Abstract
Objectives
Aims of the study were 1) to investigate the association of C-reactive protein (CRP) with lipid (i.e. total, LDL, and HDL cholesterol, triglycerides) concentrations, and to evaluate their predictive value for mortality in very old subjects.
Design
Cross-sectional and longitudinal analyses in a prospective cohort study.
Setting - Participants
Data are from 336 community-dwelling subjects aged ≥80 years old enrolled in the “Invecchiamento e Longevita' nel Sirente” (ilSIRENTE) study.
Measurements
High sensitivity CRP and lipid concentrations were measured at the baseline clinical visit. High sensitivity CRP concentrations were measured by ELISA assessment. Mortality outcome was evaluated over a 24-month follow-up.
Results
Participants had a mean age of 85.8 (SD 4.8) years old. Spearman's correlations showed significant (p values <0.01) inverse correlations between CRP and lipid parameters (except triglycerides). Adjusted linear regressions between CRP and lipid parameters concentrations showed no significant association in participants aged lower than 85 years old (all p values >0.5). In the older age group, significant inverse associations of CRP with total (p=0.002), LDL (p=0.007), and HDL cholesterol (p=0.002) were found, even after adjustment for potential confounders. Adjusted Cox proportional hazard models demonstrated that CRP was the only biomarker significantly predictive of mortality, independently of age and lipid parameters.
Conclusion
An inverse relationship of total, LDL, and HDL cholesterol with CRP is present in very old persons. The prognostic value of CRP is particularly important among very old persons whereas lipid parameters tend to lose their capacity to predict events.
Keywords: C-reactive protein, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides
INTRODUCTION
The aging process is characterized by an increase in the concentration of several inflammatory biomarkers and a progressive decrease in lipid concentrations(1). C-reactive protein (CRP), like many other inflammatory parameters, increases in concentration with age(2). Systemic inflammation characterized by elevated CRP concentrations is associated with incident cardiovascular disease(3), functional decline(4;5), physical disability(6), and mortality(4;5) in older persons. The age-related increase of inflammatory markers may also account for several pathological modifications typical of the aging process, including body composition modifications(7) and sarcopenia(8).
Several studies have demonstrated a progressive reduction of cholesterol concentrations in subjects aged ≥65 years of age. For example, in the Honolulu Heart Study, Abbott and colleagues(9) reported a longitudinal reduction in total cholesterol levels over a 20-year follow-up, even after taking into account several clinical conditions and risk factors potentially explaining this phenomenom. Authors concluded suggesting that the demonstrated reduction may be part of the normal aging process.
It has been demonstrated that inflammation (through the acute-phase response) is able to alter lipid and lipoprotein metabolism, promoting their pro-atherogenic properties(10). This phenomenon is due to the concurrent reduction of synthesis and/or increase of catabolism of these lipoproteins. However, the contribution of inflammation on changes in lipid/lipoprotein concentrations in the population is largely unknown, especially in older persons.
Systemic inflammation and plasma lipid parameters (i.e. total, low-density lipoprotein [LDL], and high-density lipoprotein [HDL] cholesterol, and triglycerides) are both associated with cardiovascular risk and mortality. Interestingly, a large body of evidence indicates a generally weaker association of traditional risk factors, including lipid profile, with negative health-related events in the elderly compared to middle-aged populations(11;12).
We hypothesized that inflammation and lipid parameters may be associated in very old subjects, but age may potentially play an important role in modifying this relationship in its strength and direction. Therefore, in the present study, we investigated the association of high sensitivity CRP, the most commonly used marker of inflammation in clinical as well as research settings, with lipid (i.e. total, LDL, and HDL cholesterol, triglycerides) concentrations in a sample of community-dwelling subjects aged 80 years and older, enrolled in the “Invecchiamento e Longevità nel Sirente” (Aging and Longevity in the Sirente geographic area, ilSIRENTE) study(13). Moreover, to provide a clinically useful meaning to the studied relationships, we tested whether CRP concentration was predictive of mortality, independently of the lipid profile.
METHODS
Data are from the ilSIRENTE, a prospective cohort study performed in community-dwelling older persons living in the Sirente geographic area (L'Aquila, Italy). Details of the design and methods of ilSIRENTE have been described elsewhere(13). Briefly, potential study participants were identified by selecting from the Registry Offices every person born before 1st January 1924 and still living in the municipalities involved in the study at the end of October 2003. A total of 364 participants were enrolled in the study. Clinical interview and functional assessment were performed at the study clinics located in each town. Home visit was performed if participant was unable to reach the study clinic. Information was obtained from the participant or, if he/she was incapable, from a proxy. The Catholic University of Sacred Heart (Rome, Italy) Ethical Committee approved the study protocol. All the participants signed an informed consent at the baseline visit.
The present analyses were conducted after exclusion of 28 participants with CRP concentrations higher than 10 mg/L. This cut-point has been indicated as suggestive of the presence of acute inflammatory state(14).
The Minimum Data Set for Home Care (MDS-HC)
The Minimum Data Set for Home Care (MDS-HC) instrument(15) was administered to all study participants. The MDS-HC contains a variety of different, multi-item summary scales, exploring socio-demographics, clinical diagnoses, and physical function status. Besides, the MDS-HC includes information about an extensive array of signs, symptoms, syndromes, and treatments. The MDS items have shown an excellent inter-rater and test-retest reliability when completed by nurses performing usual assessment duties(16;17). A questionnaire exploring family history, lifestyle, nutrition, physical activity, and other behavioral factors shared with the “Invecchiare in Chianti” (Aging in the Chianti geographic area, InCHIANTI) study(18) was additionally administered.
Mortality
Vital status of all the study participants was ascertained from the general practitioners, and confirmed by the National Death Registry until 24 months after the baseline visit. The follow-up time considered for the present analyses was calculated as the time from the date of baseline visit to the date of death (for participants who died during the follow-up), and censored to 24 months for participants who did not die during the study follow-up.
C-reactive protein
Phlebotomy was performed by a trained phlebotomist following a standardized protocol at the baseline visit. As previously described(13), blood samples were immediately centrifuged at 4°C and stored at −80°C until final analysis. CRP concentrations were determined by a high sensitivity Enzyme-Linked ImmunoSorbent Assay (ELISA) kit (Bender MedSystems, Vienna, Austria). The CRP assay had a sensitivity of 3 pg/mL. The intra-assay coefficient of variation was 6.9%.
Lipid parameters
Total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides concentrations were considered in the present analyses to evaluate their relationships with C-reactive protein levels. Standard determinations of total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides were performed by commercialy available kits suitable for use on Olympus 2700 instrumentation (Olympus, Milano, Italy).
Covariates
Covariates considered in the present analyses include: sociodemographic characteristics (age, gender, and smoking habit), body mass index (BMI), physical activity level, comorbidity, medications, and albumin levels. All the variables included in the models as potential confounders of the studied relationships have shown to be able to affect the inflammatory markers and/or lipid parameters concentrations. BMI was defined as weight (in kilograms) divided by the square of height (in meters). Physical activity was assessed by asking the participant to provide data on past and current activities involving energy expenditure, including recreational and work-related ones. For the present analyses, we considered as physically active those participants reporting at least light intensity activities (e.g. walking, dancing, fishing…) performed for no less than 2–4 hours per week during the last year. The following clinical diagnoses were assessed by a study physician on the basis of self- (or proxy-) reported history and clinical records review and considered in the adjusted analyses: coronary heart disease, congestive heart failure, cerebrovascular disease, diabetes, hypertension, peripheral artery disease, cancer. Primary care physicians collected information on up to 18 different drugs taken by each patient in the 7 days preceding the assessment. Drugs were coded using the Anatomical Therapeutic and Chemical (ATC) codes(19). For the present analyses, we considered non-steroidal anti-inflammatory drugs (including low-dosage aspirin), and statins. Serum albumin concentration, a malnutrition marker, was determined by using commercialy available kits suitable for use on Olympus 2700 instrumentation (Olympus, Milano, Italy).
Statistical analysis
Means (and standard deviations, SD), proportions (in percentage) were calculated to describe the main characteristics of the study sample. Medians (and interquartile ranges [IQR]) were reported for non-normally distributed variables. Spearman's correlations among CRP and lipid parameters were performed. Given the non-normal distribution of CRP and triglycerides concentrations, these markers were log transformed to make them normally distributed. Interactions of age and gender in the relationships of lipid parameters with CRP levels were tested by including the specific interaction term into the adjusted linear regression models. Unadjusted and adjusted linear regression models were performed to calculate the regression coefficients (and 95% confidence intervals, 95%CI) of the relationships between CRP (dependent variable) and lipid parameters levels (independent variable). Collinearity statistics (i.e. tolerance, Variance Inflation Factor, condition indices, and correlation matrix of regression coefficients) were tested for all the models as appropriate to exclude the risk of multicollinearities among the independent variables. To permit direct comparisons of lipid parameters concentrations, all the results are shown per SD increase of the biomarker. Adjusted analyses of covariance were performed to estimate (geometrically transformed) means of CRP concentrations (dependent variable) for categorical concentrations of lipid parameters. Cox proportional hazard analyses were performed to evaluate the predictive value of CRP and lipid parameters concentrations for mortality. A p value <0.05 was chosen for statistical significance for all the present analyses. All the analyses were performed using SPSS software (version 13.0, SPSS Inc., Chicago, IL).
RESULTS
Main characteristics of the study sample (n=336) are shown in Table 1. Participants had a mean age of 85.8 (SD 4.8) years old, and were predominantely women (66.7%). Very few participants were current smokers (2.4%), and the majority of the sample (60.4%) was involved in regular physically activity. Among the clinical conditions, hypertension (73.2%), diabetes (30.1%), and coronary heart disease (11.9%) were the most prevalent. The sample population was also characterized by a slightly overweight mean body size (BMI 25.7 kg/m2), the relatively low use of medications, and good concentrations of albumin and lipid parameters. A median CRP concentration of 2.9 mg/L was found in the overall sample population.
Table 1.
Main characteristics of the sample population.
| Mean ± SD, or % N=336 | |
|---|---|
| Age (years) | 85.8 ± 4.8 |
| Gender (Women) | 66.7 |
| Current smoking | 2.4 |
| Body Mass Index (kg/m2) | 25.7 ± 4.5 |
| Physical activity | 60.4 |
| Coronary heart disease | 11.9 |
| Congestive heart failure | 5.7 |
| Cerebrovascular disease | 4.2 |
| Diabetes | 30.1 |
| Hypertension | 73.2 |
| Peripheral artery disease | 2.7 |
| Cancer | 4.2 |
| Albumin (g/dL) | 4.2 ± 0.3 |
| Non-steroidal antiinflammatory drugs | 12.2 |
| Statins | 6.0 |
| Total cholesterol (mg/dL) | 197.9 ± 44.7 |
| LDL cholesterol (mg/dL) | 130.8 ± 38.8 |
| HDL cholesterol (mg/dL) | 46.2 ± 13.5 |
| Triglycerides (mg/dL)* | 137.5 (99.3–178.8) |
| C reactive protein (mg/L)* | 2.9 (1.4–5.3) |
SD: standard deviation
Expressed as median (interquartile range)
In Table 2 are shown the results from Spearman's correlation analyses performed among C-reactive protein and lipid parameters concentrations. A strong correlation (r=0.838, p value <0.001) was found between total cholesterol and LDL cholesterol levels. Furthermore, a negative correlation was found between HDL cholesterol and triglycerides levels (r=−0.228, p value <0.001). Significant (p values <0.01) inverse correlations were found between CRP and lipid parameters (except of triglycerides).
Table 2.
Spearman's correlations between C-reactive protein and lipid profile (n=336).
| C-reactive protein | Total cholesterol | LDL cholesterol | HDL cholesterol | |
|---|---|---|---|---|
| C-reactive protein | - | |||
| Total cholesterol | −0.169 p=0.002 | - | ||
| LDL cholesterol | −0.151 p=0.006 | 0.838 p<0.001 | - | |
| HDL cholesterol | −0.199 p<.001 | 0.486 p<0.001 | 0.274 p<.001 | - |
| Triglycerides | −0.032 p=0.56 | 0.360 p<0.001 | 0.341 p<.001 | −0.228 p<0.001 |
No interaction for gender between CRP and lipid parameters was found. Since possible interactions (p values for interaction terms <0.1) were found between CRP and total and HDL cholesterol for age, all the further analyses were stratified according to age groups (age 80–84.9 years old, n=188 [56.0%]; age ≥85 years old, n=148 [44%]). Participants aged ≥85 years had lower levels of total cholesterol (193.0 [SD 43.8] mg/dL vs. 201.8 [SD 45.2] mg/dL; p value=0.08), and HDL cholesterol (43.3 [SD 10.3] mg/dL vs. 48.4 [15.2] mg/dL; p value=0.001), and higher CRP levels (3.1 [IQR 1.7–6.0] mg/L vs. 2.5 [IQR 1.2–4.3] mg/L; p value=0.009) compared to participants younger than 85 years old. No significant differences were found for LDL cholesterol and triglycerides according to age groups.
Results from adjusted linear regression models between CRP and lipid parameters concentrations are presented in Table 3. No significant association was found between CRP and lipid parameters in participants aged lower than 85 years old (all p values >0.5). In the older age group (participants aged ≥85 years old), significant inverse associations of CRP with total (β=−0.244; 95%CI −0.394, −0.093; p=0.002), LDL (β=−0.203; 95%CI −0.349, −0.057; p=0.007), and HDL cholesterol (β=−0.331; 95%CI −0.534, −0.129; p=0.002) were found, even after adjustment for all the potential confounders.
Table 3.
Linear regression models (regression coefficient, 95% condifence interval) between C-reactive protein (log value, dependent variable) and lipid profile measures (indipendent variables, per SD increase).
| Age < 85 years old n=188 | Age ≥ 85 years old n=148 | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | β (95%CI) | p | |
| Total cholesterol | 0.013 (−0.119, 0.145) | 0.84 | −0.010 (−0.155, 0.135) | 0.89 | −0.241 (−0.382, −0.099) | 0.001 | −0.244 (−0.394, −0.093) | 0.002 |
| LDL cholesterol | −0.002 (−0.136, 0.132) | 0.98 | −0.016 (−0.162, 0.131) | 0.07 | −0.185 (−0.323, −0.047) | 0.009 | −0.203 (−0.349, −0.057) | 0.007 |
| HDL cholesterol | 0.045 (−0.072, 0.163) | 0.45 | 0.064 (−0.061, 0.189) | 0.32 | −0.381 (−0.561, −0.201) | <0.001 | −0.331 (−0.534, −0.129) | 0.002 |
| Triglycerides | −0.033 (−0.162, 0.096) | 0.62 | −0.058 (−0.201, 0.086) | 0.43 | 0.008 (−0.136, 0.152) | 0.91 | −0.006 (−0.160, 0.148) | 0.94 |
Model 1 adjustment: gender
Model 2 adjustment: gender, body mass index, current smoking, physical activity level, coronary heart disease, congestive heart failure, cerebrovascular disease, diabetes, hypertension, peripheral artery disease, cancer, non-steroidal antiinflammatory drugs, statins, albumin
SD: standard deviation.
Lipid profile SDs: total cholesterol 44.74 mg/dL; LDL cholesterol 38.77 mg/dL; HDL cholesterol 13.46 mg/dL; triglycerides (log value of mg/dL) 0.42.
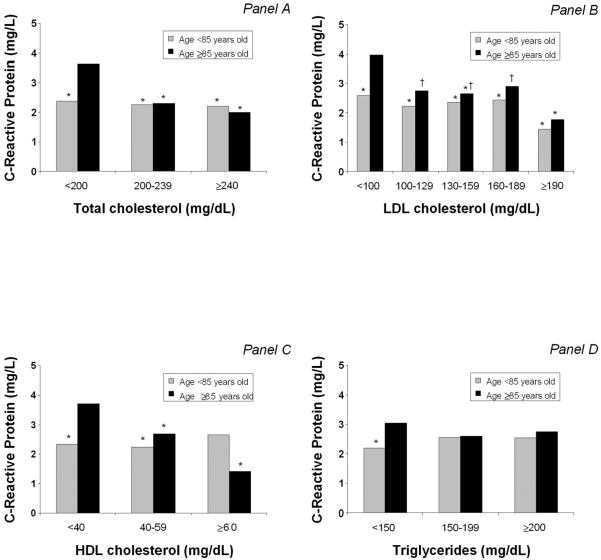
In Figure 1, results from adjusted ANCOVAs are shown. Participants aged ≥85 years old with low total cholesterol levels (<200 mg/dL) had significantly higher levels of CRP than 1) younger participants (independently of total cholesterol levels), and 2) participants in the same age group with higher total cholesterol levels. Similar findings were also reported for LDL and HDL cholesterol levels. In fact, for lower LDL and HDL cholesterol levels in participants aged ≥85 years old, CRP concentrations were higher. No significant results were found for the association between triglycerides and CRP levels, except for a significant difference of inflammatory marker concentration in participants with low triglycerides levels.
Figure 1.
Adjusted (geometric) means of C-reactive protein levels for lipid profile measures according to age. Analyses are adjusted for gender, body mass index, current smoking, physical activity level, coronary heart disease, congestive heart failure, cerebrovascular disease, diabetes, hypertension, peripheral artery disease, cancer, non-steroidal antiinflammatory drugs, statins, albumin.
Panel A: * p<0.05 when compared to participants aged ≥85 years and with total cholesterol <200 mg/dL
Panel B: * p<0.05 when compared to participants aged ≥85 years and with LDL cholesterol <100 mg/dL; † p<0.05 when compared to participants aged <85 years and with LDL cholesterol ≥190 mg/dL
Panel C: * p<0.05 when compared to participants aged ≥85 years and with HDL cholesterol <40 mg/dL
Panel D: * p<0.05 when compared to participants aged ≥85 years and with triglycerides <150 mg/dL
Finally, we longitudinally investigated the clinical meaning of the found inverse relationship between CRP and lipid parameters concentrations. Cox proportional hazard models in which CRP concentration was simultaneously included with each single lipid parameter were performed to evaluate the predictive value for mortality (mean follow-up 1.8 [SD 0.5] years; Table 4). Overall, higher values of CRP were associated with a higher risk of death, especially among older participants, independently of total cholesterol, HDL cholesterol, LDL cholesterol, or triglycerides. On the other hand, higher lipid parameters concentrations tended to be protective for mortality. In particular, total cholesterol reached a statistical significance (p=0.02), independently of CRP levels. Non statistically significant results were reported for HDL cholesterol (p=0.12), LDL cholesterol (p=0.07), and triglycerides (p=0.23). When all the studied biomarkers were simultaneously included in an exploratory adjusted Cox proportional hazard model predicting mortality, CRP was still the only biomarker showing a statistical significance in the overall sample (HR 2.92, 95%CI 1.29–6.61). No statistical significance was reported for lipid parameters. No significant interaction of CRP and lipid parameters concentrations for mortality was reported in both age groups.
Table 4.
Cox proportional hazard models (hazard ratio, 95% condifence interval)* between C-reactive protein and lipid profile measures for the prediction of mortality during the follow-up.
| Overall sample n=336 | Age < 85 years old n=188 | Age ≥ 85 years old n=148 | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| HR (95%CI) | p | HR (95%CI) | p | HR (95%CI) | p | |
| C-reactive protein | 2.97 (1.31–6.72) | 0.009 | 3.22 (0.80–12.91) | 0.10 | 3.24 (1.02–10.29) | 0.04 |
| Total cholesterol | 0.72 (0.55–0.94) | 0.02 | 0.62 (0.38–0.99) | 0.05 | 0.72 (0.50–1.04) | 0.09 |
| C-reactive protein | 3.18 (1.38–7.30) | 0.007 | 3.31 (0.79–13.90) | 0.10 | 3.69 (1.12–12.11) | 0.03 |
| HDL cholesterol | 0.78 (0.56–1.07) | 0.12 | 0.76 (0.46–1.26) | 0.29 | 0.80 (0.50–1.28) | 0.36 |
| C-reactive protein | 3.06 (1.35–6.95) | 0.008 | 3.14 (0.77–12.75) | 0.11 | 3.42 (1.08–10.92) | 0.04 |
| LDL cholesterol | 0.78 (0.60–1.02) | 0.07 | 0.77 (0.46–1.28) | 0.31 | 0.74 (0.53–1.04) | 0.09 |
| C-reactive protein | 3.21 (1.41–7.34) | 0.006 | 2.95 (0.70–12.42) | 0.14 | 4.13 (1.34–12.72) | 0.01 |
| Triglycerides | 0.85 (0.66–1.10) | 0.23 | 0.62 (0.37–1.02) | 0.06 | 0.84 (0.60–1.18) | 0.31 |
Adjusted for gender, body mass index, current smoking, physical activity level, coronary heart disease, congestive heart failure, cerebrovascular disease, diabetes, hypertension, peripheral artery disease, cancer, non-steroidal antiinflammatory drugs, statins, albumin.
Biomarker concentrations are expressed per standard deviation (SD) increase.
SDs: total cholesterol 44.74 mg/dL; LDL cholesterol 38.77 mg/dL; HDL cholesterol 13.46 mg/dL; triglycerides (log value of mg/dL) 0.42; C-reactive protein (log value of mg/L) 2.63.
C-reactive protein and each lipid profile measures are simultaneously included in separate models.
DISCUSSION
To our knowledge, this is the first study exploring the relationship of lipid parameters (i.e. total cholesterol, HDL cholesterol, LDL cholesterol, and triglycerides) with CRP in a sample of community-dwelling very old individuals. Significant differences in the association of CRP with total, HDL and LDL cholesterol levels according to age were found. In fact, in persons aged ≥85 years old, significant inverse relationships were found between levels of CRP and lipid parameters. In our longitudinal analyses, CRP represented the strongest predictor of mortality among the studied biomarkers.
Zuliani and colleagues recently demonstrated an inverse relationship of interleukin-6 with HDL cholesterol levels in the InCHIANTI study(20). Authors also showed that age is strongly and inversely correlated with cholesterol levels. Investigators from the Rancho Bernardo Study(21) reported the inverse association of cholesterol and age to be independent of (sub)clinical diseases, body weight change, and medications. Volpato and colleagues(22), confirming the inverse association between age and total cholesterol levels, argued that the age-dependent reduction of total cholesterol levels may simply mirror the effect of a poorer health status with aging. However, as also recognized by the Authors, their analyses were conducted in a sample of hospitalized older persons, in which 1) the weight of clinical conditions might be higher in affecting the studied relationship, 2) acute inflammatory conditions are more likely to be present, and 3) the average mean of cholesterol levels was very low. In all these studies, the sample populations were younger than ours.
Consistently with our findings, it has been demonstrated that persons aged 90 years and older present significant lower levels of total, HDL, and LDL cholesterol for higher levels of interleukin-6. By comparing a sample of nonagenarians with a middle-aged control population, it was shown that the relationship between interleukin-6 levels with concentrations of CRP and cholesterol differs according to age. Similarly, a further study showed that older persons presenting biohumoral signs of acute phase inflammation tend to have lower levels of total, HDL, and LDL cholesterol(23).
The relationship between inflammation and lipid parameters is very complex, especially at old age when additional pathophysiological pathways are hyperactivated and interact with this association. HDLs have shown anti-inflammatory, anti-oxidant, and pro-fibrinolytic properties. HDLs also play an important protective role by removing cholesterol from atheroma and transporting it back to the liver for excretion or re-utilization(24). At the same time, there are circumstances in which HDL may not be protective, but paradoxically promote vascular inflammation and oxidation of LDL. In particular, the pro-inflammatory action of HDL is manifested in conditions associated with chronic systemic inflammation(25;26), as it is the very old age(27;28). The strong relationship we found between CRP and lipid parameters among the oldest participants might be explained by the increasing levels of systemic inflammation associated with advancing age. The exponential increase of systemic inflammation at very advanced age (due to the increasing number of clinical and subclinical conditions) may particularly influence the lipid parameters concentrations. It is possible that relatively younger participants may have not yet reached the hypothetical threshold (in terms of burden of diseases and/or time exposure to inflammatory stimuli) activating and self-feeding the vicious cycle among lipid parameters and inflammation (possibly and partly mediated by clinical events).
The demonstrated inverse relationship between lipid parameters and CRP may, at least partly, explain some unclear clinical manifestations occurring in older age. Several studies have reported the presence of a J- or U-shaped association between total cholesterol levels and mortality. The Framingham Study demonstrated that up to 60 years of age there is a positive relationship between total cholesterol and total mortality. Nevertheless, this relationship becomes negative, and after the seventh decade of life, subjects with the highest total cholesterol levels present the highest survival(29). The weaker association between lipid parameters and mortality has shown to be independent of selective survival, disease prevalence, weight change, lifestyle habits, or medication use(9;21;30). The third factor potentially explaining this clue might be the inflammation. This hypothesis may potentially explain the unexpected higher mortality rate found in high-functioning older persons with low cholesterol levels(31).
In the present study, we demonstrated that the predictive value for mortality of CRP tends to increase with aging, independently of lipid parameters. On the other hand, these latters tend to remain stable in the strength of their prognostic value (which is modestly significant), and lend support to the existing controversy about the role played by lipid parameters as risk factors for health-related events in advanced age(32;33). The age-related weakening of traditional risk factors requires the adoption of alternative measures to identify very old subjects at increased risk of health-related events. Our findings may encorauge the CRP assessment to estimate the risk of negative outcomes in very old persons. Interestingly, inflammation is a major biological pathway for the aging process(34), and the basis for several age-related clinical and subclinical conditions (e.g. atherosclerosis(35), sarcopenia(7), physical disability(6)). In this context, the assessment of a well-established marker of inflammation (as CRP is) might provide useful information in the evaluation of overall health status of very old persons.
The age difference we found in the relationship between CRP and lipid concentrations might be found surprising since our study population enrolls only subjects aged 80 years and older. However, the two age groups we analyzed (younger than 85 years vs. 85 years and older) had a mean age difference of almost a decade (82.2 [SD 1.4] vs 90.3 [SD 3.6] years). A type I statistical error is unlikely given the consistency of the results throughout all the explored parameters. Energy intake and dietary composition are major determinants of total cholesterol levels. Although dietary records are not available in the ilSirente database, BMI and serum albumin, two markers of nutritional status, were considered in our analyses. Since the complexity of the studied biological mechanisms, the evaluation of a single inflammatory biomarker (i.e. CRP) may not be sufficient to extend our results to the entire inflammatory pathway. Finally, it is possible (as always with studies on aging) that subjects with worse inflammatory and lipid profiles might have prematurely died, leaving only individuals at lower risk of events.
In conclusion, our findings confirm the existence of an inverse relationship of total, LDL, and HDL cholesterol with CRP at very old age. Differently from lipid parameters, CRP concentrations are strongly predictive of mortality at advanced age. Our results might encourage the assessment of CRP for estimating the risk of negative outcomes in very old persons. However, further studies are needed to 1) extend our findings to different settings and biomarkers of inflammation, and 2) evaluate whether specific interventions (e.g. aspirin, statins, physical exercise) may provide clinical benefits through modifications of lipid and/or inflammatory profiles even at very old age.
ACKNOWLEDGEMENTS
The “Invecchiamento e Longevita` nel Sirente” (ilSIRENTE) study was supported by a grant from the “Comunita` Montana Sirentina” (Secinaro, L'Aquila, Italy). This work was also supported by the University of Florida Institute on Aging and the Claude D. Pepper Older Americans Independence Center (NIH grant 1P30AG028740).
Footnotes
Disclosure summary: The Authors have nothing to disclosure.
REFERENCES
- (1).Wilson PW, Anderson KM, Harris T, Kannel WB, Castelli WP. Determinants of change in total cholesterol and HDL-C with age: the Framingham Study. J Gerontol. 1994;49(6):M252–M257. doi: 10.1093/geronj/49.6.m252. [DOI] [PubMed] [Google Scholar]
- (2).Lowe GD. Circulating inflammatory markers and risks of cardiovascular and non-cardiovascular disease. J Thromb Haemost. 2005;3:1618–1627. doi: 10.1111/j.1538-7836.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- (3).Cesari M, Penninx BWJH, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and onset of cardiovascular events: results from the Health ABC study. Circulation. 2003;108(19):2317–2322. doi: 10.1161/01.CIR.0000097109.90783.FC. [DOI] [PubMed] [Google Scholar]
- (4).Reuben DB, Cheh AI, Harris TB, Ferrucci L, Rowe JW, Tracy RP, et al. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J Am Geriatr Soc. 2002;50:638–644. doi: 10.1046/j.1532-5415.2002.50157.x. [DOI] [PubMed] [Google Scholar]
- (5).Cohen HJ, Harris T, Pieper CF. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114(3):180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- (6).Ferrucci L, Harris TB, Guralnik JM, Wacholder S, Tracy RP, Corti MC, et al. Inflammation, a novel risk factor for disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- (7).Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BWJH, Lenchik L, et al. Sarcopenia, obesity and inflammation - Results from the TRAIN study. Am J Clin Nutr. 2005;82:428–434. doi: 10.1093/ajcn.82.2.428. [DOI] [PubMed] [Google Scholar]
- (8).Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 2006. 119(6):526.e9–526.e17. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- (9).Abbott RD, Yano K, Hakim AA, Burchfiel CM, Sharp DS, Rodriguez BL, et al. Changes in total and high-density lipoprotein cholesterol over 10- and 20-year periods (The Honolulu Heart Program) Am J Cardiol. 1998;82:172–178. doi: 10.1016/s0002-9149(98)00310-5. [DOI] [PubMed] [Google Scholar]
- (10).Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45(7):1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- (11).Simons LA, Simons J, Friedlander Y, McCallum J. Cholesterol and other lipids predict coronary heart disease and ischaemic stroke in the elderly, but only in those below 70 years. Atherosclerosis. 2001;159(1):201–208. doi: 10.1016/s0021-9150(01)00495-6. [DOI] [PubMed] [Google Scholar]
- (12).Casiglia E, Palatini P. Cardiovascular risk factors in the elderly. J Hum Hypertens. 1998;12(9):575–581. doi: 10.1038/sj.jhh.1000668. [DOI] [PubMed] [Google Scholar]
- (13).Landi F, Russo A, Cesari M, Barillaro C, Onder G, Zamboni V, et al. The ilSIRENTE study: a prospective cohort study on persons aged 80 years and older living in a mountain community of Central Italy. Aging Clin Exp Res. 2005;17(6):486–493. doi: 10.1007/BF03327416. [DOI] [PubMed] [Google Scholar]
- (14).Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, et al. Markers of inflammation and cardiovascular disease. Application to clinical and public health practice: a statement for healthcare professionals from the Center for Disease Control and Prevention and the American Heart Association. Circulation. 2003;177:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- (15).Morris JN, Fries BE, Bernabei R, Ikegami N, Gilgen R, Steel K, et al. RAI - Home Care assessment manual. InterRAI Coporation; Washington, DC: 1996. [Google Scholar]
- (16).Hawes C, Morris JN, Phillips CD, Mor V, Fries BE, Nonemaker S. Reliability estimates for the Minimum Data Set for nursing home resident assessment and care screening (MDS) Gerontologist. 1995;35(2):172–178. doi: 10.1093/geront/35.2.172. [DOI] [PubMed] [Google Scholar]
- (17).Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, et al. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Med Care. 2000;38(12):1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- (18).Ferrucci L, Bandinelli S, Benvenuti E, Di Iorio A, Macchi C, Harris TB, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–1625. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- (19).Pahor M, Chrischilles EA, Guralnik JM, Brown SL, Wallace RB, Carbonin P. Drug data coding and analysis in epidemiologic studies. Eur J Clin Epidemiol. 1994;10:405–411. doi: 10.1007/BF01719664. [DOI] [PubMed] [Google Scholar]
- (20).Zuliani G, Volpato S, Ble' A, Bandinelli S, Corsi AM, Lauretani F, et al. High interleukin-6 plasma levels are associated with low HDL-C levels in community-dwelling older adults: The InChianti study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2006.05.024. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Ferrara A, Barrett-Connor E, Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study 1984-1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- (22).Volpato S, Zuliani G, Guralnik JM, Palmieri E, Fellin R, the Gruppo Italiano di Farmacovigilanza nell'Anziano The inverse association between age and cholesterol level among older patients: the role of poor health status. Gerontology. 2001;47:36–45. doi: 10.1159/000052768. [DOI] [PubMed] [Google Scholar]
- (23).Volpato S, Palmieri E, Fellin R, Zuliani G. Acute phase markers are associated with reduced plasma lipid levels in a population of hospitalized elderly patients. Gerontology. 2000;46(22):27. doi: 10.1159/000022129. [DOI] [PubMed] [Google Scholar]
- (24).Spieker LE, Ruschitzka F, Luscher TF, Noll G. HDL and inflammation in atherosclerosis. Curr Drug Targets Immune Endocr Metabol Disord. 2004;4(1):51–57. doi: 10.2174/1568008043340044. [DOI] [PubMed] [Google Scholar]
- (25).Ansell BJ, Fonarow GC, Fogelman AM. High-density lipoprotein: is it always atheroprotective? Curr Atheroscler Rep. 2006;8(5):405–411. doi: 10.1007/s11883-006-0038-4. [DOI] [PubMed] [Google Scholar]
- (26).Navab M, Anantharamaiah GM, Reddy ST, Van Lenten BJ, Ansell BJ, Fogelman AM. Mechanisms of disease: proatherogenic HDL - An evolving field. Nat Clin Pract Endocrinol Metab. 2006;2(9):504–511. doi: 10.1038/ncpendmet0245. [DOI] [PubMed] [Google Scholar]
- (27).Ferrucci L, Ble' A, Bandinelli S, Lauretani F, Suthers K, Guralnik JM. A flame burning within. Aging Clin Exp Res. 2004;16(3):240–243. doi: 10.1007/BF03327390. [DOI] [PubMed] [Google Scholar]
- (28).Lehtimäki T, Ojala P, Rontu R, Goebeler S, Karhunen PJ, Jylhä M, et al. Interleukin-6 modulates plasma cholesterol and C-reactive protein concentrations in nonagenarians. J Am Geriatr Soc. 2005;53:1552–1558. doi: 10.1111/j.1532-5415.2005.53484.x. [DOI] [PubMed] [Google Scholar]
- (29).Kronmal RA, Cain KC, Ye Z, Ommen GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153:1065–1073. [PubMed] [Google Scholar]
- (30).Newschaffer CJ, Bush TL, Hale WE. Aging and total cholesterol levels: cohort, period, and survivorship effects. Am J Epidemiol. 1992;136:23–24. doi: 10.1093/oxfordjournals.aje.a116417. [DOI] [PubMed] [Google Scholar]
- (31).Hu P, Seeman TE, Harris TB, Reuben DB. Does inflammation or undernutrition explain the low cholesterol-mortality association in high-functioning older persons? MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2004;51(1):80–84. doi: 10.1034/j.1601-5215.2002.51014.x. [DOI] [PubMed] [Google Scholar]
- (32).Karlamangla AS, Singer BH, Reuben DB, Seeman TE. Increases in serum non-high-density lipoprotein cholesterol may be beneficial in some high-functioning older adults: MacArthur Studies of Successful Aging. J Am Geriatr Soc. 2004;52(4):487–494. doi: 10.1111/j.1532-5415.2004.52152.x. [DOI] [PubMed] [Google Scholar]
- (33).Krumholz HM, Seeman TE, Merrill SS, Mendes de Leon CF, Vaccarino V, Silverman DI, et al. Lack of association between cholesterol and coronary heart disease mortality and morbidity and all-cause mortality in persons older than 70 years. JAMA. 1994;272(17):1135–1140. [PubMed] [Google Scholar]
- (34).Franceschi C, Bonafe' M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging - An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–254. doi: 10.1111/j.1749-6632.2000.tb06651.x. [DOI] [PubMed] [Google Scholar]
- (35).Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]