performed. We previously found that inhibition of MMP activity resulted in auditory HC loss in vitro (Setz et al. 2011). However, the effect of MMP inhibition has not been investigated in SGNs.
The aim of the present study was to evaluate the effect of MMP inhibitor on SGNs in vitro. We used MMP inhibitors known to block MMPs involved in axonal guidance, neurite elongation, and apoptosis in other neuronal systems. The effect on SG neuronal survival, neuritogenesis, and neurite elongation of the general MMP inhibitor GM6001, as well as specific MMP-2 and MMP-9 inhibitors was analyzed.
Materials and Methods
Preparation of Tissue Culture Plates
To prepare uniformly coated 24-well cell culture plates (Costar®, Corning Inc, Acton, MA, USA), wells were filled with 300 μl of 5 μg/ml poly-l-lysine (PLL) (Sigma-Aldrich, St. Louis, USA) in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco by Invitrogen, Carlsbad, USA) and incubated at 37 °C for 1 h. The wells were then washed twice with phosphate buffered saline (PBS). The prepared wells were then filled with 170 μl of primary attachment medium, containing DMEM (Gibco), 10 % fetal bovine serum (Sigma-Aldrich), 25 mM HEPES buffer (Gibco) ,and 300 U/ml penicillin (Sigma-Aldrich).
SG Dissection
All animal procedures were carried out according to an approved animal research protocol (Kantonales Veterinaeramt, Basel, Switzerland and the IACUC of the San Diego VA Medical Center, USA). Neonatal 5-day-old Wistar rats (Harlan, Indianapolis, USA) were euthanized. The temporal bones were removed and further dissected similar to the method described by Van de Water and Ruben (1971). Briefly, the cochlear capsule was opened, and the membranous labyrinth was removed from the modiolus. The spiral lamina containing the SG was carefully separated from the modiolus and transferred immediately into primary cell culture medium, where it was then cut into equal portions of 300–500 μm before being transferred to the prepared culture plates. Each explant was cultured in a separate culture well.
Cell Culture
The explants were first incubated for 24 h at 37o C in primary attachment medium, and the culture medium was subsequently changed to serum-free maintenance medium [DMEM (Gibco), 25 mM Hepes Buffer (Gibco), 6 mg/ml glucose (Gibco), 300 U/ml penicillin (Sigma-Aldrich), and 30 μl/ml N2-supplement (Gibco)]. Maintenance medium was supplemented with 10 ng/ml of recombinant BDNF for trophic support of SGN survival and optimization of neurite outgrowth (R&D Systems, Minneapolis, MN, USA). Cultures were kept in a humidified incubator at 5 % CO2 and 37 °C for 72 h.
Experimental cultures received various concentrations of MMP inhibitors. This included 10 or 50 µM of the general MMP inhibitor GM6001 (Calbiochem). GM6001 is a potent, cell-permeable, broad-spectrum hydroxamic acid inhibitor of MMPs (K i = 400 pM for MMP-1, 500 pM for MMP-2, 27 nM for MMP-3, 100 pM for MMP-8, and 200 pM for MMP-9). An MMP-2 inhibitor (Calbiochem) was used at 5, 20, or 50 µM. This MMP-2 inhibitor is a cell-permeable, potent inhibitor of MMP-2 that acts in a dose-dependent manner (K i = 1.7 µM). An MMP-9 inhibitor was used at 5, 50, or 500 nM. This MMP-9 inhibitor is a cell-permeable, potent, selective, and reversible MMP-9 inhibitor (IC50 = 5 nM), which inhibits MMP-1 (IC50 = 1.05 µM) and MMP-13 (IC50 = 113 nM) only at much higher concentrations. Detailed information about the selectivity of the inhibitors used is available at the suppliers website (Calbiochem, www.calbiochem.com/inhibitors/MMP).
A GM6001 negative control (GM6001NC; N-t-butoxy- carbonyl-l-leucyl-l-tryptophan methylamide) (Calbiochem), a closely related compound without MMP inhibitor activity, was also prepared and used at a concentration of 50 µM (Webber et al. 2002). Additional control media for the MMP-2 inhibitor and MMP-9 inhibitor experiments contained dimethyl sulfoxide (DMSO) alone (Sigma-Aldrich), since MMP inhibitors were solubilized in DMSO (Sigma-Aldrich). The concentration of DMSO (Sigma-Aldrich) used in controls was the same as the highest concentration of the specific inhibitor used.
Immunohistochemistry
After fixation with 4 % paraformaldehyde for 20 min at room temperature (RT) and two washes with PBS (Gibco), the explants were permeabilized with 5 % triton X-100 (Sigma-Aldrich) for 10 min, washed twice with PBS, and blocked for non-specific antibody binding with 5 % donkey serum (Sigma-Aldrich). Neurites were labeled for neurofilament using a mouse polyclonal 200 kDa anti-neurofilament primary antibody (1:400; Sigma-Aldrich). After primary antibody incubation overnight at 4 °C, followed by two PBS washes, the neurites were visualized by 2.5 h of incubation with fluorescein isothiocyanate (FITC)-conjugated secondary antibodies (1:100; Jackson Immunoresearch, West Grove, PA, USA) against mouse antibody. Specificity of staining was confirmed by a series of negative control staining without primary antibodies.
Quantification of Neuronal Survival
To assess effects on neuronal survival, half turn SG explants were cultured as above for 72 h, except that the explants were grown on glass cover slips. The explants were fixed as above, treated with 0.5 % peroxide in methanol to block endogenous peroxidases, reacted with a mouse monoclonal antibody IgG against rat neurofilament 200 (Sigma- Aldrich), followed by a biotinylated secondary anti-mouse IgG, and developed by an avidin and DAB procedure (Vector Laboratories, Burlingame, CA, USA). The tissue was cleared with citrosol (Fischer Scientific, Waltham, MA, USA) to allow the visualization of the cell somas for the evaluation of neuronal survival. 36 SG explants were studied for each condition.
Data Analysis
Digital images for immunohistochemistry were obtained on a fluorescence microscope (Olympus IX71, Center Valley, PA, USA) and photographed with an AxioCam (Zeiss, San Diego, USA). Digital images for the quantification of neuronal survival were obtained on an inverted microscope (Olympus FSX100, Center Valley, PA, USA). For publication in this manuscript, images were optimized to achieve uniform brightness and contrast using Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA). Neurite outgrowth from the SG was evaluated by measuring the number and lengths of the processes. Growth of supporting cells was evaluated by measuring the area of the skirt surrounding the SGN. Images of the immunostained cultures were analyzed by using ImageJ software (NIH, Bethesda, MD, USA). Each neurite was traced, and number of neurites, average lengths of neurites, and area of the supporting cells per explant were analyzed. Neuronal survival was analyzed by evaluating the number of neurons per 100 µm. Statistical analysis was performed using a one-way analysis of variance (ANOVA) followed by Tukey's least-significant-difference post hoc test with Bonferroni correction. Data presented in the text and figures are the means and standard deviations. Results were considered to be significant when the likelihood for a type 1 error was less than 5 % (p < 0.05). 20 SG explants were studied for each condition.
RNA Extraction
6 SGNs of 5-day-old WS rat pups for each experimental condition were cultured and treated with 500 nM MMP-9 inhibitor (Calbiochem) as described above for 3 h and placed separately in RNAlater (Qiagen, Hombrechtikon, Switzerland). RNA isolation was performed using the RNAeasy Minikit (Qiagen, Switzerland) including DNase treatment according to the supplier’s instructions. An Ultra-Turrax T8 homogenizer (IKA- Werke, Staufen, Germany) was used to homogenize the tissues. The quantity and quality of the isolated RNA were determined with NanoDrop ND 1000 (NanoDrop Technologies, DE, USA). The 260/280 nm ratio of all our samples was between 1.8 and 2.1.
Real-Time PCR
Total RNA (500 ng) was reverse transcribed into cDNA with the first strand cDNA synthesis kit (Roche Applied Biosciences, Rotkreuz, Switzerland) according to the supplier’s instructions. The reaction took place in an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, USA) using a Fast Start Universal SYBR Green Master (Rox) (Roche Applied Biosciences, Rotkreuz, Switzerland). The primer sequences were MMP-9 reverse 5′-GGTCAGGTT-TAGAGCCACGA-3′ (Microsynth, St. Gallen, Switzerland). Each reaction contained 300 nM of primer. The cycling parameters were 10 min at 95 °C, then 40 cycles of 15 s at 95 °C, and 60 s at 60 °C. We calculated the relative quantities of specifically amplified cDNA with the comparative threshold cycle method. GAPDH acted as an endogenous reference. Relative expression of MMP-9 mRNA was compared to DMSO control. No-template and no-reverse-transcription controls served as negative controls and excluded DNA contamination. All experiments were repeated three times in triplicate.
Results
General Inhibition of MMP Activity Results in Decreased SG Neurite Number
Treatment of neonatal SG explants with the general MMP inhibitor GM6001 (10 or 50 µM, respectively) decreased the number of neurites per SG explant compared to the negative control (50 µM GM6001NC) (Fig. 1b, ANOVA, p < 0.05 for both conditions). Figure 1a shows a representative image of SG explants treated with the different concentrations of the general MMP inhibitor GM6001 compared to 50 µM GM6001 negative control (NC).
Fig. 1.
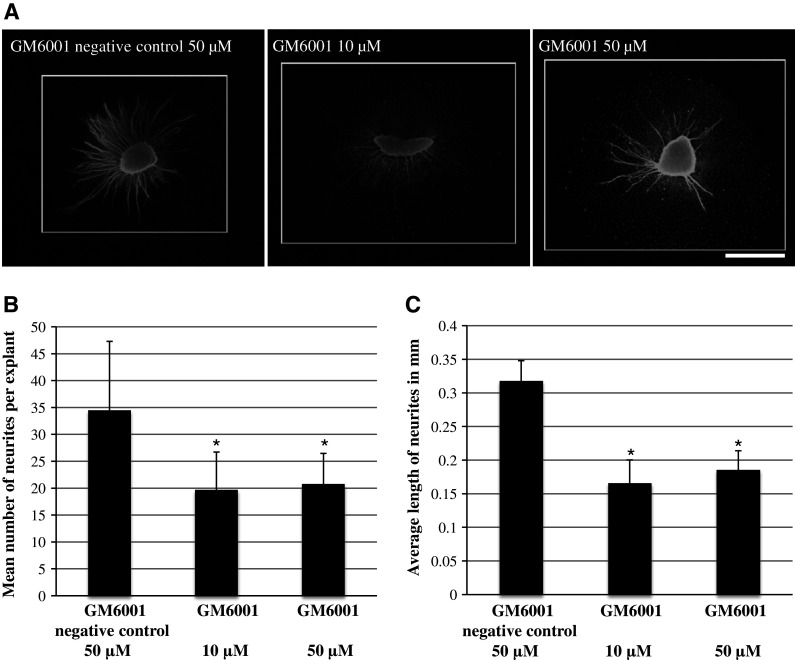
a Representative SG explants stained with anti-200 kDa neurofilament antibody after treatment with 50 µM GM6001 negative control, 10 µM GM6001, and 50 µM GM6001. Scale bar 300 µm. b Average number of SG neurites observed on SG explants. The number of neurites observed with 50 µM GM6001 negative control is compared to that seen with two different concentrations of the general MMP inhibitor GM6001. GM6001-treated samples were significantly different from control in both concentrations used. c Average length of SG neurites observed on SG explants. The length of neurites observed in control samples (50 µM GM6001 negative control) are compared to that seen with two different concentrations of the general MMP inhibitor GM6001. GM6001-treated samples were significantly different from control in both concentrations used. Asterisks denote statistical difference compared to control. Data are represented as mean + SD. N = 20 for each experimental condition
General Inhibition of MMP Activity Results in Decreased Length of SG Neurites
The general MMP inhibitor GM6001 (10 or 50 µM, respectively) also significantly decreased the length of SG neurites compared to the negative control (50 µM GM6001NC) (Fig. 1c, ANOVA, p < 0.05 for both conditions). Figure 1a shows a representative image of SG explants treated with the different concentrations of the general MMP inhibitor GM6001 versus 50 µM GM6001NC.
General Inhibition of MMP Activity Results in Decreased Area of Supporting Cells
The general MMP inhibitor GM6001 (10 µM) also significantly decreased the area of non-neuronal cells, which we have previously identified as fibroblasts and Schwann cells (Aletsee et al. 2001; Brors et al. 2002), growing around the explant, as compared to the negative control (50 µM GM6001NC) (Fig. 4b, ANOVA, p < 0.05). Figure 4a shows a representative image of SG explants treated with the general MMP inhibitor GM6001 versus GM6001NC.
Fig. 4.
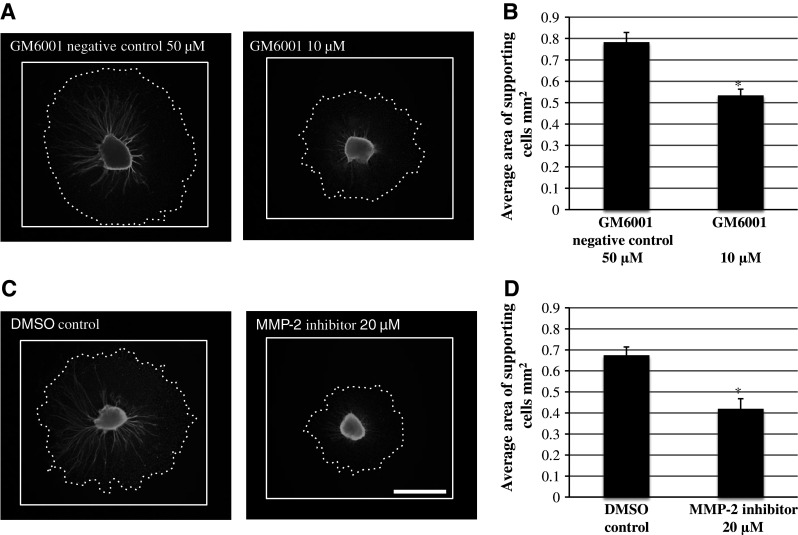
a Representative SG explants stained with anti-200 kDa neurofilament antibody after treatment with 50 µM GM6001 negative control and 10 µM GM6001. c Representative SG explants stained with anti-200 kDa neurofilament antibody after treatment with 20 µM MMP-2 inhibitor and DMSO control. Scale bar 300 µm. b Average area of supporting cells observed around SG explants. GM6001 treatment resulted in significantly smaller area of supporting cells around the SG explant compared to GM6001 negative control. d Average area of supporting cells observed around SG explants. MMP-2 inhibitor treatment resulted in significantly smaller area of supporting cells around the SG explant compared to DMSO negative control. Asterisks denote statistical difference compared to control. Data are represented as mean + SD. N = 20 for each experimental condition
Specific Inhibition of MMP 2 Activity Results in Decreased SG Neurite Number in a Dose-Dependent Manner
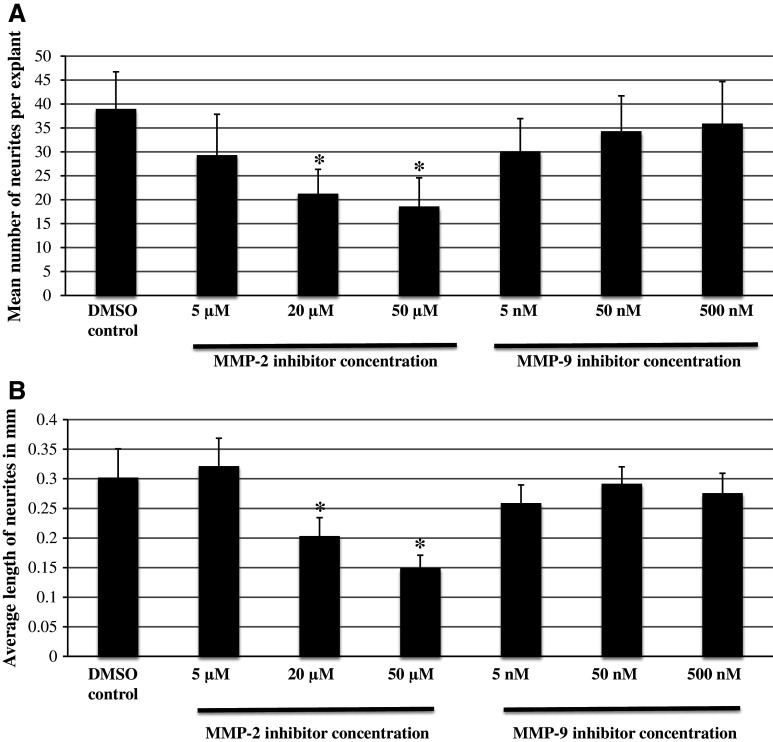
Treatment of neonatal SG explants with a MMP-2 inhibitor (5, 20, or 50 µM, respectively) significantly decreased the number of neurites per SG explant compared to DMSO control in a dose-dependent manner (Fig. 3a, ANOVA, p < 0.05 for all conditions). Figure 2 shows a representative image of SG explants treated with the different concentrations of the MMP-2 inhibitor compared to a DMSO control.
Fig. 3.
a Average number of SG neurites observed on SG explants. The number of neurites observed on control (DMSO only) is compared to those seen with three different concentrations of the MMP-2 and MMP-9 inhibitor. MMP-2 inhibitor-treated samples were significantly different from control at the two highest concentrations used. MMP-9 inhibitor treatment had no influence on the average number of SG neurites observed on SG explants. Asterisks denote statistical difference compared to control. Data are represented as mean + SD. N = 20 for each experimental condition. b The average length of SG neurites observed on SG explants. The length of neurites observed in control samples (DMSO only) are compared to those seen at the three different concentrations of the MMP-2 and MMP-9 inhibitor. MMP-2 inhibitor-treated samples were significantly different from control at the two highest concentrations. MMP-9 inhibitor treatment had no influence on neurite length. Asterisks denote statistical difference compared to control. Data are represented as mean + SD. N = 20 for each ex
perimental condition
Fig. 2.
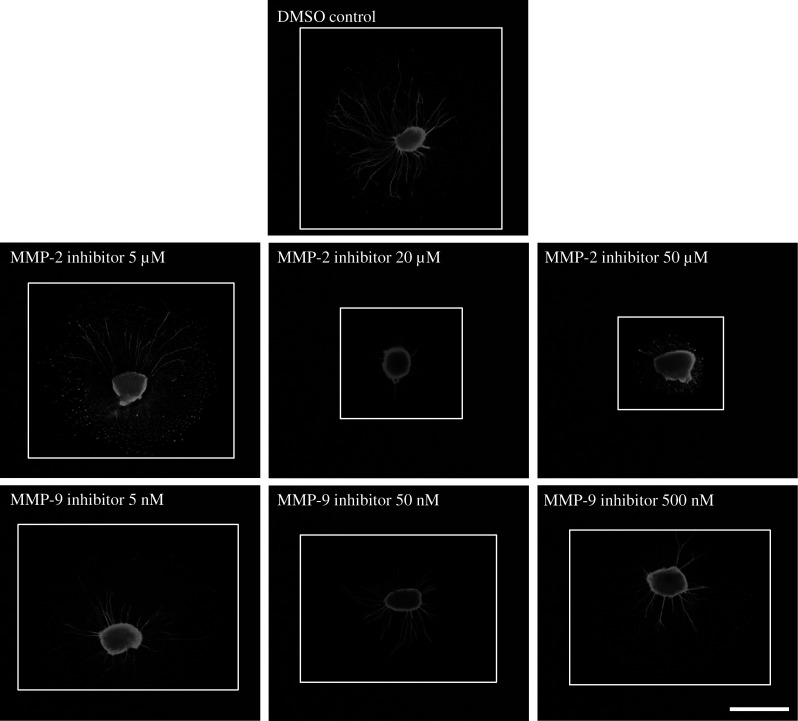
Representative SG explants stained with anti-200 kDa neurofilament antibody after treatment with DMSO only, 5 µM MMP-2 inhibitor, 20 µM MMP-2 inhibitor, 50 µM MMP-2 inhibitor, 5 nM MMP-9 inhibitor, 50 nM MMP-9 inhibitor, and 500 nM MMP-9 inhibitor. Scale bar 300 µm
Specific Inhibition of MMP-2 Activity Results in Decreased Length of SG Neurites in a Dose-Dependent Manner
Treatment of neonatal SG explants with a MMP-2 inhibitor (5, 20, or 50 µM, respectively) also decreased the length of SG neurites compared to DMSO control (Fig. 3b, ANOVA, p < 0.05 for all conditions). Figure 2 shows a representative image of SG explants treated with the different concentrations of the MMP-2 inhibitor compared to DMSO control.
Specific Inhibition of MMP-2 Activity Results in Decreased Area of Supporting Cells
The specific MMP-2 inhibitor (20 µM) also significantly decreased the area of non-neuronal cells around the SGN compared to the DMSO control (Fig. 4d, ANOVA, p < 0.05). Figure 4c shows a representative image of SG explants treated with the specific MMP-2 inhibitor versus DMSO control.
Specific Inhibition of MMP-9 Activity Does not Influence Number or Length of SG Neurites
Treatment of neonatal SG explants with a MMP-9 inhibitor (5, 50, or 500 nM, respectively) had no effect on either the number of neurites per SG explant (Fig. 3a, ANOVA, p > 0.05 for all conditions) or the length of SG neurites (Fig. 3b, ANOVA, p > 0.05 for all conditions) when compared to controls. Figure 2 shows a representative image of SG explants treated with the different concentrations of the MMP-9 inhibitor versus the DMSO control.
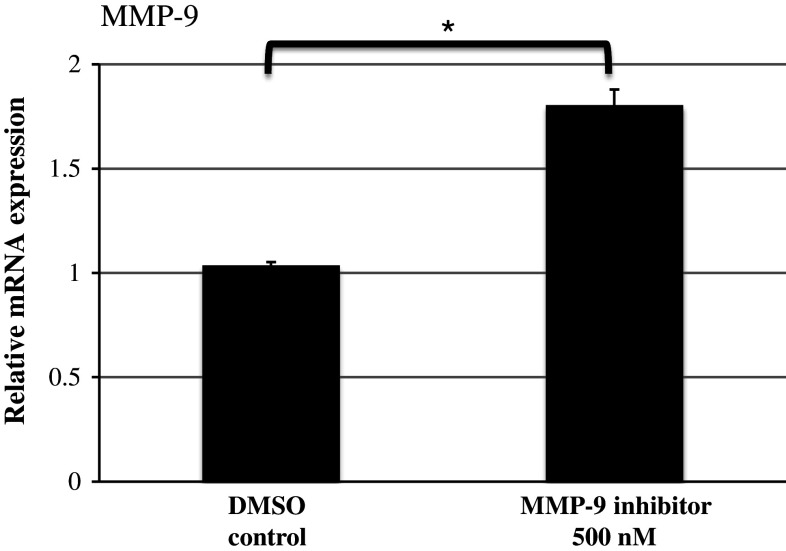
MMP-9 mRNA relative expression of SG explants treated with the highest concentration used in the experiments compared to DMSO control was analyzed. Treatment with 500 nM MMP-9 inhibitor increased MMP-9 mRNA expression compared to DMSO control (Fig. 5, ANOVA, p < 0.05). The change in mRNA expression served as positive control, since no effect of MMP-9 inhibitor treatment on SGN neurites was observed.
Fig. 5.
MMP-9 mRNA relative expression in SG explants after treatment with and without 500 nM MMP-9 inhibitor. MMP-9 mRNA relative expression in control SG explants (DMSO control) has a value of 1 and was used as a comparison parameter. Expression was measured by quantitative real-time PCR with GAPDH as an endogenous control. Histogram and bars represent mean + SD. *p < 0.05 versus DMSO control. The experiment was repeated three times in triplicates. N = 6 for each experimental condition
General Inhibition of MMP Activity and Specific Inhibition of MMP-2 Activity Does not Influence Neuronal Survival
The decreased number of neurites extending from SG explants could reflect the altered survival and/or neuritogenesis of SGNs. To assess this, we evaluated the survival of SGN cell bodies within explants treated with dosages of the general MMP inhibitor GM6001 (10 µM) or the specific MMP-2 inhibitor (20 µM) that altered the neurite number. However, neither treatment influenced SG neuronal survival when compared to controls. (Figure 6b, c, ANOVA, p > 0.05 for both conditions). Figure 6a shows the representative images of SG explants treated with 10 µM of the general MMP inhibitor GM6001 compared to 10 µM GM6001 negative control and SG explants treated with 20 µM of a specific MMP-2 inhibitor compared to DMSO control.
Fig. 6.
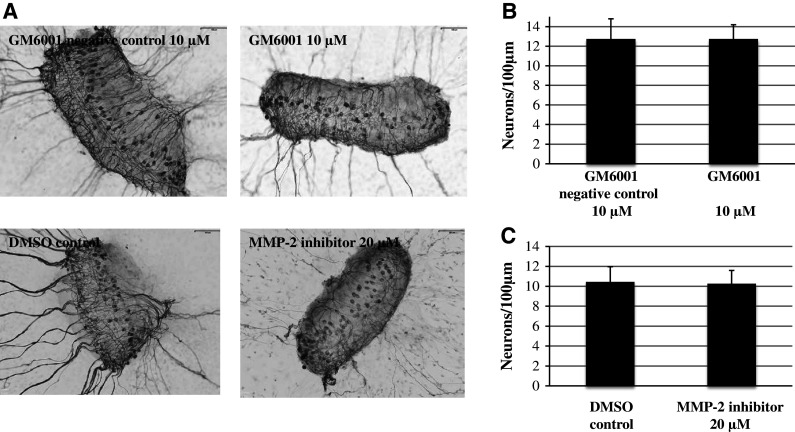
Effects of MMP inhibition on neuronal survival from SG explants. a Representative SG explants after treatment with 10 µM GM6001 negative control, 10 µM GM6001, DMSO control, and 20 µM MMP-2 inhibitor. b Neither GM6001 nor MMP-2 inhibitor treatment showed a difference in SG neuron survival compared to controls. Scale bar 100 μm. Data are represented as mean + SD. N = 36 for each experimental condition
Discussion
At present, information about the function of MMPs in the inner ear is limited. Hu et al. (2012) showed that MMPs and their related genes participate in the regulation of cochlear response to acoustic overstimulation. The authors suggested that the modulation of MMP activity can serve as a novel therapeutic target for the reduction of noise-induced cochlear damage. However, Hu et al. (2012) focused on the sensory epithelium of the cochlea and not on SGNs. Recently, our group demonstrated that general inhibition of MMPs resulted in auditory HC loss in vitro (Setz et al. 2011). The data of the present study indicates that MMP inhibition not only affects the auditory HCs, but also has a negative influence on SGNs. These findings suggest that MMPs play an important role in regulating the homeostasis of the ECM in the inner ear. Interestingly, the effect of the general inhibitor GM6001 on SGNs was similar to the specific MMP-2 inhibitor, while inhibition of MMP-9 had no effect. This indicates that the effects on SGNs can be attributed primarily to MMP-2 inhibition, although the possibility that additional MMPs may also participate cannot be excluded.
SG explants treated with the general MMP inhibitor GM6001 and the specific MMP-2 inhibitor showed a significant reduction in the number of neurites per SG explant. In order to clarify whether the reduction in number of neurites per SG explants reflects the failure of neurite initiation or diminished neuronal survival, we evaluated SG neuronal survival by visualization of the cell soma of the SGNs. General MMP inhibitor GM6001 and specific MMP-2 inhibitor treatment did not influence SG neuronal survival. Therefore, the reduction in number of neurites per SG explant represents neuritogenesis and not SG neuronal survival.
This difference in response to MMP-2 and MMP-9 inhibition could be related to the substrate specificities of the two MMPs. MMP-2 actively degrades not only collagens, but also fibronectin and laminin. In contrast, MMP-9 degrades collagens and fibronectin but not laminin. As noted above, we have previously shown that laminin increases both SG neurite number and length in vitro (Aletsee et al. 2002). If MMP-2 exposes laminin epitopes deposited on the growth surface by cells of the explant, it could increase signaling from this ECM, while MMP-9 would not be expected to do so. Cleavage of laminins by MMP-2 has been shown to enhance the laminin responses of other cell types (Giannelli et al. 1997). Since we also found that a uniform fibronectin surface increases SG neurite number and length (Evans et al. 2007), the lack of effect of MMP-9 may imply that FN is less important than laminin SG neurite growth in our system, or that processing by MMPs is not required for the neurotropic effects of fibronectin.
However, it should be noted that with the exception of MMP-2, which is constitutively expressed in various tissues, MMPs are induced in response to exogenous signals such as growth factors and cytokines (Chintala 2006). A possible explanation of our findings is that under non-pathological conditions, the level of active MMPs other than MMP-2 is very low and, therefore, their inhibition does not influence the SGNs in vitro.
Our findings are in line with a prior study showing that homeostasis of MMP-2 is important in the inner ear. Reuter et al. (1998) demonstrated that a strong increase in MMP-2 expression in the inner ear causes degeneration of various inner ear structures, including a loss of SGNs and degeneration of the organ of Corti along with hearing loss. Our data indicates that a basal level of MMP-2 activity is required for normal SG neurite outgrowth and survival. Reuter et al. (1998) and our results suggest that homeostasis of MMP-2 must be tightly regulated in the inner ear. How can the negative effect of both MMP-2 inhibition and MMP-2 overexpression on SGNs be explained? A study of endothelial cells showed that the mitogen-activated kinase (MAPK) and jun N-terminal kinase (JNK) regulate MMP-2 mRNA expression, while phosphatidylinositol-3-kinase (PI3K) regulates protein levels of MMP-2 (Ispanovic and Haas 2006). We and others have demonstrated that PI3K-Akt signaling mediates the formation of neurites (Mullen et al. 2012; Lallemend et al. 2005; Hansen et al. 2001). However, we recently showed that Rac/cdc42/JNK signaling reduces the formation of neurites (Mullen et al. 2012). Inhibition and overexpression of MMP-2 may alter this balance between competing intracellular signaling pathways. While this hypothesis is perhaps too complex to be attractive without additional supporting data, it is at least consistent with our observations. Further studies will be needed to clarify this issue.
In our experiments, we used organotypic explants from the cochlear SG. Besides neurons, the explants contained supporting cells, including fibroblasts and Schwann cells, which reside between the neurons in vivo. Both cells are known to provide guidance cues to advancing neurites and growth cones, and, therefore, might have influenced the observed reduction in neuritogenesis and length of neurites in our experiments. SG explants treated with the general MMP inhibitor GM6001 and the specific MMP-2 inhibitor showed not only decreased in number of neurites per SG explant and reduction in length of neurites, but also a significant decrease of the area of the supporting cells surrounding the SG explant. The inhibition of non-neuronal cell growth was somewhat less than the inhibition of neurite length. However, this finding suggests that the reduction of SG neurite elongation due to MMP inhibitors may be mediated, at least in part, via Schwann cells or fibroblasts. However, the possibility of directly mediated effects must still be considered. For example, MMP-2 can enhance neurite outgrowth directly by cleaving and inactivating the neurite growth inhibitor CD44 (Zhang et al. 2007). CD44 is present in the developing cochlea, including in the organ of Corti (Hertzano et al. 2010).
It should be noted that we could not distinguish between the dendrites and axons of SGNs, since we have not found markers that distinguish between the two in explants. Similarly, we could not distinguish between type I and type II SGN neurites, since peripherin labeling in the rat does not distinguish between these two classes of neurons, due to the up-regulation of peripherin in Type I neurons in vitro (Lallemend et al. 2007). However, since 95 % of SGNs are type I cells, it seems likely that this class of neuron dominates our results. In our study, we used 5-day-old rat SGNs. Onset of hearing in the rat cochlea approximately occurs on postnatal day 10 (Henley et al. 1989; Rybak et al. 1992). Prehearing neurons were studied, since older neurons are more difficult to culture and neurite development is ongoing at this age (Ernfors et al. 1995; Echteler and Nofsinger 2000).
In summary, we have shown that MMP-2 can modulate neonatal SG neuritogenesis and neurite elongation in vitro, suggesting that this enzyme may play a role in the development of the cochlear innervation.
Acknowledgments
This study is supported by Medizinische Abteilung der Margarete und Walter Lichtsteiner-Stiftung, Basel, Switzerland.
Conflict of interest
All authors report no conflicts of interest.
Footnotes
Michael Sung and Eric Wei have contributed equally to this study.
References
- Agrawal SM, Lau L, Yong VW (2008) MMPs in the central nervous system: where the good guys go bad. Semin Cell Dev Biol 19:42–51 [DOI] [PubMed] [Google Scholar]
- Aletsee C, Mullen L, Kim D, Pak K, Brors D, Dazert S, Ryan AF (2001) The disintegrin kistrin inhibits neurite extension from spiral ganglion explants cultured on laminin. Audiol Neurootol 6:57–65 [DOI] [PubMed] [Google Scholar]
- Aletsee C, Brors D, Palacios S, Pak K, Mullen L, Dazert S, Ryan AF (2002) The effects of laminin-1 onganglion neurons are dependent on the MEK/ERK signaling pathway and are partially independent of Ras. Hear Res 164:1–11 [DOI] [PubMed] [Google Scholar]
- Brors D, Aletsee C, Schwager K, Mlynski R, Hansen S, Schäfers M, Ryan AF, Dazert S (2002) Interaction of spiral ganglion neuron processes with alloplastic materials in vitro. Hear Res 167:110–121 [DOI] [PubMed] [Google Scholar]
- Chang DI, Hosomi N, Lucero J, Heo JH, Abumiva T, Mazar AP, del Zoppo GJ (2003) Activation systems for latent matrix metalloproteinase-2 are upregulated immediately after focal cerebral ischemia. J Cereb Blood Flow Metab 23:1408–1419 [DOI] [PubMed] [Google Scholar]
- Chintala SK (2006) The emerging role of proteases in retinal ganglion cell death. Exp Eye Res 82:5–12 [DOI] [PubMed] [Google Scholar]
- Chintala SK, Zhang X, Austin JS, Fini ME (2002) Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J Biol Chem 277:47461–47468 [DOI] [PubMed] [Google Scholar]
- Clark AW, Krekoski CA, Bou SS, Chapman KR, Edwards DR (1997) Increased gelatinase A (MMP-2) and gelatinase B (MMP-9) activities in human brain after focal ischemia. Neurosci Lett 238:53–56 [DOI] [PubMed] [Google Scholar]
- Echteler SM, Nofsinger YC (2000) Development of ganglion cell topography in the postnatal cochlea. J Comp Neurol 425:436–446 [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R (1995) Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron 14:1153–1164 [DOI] [PubMed] [Google Scholar]
- Evans AR, Euteneuer S, Chavez E, Mullen LM, Hui EE, Bhatia SN, Ryan AF (2007) Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol 67:1721–1730 [DOI] [PubMed]
- Faissner A, Pyka M, Geissler M, Sobik T, Frischknecht R, Gundelfinger ED, Seidenbecher C (2010) Contributions of astrocytes to synapse formation and maturation: potential functions of the perisynaptic extracellular matrix. Brain Res Rev 63:26–38 [DOI] [PubMed] [Google Scholar]
- Fredrich M, Illing RB (2011) Deafferentation-induced redistribution of MMP-2, but not MMO-9, depends on the emergence of GAP-43 positive axons in the adult cochlear nucleus. Neural Plast 2011:859359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka H, Dairyo Y, Yasunaga K, Emoto K (2012) Neural functions of matrix metalloproteinases: plasticity, neurogenesis and disease. Biochem Res Int 2012:789083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genepaint (2013) http://www.genepaint.org. Accessed 30 June 2013
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V (1997) Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science 277:225–228 [DOI] [PubMed] [Google Scholar]
- Hansen MR, Zha XM, Bok J, Green SH (2001) Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro. J Neuorosci 21:2256–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley CM, Owings MH, Stagner BB, Martin GK, Lonsbury-Martin BL (1989) Postnatal development of 2f1–f2 otoacoustic emissions in pigmented rat. Hear Res 43:141–148 [DOI] [PubMed] [Google Scholar]
- Hertzano R, Puligilla C, Chan SL, Timothy C, Depireux DA, Ahmed Z, Wolf J, Eisenman DJ, Friedman TB, Riazuddin S, Kelley MW, Strome SE (2010) CD44 is a marker for the outer pillar cells in the early postnatal mouse inner ear. J Assoc Res Otolaryngol 11:407–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu BH, Cai Q, Hu Z, Patel M, Bard J, Jamison J, Coling D (2012) Metalloproteinases and their associated genes contribute to the functional integrity and noise-induced damage in the cochlear sensory epithelium. J Neurosci 32:14927–14941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ispanovic E, Haas TL (2006) JNK and PI3K differentially regulate MMP-2 and MT1-MMP mRNA and protein in response to actin cytoskeleton reorganization in endothelial cells. Am J Physiol Cell Physiol 291:579–588 [DOI] [PubMed] [Google Scholar]
- Kundu S, Tyagi N, Sen U, Tyagi SC (2009) Matrix imbalance by inducing expression of metalloproteinase and oxidative stress in cochlea of hyperhomocysteinemic mice. Mol Cell Biochem 332:215–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallemend K, Hadjab S, Hans G, Moonen G, Lefebvre PP, Malgrange B (2005) Activation of protein kinaseCbetal constitutes a new neurotrophic pathway for deafferented spiral ganglion neurons. J Cell Sci 118:4511–4525 [DOI] [PubMed] [Google Scholar]
- Lallemend F, Vandenbosch R, Hadjab S, Bodson M, Breuskin I, Moonen G, Lefebvre PP, Malgrange B (2007) New insights into peripherin expression in cochlear. Neurons Neurosci 150:212–222 [DOI] [PubMed] [Google Scholar]
- Mullen LM, Pak KK, Chavez E, Kondo K, Brand Y, Ryan AF (2012) Ras/p38 and PI3K/Akt but not Mek/Erk signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res 1430:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald J, Werb Z (2007) Matrix metalloproteinases and the regulation of tissue remoldeling. Nat Rev Mol Cell Biol 8:221–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter A, Nestl A, Zwacka RM, Tuckermann J, Waldherr R, Wagner EM, Meyer zum Gottesberge AM, Angel P, Weiher H (1998) Expression of the recessive glomerulosclerosis gene Mpv17 regulates MMP-2 expression in fibroblasts, the kidney, and the inner ear of mice. Mol Biol Cell 9:1675–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg GA (2009) Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol 8:205–216 [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth C, Scott V (1992) Development of endocochlear potential and compound action potential in the rat. Hear Res 59:189–194 [DOI] [PubMed] [Google Scholar]
- Setz C, Brand Y, Radojevic V, Hanusek C, Mullen PJ, Levano S, Listyo A, Boderm D (2011) Matrix metalloproteinases 2 and 9 in the cochlea: expression and activity after aminoglycoside exposition. Neuroscience 181:28–39 [DOI] [PubMed] [Google Scholar]
- Sorokin L (2010) The impact of extracellular matrix on inflamation. Nat Rev Immunol 10:712–723 [DOI] [PubMed] [Google Scholar]
- Vaillant C, Meissirel C, Mutin M, Belin MF, Lund RF, Thomasset N (2003) MMP-9 deficiency affects axonal outgrowth, migration, and apoptosis in the developing cerebellum. Mol Cell Neurosci 24:395–408 [DOI] [PubMed] [Google Scholar]
- Van de Water TR, Ruben RJ (1971) Organ culture of the mammalian inner ear. Acta Otolaryngol 71:303–312 [DOI] [PubMed] [Google Scholar]
- Webber CA, Hocking JC, Yong VW, Stange CL, McFarlane S (2002) Metalloproteases and guidance of retinal ganglion axons in the developing visual system. J Neurosci 22:8091–8100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlon DS, Zhang X, Pecelunas K, Greiner MA (1999a) A temporospatial map of adhesive molecules in the organ of Corti of the mouse cochlea. J Neurocytol 28:955–968 [DOI] [PubMed] [Google Scholar]
- Whitlon DS, Zhang X, Kusakabe M (1999b) Tenascin-C in the cochlea of the developing mouse. J Comp Neurol 406:361–374 [DOI] [PubMed] [Google Scholar]
- Woolf NK, Koehrn FJ, Ryan AF (1992) Immunohistochemical localization of fibronectin-like protein in the inner ear of the developing gerbil and rat. Dev Brain Res 65:21–33 [DOI] [PubMed] [Google Scholar]
- Yang Y, Estrada EY, Thompson JF, Liu W, Rosemberg GA (2007) Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J Cereb Blood Flow Metab 27:697–709 [DOI] [PubMed] [Google Scholar]
- Yong VW (2005) Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci 6:931–944 [DOI] [PubMed] [Google Scholar]
- Zhang X, Chintala SK (2004) Influence of interleukin-1 beta induction and mitogen-activated protein kinase phosphorylation on optic nerve ligation-induced matrix metalloproteinase-9 activation in the retina. Exp Eye Res 78:849–860 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Klassen HJ, Tucker BA, Perez MT, Young MJ (2007) CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J Neurosci 27:4499–4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Chang M, Hansen CM, Basso DM, Noble-Haeusslein LJ (2011) Role of matrix metalloproteinases and therapeutic benefits of their inhibition in spinal cord injury. Neurotherapeutics 8:206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]