Abstract
Osteosarcoma is the most common bone cancer in children and adolescents with a five-year survival rate of about 70%. In this study, we have evaluated the preclinical therapeutic efficacy of the novel synthetic drug, Minnelide, a prodrug of triptolide on osteosarcoma. Triptolide was effective in significantly inducing apoptosis in all osteosarcoma cell lines tested but had no significant effect on the human osteoblast cells. Notably, Minnelide treatment significantly reduced tumor burden and lung metastasis in the orthotopic and lung colonization models. Triptolide/Minnelide effectively downregulated the levels of pro-survival proteins such as heat shock proteins, cMYC, survivin and targets NF-κB pathway.
Keywords: Triptolide, Minnelide, osteosarcoma, heat shock proteins, NF-κB
1. INTRODUCTION
Osteosarcoma is the most common malignant bone cancers in children and adolescents [1,2]. The health impact and social burden of this cancer is high: even with recent advances in multimodal treatments the 5-year survival of osteosarcoma patients is less than 70%. About 50% of patients with an initial diagnosis of non-metastatic disease subsequently develop metastases [3,4]. Patients who present with metastases at diagnosis have a poor prognosis, with overall survival rates of less than 20%. Thus, the clinical outcomes continue to be dismal and have identified osteosarcoma among the ‘most wanted’ for development of new, effective therapies. Hence, potent drugs for treating osteosarcoma that effectively target key oncogenic genes and pathways are greatly needed.
Triptolide, an anti-inflammatory diterpene epoxide derived from the Chinese plant Trypterygium wilfordii, was identified as part of a small molecule screen as regulators of the heat shock gene transcription [5,6]. Triptolide has been shown to inhibit cell proliferation and induce apoptosis in many cancer cell lines by downregulating the levels of HSP70 [7]–13]. Upregulation of heat shock proteins including HSP70 have been classically associated with osteosarcoma [8–10]. Hence, triptolide can be investigated as a potential novel agent in the treatment of osteosarcoma. However, the use of triptolide in in vivo models has been limited owing to its sparse solubility in aqueous medium. To address this issue, we have recently developed a water soluble prodrug of triptolide and demonstrated its effectiveness in various preclinical models of pancreatic cancer [11].
Osteosarcoma is characterized by elevated expression of pro-survival genes. Elevated expression of HSP70 in osteosarcoma has been reported to be anti-apoptotic [12]. Further, proteomic analysis of osteosarcoma and primary osteoblastic cells has identified overexpression of other members of the heat shock proteins such as HSF1 and HSP27 [10]. Wnt signaling is another pro-survival pathway in osteosarcoma; aberrant activation of canonical Wnt signaling is associated with human and canine osteosarcoma progression [13, 14]. Aberrant Wnt signaling in osteosarcoma also leads to dysregulation cMyc and NF-κB, transcription factors known to be associated with cancer progression [15]. Thus, drugs targeting pro-survival genes and Wnt signaling pathway genes including cMYC and NF-κB are predicted to have therapeutic potential in osteosarcoma. Here, we show that triptolide/Minnelide treatment in osteosarcoma cell lines, orthotopic and lung colonization models of mouse osteosarcoma effectively induces cell death, reduces tumor burden and prevents metastasis. We also demonstrate that triptolide/Minnelide affects a number of pro-survival genes and pathways in osteosarcoma.
2. MATERIALS AND METHODS
2.1 Cell lines and cell culture
Osteosarcoma cell lines SaOS2, U2OS and HOS were obtained from ATCC and subcultured according to ATCC protocols. Osteosarcoma cell line K7M2 was a kind gift from Dr. Chand Khanna and was subcultured in DMEM low glucose supplemented with 10% FBS and antibiotics [16]. MG63.2 was a kind gift from Dr. Hue Luu’s laboratory and was subcultured in DMEM high glucose supplemented with 10% FBS with antibiotics [17]. Human osteoblast cells (hFOB19.9) were cultured in 1:1 mixture of Ham’s F12 Medium and Dulbecco’s Modified Eagle’s Medium, with 2.5 mM glutamine supplemented with 10% FBS and antibiotics. SaOS2, U2OS and HOS cells were authenticated by their miRNA expression patterns compared to OS tumor tissues. hFOB19.9 cells were authenticated by their differentiation potential to mature osteoblasts. Genotypes of the osteosarcoma cell lines used in this study are given in supplemental Table 1.
2.2 Triptolide treatment of osteosarcoma and osteoblast cells
Triptolide (Calbiochem, EMD Chemicals, Inc., Gibbstown,NJ) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a concentration of 1mg/mL. For cell viability and caspase assays, cells were seeded in serum-containing media in 96-well plates at densities of 1×103 cells per well for SaOS2 and 2×103 cells per well for MG63.2 and human osteoblast cells. For extraction of protein and RNA, cells were seeded in 6-well plates in serum-containing media at densities of 2.5×105 cells per well for SaOS2 and 5×105 cells per well for MG63.2 and human osteoblast. For Annexin-V staining, cells were plated similarly, at densities of 1.25×105 (SaOS2) and 2.5×105 (MG63.2 and human osteoblast) cells per well. Following incubation of 48h, cells were treated with varying concentrations of triptolide (0, 25nM, 50nM, 100nM and 200nM) in serum-free media and treated for 24, 48 or 72h at 37°C. Controls were treated with serum-free media.
2.3 Cell viability assay
Cell viability was determined by Dojindo Cell Counting Kit-8. Following treatment with triptolide at various concentrations (0, 25nM, 50nM, 100nM and 200nM) for 24, 48 and 72h, 10μL of the tetrazolium substrate was added to each well of the plate. Plates were incubated at 37°C for 1 h, after which the absorbance at 450nm was measured. All experiments were done in triplicate and repeated four independent times.
2.4 Caspase assay
Caspase-3/7 and caspase-9 activities were analyzed using the Caspase-Glo luminescent-based assays according to the manufacturer’s instructions. Following treatment, 100μL of the appropriate Caspase-Glo reagent was added to each well containing 100μL of blank, negative control, or treated cells in culture medium. After incubation at room temperature for 1h, the luminescence was then read in a luminometer (Biotek). The corresponding 96-well clear plate was used to measure the number of viable cells with the CCK-8 reagent. Caspase activity was normalized to the cell viability measurements.
2.5 Annexin assay
Cells were seeded in a 6-well plate and treated with triptolide and phosphatidylserine externalization was analyzed using the Guava Nexin Kit by flow cytometry, according to manufacturer’s instruction.
2.6 Orthotopic intra-tibial mouse model of osteosarcoma
All animal procedures were carried out according to the guidelines of the University of Minnesota Institutional Animal Care and Use Committee (IACUC). Four to six week old athymic female nude mice (NCr-nu/nu, NCI# 01B74) were purchased from NCI and anaesthetized by intra-peritoneal injection of 200μl of xylazine/ketamine mixture. K7M2 osteosarcoma cells were implanted through intra-tibial injection. A drill was made in the tibia just above the calcaneum with 29 gauge sterile needled 0.3 ml syringe (B&D) and K7M2 cells (7.5×104 in 10 μl PBS) were injected into the tibia. Twenty four animals were intra-tibially implanted with osteosarcoma cells. Animals were weighed and monitored for tumor onset by inspection of the injection site by palpation. When the animals developed tumors, they were measured by a caliper and tumor volume was calculated (V= L×W2×0.52). In order to comply with the institutional regulations, experiment was ended when tumor size in any of the experimental group reached the permissible threshold tumor burden of 1.0 cm3.
Animals (n=12) were administered with Minnelide (0.42mg/kg body weight) through intra-peritoneal injection from 7th day of post tumor cells implantation, until the last day of completion of the experiment. Control animals (n=12) received equal volume of carrier (saline). Tumor progression in these orthoptopic models was calculated by measuring tumor volumes.
2.7 Colonization models for osteosarcoma lung metastasis
Four to six weeks old female nude mice (Charles River Laboratories) were used for the lung colonization model of osteosarcoma metastasis as described by Khanna et al [16]. Briefly, 1×106 MG 63.2 cells/100μl PBS were injected into the lateral tail vein of 17 mice. After 7 days, mice were randomized, eight mice were treated everyday with Minnelide (0.42mg/kg body weight) while remaining 9 mice were injected with saline (vehicle). The treated and untreated mice were sacrificed 6 weeks after introduction of MG63.2 cells. The extent of metastasis was estimated by counting the number of visible nodules on the lungs of both treated and untreated mice.
2.8 Histopathology and immunohistochemistry analysis
On completion of the experiment, animals were sacrificed and tumor tissues were resected. Tumor and lungs were dissected from control and Minnelide treated animals and subjected to histopathological examinations. Briefly, tissue specimens were fixed in 10% formalin solution followed by 80% ethyl alcohol. Tumor tissues were decalcified using Decal (Cal-Ex, Fisher Scientific, Pittsburg, Pennsylvania) for a day and washed with water for 3h before fixing and paraffin embedding.
Paraffin embedded tissue specimens were deparaffinized and rehydrated in alcohol solution. Antigen was retrieved by steam cooking with citrate buffer pH 6.0 (Biocare #RV1000M) followed by permeabilization and blocking with Serum Free block (Dako, Carpinteria, CA). Slides were incubated with HSP70 antibody (Abcam #ab31010, 1:100) or Ki67 antibody (Thermofisher # PA1-21520) in Background Sniper (Biocare # BS966L) and incubated for overnight at 4°C. Slides were incubated with secondary antibody (Rabbit on rodent HRP-polymer, Biocare #RMR622H) and developed by DAB reagent (Vector labs) and counter stained with heamtoxylin for 30 sec.
Expression of Ki67 and HSP70 were calculated using Aperio positive pixel count algorithm by measuring the hue intensity at six representative squares. Ki67 index was calculated by Positivity = NPositive/NTotal. HSP70 expression was calculated by intensity average algorithm [Iavg = (Iwp+Ip+Isp)/(Nwp+Np+Nsp)]. The number and intensity-sum in each intensity range was calculated along with average intensity, ratio of strong/total number, and average intensity of weak + positive pixels.
TUNEL staining was performed on formaldehyde fixed tissue sections using in situ cell death determination kit (Roche) according to manufacturer’s protocol.
2.9 qRT-PCR
The expression levels of different genes were examined in osteosarcoma cells after they were treated with triptolide using qRT-PCR. Briefly, RNA was extracted using the Qiagen RNeasy Mini Kit according to the manufacturer’s instructions. Total RNA (1μg) was reverse transcribed. qRT-PCR was done using the Quantitect SYBR green PCR kit (Qiagen) according to the manufacturer’s instructions using an Applied Biosystems 7300 real-time PCR system. Primers for the different genes are tabulated, in supplementary Table 2.
2.10 Western blotting
Cell lysates were prepared by re-suspending cells in RIPA buffer (Boston Bioproducts) supplemented with protease inhibitor cocktail (Roche) for 30 min at 4°C and cleared by centrifugation for 30 min at 13,000×g. Supernatants were collected and total protein concentration was determined using the Bicinchoninic Acid Assay (Thermo Scientific). Equal protein loading was confirmed by staining with Ponceau S [0.1% Ponceau S (w/v) in 5% acetic acid (v/v)]. Actin expression was used as an internal control. Antibody against HSP70 and HSP27 were obtained from Assay Designs Inc (Farmingdale, NY), Antibodies against cMYC, Cyclin D1, Survivin, HSF1 were obtained from Cell signaling Technology (Danvers, MA).
2.11 NF-κB activity assay
NF-κb activity assay was performed using the Transcription Factor Assay kit (ThermoScientific) according to manufacturer’s instruction. Briefly, nuclei were isolated from treated and untreated OS cells and osteoblast cells using NE-PER nuclear and cytosolic protein extraction kit (Thermo Scientific). For tumor samples, protein was extracted from saline and Minnelide-treated tumors (n=3) using RIPA buffer. Nuclear extracts from treated and untreated cells and protein extracts from tumors were used in this ELISA based assay. Luminiscence values obtained were normalized to amount of protein used in the assay. Resulted was expressed either as fold change of control or as fold change over osteoblast cells. The assay was repeated three times.
2.12 Statistical Analysis
Values are expressed as the mean ± SEM. All in vitro experiments were performed at least three times. The significance of the difference between any two samples was analyzed by unpaired Student’s t-test; values of p <0.05 were considered statistically significant.
3. RESULTS
3.1 Triptolide induces apoptotic cell death in osteosarcoma cells but does not affect human osteoblasts
The molecular heterogeneity in osteosarcoma is attributed to our limited understanding of its pathobiology. In order to study the effect of triptolide on a broad spectrum of osteosarcoma cell lines (SaOS2, HOS, U2OS and MG63.2 and K7M2) differing in their genetic background, we treated these cell lines in vitro with varying triptolide concentrations at different time points. Treatments with triptolide significantly reduced the cell viability of all five osteosarcoma cell lines tested in a concentration – and time-dependent manner (Figure 1A). SaOS2 and U2OS were found to be most responsive to triptolide treatment as early as 24h at 50nM drug concentration showing viability of only 40% cells compared to control (Figure 1A). However, HOS, K7M2 and MG63.2 responded to triptolide showing a viability of 40% of control after 48h of treatment with 50nM triptolide (Figure 1A). All five osteosarcoma cell lines tested, showed significant cell death (~20% viability after 72h treatment). Notably, the viability of human osteoblast cells hFOB1.19 was less affected (70% of untreated in hFOB vs.20–30% of untreated in the osteosarcoma) even after 72h of drug treatment at 200nM (Figure 1A).
Figure 1. Viability of OS cells in response to triptolide treatment.
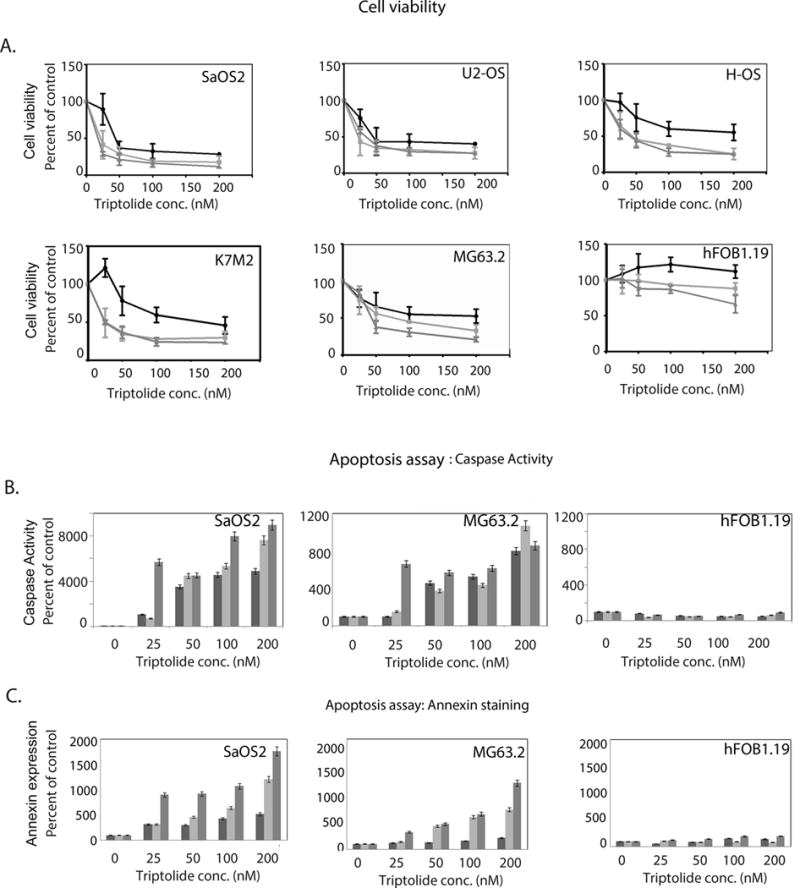
A) Osteosarcoma cells SaOS2 U2OS, HOS, K7M2, and MG63.2 and human osteoblast cells (fOB1.19) were treated with triptolide for 24h, 48h and 72h. Cell viability was determined by CCK assay and plotted after normalizing to untreated controls. Treatment with triptolide induced apoptosis in both all osteosarcoma cell lines tested but not in the osteoblast cells.
B) Caspase-3 was induced in SaOS2 and MG63.2 cells in response to triptolide whereas human osteoblasts did not show any caspase activity.
C) Annexin V positive cells confirmed apoptosis specifically in SaOS2 and MG63.2 in response to triptolide.
To evaluate whether triptolide induced apoptosis in osteosarcoma cells, we assayed the activity of caspase-3/7 in these cells. SaOS2, MG63.2 and human osteoblast cells were treated with triptolide at varying concentrations for 24h, 48h and 72h. Triptolide treatment induced significant activation of caspase 3 in osteosarcoma cells in a concentration – and time-dependent manner, as compared with untreated cells (Figure 1B). Activation of caspase-9, an earlier player in the apoptotic pathway, was also activated at 24h with 100nM triptolide and showed increased activity till 48h (Supplementary Figure 1). These results indicate that decreased osteosarcoma cell viability after treatment with triptolide was due to activation of caspase-dependent apoptotic pathway. No significant caspase-3 activity was observed in human osteoblast cells.
Phosphatidylserine externalization is another parameter of apoptosis, which is measured by Annexin V staining. Triptolide significantly increased Annexin V positive cells in SaOS2 and MG63.2 cells in a concentration-dependent manner in but not in the human osteoblast cells (Figure 1C). These results show that osteosarcoma cell death induced by triptolide is facilitated by the induction of apoptosis and may be tumor cell specific.
3.2 Minnelide reduces tumor burden in orthotopic mouse model of osteosarcoma
Since triptolide significantly induced osteosarcoma cell death in vitro, we investigated its in vivo therapeutic efficacy using preclinical orthotopic models of osteosarcoma. Due to the limited solubility of triptolide in aqueous medium, we used Minnelide, a water-soluble prodrug of triptolide, in these studies [11]. Three weeks old female nude mice were intra-tibialy injected with osteosarcoma cells (K7M2). Based on the orthotopic tumor development in these mice, we determined that the tumor take was >95%. Twenty mice injected with OS cells were randomized and grouped as control and treatment sets. All the mice in the control group developed larger tumors, which grew from bone to surrounding muscle tissue (Supplementary Figure 2A). In order to comply with institutional guidelines on tumor burden, experiments were terminated on day 33 post tumor cell implantation when the tumor in control treated animals reached the permissible threshold of 1cm3. Notably, Minnelide treated animals showed late tumor onset and significantly slower tumor progression relative to saline treated (p <0.001) (Figure 2A and B) resulting in significant difference in tumor volume with an average of 4.2 times reduced tumor volume (p <0.001) at the completion of the experiment.
Figure 2. Minnelide, causes tumor regression in an orthotopic mouse model of osteosarcoma.
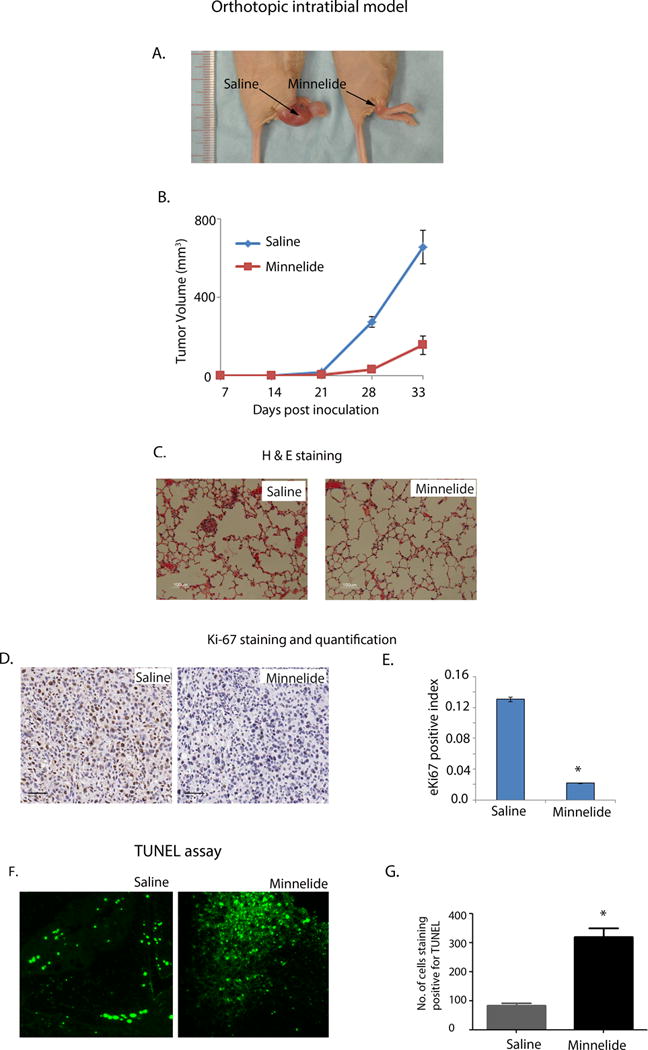
A) Representative mice treated with saline and Minnelide on 33rd day of post tumor cells implantation. Minnelide significantly decreased tumor size.
B) Tumor volume in saline and Minnelide treated OS mice on 33rd day of post tumor cells implantation. Minnelide treated animals showed late tumor onset and significantly reduced tumor progression resulting significant difference in tumor size (cumulative average of 4.2 times, p <0.001).
C) H&E staining of lung tissue from saline and Minnelide treated OS mice (10× mag). Minnelide decreased the formation of pulmonary micrometastasis relative to saline treatment.
D) Ki67 expression in the tumor tissues of saline and Minnelide treated OS mice (20× mag, scale 50μM).
E) Quantitation of Ki-67 staining showed decreased proliferation in Minnelide treated tumor as seen in Ki67 positive index of saline and Minnelide treated mice (44-fold, p<0.001).
F) TUNEL staining showing increased apoptotic cells in Minnelide treated tumors compared to saline treatment.
G) Quantitation of TUNEL staining in saline and Minnelide treated tumors (p<0.001).
Strikingly, 95% of saline treated animals had developed pulmonary micro-metastasis, whereas the Minnelide treated animals showed no pulmonary metastasis (Figure 2C). Histological examination of tumors from the control group showed higher nuclear cytoplasmic index with multiple nucleoli (Supplementary Figure 2B). Further, positive pixel counting for Ki67 staining in saline and Minnelide treated mice revealed an average of 44-fold (p <0.001) difference (Figure 2D and E). Results from these animal experiments are summarized in Table 1.
Table 1.
Effect of Minnelide on orthotopic mouse model of osteosarcoma.
| Saline | Minnelide | |
|---|---|---|
| Number of animals included (n) | 10 | 10 |
| Tumor latency* (days ± SD) | 17.5 ± 4 | 22 ± 4 |
| Tumor volume at the completion (average mm3 ± SE) | 657.77 ± 85.55 | 154.40 ± 48.27 |
| Percent pulmonary metastasis | 95 % | 0 % |
| Ki67 positivity index (pixels) (average ± SE) | 0.1307 ± 0.0029 | 0.0217 ± 0.0004 |
| HSP70 intensity average (pixels) (average ± SE) | 176.8676 ± 28.7018 | 87.0316 ± 17.2188 |
days between tumor implantation to tumor detection
To determine whether the decrease in tumor volume in Minnelide treated tumors was due to cell death, we performed TUNEL assay on representative tumor tissue sections obtained from treated and untreated mice. Minnelide treated tumors showed a mean of 320 TUNEL positive events compared to 83 positive events in tumors in the saline group. Since TUNEL assay specifically determines apoptotic cell death, we concluded that decrease in tumor volume in the Minnelide treated mice was predominantly due to apoptotic cell death (Figure 2F and G).
3.3 Colonization models for osteosarcoma lung metastasis
Next we investigated the effect of Minnelide in a lung colonization model of osteosarcoma metastasis. 1×106 MG63.2 OS cells were injected through lateral tail vein of mice (n=17). Animals were randomized on day 7 and treatment was started (saline n=9; Minnelide n=8, 0.42mg/kg body weight, ip, QD). Both groups were closely monitored for any behavioral changes resulting from respiratory distress. Six weeks after starting Minnelide treatment, both the control and treated mice were sacrificed and their organs were examined for metastasis. We observed number of visible metastatic nodules in lungs of mice from the control group (Figure 3A). Visible metastatic nodules in lungs were counted in control as well as treated animals. The number of visible metastatic nodules in the treated animals was found to be significantly less than those in the control group (Figure 3B). It is noteworthy that 3 of 8 mice in the Minnelide treated group did not have any visible metastatic nodules in their lungs, the presence of 2 to 3 nodules in the others indicated that metastatic nodules developed in all the animals in this model and treatment resulted in reducing the progression of nodules in the lungs.
Figure 3. Minnelide reduces the number of metastatic nodules in the lung colonization model for osteosarcoma.
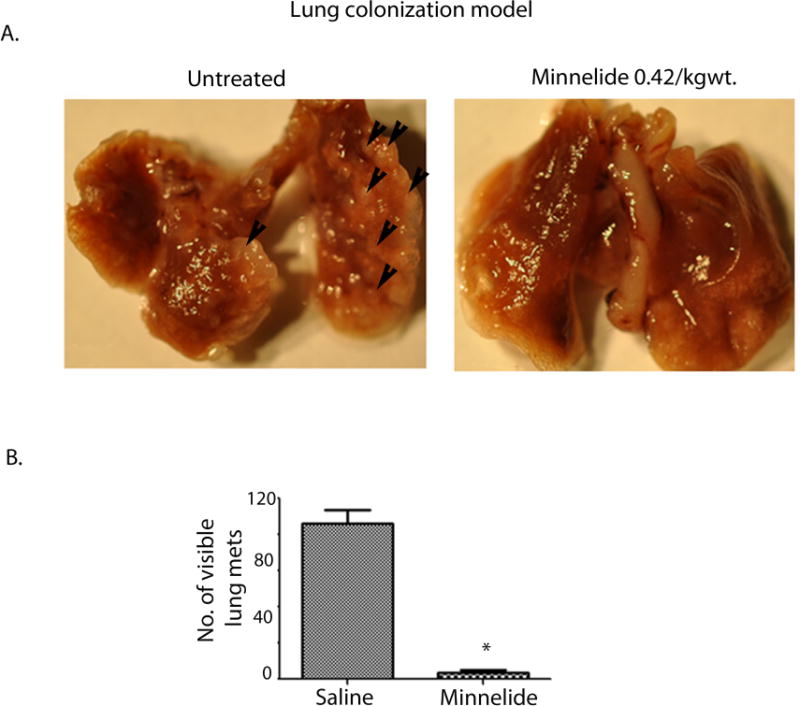
A) Representative mouse lungs show metastasizing nodules injected in saline, which decrease with Minnelide treatment.
B) Visible nodules were counted and plotted in treated and untreated animals.
3.4 Minnelide treatment significantly reduces heat shock proteins
Genes associated with pro-survival signaling pathways are aberrantly expressed in OS cells. Of these, the heat shock genes such as HSF1, HSP27 and HSP70 have been reported to be overexpressed in osteosarcoma. We studied the effect of triptolide on the expression of these genes in SaOS2, MG63.2 and human osteoblast cells by qRT-PCR and Western blotting. Expression levels of HSP70, HSP27 and their major transcription factor HSF1 were overexpressed in osteosarcoma cells compared to the osteoblasts (Supplementary figure 3). Triptolide (100nM) significantly decreased transcript levels for HSP70, HSP27, and HSF1 as early as 24h after treatment as compared to control cells (Figure 4A). Human osteoblast cells, however, did not show a significant difference in transcript expression levels of these heat shock genes. In agreement with the transcript expression, HSP70, HSP27 and HSF1 protein levels significantly decreased in both triptolide-treated SaOS2 and MG63.2 cells (Figure 4B). We further evaluated tumor tissues from treated and untreated mice of the orthotopic osteosarcoma models for their HSP70 expression level. The analysis showed that HSP70 staining to be about 2-fold different (p <0.001) in Minnelide treated group compared with the untreated group (Figure 4C and D). Taken together, these results suggest that triptolide/Minnelide treatment significantly inhibits the expression of HSP70 that may contribute to apoptosis of osteosarcoma cells.
Figure 4. Triptolide decreases HSP70 expression in osteosarcoma cells.
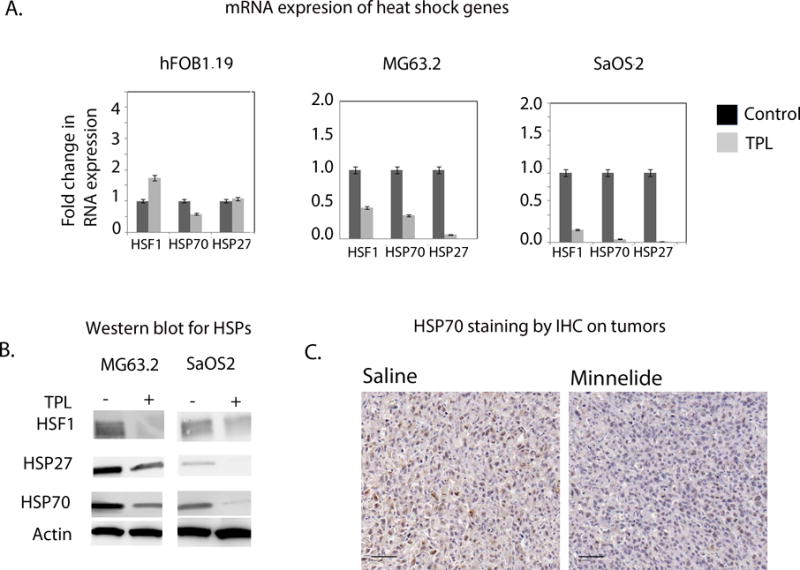
A) Heat shock family of genes showed lower mRNA expression in triptolide treated SaOS2 and MG63.2 compared to human osteoblast (fOB1.19) cells.
B) Decreased protein expression for HSP70, HSP27 and HSF1 was observed in triptolide treated SaOS2 and MG63.2 cells.
C) HSP70 expression in saline and Minnelide treated mice (20×mag, scale 50μM). The in vivo intratibial model for osteosarcoma also showed decreased HSP70 expression in Minnelide treated mice.
D) Minnelide treated mice show significantly reduced (2 fold, p <0.001) HSP70 expression compared to the saline treated.
3.5 Minnelide treatment downregulate pro-survival genes
Wnt signaling plays a major role in survival of tumor cells [15]. β-catenin and genes activated by Wnt (cMYC, cyclinD1 and survivin) are overexpressed in osteosarcoma compared to osteoblasts (Supplementary figure 4). Similarly, RUNX2, a transcription factor controlling bone development, was also upregulated in osteosarcoma (Supplementary figure 4). To evaluate the effect of triptolide on the expression of these pro-proliferative genes, we examined their transcript and protein expression levels in triptolide treated osteosarcoma cells. We observed that triptolide treated SaOS2 and MG63.2 cells showed significantly reduced β-catenin transcript as wells as protein levels (Figure 5A and B). β-catenin regulates cMYC, which in turn, can control the transcription of cyclin D1. Notably, we observed a significant reduction in the expression of both cMYC and Cyclin D1 at the transcriptional and translational level in both of the triptolide treated OS cell lines. Survivin, another pro-survival protein regulated by β-catenin mediated transcription, was found to be downregulated with triptolide treatment as well (Figure 5A and B). Runx2 was also downregulated by triptolide (Figure 4C). Levels of all these genes tested remained unchanged in the treated human osteoblast cells.
Figure 5. Triptolide reduces expression of survival genes and results in decreased NF-κB activity in osteosarcoma.
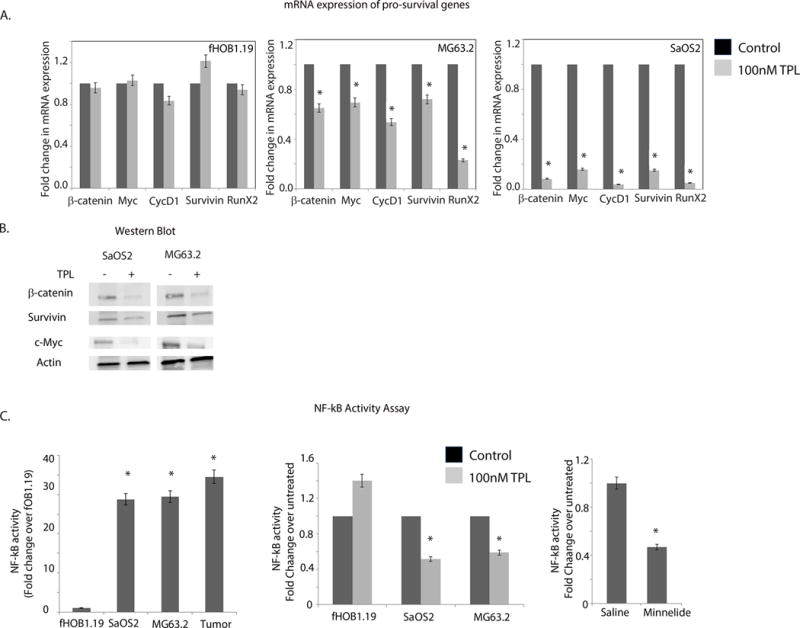
A) Decreased mRNA expression of regulatory genes in OS like of β-catenin, cMYC, Cyclin D1, survivin and RunX2 was observed in SaOS2 and MG63.2 after treatment with 100nM triptolide but no change in expression was noticed in normal osteoblast cells.
B) Reduced protein expression of b-catenin, cMYC, Cyclin D1 and survivin was observed in triptolide treated SaOS2 and MG63.2 but not in normal osteoblast cells.
C) DNA binding activity by NF-κB in response to triptolide treatment was studied by ELISA based assay in SaOS2, MG63.2 and fOB1.19 cell lines as well as the intratibial orthotopic model for OS. In SaOS2, MG63.2 and the tumors, NF-κB activity was 25-to 30-fold higher compared to the osteoblast.
D) NF-κB DNA binding was reduced following 24h treatment with 100nM triptolide in both SaOS2 and MG63.2 cells but no change in DNA binding was seen in osteoblast fOB1.19 cells. (E) NF-κB activity was significantly decreased in the orthotopic tumors treated with Minnelide.
3.6 Minnelide regulates NF-κB activity
Another pro-survival pathway activated in a number of cancers including osteosarcoma is NF-κB mediated signaling [18]. Triptolide has previously been shown to reduce NF-κB activity in bronchial epithelial cells without having any significant effect on DNA binding [19]. To assess the influence of triptolide on NF-κB signaling in osteosarcoma and osteoblast cells, the DNA binding ability of NF-κB was assessed in these cells by ELISA. The nuclear fraction of the osteosarcoma cell lines and tumor tissues obtained from treated and control groups of orthotopic mouse models were used for the assay. NF-κB activity (DNA binding ability of p50 subunit) found to be 25–30 fold higher in the OS cell lines and tumors compared to the osteoblasts (Figure 5C). We next assayed for NF-κB oligo binding after triptolide treatment. For this, nuclear extracts of osteosarcoma cell lines and osteoblasts were treated with triptolide (100nM) for 24h were used. We noticed a significant decrease in NF-κB in both SaOS2 and MG63.2 after 24h treatment, but NF-κB remained unchanged in osteoblast cells (Figure 5D). To see if NF-κB binding was downregulated in the Minnelide-treated tumors, protein from tumor lysate was used for the binding assay. NF-κB DNA binding activity was lower in the Minnelide treated group compared to the saline-treated group (Figure 5E). Our results show that NF-κB is activated in osteosarcoma and that the DNA binding activity of NF-κB is reduced on treatment with Minnelide.
4. DISCUSSION
Osteosarcoma is an aggressive childhood cancer; the 5-year overall survival is less than 70% and has remained static for the past two decades. This is may be attributed to the fact that current therapies ultimately fail to prevent relapse and/or metastasis for most patients. A number of targeted therapies including doxorubicin and cisplatin (DNA intercalating drugs) are currently being used in the treatment of osteosarcoma [20]. The drugs most often used to treat osteosarcoma in young adults are methotrexate, an anti-metabolite in combination with leucovorin; etoposide, a topoisomerase inhibitor and alkylating agents like Ifosfamide and cyclophosphamide that are anti-proliferative for cancer cells. However, these chemotherapeutic agents also have increased side effects that include decreased production of blood cells resulting in infection. Some of the alkylating agents cause damage to the bladder and kidney. Platinum containing drugs, though effective in controlling tumor growth, often result in nerve damage. These side effects can also be long lasting, often affecting the quality of life in the young patients. Thus, there is a critical need to identify and develop novel therapeutic agents that have no or limited side effects. Further, less than 40% of osteosarcoma patients, mostly children and adolescents with metastasis, survive beyond ten years. Thus it is imperative that novel drugs being tested for osteosarcoma are able to treat and prevent metastasis. Osteosarcoma is characterized by a complex karyotype, aneuploidy, and dysregulation of numerous pathways and genes. Thus the novel therapeutic agent against osteosarcoma should be effective in targeting multiple dysregulated pathways, specifically in cancer cells. Triptolide, a diterpenoid triepoxide, has been found to be an effective drug for a number of cancer types [21–23]. Recently, we have developed a water soluble prodrug of triptolide, Minnelide, which has shown tremendous promise in preclinical studies for pancreatic cancer with minimal toxicity effect on normal cells at therapeutic doses [24]. One of the major effects of triptolide treatment is the significant reduction in the expression of heat shock protein 70 (HSP70) levels [21,25]. Further, this compound has been shown to act on multiple cancer signaling pathways [22,24,26].
In the current study, we evaluated the efficacy of Minnelide using an orthotopic mouse model of osteosarcoma. This in vivo model showed significant decrease in tumor volume following treatment. Along with the primary tumor, a decrease in the lung metastasis was also observed following Minnelide treatment (Figure 2). To further confirm the effect of Minnelide on metastasis, an experimental lung colonization model using MG63.2 osteosarcoma cell line was carried out. In line with our orthotopic model experiments, Minnelide significantly decreased the number of metastatic lung nodules in the colonization model compared to the control group (Figure 3A, B). Notably, in vitro experiments also demonstrated that triptolide had little effect on the viability of osteoblast cells. Since Minnelide has all three desirable qualities of novel agents needed for osteosarcoma treatment such as minimal toxicity to normal cells, ability to regress tumors and ability to target multiple pro-survival pathways, it can be developed as an effective novel agent for treatment of osteosarcoma.
Heat shock proteins such as HSP70 and HSP27 are overexpressed in osteosarcoma [8,9]. In agreement with this, we also observed that HSP70 and HSP27 were overexpressed in OS cells lines (MG63.2 and SaOS2) compared to the human osteoblasts (fOB1.19). Moreover, HSP70 overexpression is associated with poor prognosis [27,28] and HSP70 de novo expression correlates with a good response to neoadjuvant chemotherapy [29]. The expression levels of HSP70 and HSP27 proteins were dramatically reduced by triptolide treatment. HSF1, a transcription factor that regulates the expression of these heat shock proteins was also reduced by triptolide specifically in tumor cells (Figure 4A). These observations suggest that triptolide inhibits heat shock protein mediated survival mechanism in osteosarcoma.
Our results show that triptolide treatment in OS reduces transcript levels of genes involved in proliferation, cell cycle and survival. This is in agreement with previous reports that triptolide can limit the expression of cyclins, cMYC and Wnt pathway genes [30] [26]. We also observed that β-catenin expression level was downregulated in triptolide treated OS cells. β-catenin mediates the expression Wnt signaling genes [31] and subsequent activation of targets genes relevant to tumor initiation and progression. Transcriptional targets of Wnt signaling includes genes such as cyclins, cMYC and survivin [13]. Osteogenic factor like RUNX2 was also downregulated by triptolide is this study. Interestingly, no such effect was observed in the osteoblasts suggesting that this compound had minimal effect on these regulating pathways in non-tumor tissues (Figure 5 A and B).
Constitutive activation of NF-κB has been linked to cancer. NF-κB has been shown to regulate an array of genes implicated in angiogenesis, invasion and metastasis [32], [33,34]. β-catenin can physically interact with NF-κB and inhibit its activity. Therefore, suppressed NFκB activity and NFκB target gene expression can be found in cells expressing high levels of β-catenin [35]. Further, NF-κB is reported to control tumorigenesis is osteosarcoma [18]. In this study, we observed that the DNA binding property of NF-κb in osteosarcoma cells was significantly decreased at 24h in response to triptolide treatment in the OS cells, whereas this activity remained unaltered in human osteoblast cells. A similar effect was seen in the tumors derived from orthotopic models (Figure 4C). This view is also strengthened by the observation that NF-κB regulates the transcription of a number of anti-apoptotic genes (survivin) and proliferation genes (cMYC and cyclin D1). Our results also showed a decreased level of cMYC, cyclin D1 and surviving, all regulated by NF-κB. A previous study has shown that NF-κB levels were affected by triptolide in multiple myeloma cells [36]. Further, inhibitors of NF-κB pathway used on different OS cell lines have also shown significant cytotoxicity [37–39] in other studies. In osteosarcoma cells, NF-κB regulated pathways have often been reported as a potential drug target [38,40–42]. Recent reports show that osteosarcoma metastasis is dependent on NF-κB activity [43]. Our data show that Minnelide inhibits NF-κB activity and prevents metastasis. It is possible that the inhibition of metastasis by Minnelide being orchestrated through this pathway. Triptolide acts in a multipronged manner, i) inhibiting the DNA binding activity of NF-κb resulting in decreased transcription of cell proliferation genes, ii) acting on the ‘survival’ machinery of the cells inhibiting the activity of the HSF1 transcription factor and thus lowering the expression of the heat shock protein family members, and iii) inhibiting the Wnt signaling pathway by deregulating the β-catenin mediated transcription machinery.
In conclusion, this study reveals the potential of Minnelide in efficiently reducing tumor burden and reducing metastasis of osteosarcoma with minimal effect on osteoblasts, thus opening a possibility of developing this compound into a very efficient chemotherapeutic agent against osteosarcoma. These observations also paves the way for further evaluation of this compound in treatment for spontaneous canine osteosarcoma as preclinical models either as a stand-alone compound or in combination with the current standard of care therapy for this disease. These studies in preclinical models will establish the potential for Minnelide as a novel drug in treating human osteosarcoma.
Supplementary Material
Acknowledgments
This study was supported by grants R01CA124723 and R01CA170496 from the NIH (AKS), by a Faculty Research and Development grant from the University of Minnesota Academic Health Center, by a translational research grant from the Masonic Cancer Center, University of Minnesota, by grants from the Wyckoff Rein in Sarcoma (SS).
ROLE OF FUNDING SOURCES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
ABBREVIATION
- OS
Osteosarcoma
- QD
qua’que di’e (everyday)
- ELISA
Enzyme Linked Immunosorbent Assay
- RTPCR
Real Time Polymerase Chain Reaction
Footnotes
CONFLICT OF INTEREST
University of Minnesota has filed a patent for Minnelide, which has been licensed to Minneamrita Therapeutics, LLC. AKS have financial interests in this company. AKS is also one of the inventors on this patent. The other authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
SB, VT, TM, VS, AS and SS conceived and designed the experiments; AS and SS Contributed reagents/materials/analysis tools; SB, VT, VS, performed the experiments and analysis; SB, VT, AS and SS wrote the paper.
References
- 1.Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26:1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- 2.Marulanda GA, Henderson ER, Johnson DA, Letson GD, Cheong D. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15:13–20. doi: 10.1177/107327480801500103. [DOI] [PubMed] [Google Scholar]
- 3.Basu-Roy U, Basilico C, Mansukhani A. Perspectives on cancer stem cells in osteosarcoma. Cancer Lett. 2012 doi: 10.1016/j.canlet.2012.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis VO. What’s new in musculoskeletal oncology. J Bone Joint Surg Am. 2007;89:1399–1407. doi: 10.2106/JBJS.G.00075. [DOI] [PubMed] [Google Scholar]
- 5.Westerheide SD, Kawahara TL, Orton K, Morimoto RI. Triptolide, an inhibitor of the human heat shock response that enhances stress-induced cell death. J Biol Chem. 2006;281:9616–9622. doi: 10.1074/jbc.M512044200. [DOI] [PubMed] [Google Scholar]
- 6.Dudeja V, Chugh RK, Sangwan V, Skube SJ, Mujumdar NR, Antonoff MB, Dawra RK, Vickers SM, Saluja AK. Pro-survival role of heat shock factor 1 (HSF1) in the pathogenesis of PancreatoBiliary tumors. Am J Physiol Gastrointest Liver Physiol. 2011 doi: 10.1152/ajpgi.00346.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tengchaisri T, Chawengkirttikul R, Rachaphaew N, Reutrakul V, Sangsuwan R, Sirisinha S. Antitumor activity of triptolide against cholangiocarcinoma growth in vitro and in hamsters. Cancer Lett. 1998;133:169–175. doi: 10.1016/s0304-3835(98)00222-5. [DOI] [PubMed] [Google Scholar]
- 8.Haskin CL, Athanasiou KA, Klebe R, Cameron IL. A heat-shock-like response with cytoskeletal disruption occurs following hydrostatic pressure in MG-63 osteosarcoma cells. Biochem Cell Biol. 1993;71:361–371. doi: 10.1139/o93-054. [DOI] [PubMed] [Google Scholar]
- 9.Song LN. Effects of heat shock on glucocorticoid receptor. Sci China B. 1994;37:557–562. [PubMed] [Google Scholar]
- 10.Liu X, Zeng B, Ma J, Wan C. Comparative proteomic analysis of osteosarcoma cell and human primary cultured osteoblastic cell. Cancer Invest. 2009;27:345–352. doi: 10.1080/07357900802438577. [DOI] [PubMed] [Google Scholar]
- 11.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: Anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- 13.Barker N, Clevers H. Mining the wnt pathway for cancer therapeutics. Nat Rev Drug Discov. 2006;5:997–1014. doi: 10.1038/nrd2154. [DOI] [PubMed] [Google Scholar]
- 14.Selvarajah GT, Kirpensteijn J, van Wolferen ME, Rao NA, Fieten H, Mol JA. Gene expression profiling of canine osteosarcoma reveals genes associated with short and long survival times. Mol Cancer. 2009;8:72. doi: 10.1186/1476-4598-8-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modder UI, Oursler MJ, Khosla S, Monroe DG. Wnt10b activates the wnt, notch, and NFkappaB pathways in u2os osteosarcoma cells. J Cell Biochem. 2011;112:1392–1402. doi: 10.1002/jcb.23048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna C, Khan J, Nguyen P, Prehn J, Caylor J, Yeung C, Trepel J, Meltzer P, Helman L. Metastasis-associated differences in gene expression in a murine model of osteosarcoma. Cancer Res. 2001;61:3750–3759. [PubMed] [Google Scholar]
- 17.Su Y, Luo X, He BC, et al. Establishment and characterization of a new highly metastatic human osteosarcoma cell line. Clin Exp Metastasis. 2009;26:599–610. doi: 10.1007/s10585-009-9259-6. [DOI] [PubMed] [Google Scholar]
- 18.Tang QL, Xie XB, Wang J, et al. Glycogen synthase kinase-3beta, NF-kappaB signaling, and tumorigenesis of human osteosarcoma. J Natl Cancer Inst. 2012;104:749–763. doi: 10.1093/jnci/djs210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 20.Chou AJ, Geller DS, Gorlick R. Therapy for osteosarcoma: Where do we go from here? Paediatr Drugs. 2008;10:315–327. doi: 10.2165/00148581-200810050-00005. [DOI] [PubMed] [Google Scholar]
- 21.Aghdassi A, Phillips P, Dudeja V, Dhaulakhandi D, Sharif R, Dawra R, Lerch MM, Saluja A. Heat shock protein 70 increases tumorigenicity and inhibits apoptosis in pancreatic adenocarcinoma. Cancer Res. 2007;67:616–625. doi: 10.1158/0008-5472.CAN-06-1567. [DOI] [PubMed] [Google Scholar]
- 22.Antonoff MB, Chugh R, Borja-Cacho D, Dudeja V, Clawson KA, Skube SJ, Sorenson BS, Saltzman DA, Vickers SM, Saluja AK. Triptolide therapy for neuroblastoma decreases cell viability in vitro and inhibits tumor growth in vivo. Surgery. 2009;146:282–290. doi: 10.1016/j.surg.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Clawson KA, Borja-Cacho D, Antonoff MB, Saluja AK, Vickers SM. Triptolide and TRAIL combination enhances apoptosis in cholangiocarcinoma. J Surg Res. 2010;163:244–249. doi: 10.1016/j.jss.2010.03.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chugh R, Sangwan V, Patil SP, et al. A preclinical evaluation of minnelide as a therapeutic agent against pancreatic cancer. Sci Transl Med. 2012;4:156ra139. doi: 10.1126/scitranslmed.3004334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antonoff MB, Chugh R, Skube SJ, Dudeja V, Borja-Cacho D, Clawson KA, Vickers SM, Saluja AK. Role of hsp-70 in triptolide-mediated cell death of neuroblastoma. J Surg Res. 2010;163:72–78. doi: 10.1016/j.jss.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu J, Shen M, Yue Z, Yang Z, Wang M, Li C, Xin C, Wang Y, Mei Q, Wang Z. Triptolide inhibits colon-rectal cancer cells proliferation by induction of G1 phase arrest through upregulation of p21. Phytomedicine. 2012;19:756–762. doi: 10.1016/j.phymed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Uozaki H, Ishida T, Kakiuchi C, Horiuchi H, Gotoh T, Iijima T, Imamura T, Machinami R. Expression of heat shock proteins in osteosarcoma and its relationship to prognosis. Pathol Res Pract. 2000;196:665–673. doi: 10.1016/S0344-0338(00)80118-1. [DOI] [PubMed] [Google Scholar]
- 28.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: Chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 29.Trieb K, Lechleitner T, Lang S, Windhager R, Kotz R, Dirnhofer S. Heat shock protein 72 expression in osteosarcomas correlates with good response to neoadjuvant chemotherapy. Hum Pathol. 1998;29:1050–1055. doi: 10.1016/s0046-8177(98)90412-9. [DOI] [PubMed] [Google Scholar]
- 30.Johnson SM, Wang X, Mark Evers B. Triptolide inhibits proliferation and migration of colon cancer cells by inhibition of cell cycle regulators and cytokine receptors. J Surg Res. 2009 doi: 10.1016/j.jss.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willert K, Jones KA. Wnt signaling: Is the party in the nucleus? Genes Dev. 2006;20:1394–1404. doi: 10.1101/gad.1424006. [DOI] [PubMed] [Google Scholar]
- 32.Hansen SK, Nerlov C, Zabel U, Verde P, Johnsen M, Baeuerle PA, Blasi F. A novel complex between the p65 subunit of NF-kappa B and c-rel binds to a DNA element involved in the phorbol ester induction of the human urokinase gene. EMBO J. 1992;11:205–213. doi: 10.1002/j.1460-2075.1992.tb05043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yokoo T, Kitamura M. Dual regulation of IL-1 beta-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-kappa B and AP-1. Am J Physiol. 1996;270:F123–30. doi: 10.1152/ajprenal.1996.270.1.F123. [DOI] [PubMed] [Google Scholar]
- 34.Yoshida S, Ono M, Shono T, Izumi H, Ishibashi T, Suzuki H, Kuwano M. Involvement of interleukin-8, vascular endothelial growth factor, and basic fibroblast growth factor in tumor necrosis factor alpha-dependent angiogenesis. Mol Cell Biol. 1997;17:4015–4023. doi: 10.1128/mcb.17.7.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deng J, Miller SA, Wang HY, Xia W, Wen Y, Zhou BP, Li Y, Lin SY, Hung MC. Beta-catenin interacts with and inhibits NF-kappa B in human colon and breast cancer. Cancer Cell. 2002;2:323–334. doi: 10.1016/s1535-6108(02)00154-x. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Shen JK, Huang J, Du HP, Ma QL, Jin J. Interleukin-6-independent expression of glucocorticoid receptor is upregulated by triptolide in multiple myeloma. Leuk Lymphoma. 2009;50:802–808. doi: 10.1080/10428190902801838. [DOI] [PubMed] [Google Scholar]
- 37.Hafeez BB, Ahmed S, Wang N, Gupta S, Zhang A, Haqqi TM. Green tea polyphenols-induced apoptosis in human osteosarcoma SAOS-2 cells involves a caspase-dependent mechanism with downregulation of nuclear factor-kappaB. Toxicol Appl Pharmacol. 2006;216:11–19. doi: 10.1016/j.taap.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 38.Kishida Y, Yoshikawa H, Myoui A. Parthenolide, a natural inhibitor of nuclear factor-kappaB, inhibits lung colonization of murine osteosarcoma cells. Clin Cancer Res. 2007;13:59–67. doi: 10.1158/1078-0432.CCR-06-1559. [DOI] [PubMed] [Google Scholar]
- 39.White DE, Burchill SA. BAY 11–7082 induces cell death through NF-kappaB-independent mechanisms in the ewing’s sarcoma family of tumours. Cancer Lett. 2008;268:212–224. doi: 10.1016/j.canlet.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura A, Akeda K, Matsubara T, Kusuzaki K, Matsumine A, Masuda K, Gemba T, Uchida A, Sudo A. Transfection of NF-kappaB decoy oligodeoxynucleotide suppresses pulmonary metastasis by murine osteosarcoma. Cancer Gene Ther. 2011;18:250–259. doi: 10.1038/cgt.2010.75. [DOI] [PubMed] [Google Scholar]
- 41.Chen L. Okadaic acid induces apoptosis through the PKR, NF-kappaB and caspase pathway in human osteoblastic osteosarcoma MG63 cells. Toxicol in Vitro. 2011;25:1796–1802. doi: 10.1016/j.tiv.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 42.Grotterod I, Maelandsmo GM, Boye K. Signal transduction mechanisms involved in S100A4-induced activation of the transcription factor NF-kappaB. BMC Cancer. 2010;10:241. doi: 10.1186/1471-2407-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomonaga M, Hashimoto N, Tokunaga F, Onishi M, Myoui A, Yoshikawa H, Iwai K. Activation of nuclear factor-kappa B by linear ubiquitin chain assembly complex contributes to lung metastasis of osteosarcoma cells. Int J Oncol. 2012;40:409–417. doi: 10.3892/ijo.2011.1209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


