Abstract
The discovery of a stable latent reservoir for HIV-1 in resting memory CD4+ T cells provides a mechanism for lifelong persistence of HIV-1. The long-lived latently infected cells persist in spite of prolonged highly active antiretroviral therapy and present a major barrier to a cure of HIV-1 infection. In this review, we discuss the current understanding of HIV-1 persistence and latent viral infection in the context of effective antiretroviral therapy and the recent progress in purging viral latent reservoir. Recent studies demonstrate that reactivation of latent HIV-1 is a promising strategy for the depletion of this viral reservoir. A thorough evaluation of the anti-latency activity of drug candidates should include the measurement of changes in intracellular viral RNA, plasma virus levels, and the size of viral latent reservoir, as well as potential adverse effects. Currently, there are several technical barriers to the evaluation of anti-latency drugs in vivo. We also discuss these challenging issues that remain unresolved.
Introduction
Human immunodeficiency virus-1 (HIV-1) is the causative agent of acquired immunodeficiency syndromes (AIDS). Highly active antiretroviral therapy (HAART) can reduce plasma HIV-1 RNA level to less than 50 copies ml−1, which is below the detection limit of clinical assays. Previous studies of viral dynamics predicted a cure of HIV-1 infection within 2 to 3 years of treatment (1). However, a low level residual viremia can be consistently detected by ultrasensitive assays from most of the patients even after years of treatment (2-5). Rapid viral rebound after treatment interruptions was observed from patients on efficacious antiretroviral therapy who had no clinically detectable viremia (6). A stable latent reservoir for HIV-1 in resting CD4+ T cells which is not affected by HAART is a major reason for viral persistence (7-9). Therefore, HAART does not cure HIV-1 infection, but rather converts HIV-1 infection from a “death sentence” into chronic infectious disease. Lifelong HAART is required for every infected individual to keep residual HIV-1 under control. Despite its great success, lifelong HAART can have adverse effects. In addition, providing lifelong antiretroviral therapy for tens of millions of infected individuals poses a great economic challenge. Strategies aiming for a cure of HIV-1 infection are therefore needed. Attempts to cure HIV-1 infection will likely be made in patients on HAART.
HIV-1 persists despite long-term antiretroviral therapy
HAART effectively suppresses HIV-1 replication. Multiphasic decay of plasma virus levels, usually from above 10,000 to below 50 copies ml−1, is achieved in vast majority of patients after the initiation of HAART. Since current antiretroviral drugs do not block viral budding from infected cells, but rather block new infection, the decay rate of viremia corresponds to the lifespan of infected cells. During the first and most rapid phase of decay, the plasma virus levels are reduced exponentially by two orders of magnitude. Free virus has a very short half-life (minutes to hours), and the first phase of decay, which has a half-life of one to two days, corresponds to the half-life of HIV-1 infected CD4+ T lymphoblasts (10). Infected CD4+ T lymphoblasts die quickly due to viral cytopathic effects (CPE) or host immune responses. The second phase of decay has a half-life of two weeks (1), which reflects the death of cells with a longer lifespan. These cells could by macrophages, dendritic cells or perhaps partially activated CD4+ T cells. After the second phase of decay, plasma virus levels decrease to below 50 copies ml−1, with a median viral load of 3 copies ml−1 (5). However, in the next phase (third phase), this low level of residual viremia stabilizes and can be consistently detected despite of years of continuous treatment (2-5). Some study suggests that the third phase can be further divided into two separate phases based on their different rates of decay (5). Given the fact that the half-life of cell-free virions is measured in unit of minutes or hours (10), the residual viremia from patients under long-term antiretroviral therapy must reflect continuous virus production. Therefore, it is of great importance to understand where residual viremia arises from.
There are currently two prevailing views regarding to the third phase of viral decay which lasts indefinitely. One explanation is that HAART cannot completely block viral replication in vivo but only reduces plasma virus levels to a stable set point. The residual viremia stems from a low degree of ongoing viral replication. The belief in ongoing viral replication has been greatly challenged by several lines of evidence. Given the error rate of HIV-1 reverse transcriptase (11), viral evolution is inevitable and drug resistance mutations may eventually arise if ongoing rounds of viral replication are sustained for years. The most convincing evidence against ongoing viral replication is that patients with good adherence to HAART regimens do not experience treatment failure, which is in agreement with the lack of viral evolution in the persistent viremia (12-15). In addition, no effect on residual plasma viremia was reproducibly observed upon intensification of treatment with additional antiretroviral drugs. This also argues against ongoing viral replication. In these studies, a fourth drug was added into the three-drug regimen. In one study, a boosted protease inhibitor atazanavir/ritonavir or lopinavir/ritonavir, or a nonnucleoside reverse transcriptase inhibitor efavirenz was combined with original regimen for a four-week intensification period. No decrease in plasma virus levels was observed (16). The lack of effect of treatment intensification on residual viremia was also observed in other intensification studies (17-22). In some intensification studies with the integrase inhibitor raltegravir, although no decay of viremia is observed, an increase of 2-LTR circles was seen (18, 19). In another intensification study with the integrase inhibitor raltegravir, reduction in the size of latent reservoir for HIV-1 was observed (23). The results from these studies suggest that raltegravir as the additional antiretroviral drug might further inhibit viral replication, but the underlying mechanism is not clear. Recent pharmacodynamic studies demonstrate that HIV-1 infection can be completely inhibited by three-drug combination therapy (24). Previous studies on HIV-1 persistence primarily focused on plasma viremia from patient on HAART. However, the possibility of ongoing viral replication in patients under efficacious antiretroviral therapy cannot be completely ruled out by the aforementioned clinical studies on patient plasma virus levels. Certain anatomical sites with poor drug penetration, such as the central nervous system (CNS) or the genital tract, may support compartmentalized viral replication. Further understanding of viral dynamics in these putative drug sanctuaries is hindered due to the technical barriers to the measurement of local free drug and intracellular drug concentrations.
The second view is that the low-level residual viremia reflects release of virus from a long lived cellular reservoir(s) which is established prior to the initiation of HAART. Viruses produced from the cellular reservoir do not initiate new cycles of infection due to the presence of HAART. In 1997, three groups reported the finding of a stable reservoir for HIV-1 in resting CD4+ T cells. These cells could produce replication competent viruses in vitro upon stimulation (7-9). Other reservoirs for HIV-1 have been proposed, including in multipotent hematopoietic progenitor cells (25). However, another study failed to detect HIV-1 DNA in multipotent progenitor cells patient from patients on HAART (26). Therefore, the presence of this reservoir is still debatable. To date, latently infected resting memory CD4+ T cells are the most studied and best characterized reservoir for HIV-1. As shown in Figure 1, in this cellular reservoir, HIV-1 is integrated within the transcription unit of the host genome (27, 28) and is transcriptionally silent (29, 30). The stability of latent HIV-1 is not affected by HAART because no antiretrovirals eliminate proviruses integrated into the cellular genome. Since no viral protein is produced, latently infected resting CD4+ T cells avoid both viral cytopathic effects and host immune clearance. Numerous studies have explored the correlation between HIV-1 persistence and the latent viral reservoir. It is estimated that in patients on HAART up to 10 million resting CD4+ T cells are latently infected with HIV-1 (31). Longitudinal analysis demonstrated that the latent viral reservoir is extremely stable, which provides a mechanism for lifelong persistence of HIV-1 infection (32-34). A long-term follow-up study by Siliciano et al. closely monitored patients on HAART for up to seven years and found that the size of latent reservoir in those patients did not change over time. In this study, the measured half-life of latently infected resting CD4+ T cells was 44 months, indicating that it would take up to 70 years to allow this stable viral reservoir to fully decay (33). Phylogenetic analysis revealed that in patients under long-term efficacious antiretroviral therapy, rebounding plasma viruses after structured treatment interruptions (STI) were from long-lived latently infected resting CD4+ T cells (35, 36). It was also demonstrated that viral variants rebounding during STI were already present before the initiation of HAART, suggesting that a stable reservoir of HIV-1 is established early during infection (36). Early establishment of the viral latent reservoir was supported by several studies. In one study, patients were treated with HAART within 120 days of the onset of symptoms of newly acquired HIV-1 infection and virus rebound occurred in all subjects after treatment was discontinued (37). Taken together, these results suggest that a viral latent reservoir in resting CD4+ T cells is established early during infection. Low level residual viremia appears to reflect the release of viruses from this stable reservoir. The presence of a stable latent reservoir for HIV-1 which is able to rekindle infection after treatment cessation provides the best explanation for HIV-1 persistence.
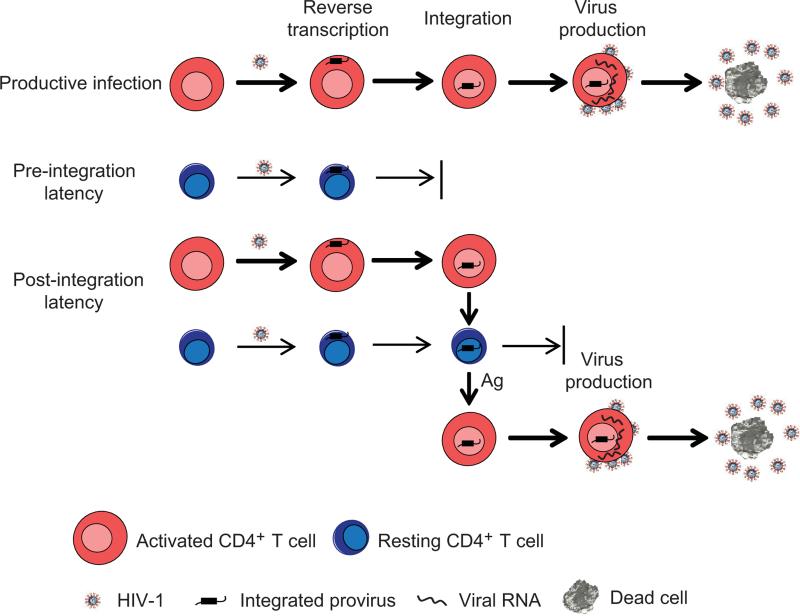
Figure 1. HIV-1 latent infection.
In productively infected cells, HIV-1 rapidly completes all the steps including entry, reverse transcription, integration, viral gene transcription and virus production. There are two forms of latent infection: post-integration latency and pre-integration latency. Post-integration latency can be generated when some activated CD4+ T cells survives and reverts back to a resting state. In these cells, HIV-1 completes integration but its gene transcription is silenced. Post-integration latency can also be generated when HIV-1 infects resting CD4+ T cells and completes all the steps in the life cycle through integration. However, infection of resting CD4+ T cells is less efficient because of the blocks at the steps of reverse transcription and integration. Latently infected resting CD4+ T cells with integrated provirus can produce viruses after encountering its cognate antigen. Pre-integration latency can be generated when HIV-1 completes reverse transcription in resting CD4+ T cells but fails to integrate into the host genome. Pre-integration latency is a labile form of latency that decays rapidly due to the short half life of HIV-1 double-stranded DNA.
HIV-1 establishes stable latent infection in resting CD4+ T cells
Since latent HIV-1 infection allows lifelong persistence of replication-competent viruses in patients on HAART, eliminating the latent reservoir is a key step toward a cure. To date, it remains unclear how HIV-1 establishes latent infection in resting CD4+ T cells in vivo. The majority of evidence suggests that resting CD4+ T cells are relatively non-permissive to HIV-1 infection due to low levels of the HIV-1 co-receptor CCR5, inefficient reverse transcription (38, 39) and defective nuclear import of pre-integration complex (40). Therefore, HIV-1 latency is often viewed as an accidental consequence of viral tropism for activated CD4+ T cells. One hypothesis is that latently infected cells arise when activated CD4+ T cells become infected and a small fraction of them survive and revert to a resting memory state (41). This idea is supported by the studies in which latent HIV-1 was found primarily in resting memory CD4+ T cells but not in naïve cells (31, 42-44). Other studies suggest that HIV-1 can establish latent infection by directly infecting resting CD4+ T cells (45-48). In these studies, despite of the low efficiency of reverse transcription and integration, integrated proviral DNA was detected using a sensitive PCR assay, and silently infected cells could produce viruses after stimulation. In addition, chemokines including CCL19, CCL20, CXCL9 and CXCL10 increase the efficiency of HIV-1 nuclear localization and integration but not viral gene expression in resting CD4+ T cells, and thus facilitate the establishment of HIV-1 latent infection (49). Nonetheless, both views agree that resting CD4+ T cells support establishment and maintenance of latent HIV-1 infection because the host factors indispensable for viral gene expression are either insufficiently expressed or sequestered in an inactive form in resting CD4+ T cells (41, 50).
To eliminate latent HIV-1, we must understand how the viral latent reservoir is stably maintained in vivo (Figure 2A). In patients on long-term antiretroviral therapy, the latent reservoir is unlikely to be replenished by continuous rounds of infection, but, rather, stably maintained in the cells which are latently infected before HAART is initiated. The lifespan of cells harboring latent HIV-1 should be similar to that of uninfected resting memory CD4+ T cells, which are long lived and not subject to activation-induced cell death (51). Stochastic activation of latently infected resting CD4+ T cells reactivates the latent virus and leads to virus production which provides an explanation for the presence of low-level residual viremia. The antiretroviral drugs prevent the released virus from infecting more cells. In addition, recent studies demonstrated that the viral latent reservoir was maintained through homeostatic proliferation of latently infected resting CD4+ T cells driven by common γ-chain cytokines Interleukin-7 and Interleukin-15 (44, 52).
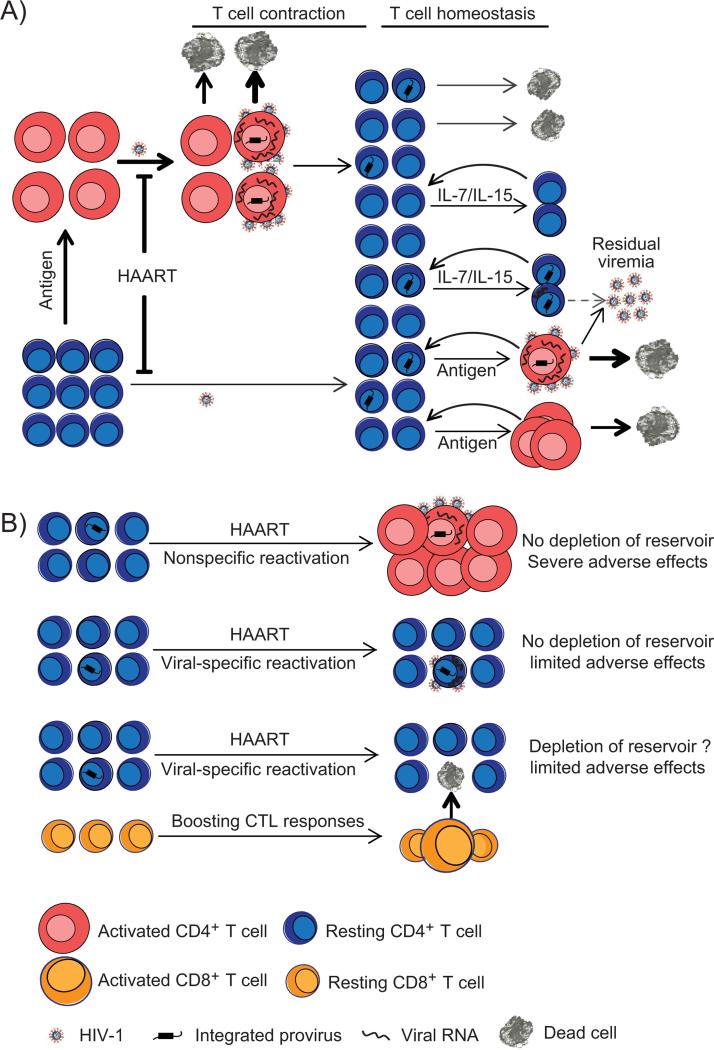
Figure 2. (A) Establishment and maintenance of latent reservoir for HIV-1.
Latently infected cells appear to arise when activated CD4+ T cells become infected and a small fraction of them survive and revert to a resting memory state. In patient under suppressive antiretroviral therapy, ongoing viral infection of activated and resting CD4+ T cells is inhibited by antiretroviral drugs. The viral latent reservoir is established prior to the initiation of HAART. Due to the lack of viral gene expression, latently infected resting CD4+ T cells avoid viral CPE or host immune response. When encountering their cognate antigen, resting CD4+ T cells harboring latent virus become activated and induce viral gene expression and virus production, which appears to be the major source of residual plasma viremia. Latently infected resting CD4+ T cells also undergo homeostatic proliferation driven by IL-7 or IL-15, thus replenish the latent reservoir. (B) Strategies for purging viral latent reservoir. Nonspecific reactivation of latent HIV-1 using interleukin-2 plus anti-CD3 antibodies did not deplete the viral reservoir for unknown reasons. Such strategy caused global T cell activation, massive cytokine release and severe adverse effects. Compounds that reactivate latent HIV-1 but avoid activating the host cells are identified using cell models of HIV-1 latency. However, virus reactivation does not cause the death of infected resting cells due to insufficient viral CPE and defective CTL responses. Priming CTL response prior to virus reactivation is a promising strategy to enhance killing of resting CD4+ T cells in which the latent infection is reversed.
HIV-1 latent infection can be reversed by pharmacological approaches
Several approaches to purging the latent reservoir have been discussed. Though not the only routes, the most studied approaches to elimination of the viral reservoir rely on reversal of HIV-1 latency in resting CD4+ T cells (Figure 2B). For example, the activation of latently infected cells leads to reactivation of latent viruses (7-9) which consequently causes death of the host cells. Since there is no feasible strategy to selectively activate resting CD4+ T cells that harbor latent HIV-1, initial attempts focused on interleukin-2 or interleukin-2 plus anti-CD3 antibodies which induced global T cell activation. Although increased plasma virus levels were observed in some of the patients in several clinical trials, such a strategy failed to deplete the latent reservoir for HIV-1 (53-57). It is also worth mentioning that non-selective T cell activation induced massive cytokine release and caused significant adverse effects (54, 55). The failure of this strategy is not well understood. One possible explanation is that global T cell activation induced clonal expansion of latently infected CD4+ T cells. Although the majority of these activated cells infected with HIV-1 died quickly, a small fraction of these cells survived and reverted back to resting memory state, thus replenishing the latent reservoir. Interleukin-7 was also investigated as a potential anti-latency agents but its role in the T cell survival and homeostatic proliferation makes it a controversial candidate (44). Thus, strategies that specifically reactivate latent virus without inducing T cell activation are more attractive. An early attempt to eliminate the latent reservoir involved reactivating latent HIV-1 with valproic acid, which was approved by the US Food and Drug Administration (FDA) for the treatment of certain types of seizures. As a histone deacetylase inhibitor, valproic acid was found to specifically reactivate latent HIV-1 by promoting histone acetylation of the integrated proviral promoter in vitro (58). However, administration of valproic acid in order to reactivate latent HIV-1 in patients on HAART failed to affect the stability of latent reservoir in several clinical trials (59-64). The failure of these clinical trials is not well understood. The inefficient reactivation of latent HIV-1 by valproic acid in vivo is a potential reason. Renewed optimism was recently brought by the interesting case of a patient who is considered to be the first and the only patient cured of HIV-1 infection. In this case, the patient - the so-called “Berlin patient” - was doing well on HAART when he developed acute myelogenous leukemia (AML). As part of the treatment of his leukemia, he was given myeloablative therapy and hematopoietic stem cell transplantation from a donor homozygous for a 32 base pair deletion mutation (Δ32) in the HIV-1 co-receptor CCR5. HAART was stopped following the transplant, and the patient has not experienced viral rebound for several years (65). In addition, studies performed in different laboratories confirmed that no HIV RNA, DNA or latent reservoir was detected in this patient. Although this aggressive approach is very hard to generalize, thorough elimination of latent HIV-1 was achieved in this case. However, it is not clear whether myeloablative therapy along could cure HIV-1 infection, or the transplant donor homozygous for CCR5Δ32 is needed.
Successful elimination of the latent reservoir requires a better understanding of HIV-1 latency in resting CD4+ T cells. The viral latent reservoir is very hard to study for two reasons. First, latently infected cells are rare in vivo. In patients on HAART, only one per million resting CD4+ T cells carries integrated latent provirus that can produce replication-competent viruses upon stimulation (31). Due to the rarity of latently infected cells, current assays that measure size of the latent reservoir require large amount of patient blood and only have limited dynamic range (66). Second, latently infected resting CD4+ T cells harboring integrated proviral DNA are technically indistinguishable from the uninfected cells. It would be a major breakthrough if any unique markers, preferably cell-surface markers of latently infected cells could be identified to help distinguish them from other uninfected cells. Recently, development of in vitro primary cell models (67-73) and cell line models (74) of HIV-1 latency that capture the key characteristics of the latent reservoir in vivo have significantly advanced the understanding of HIV-1 latency and facilitated the development of strategies to eliminate the viral reservoir. From high throughput drug screening, recent studies have identified many small compounds that can reverse latent HIV-1 without causing T cell activation. Interestingly, these include two drugs, disulfiram and vorinostat, which are approved by the US FDA for the clinical treatment of chronic alcoholism and cutaneous lymphoma, respectively. They are promising candidates for future eradication clinical trials. Vorinostat (SAHA; suberoylanilide hydroxamic acid), a histone deacetylase inhibitor (HDACi), has been investigated in several studies. Vorinostat is a much more potent inhibitor than valproic acid against class I histone deacetylases, the major isoform of histone deacetylase responsible for repression of viral transcription (75) (IC50=0.01μM and 171μM, respectively (76)). Earlier ex vivo studies demonstrated that vorinostat induced virus production in resting CD4+ T cells from patients under long-term antiretroviral therapy (77-79). Besides vorinostat, multiple HDACis have been evaluated for potency in reversing latent HIV in in vitro primary cell or cell line models (80). Disulfiram was also found to reactivate latent HIV-1 in an in vitro primary cell model (81). To evaluate and improve the effectiveness of these compounds, a recent study compared different anti-latency agents in both transformed cell line and primary cell models and reported that single-agent latency reactivation therapy is ineffective against most HIV-1 subtypes, while combinations of two drugs achieved better virus reactivation (82).
Critical issues in HIV/AIDS cure research remain unresolved
Despite the recent success in finding anti-latency agents, it is not completely understood how well in vitro cell models of HIV-1 latency recapitulate the in vivo situation. In the future clinical trials investigating strategies for viral eradication, a thorough evaluation of the anti-latency activity of drug candidates should include the measurement of changes in intracellular viral RNA, plasma virus levels, and the size of viral latent reservoir, as well as potential adverse effects. These studies will be performed when patients are on HAART. The final step of evaluation, which can potentially provide the ultimate proof of a complete depletion of the latent reservoir, is monitoring rebound in viremia after the withdrawal of HAART. Convincing evidence for the complete depletion of the latent HIV-1 must be acquired before initiating the final step because withdrawal of HAART will likely put patients at some risk of adverse effects. Here we discuss some of the key issues that need to be resolved before taking the final step.
1. How to quantitatively measure virus reactivation in vivo?
There are several technical barriers to evaluating the in vivo efficiency of anti-latency drugs which hamper the progress in the search for the cure for HIV-1 infection. For example, we do not know how to quantitatively measure virus reactivation in vivo. A recent clinical study directly investigated the effect of vorinostat in vivo and demonstrated that a single oral dose of vorinostat induced an increase in intracellular viral RNA levels in most of the subjects (83). However, detection of intracellular viral RNA only confirms that the drug has in vivo effect, but does not provide an answer to how completely latently infected resting CD4+ T cells can be induced to produce viral RNA. Incomplete reactivation of latent HIV-1 will not lead to a cure since a small number of latently infected cells can fully rekindle infection. To date, quantitative measurement of virus reactivation can only be achieved in in vitro systems where T cell activating agents, such as α-CD3 plus α-CD28 antibodies, phytohaemagglutinin (PHA) or phorbol 12-myristate 13-acetate (PMA) plus ionomycin can be used as controls. Although there is no in vitro model that can perfectly mimic the in vivo situation, testing and comparing drugs in different in vitro systems can provide some indirect evidence of how efficient these drugs and drug combinations are in vivo.
In addition to detecting intracellular viral RNA, measuring plasma viral RNA in patients treated with anti-latency drugs is a useful strategy to monitor virus reactivation. In previous clinical trials, increased plasma virus levels were observed in some of the patients treated with α-CD3 antibody (54). A recent clinical study demonstrated that the level of intracellular viral RNA increased after administration of vorinostat but no increase in plasma HIV-1 RNA levels was observed (83). It is possible that current viral quantification assay may not be able to detect a small change in residual viremia if viruses are produced from the latent reservoir after vorinostat treatment. It is important to understand how efficient viruses can be produced from resting CD4+ T cells in which latent infection has been reversed and whether virus production from resting CD4+ T cells can cause an increase in plasma virus levels that can be detected by current assays. Previous studies in in vitro primary cell models primarily focused on induction of viral gene transcription but not virus release from latently infected resting CD4+ T cells, partially due to the use of replication-defective viruses in those systems (71, 72). Modified systems with primary resting CD4+ T cells using budding-competent viruses can determine whether anti-latency drugs can induce virus production from resting CD4+ T cells. If so, viral burst size in resting CD4+ T cells has to be measured so that we know whether a significant increase in plasma viremia can be expected. It is possible that resting CD4+ T cells cannot support efficient virus production. This is suggested by certain studies in which production of viral RNA and viral proteins but not cell-free viruses can be detected from HIV-1-infected resting CD4+ T cells. These findings suggest that there are some barriers at later steps in HIV-1 life cycle (48, 84). Even if release of free virus is inefficient, viral protein expression can expose latently infected cells to virus- or immune-mediated killing.
2. How to measure the size of latent reservoir for HIV-1?
Although viral gene transcription and virus production are considered to be evidence for virus reactivation, additional studies are required to determine whether the reversal of latency actually leads to the elimination of latently infected cells. A reduction in the number of resting CD4+ T cells harboring latent virus in vivo is often considered direct evidence for elimination of latent HIV-1. However, there is no simple assay to measure the size of latent reservoir for HIV-1. In patients on HAART, the frequency of cells carrying HIV-1 DNA is about 100-fold greater than the frequency of cells that produce replication-competent virus in the viral outgrowth assay. This is probably because the majority of integrated proviruses are defective (31). Although assays using PCR to quantify viral DNA are simple and fast, the measured amount of viral DNA is not necessarily correlated with the size of latent reservoir. Therefore, such assays cannot reliably predict the size of latent reservoir in vivo. The current assay that best measures the frequency of latently infected resting CD4+ T cells relies on in vitro reactivation of resting CD4+ T cells with the mitogen PHA and expansion of the viruses that are produced by coculture with CD4+ lymphoblasts from normal donors (66). One of the major advantages of this assay is that it only measures cells harboring replication-competent HIV-1, not cells with defective viruses. Its disadvantages include the cost and length of time required for the assay. In addition, due to the low frequency of latently infected cells (one per one million), large amount of patient blood is needed in order to obtain the millions of resting CD4+ T cells needed for this assay. The dynamic range of this assay is limited because only a few latently infected cells can be detected in one sample, which suggests that small changes in the size of latent reservoir may not be identified by this assay. Larger amounts of cells can be obtained from leukapheresis to increase the dynamic range of this assay. Such strategy is not ideal for large-scale clinical trials. Studies to simplify the current assay meanwhile increase the dynamic range of the assay need to be done in the future.
3. How to kill resting CD4+ T cells in which latent HIV-1 has been reactivated?
A critical issue in viral eradication is how to kill infected resting CD4+ T cells in which latent HIV-1 has been reactivated. It was often assumed that reactivation of latent HIV-1 would kill the infected cells either through viral CPE or host immune responses, in combination with HAART to prevent new infections. However, the validity of this assumption has been questioned. Previous studies mainly focused on transformed cell lines and activated CD4+ T cells and demonstrated that viral CPE caused cell death through caspase-dependent or independent mechanisms (85-88). Since current efforts are focused on finding latency reversing agents that do not cause T cell activation, it is important to know whether resting CD4+ T cells are susceptible to viral cytopathic effects (CPE). In addition to viral CPE, CD8+ cytolytic T-lymphocytes (CTL) are a major component of adaptive immunity against viral infection. Some studies have demonstrated that functions of HIV-1-specific CTLs in patients are defective and are not restored by HAART (89, 90). To understand whether the reversal of viral latency causes cell death in resting CD4+ T cells or not, a recent study examined the killing of latently infected cells by viral CPE and HIV-1-specific CD8+ T cells in an in vitro system (91). In this study, viral latent infection was generated in patient CD4+ T cells by superinfection with HIV-1 reporter virus in vitro. After latent virus was reactivated by vorinostat, infected resting CD4+ T cells survived. The inefficient killing of resting CD4+ T cells by viral CPE is partly due to inefficient viral gene expression in resting cells. In addition, resting cells at a quiescent G0 state are less susceptible to viral CPE mediated by HIV-1 vif and vpr, which cause cell cycle arrest in activated CD4+ T cells. This study also demonstrated that autologous CTLs from patients on HAART failed to kill latently infected resting CD4+ T cells in which latent infection had been reversed. Overall, these results suggest that resting CD4+ T cells harboring latent HIV-1 cannot be eliminated by either viral CPE or cytolytic T-lymphocyte responses after the reversal of HIV-1 latent infection. The limitation of this study is that Bcl-2 overexpression in the in vitro model could potentially interfere with viral CPE.
There are several strategies proposed to improve the killing of HIV-1-infected resting CD4+ T cells. Studies in an in vitro system demonstrated that stimulating autologous CTLs with HIV-1 Gag peptides prior to virus reactivation led to efficient killing of latently infected cells (91). However, in vitro stimulation of HIV-1-specific CTLs from patients on HAART with viral peptides did not fully restore their proliferative and cytolytic capacities, or other functions such as cytokine production (89-91). High-level proliferative and cytotoxic capacities of HIV-1-specific CTLs can be re-stimulated in vitro with phorbol esters and calcium ionophores, which are potent stimuli that cannot be administrated in vivo (89). Thus, how to efficiently restore CTL functions in vivo is an important area for future studies. In other studies, antibody-conjugated bacterial toxins were designed to target and kill cells expressing HIV-1 Env protein after virus reactivation (92). It is possible that some anti-latency agents can induce much higher level of HIV-1 gene expression than most of the current candidates so that viral CPE can be largely enhanced efficiently cause the death of host cells. In addition, it will be very interesting to find drugs that have synergistic effect with viral CPE to kill host cells.
Conclusion
Despite recent advances in the understanding of HIV-1 latency and its clinical ramifications, achieving a cure for HIV-1 infection remains a great challenge for the scientific community. Reactivation of latent virus while patients stay on HAART is currently one of the best strategies for the depletion of viral latent reservoir. Intensive efforts have been made in the past several years to identify compounds that can reactivate latent HIV-1 without causing T cell activation in in vitro cell models. However, there are multiple barriers to the accurate evaluation of in vivo effectiveness of anti-latency drugs. Furthermore, strategies such as priming HIV-1-specific CTL response to enhance killing of resting CD4+ T cells in which latent infection has been reversed are needed for the elimination of latent reservoir. The search for a cure will be a prolonged process, and success is not guaranteed.
Acknowledgments
R.F.S. is supported by the NIH Martin Delaney CARE Collaboratory, grant AI043222, the Foundation for AIDS Research (amFAR), and the Howard Hughes Medical Institute.
Footnotes
Conflict of interest statement
No conflict of interest was declared.
References
- 1.Perelson AS, Essunger P, Cao Y, Vesanen M, et al. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387:188–91. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 2.Dornadula G, Zhang H, VanUitert B, Stern J, et al. Residual HIV-1 RNA in blood plasma of patients taking suppressive highly active antiretroviral therapy. JAMA. 1999;282:1627–32. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 3.Havlir DV, Koelsch KK, Strain MC, Margot N, et al. Predictors of residual viremia in HIV-infected patients successfully treated with efavirenz and lamivudine plus either tenofovir or stavudine. J Infect Dis. 2005;191:1164–8. doi: 10.1086/428588. [DOI] [PubMed] [Google Scholar]
- 4.Palmer S, Wiegand AP, Maldarelli F, Bazmi H, et al. New real-time reverse transcriptase-initiated PCR assay with single-copy sensitivity for human immunodeficiency virus type 1 RNA in plasma. J Clin Microbiol. 2003;41:4531–6. doi: 10.1128/JCM.41.10.4531-4536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmer S, Maldarelli F, Wiegand A, Bernstein B, et al. Low-level viremia persists for at least 7 years in patients on suppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105:3879–84. doi: 10.1073/pnas.0800050105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey RT, Jr, Bhat N, Yoder C, Chun TW, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A. 1999;96:15109–14. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finzi D, Hermankova M, Pierson T, Carruth LM, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 8.Wong JK, Hezareh M, Gunthard HF, Havlir DV, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 9.Chun TW, Stuyver L, Mizell SB, Ehler LA, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94:13193–7. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perelson AS, Neumann AU, Markowitz M, Leonard JM, et al. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 11.Mansky LM, Temin HM. Lower in vivo mutation rate of human immunodeficiency virus type 1 than that predicted from the fidelity of purified reverse transcriptase. J Virol. 1995;69:5087–94. doi: 10.1128/jvi.69.8.5087-5094.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frenkel LM, Wang Y, Learn GH, McKernan JL, et al. Multiple viral genetic analyses detect low-level human immunodeficiency virus type 1 replication during effective highly active antiretroviral therapy. J Virol. 2003;77:5721–30. doi: 10.1128/JVI.77.10.5721-5730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieffer TL, Finucane MM, Nettles RE, Quinn TC, et al. Genotypic analysis of HIV-1 drug resistance at the limit of detection: virus production without evolution in treated adults with undetectable HIV loads. J Infect Dis. 2004;189:1452–65. doi: 10.1086/382488. [DOI] [PubMed] [Google Scholar]
- 14.Bailey JR, Sedaghat AR, Kieffer T, Brennan T, et al. Residual human immunodeficiency virus type 1 viremia in some patients on antiretroviral therapy is dominated by a small number of invariant clones rarely found in circulating CD4+ T cells. J Virol. 2006;80:6441–57. doi: 10.1128/JVI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Evering TH, Mehandru S, Racz P, Tenner-Racz K, et al. Absence of HIV-1 evolution in the gut-associated lymphoid tissue from patients on combination antiviral therapy initiated during primary infection. PLoS Pathog. 2012;8:e1002506. doi: 10.1371/journal.ppat.1002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinoso JB, Kim SY, Wiegand AM, Palmer SE, et al. Treatment intensification does not reduce residual HIV-1 viremia in patients on highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 2009;106:9403–8. doi: 10.1073/pnas.0903107106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMahon D, Jones J, Wiegand A, Gange SJ, et al. Short-course raltegravir intensification does not reduce persistent low-level viremia in patients with HIV-1 suppression during receipt of combination antiretroviral therapy. Clin Infect Dis. 2010;50:912–9. doi: 10.1086/650749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzon MJ, Massanella M, Llibre JM, Esteve A, et al. HIV-1 replication and immune dynamics are affected by raltegravir intensification of HAART-suppressed subjects. Nat Med. 2010;16:460–5. doi: 10.1038/nm.2111. [DOI] [PubMed] [Google Scholar]
- 19.Yukl SA, Shergill AK, McQuaid K, Gianella S, et al. Effect of raltegravir-containing intensification on HIV burden and T-cell activation in multiple gut sites of HIV-positive adults on suppressive antiretroviral therapy. AIDS. 2010;24:2451–60. doi: 10.1097/QAD.0b013e32833ef7bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi RT, Bosch RJ, Aga E, Albrecht M, et al. No evidence for decay of the latent reservoir in HIV-1-infected patients receiving intensive enfuvirtide-containing antiretroviral therapy. J Infect Dis. 2010;201:293–6. doi: 10.1086/649569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gandhi RT, Zheng L, Bosch RJ, Chan ES, et al. The effect of raltegravir intensification on low-level residual viremia in HIV-infected patients on antiretroviral therapy: a randomized controlled trial. PLoS Med. 2010;7:e1000321. doi: 10.1371/journal.pmed.1000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yilmaz A, Verhofstede C, D'Avolio A, Watson V, et al. Treatment intensification has no effect on the HIV-1 central nervous system infection in patients on suppressive antiretroviral therapy. J Acquir Immune Defic Syndr. 2010;55:590–6. doi: 10.1097/QAI.0b013e3181f5b3d1. [DOI] [PubMed] [Google Scholar]
- 23.Vallejo A, Gutierrez C, Hernandez-Novoa B, Diaz L, et al. The effect of intensification with raltegravir on the HIV-1 reservoir of latently infected memory CD4 T cells in suppressed patients. AIDS. 2012;26:1885–94. doi: 10.1097/QAD.0b013e3283584521. [DOI] [PubMed] [Google Scholar]
- 24.Jilek BL, Zarr M, Sampah ME, Rabi SA, et al. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med. 2012;18:446–51. doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter CC, Onafuwa-Nuga A, McNamara LA, Riddell J, 4th, et al. HIV-1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446–51. doi: 10.1038/nm.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durand CM, Ghiaur G, Siliciano JD, Rabi SA, et al. HIV-1 DNA is detected in bone marrow populations containing CD4+ T cells but is not found in purified CD34+ hematopoietic progenitor cells in most patients on antiretroviral therapy. J Infect Dis. 2012;205:1014–8. doi: 10.1093/infdis/jir884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroder AR, Shinn P, Chen H, Berry C, et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–9. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Lassen K, Monie D, Sedaghat AR, et al. Resting CD4+ T cells from human immunodeficiency virus type 1 (HIV-1)-infected individuals carry integrated HIV-1 genomes within actively transcribed host genes. J Virol. 2004;78:6122–33. doi: 10.1128/JVI.78.12.6122-6133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hermankova M, Siliciano JD, Zhou Y, Monie D, et al. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77:7383–92. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chun TW, Justement JS, Lempicki RA, Yang J, et al. Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc Natl Acad Sci U S A. 2003;100:1908–13. doi: 10.1073/pnas.0437640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chun TW, Carruth L, Finzi D, Shen X, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–8. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 32.Finzi D, Blankson J, Siliciano JD, Margolick JB, et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5:512–7. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 33.Siliciano JD, Kajdas J, Finzi D, Quinn TC, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–8. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 34.Strain MC, Gunthard HF, Havlir DV, Ignacio CC, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A. 2003;100:4819–24. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Chung C, Hu BS, He T, et al. Genetic characterization of rebounding HIV-1 after cessation of highly active antiretroviral therapy. J Clin Invest. 2000;106:839–45. doi: 10.1172/JCI10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joos B, Fischer M, Kuster H, Pillai SK, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A. 2008;105:16725–30. doi: 10.1073/pnas.0804192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markowitz M, Jin X, Hurley A, Simon V, et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J Infect Dis. 2002;186:634–43. doi: 10.1086/342559. [DOI] [PubMed] [Google Scholar]
- 38.Zack JA, Arrigo SJ, Weitsman SR, Go AS, et al. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell. 1990;61:213–22. doi: 10.1016/0092-8674(90)90802-l. [DOI] [PubMed] [Google Scholar]
- 39.Pierson TC, Zhou Y, Kieffer TL, Ruff CT, et al. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J Virol. 2002;76:8518–31. doi: 10.1128/JVI.76.17.8518-8531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bukrinsky MI, Sharova N, Dempsey MP, Stanwick TL, et al. Active nuclear import of human immunodeficiency virus type 1 preintegration complexes. Proc Natl Acad Sci U S A. 1992;89:6580–4. doi: 10.1073/pnas.89.14.6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lassen K, Han Y, Zhou Y, Siliciano J, et al. The multifactorial nature of HIV-1 latency. Trends Mol Med. 2004;10:525–31. doi: 10.1016/j.molmed.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Pierson T, Hoffman TL, Blankson J, Finzi D, et al. Characterization of chemokine receptor utilization of viruses in the latent reservoir for human immunodeficiency virus type 1. J Virol. 2000;74:7824–33. doi: 10.1128/jvi.74.17.7824-7833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenchley JM, Hill BJ, Ambrozak DR, Price DA, et al. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–8. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomont N, El-Far M, Ancuta P, Trautmann L, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swiggard WJ, Baytop C, Yu JJ, Dai J, et al. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J Virol. 2005;79:14179–88. doi: 10.1128/JVI.79.22.14179-14188.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Agosto LM, Yu JJ, Dai J, Kaletsky R, et al. HIV-1 integrates into resting CD4+ T cells even at low inoculums as demonstrated with an improved assay for HIV-1 integration. Virology. 2007;368:60–72. doi: 10.1016/j.virol.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vatakis DN, Kim S, Kim N, Chow SA, et al. Human immunodeficiency virus integration efficiency and site selection in quiescent CD4+ T cells. J Virol. 2009;83:6222–33. doi: 10.1128/JVI.00356-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pace MJ, Graf EH, Agosto LM, Mexas AM, et al. Directly infected resting CD4+T cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012;8:e1002818. doi: 10.1371/journal.ppat.1002818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cameron PU, Saleh S, Sallmann G, Solomon A, et al. Establishment of HIV-1 latency in resting CD4+ T cells depends on chemokine-induced changes in the actin cytoskeleton. Proc Natl Acad Sci U S A. 2010;107:16934–9. doi: 10.1073/pnas.1002894107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams SA, Greene WC. Regulation of HIV-1 latency by T-cell activation. Cytokine. 2007;39:63–74. doi: 10.1016/j.cyto.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yusuf I, Fruman DA. Regulation of quiescence in lymphocytes. Trends Immunol. 2003;24:380–6. doi: 10.1016/s1471-4906(03)00141-8. [DOI] [PubMed] [Google Scholar]
- 52.Bosque A, Famiglietti M, Weyrich AS, Goulston C, et al. Homeostatic proliferation fails to efficiently reactivate HIV-1 latently infected central memory CD4+ T cells. PLoS Pathog. 2011;7:e1002288. doi: 10.1371/journal.ppat.1002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chun TW, Engel D, Mizell SB, Hallahan CW, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–5. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- 54.Prins JM, Jurriaans S, van Praag RM, Blaak H, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–10. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 55.van Praag RM, Prins JM, Roos MT, Schellekens PT, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21:218–26. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- 56.Kulkosky J, Nunnari G, Otero M, Calarota S, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186:1403–11. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- 57.Stellbrink HJ, van Lunzen J, Westby M, O'Sullivan E, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial). AIDS. 2002;16:1479–87. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- 58.Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, et al. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–8. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- 59.Lehrman G, Hogue IB, Palmer S, Jennings C, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–55. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Steel A, Clark S, Teo I, Shaunak S, et al. No change to HIV-1 latency with valproate therapy. AIDS. 2006;20:1681–2. doi: 10.1097/01.aids.0000238421.36313.fa. [DOI] [PubMed] [Google Scholar]
- 61.Siliciano JD, Lai J, Callender M, Pitt E, et al. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–6. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- 62.Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, et al. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–9. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- 63.Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, et al. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22:1131–5. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Archin NM, Cheema M, Parker D, Wiegand A, et al. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One. 2010;5:e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hutter G, Nowak D, Mossner M, Ganepola S, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–8. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 66.Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- 67.Brooks DG, Kitchen SG, Kitchen CM, Scripture-Adams DD, et al. Generation of HIV latency during thymopoiesis. Nat Med. 2001;7:459–64. doi: 10.1038/86531. [DOI] [PubMed] [Google Scholar]
- 68.Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sahu GK, Lee K, Ji J, Braciale V, et al. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355:127–37. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 70.Marini A, Harper JM, Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol. 2008;181:7713–20. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- 71.Yang HC, Xing S, Shan L, O'Connell K, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–86. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–37. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Saleh S, Wightman F, Ramanayake S, Alexander M, et al. Expression and reactivation of HIV in a chemokine induced model of HIV latency in primary resting CD4+ T cells. Retrovirology. 2011;8:80. doi: 10.1186/1742-4690-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–77. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Keedy KS, Archin NM, Gates AT, Espeseth A, et al. A limited group of class I histone deacetylases acts to repress human immunodeficiency virus type 1 expression. J Virol. 2009;83:4749–56. doi: 10.1128/JVI.02585-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huber K, Doyon G, Plaks J, Fyne E, et al. Inhibitors of histone deacetylases: correlation between isoform specificity and reactivation of HIV type 1 (HIV-1) from latently infected cells. J Biol Chem. 2011;286:22211–8. doi: 10.1074/jbc.M110.180224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Contreras X, Schweneker M, Chen CS, McCune JM, et al. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–9. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Archin NM, Espeseth A, Parker D, Cheema M, et al. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25:207–12. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses. 2009;25:883–7. doi: 10.1089/aid.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wightman F, Ellenberg P, Churchill M, Lewin SR. HDAC inhibitors in HIV. Immunol Cell Biol. 2012;90:47–54. doi: 10.1038/icb.2011.95. [DOI] [PubMed] [Google Scholar]
- 81.Xing S, Bullen CK, Shroff NS, Shan L, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85:6060–4. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burnett JC, Lim KI, Calafi A, Rossi JJ, et al. Combinatorial latency reactivation for HIV-1 subtypes and variants. J Virol. 2010;84:5958–74. doi: 10.1128/JVI.00161-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482–5. doi: 10.1038/nature11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lassen KG, Ramyar KX, Bailey JR, Zhou Y, et al. Nuclear retention of multiply spliced HIV-1 RNA in resting CD4+ T cells. PLoS Pathog. 2006;2:e68. doi: 10.1371/journal.ppat.0020068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roshal M, Zhu Y, Planelles V. Apoptosis in AIDS. Apoptosis. 2001;6:103–16. doi: 10.1023/a:1009636530839. [DOI] [PubMed] [Google Scholar]
- 86.Shedlock DJ, Hwang D, Choo AY, Chung CW, et al. HIV-1 viral genes and mitochondrial apoptosis. Apoptosis. 2008;13:1088–99. doi: 10.1007/s10495-008-0239-0. [DOI] [PubMed] [Google Scholar]
- 87.Bolton DL, Hahn BI, Park EA, Lehnhoff LL, et al. Death of CD4(+) T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J Virol. 2002;76:5094–107. doi: 10.1128/JVI.76.10.5094-5107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci U S A. 2006;103:3369–74. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Migueles SA, Osborne CM, Royce C, Compton AA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–21. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Migueles SA, Weeks KA, Nou E, Berkley AM, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–89. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shan L, Deng K, Shroff NS, Durand CM, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brooks DG, Hamer DH, Arlen PA, Gao L, et al. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19:413–423. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]