Abstract
The circadian system regulates many physiological functions including inflammatory responses. For example, mortality caused by lipopolysaccharide (LPS) injection varies depending on the time of immunostimulation in mammals. The effects of more subtle challenges on the immune system and cellular mechanisms underlying circadian differences in neuroinflammatory responses are not well understood. Here we show that adult male Sprague-Dawley rats injected with a sub-septic dose of LPS during the light phase displayed elevated sickness behaviors and hippocampal cytokine production compared to rats injected during the dark phase. Microglia are the primary central nervous system (CNS) immune cell type and may mediate diurnal differences in sickness response, thus we explored whether microglia demonstrate temporal variations in inflammatory factors. Hippocampal microglia isolated from adult rats rhythmically expressed inflammatory factors and circadian clock genes. Microglia displayed robust rhythms of TNFα, IL1β and IL6 mRNA, with peak cytokine gene expression occurring during the middle of the light phase. Microglia isolated during the light phase were also more reactive to immune stimulation; such that, ex vivo LPS treatment induced an exaggerated cytokine response in light phase-isolated microglia. Treating microglia with corticosterone ex vivo induced expression of the circadian clock gene Per1. However, microglia isolated from adrenalectomized rats maintained temporal differences in clock and inflammatory gene expression. This suggests circadian clock gene expression in microglia is entrained by, but oscillates in the absence of, glucocorticoids. Taken together, these findings demonstrate that microglia possess a circadian clock that influences inflammatory responses. These results indicate time-of-day is an important factor to consider when planning inflammatory interventions such as surgeries or immunotherapies.
Keywords: circadian, clock gene, inflammation, microglia, glucocorticoids, cytokines
Introduction
Circadian rhythms have evolved in response to the consistent 24 h light cycle and allow animals to anticipate predictable daily events such as food availability, fluctuations in predation, and sleep opportunity (Hut and Beersma, 2011). Importantly, these activities are associated with time of day variations in risk for encountering pathogens, infection, and tissue damage to the host (Curtis et al., 2014). Thus, it follows that several aspects of the immune system are regulated by the circadian system and disruption of the circadian system is linked to inflammatory pathologies including cancer, metabolic disorder, and premature aging (Evans and Davidson, 2013, Fonken and Nelson, 2014).
In mammals, circadian rhythms are initiated in the suprachiasmatic nuclei (SCN) of the hypothalamus. Within SCN neurons, rhythms are driven by an autoregulatory feedback loop of transcriptional activators and repressors (Reppert and Weaver, 2002). The transcriptional activators, circadian locomotor output cycles kaput (clock) and brain and muscle arnti-like protein 1 (bmal1), form heterodimers that induce expression of the period (per) and cryptochrome (cry) genes through E-box enhancers. Per and cry proteins accumulate in the cytoplasm and upon reaching critical levels form a complex that translocates back to the nucleus to interact with clock and bmal1 to inhibit their own transcription. This process takes approximately 24 h. While the SCN is the master circadian oscillator in mammals, the molecular machinery necessary for generating circadian rhythms is expressed in many tissues and cells throughout the body (Mohawk et al., 2012). Indeed, circadian clocks persist in several immune cells including macrophages/monocytes (Boivin et al., 2003, Hayashi et al., 2007, Keller et al., 2009, Silver et al., 2012a), T cells (Bollinger et al., 2011), NK cells (Arjona and Sarkar, 2006), dendritic cells (Silver et al., 2012a), and B cells (Silver et al., 2012a).
In addition to driving circadian rhythms, clock genes are involved in regulating immunological activities. For example, the circadian clock gene Rev-erb represses macrophage gene expression (Lam et al., 2013) and targets inflammatory function of macrophages through the direct regulation of Ccl2 (Sato et al., 2014). Bmal1 controls rhythmic trafficking of inflammatory monocytes to sites of inflammation (Nguyen et al., 2013). Additionally, the CLOCK:BMAL1 heterodimer binds E-boxes in the TLR9 promoter (Silver et al., 2012b) and clock protein complexes with the NF-kB subunit p65 (RELA), leading to enhanced transcriptional activity of the NF-kB complex (Spengler et al., 2012). The relationship between circadian clock genes and immune function also appears bi-directional with immune activation altering circadian rhythms (O'Callaghan et al., 2012).
Circadian differences in immune regulation have important physiological consequences in mammals. For example, outcome following global cerebral ischemia varies depending on the time of day at which the ischemic event occurs (Weil et al., 2009). Furthermore, mortality following bacterial challenge varies depending on the time of immunostimulation. Halsberg et al. first demonstrated that a dose of E. coli endotoxin that is non-lethal in most mice when given during the dark (active) period of the day, is highly lethal when administered 8-12h earlier (Halberg et al., 1960). Subsequent studies revealed that lipopolysaccharide (LPS) produces similar responses, with peak mortality in rodents occurring when LPS is administered during the light phase (Marpegan et al., 2009, Spengler et al., 2012).
Peripheral immune cells mediate LPS lethality. However, peripherally administered LPS also impacts centrally mediated processes. For example, there are diurnal variations in LPS induced alterations in sleep and body temperature (Morrow and Opp, 2005). Importantly, circadian regulation of sickness behaviors (outside of the context of sleep) and neuroinflammatory responses has not been well characterized. Peripheral macrophages are under circadian control (Keller et al., 2009) and there is evidence that microglia express clock genes (Nakazato et al., 2011, Hayashi et al., 2013). Furthermore, circadian system disruptions exacerbate inflammatory responses in both the periphery (Castanon-Cervantes et al., 2010) and CNS (Fonken and Nelson, 2013). Thus, we hypothesized that there are temporal differences in coping with an immune challenge and that circadian variations in the sickness response are mediated by microglia.
Methods
Animals
Male Sprague-Dawley rats (60-90 days old; Harlan Sprague-Dawley, Inc, Indianapolis, IN, USA) were pair-housed (unless otherwise specified) with food and water available ad libitum at an ambient temperature of 22±2°C. Rats were given at least two weeks to acclimate to colony conditions before experimentation began. All rats were maintained on a 12:12 light cycle with lights on either at 0700 or 1700 h. All experimental procedures were conducted in accordance with the University of Colorado Institutional Animal Care and Use Committee.
Experimental design
To assess temporal changes in hippocampal cytokine responses and sickness behavior rats received a single IP injection of vehicle (sterile saline) or lipopolysaccharide (LPS) (100 μg/kg; E. coli serotype 0111:B4), either during the middle of the light (ZT6) or dark (ZT16) phase. In order to evaluate cytokine responses, hippocampal tissue was collected 3 or 24 h following the injection. Rats were saline perfused prior to tissue collection in order to remove peripheral immune cells. Hippocampal tissue was then excised and flash frozen. Sickness responses were evaluated as described below.
Sickness behavior
To determine sucrose preference, rats were provided with two solutions, water or water supplemented with 2% sucrose. On day 1, rats were singly housed and water in the home cage was replaced with a 2% sucrose solution for 8 h at the onset of the dark phase in order to habituate rats to the novel solution. On days 2-4, baseline levels of sucrose intake were established. Rats were provided two standard bottles; one containing water and the other contained the 2% sucrose solution, for 8h beginning at either ZT6 or ZT16. On day 5, rats received either an IP vehicle (sterile saline) or LPS (100 μg/kg) injection and were again provided the two-bottle choice test for 8 h. Animals did not have access to bottles during the social investigation testing.
To assess the motivation to engage in social exploratory behavior, a novel juvenile conspecific was introduced to the test subject in a novel cage for a 5 min session. Rats were acclimated to the cage for 30 min prior to testing. Behavior took place under dim red illumination and was scored for the total time the experimental rat engaged in social investigation. Baseline social behavior was established 24 h prior to saline or LPS injection. Social investigation was repeatedly assessed at 3 h, 8 h, and 24 h following the injections.
Adrenalectomy (ADX)
Bilateral ADX was aseptically performed under isoflurane anesthesia as previously described (Frank et al., 2012). All tissue was examined immediately following removal to confirm complete excision of the adrenal gland and serum corticosterone (CORT) was measured at the conclusion of the study (CORT concentrations were uniformly very low in ADX animals; Fig. S2). Sham-operated animals received the same surgical manipulations, except the adrenal gland was visualized and gently manipulated with forceps, but not removed. Rats were treated post-operatively with a topical triple antibiotic ointment (Kroger brand) and 5 mg/kg i.p. meloxicam, and were given one week to recover from surgery prior to additional experimental manipulations. Immediately following the surgeries, ADX animals received basal CORT replacement (25 ug/mL dissolved in 0.4% ETOH containing 0.9% saline; Sigma, St. Louis, MO) in their drinking water and were maintained on the CORT water until 24h prior to microglia isolations. Sham animals received vehicle water. Cardiac blood was taken during tissue collection to assess serum corticosterone concentrations.
ELISA and multiplex array
Cardiac blood was centrifuged (14,000xg for 10 min at 4°C) and serum collected. Hippocampal samples were sonicated on ice using a tissue extraction reagent (Invitrogen) supplemented with protease inhibitor cocktail (Sigma). Homogenates were centrifuged (14,000xg for 10 min at 4°C) and supernatant collected and stored at −20°C. Total protein was quantified using a Bradford assay.
An ELISA for CORT (Assay Designs, Inc., Ann Arbor, MI) was run in duplicate according to the manufacturer's instructions. A 4-Plex array for detecting IL1β, TNFα, IL10, and IL6 was also run in duplicate according to the manufacturer's instructions (Aushon, Billerica, MA).
Quantitative real-time PCR (qPCR)
Primers were designed using Genbank at the National Center for Biotechnology Information (NCBI), the Operon Oligo Analysis Tool, and the Basic Local Alignment Search Tool at NCBI and obtained from Invitrogen. Primers were designed to span exon/exon boundaries and thus exclude amplification of genomic DNA (see Table S1). Primer specificity was verified by melt curve analysis. PCR amplification of cDNA was performed using the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA) with a MyiQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA). Gene expression was determined in duplicate and is expressed relative to β-actin.
Microglia isolations and ex vivo treatments
Hippocampal microglia were isolated using a Percoll density gradient as previously described (Frank et al., 2006). Our lab has previously demonstrated that this isolation procedure yields highly pure microglia (Iba-1+/MHCII+/CD163-/GFAP-) and we confirmed immunophenotype and purity of microglia with qPCR in this experiment (data not shown). Following isolations, microglia were suspended in DMEM+10%FBS and microglia concentration determined by trypan blue exclusion. Microglia concentration was adjusted to a density of 8,000 cells/100 uL and plated in a 96-well v-bottom plate. To assess microglia cytokine responsiveness, cells were challenged ex vivo with 10 uL lipopolysaccharide (LPS; E. coli serotype 0111:B4; Sigma) at a concentration of 10 or 100 ng/mL or media alone for 3 h at 37°C, 5% CO2. To determine the influence of CORT on microglial clock genes, microglia were treated with 10uL of CORT at a concentration of 0, 10, 100, or 1000nM for 2 h. Following ex vivo manipulations, the plates were centrifuged at 1000xg for 10 min at 4°C to pellet cell and supernatant was removed and discarded. Cells were then washed with 0.1M ice cold PBS and centrifuged at 1000xg for 10min at 4°C. Cell lysis and cDNA synthesis was performed using SuperScript III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA) following the manufacturer's protocol.
Statistical analyses
To ensure a normal distribution, data were subjected to a Shapiro-Wilk test. Sickness behaviors were analyzed using repeated-measures ANOVA with ZT and LPS as the between subjects factors. Gene, protein, and hormone results were analyzed using one- (ZT), two- (ZT × LPS; ZT × CORT), or three-way (ZT × ADX × LPS) ANOVAs. Following a significant F score, multiple comparisons were conducted using Tukey's HSD. Statistical analyses were performed using StatView (v.5.0.1, Cary, NC) and Prism software. In all cases, differences between group means were considered statistically significant if p < 0.05
Results
Daily variations in sickness behavior are associated with rhythmic changes in hippocampal cytokine expression
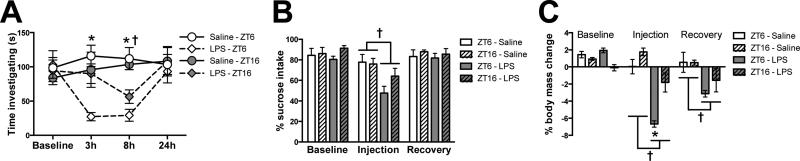
To test whether there are circadian differences in sickness responses to a sub-septic immune stimulation, rats were injected with 100 ug/kg LPS (E. coli serotype 0111:B4; Sigma) either during the middle of the light phase (Zeitgeber time 6, ZT6; 12:12 light/dark cycle with lights on at ZT0) or during the dark phase (ZT16; n = 4/group). Rats injected with LPS during the dark (active) phase displayed a blunted sickness responses compared to those injected during the light (rest) phase. There were no time-of-day differences in baseline social exploration between groups and rats significantly reduced social exploration following LPS injection (Fig. 1A). However, decreases in social exploration were blunted in LPS-treated rats injected in the dark as compared to the light phase (Interaction: F9,48 = 8.906, followed by post hoc at 3 h and 8 h: p < 0.01). Three hours post-LPS, animals injected during the light phase had greatly reduced social exploration, whereas rats that received LPS during the dark phase showed similar activity to saline-injected rats. Rats injected with LPS during the dark phase did not merely delay their sickness response, as 8 h following immunostimulation, dark phase injected rats still showed a reduced sickness response and the effects of LPS on social investigation recovered to baseline in both groups by 24 h post-injection (Fig. 1A). LPS injection also reduced sucrose consumption in a sucrose anhedonia test (main effect of injection: F1,12 = 10.64, p < 0.01; Fig. 1B). Reductions in sucrose intake were decreased in rats injected with LPS during the dark phase as compared to rats injected during the middle of the light phase but this effect was not statistically significant (post hoc, p > 0.05). Rats injected with LPS during the dark phase also displayed less weight loss 24 h following LPS administration (main effect of injection and group: F1,12 = 46.32 and 18.83, p < 0.001; Fig. 1C).
Figure 1. Circadian timing of LPS injection affects sickness behavior.
Adult male Sprague-Dawley rats were injected with LPS (100 ug/kg) at either ZT6 or ZT16 and (A) social investigation, (B) sucrose anhedonia, and (C) changes in body mass were evaluated (n=4). Data are expressed as mean ± SEM. *p<0.05 between ZT16 and ZT6 LPS groups, †p<0.05 between LPS and saline
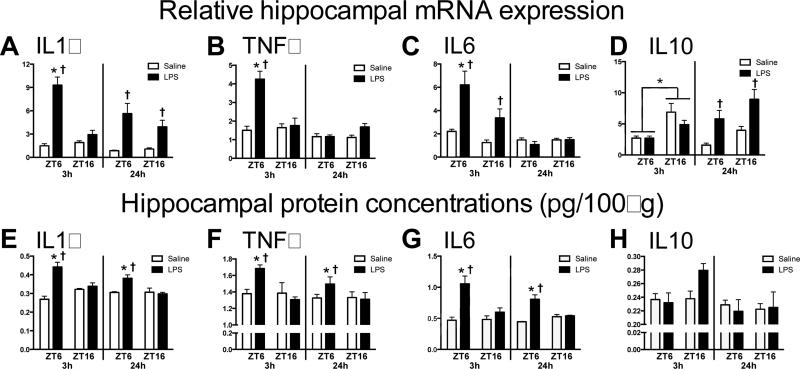
Sickness behaviors are induced by cytokine synthesis in the brain, particularly in the hippocampus (Dantzer et al., 2008). Therefore, we evaluated hippocampal cytokine responses in rats injected with LPS in the light versus the dark phase (n = 4/group). Hippocampal pro-inflammatory cytokine expression was elevated in rats injected during the middle of the light phase compared to rats injected during the dark phase (Fig 2A-C). There was an overall main effect of LPS on hippocampal IL1β mRNA expression 3 h after injection (main effect of LPS: F1,12 = 51.37, p < 0.0001). However, the IL1β response was blunted in rats that received LPS during the dark phase (Interaction: F1,12 = 30.5, p < 0.0001; Fig. 2A). Moreover, TNFα expression was only increased in rats that received LPS during the light phase; rats injected with LPS or saline during the dark phase had comparable TNFα mRNA expression at 3 h post-injection (Interaction: F1,12 = 15.82, p < 0.005, post hoc p < 0.05; Fig. 2B). IL6 mRNA expression was also upregulated by LPS injection, but more so in rats injected during the light phase (Interaction: F1,12 = 6.997, p < 0.05; Fig. 2C). Interestingly, the anti-inflammatory cytokine IL10 was altered by time of injection but in the opposite direction of the pro-inflammatory cytokines. IL-10 mRNA expression was elevated in rats injected with either saline or LPS during the dark, as compared to the light phase (main effect of ZT at 3h: F1,12 = 14.66, p < 0.05; Fig. 2D). Differences in pro-inflammatory mRNA expression were reflected in protein concentrations as hippocampal IL1β (Interaction at 3h and 24h: F1,11 = 14.68 and F1,12 = 7.943, p < 0.05), TNFα (Interaction at 3h: F1,11 = 5.834, p < 0.05, and IL6 (Interaction at 3h and 24h: F1,11 = 7.609 and F1,12 = 20.01, p < 0.05) protein were also elevated in rats injected with LPS during the light, as compared to the dark phase (Fig. 2E-G). In contrast, IL-10 protein concentrations did not mirror mRNA results. IL-10 was only induced 3 h post-LPS in rats injected during the dark phase and this effect was not significant (p = 0.07; Fig. 2H).
Figure 2. Temporal differences in hippocampal cytokine expression following an LPS injection.
Adult male Sprague-Dawley rats were injected with LPS (100 ug/kg) at either ZT6 or ZT16 and hippocampal tissue was collected after 3 or 24 h to evaluate (A) IL1β, (E) TNFα, (C) IL6, (D) IL10 mRNA expression and (E) IL1β, (F) TNFα, (G) IL6, (H) IL10 protein concentrations (n=4). Data are expressed as mean ± SEM. *p<0.05 between ZT16 and ZT6, †p<0.05 between LPS and saline.
In addition to evaluating cytokine expression, we measured several inflammatory pathway genes including NLRP3 (rate-limiting protein in NLRP3 inflammasome assembly) and TLR4 (pattern recognition receptor for LPS). These genes were specifically selected because they regulate the IL1β response as well as the production of other cytokines. We also evaluated MHC II mRNA expression because it is an indicator of microglia activation state. MHC II mRNA was increased during the light, as compared to the dark phase and unaffected by LPS injection (main effect of ZT at 3h: F1,11 = 13.48; Fig. S1A). There was no effect of time of LPS injection on either TLR4 or NLRP3 mRNA expression (Fig. S1B&C). These results suggest that there are time-of-day differences in sickness response and that the severity of the inflammatory response in the hippocampus correlates with circadian differences in sickness behavior.
Circadian clock genes are rhythmically expressed in hippocampal microglia
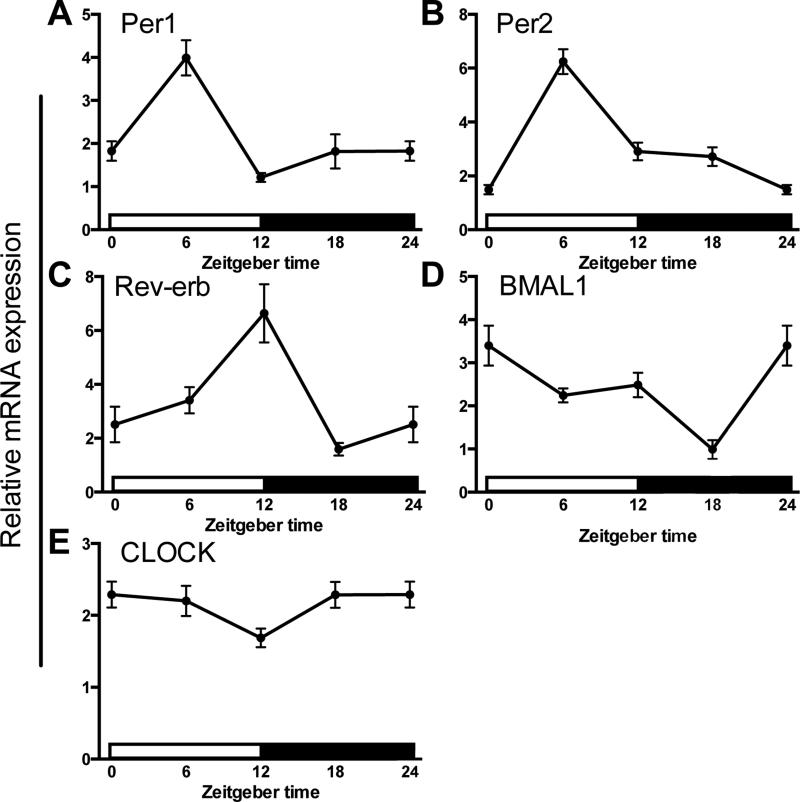
Microglia are the primary innate immune cell of the CNS and the dominant source of cytokines in the brain following a sub-septic peripheral immune challenge (Saijo and Glass, 2011). Circadian clock genes are rhythmically expressed in various immune cells. However, the presence and function of rhythmic clock gene expression in microglia are not well established. To determine whether hippocampal microglia demonstrate rhythmic expression of circadian clock genes, we isolated hippocampal microglia every 6 h from adult male Sprague-Dawley rats maintained on a 12:12 light/dark cycle (n=5 per time point). Microglia displayed time of day differences in expression of several core clock genes including Per1 (F3,16 = 43.7, p < 0.0001), Per2 (F3,15 = 17.61, p < 0.0001), Rev-erb (F3,15 = 11.62, p < 0.0005) and Bmal1 (F3,16 = 10.65, p < 0.0005; Fig. 3). Expression of Per1 and Per2 peaked during the middle of the light phase (Fig. 3A&B). Rhythmic Per1 expression was similar to Per1 rhythms described in macrophages, although Per2 peaked slightly later in macrophages (Hayashi et al., 2007, Keller et al., 2009). Rev-erb showed a similar expression pattern to Per1 and Per2; however, peak levels occurred at the end of the light phase (Fig. 3C). Interestingly, rhythmic expression of Rev-erb is also similar to expression patterns described in macrophages (Keller et al., 2009). Peak Bmal1 expression occurred at the onset of the light phase and decreased throughout the light phase and into the middle of the dark phase (Fig. 3D). The expression pattern of CLOCK, a dimerization partner of BMAL1, was relatively constant throughout the day (Fig. 3E). This is not atypical as previous research demonstrated that circadian rhythmicity in CLOCK mRNA is often minimal or nonexistent (Bjarnason et al., 2001, Takata et al., 2002, Sumova et al., 2003), including in macrophages (Hayashi et al., 2007, Keller et al., 2009).
Figure 3. Circadian clock gene expression in microglia.
Hippocampal microglia were isolated from adult male Sprague-Dawley rats every 6h (n=5). Microglia were plated for 3 h prior to RNA extraction. mRNA concentrations are expressed relative to β-actin and presented as mean ± SEM.
Inflammatory genes are rhythmically expression in hippocampal microglia
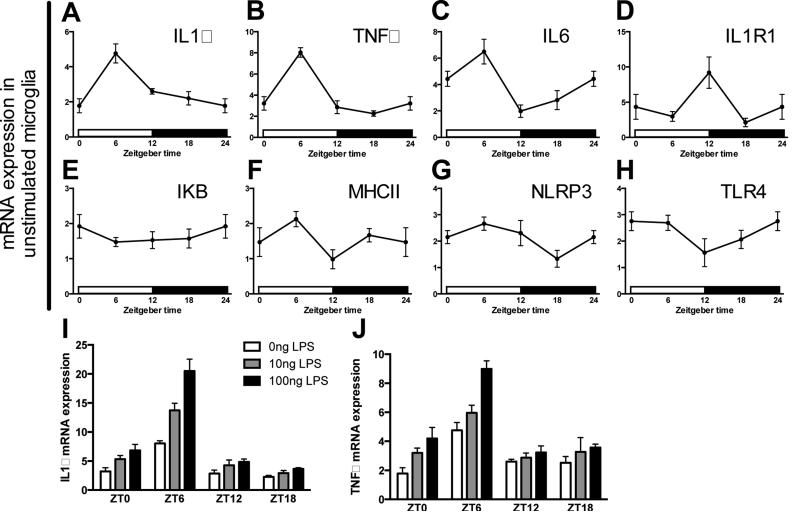
Peripheral clocks typically have local regulatory roles in tissues in which they are expressed (for example, see (Lamia et al., 2008)). Thus, we next sought to determine whether microglia display circadian rhythms in inflammatory genes that may mediate time of day differences in sickness response. There was a significant effect of time of day on IL1β (F3,15 = 9.697, p < 0.001; Fig. 4A), TNFα (F3,15 = 29.28, p < 0.0001; Fig. 4B), IL6 (F3,15 = 8.288, p < 0.005; Fig. 4C), and IL1R1 (F3,15 = 4.168, p < 0.05; Fig. 4D) mRNA expression but not several inflammatory pathway genes including IkB (F3,15 = 0.6653, p = 0.5862; Fig. 4E), MHCII (F3,15 = 2.703, p = 0.0826; Fig. 4F), NLRP3 (F3,15 = 2.478, p = 0.1011; Fig. 4G), and TLR4 (F3,15 = 2.104, p = 0.1426; Fig. 4H). Both IL1β and TNFα mRNA were specifically elevated in microglia isolated during the middle of the light phase.
Figure 4. Circadian gene expression of inflammatory factors in microglia.
Hippocampal microglia were obtained from adult male Sprague Dawley rats every 6 h (n=5). Microglia were then plated with media for 3 h and expression of (A) IL1β, (B) TNFα, (C) IL6, (D) IL1R1, (E) IKB, (F) MHCII, (G) NLRP3, and (H) TLR4 mRNA were evaluated. To assess microglia priming (I) IL1β and (J) TNFα mRNA expression were also evaluated in microglia stimulated for 3 h ex vivo with 0, 10, or 100 ng of LPS. mRNA concentrations are expressed relative to β-actin and presented as mean ± SEM.
Microglia appear more reactive during the middle of the light as compared to the dark phase. Microglia isolated during the light phase exhibited a more robust innate immune response to ex vivo challenge with LPS (TNFα interaction of ZT and dose: F3,45 = 2.821, IL1β interaction of time and dose: F3,45 = 7.881, p < 0.05; Fig. 4I&J). Whereas 100 ng of LPS elicited a robust IL1β and TNFα mRNA expression in microglia isolated during the light phase, this effect was greatly attenuated in microglia isolated at ZT18 (Fig. 4I&J). Importantly, these results establish that circadian rhythms in inflammation are an intrinsic property of microglia as microglia display time-of-day differences in LPS sensitivity in the absence of other cell types.
Circadian clock expression in microglia is likely entrained by, but not dependent on, glucocorticoids
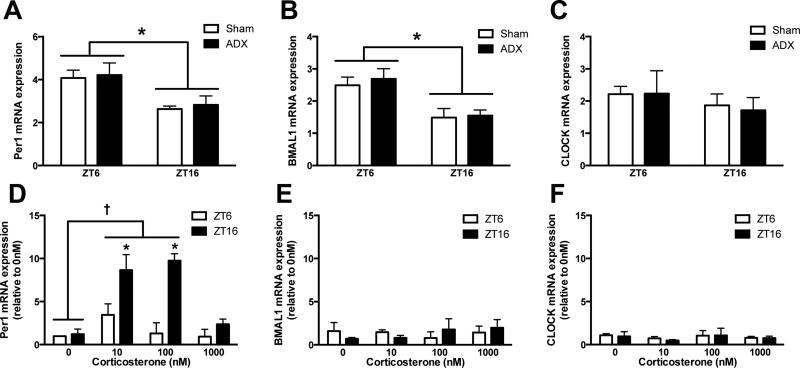
Glucocorticoids are well known to vary in a circadian fashion and to directly alter the responsivity of immune cells. To determine whether circadian clock gene expression in microglia is dependent on glucocorticoid signaling, rats either underwent an adrenalectomy (ADX) or sham surgery and hippocampal microglia were isolated one week later at either ZT6 or ZT16 (n = 4/group; confirmed that ADX ablated glucocorticoids with ELISA; Fig. S2). CORT was replaced in ADX rats’ drinking water until 24 h prior to microglia isolations to maintain the health of the rats. The results demonstrate that microglial rhythms persisted in the absence of glucocorticoids for 24 h. Per1 (main effect of time: F1,12 = 12.35, p < 0.005 but not surgery: F1,12 = 0.1682), Bmal1 (main effect of time: F1,12 = 16.88, p < 0.005 but not surgery F1,12 = 0.2542), and Clock (no effect of time or surgery: F1,12 = 0.8925 and 0.02072, respectively) mRNA expression did not differ between ADX or sham rats at either ZT6 or ZT16 (Fig. 5A-C).
Figure 5. Microglia rhythms in circadian clock genes are entrained by but not dependent on glucocorticoids.
Hippocampal microglia were isolated at ZT6 or ZT16 from rats that underwent either an ADX or sham surgery (n=4). Microglia were plated with media for 3 h and mRNA expression of (A) Per1, (B) BMAL1, and (C) CLOCK were evaluated. In a separate cohort of rats hippocampal microglia were isolated at ZT6 or ZT16 and stimulated ex vivo with 0, 10, 100, or 1000 nM CORT for 2 h prior to extracting mRNA to measure whether CORT induces (D) Per1, (E) BMAL1, and (F) CLOCK expression (n=3). mRNA concentrations are expressed relative to β-actin and presented as mean ± SEM. *p<0.05 between ZT6 and ZT16, †p<0.05 between CORT concentrations.
Previous work indicates that glucocorticoids are an entraining signal for peripheral oscillators (Balsalobre et al., 2000, Conway-Campbell et al., 2010). To establish whether glucocorticoids entrain microglial rhythms, microglia were isolated and plated with increasing concentrations of corticosterone (CORT; n = 3/group). Ex vivo CORT treatment specifically induced Per1 mRNA expression (main effect of CORT: F3,16 = 8.958, p < 0.001; Fig. 5D). CORT did not alter BMAL1 or CLOCK expression (Fig. 5E&F). These results are consistent with previous findings indicating that there is a glucocorticoid response element in the promoter region of Per1 (Conway-Campbell et al., 2010). Interestingly, CORT exerted a greater influence on Per1 expression in microglia isolated during the dark (when Per1 expression is lower), as compared to the light phase (Fig. 5D).
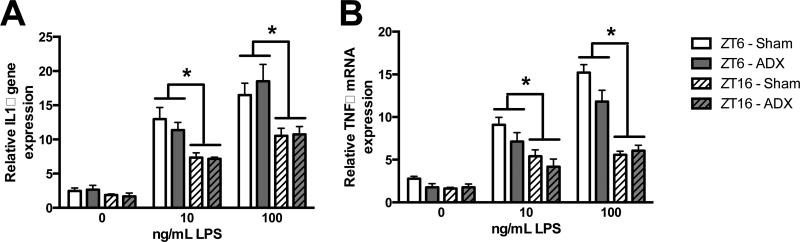
To evaluate whether diurnal differences in inflammatory potential also occur independently of glucocorticoids, microglia were isolated from ADX and sham rats either during the middle of the light or the dark phase and stimulated ex vivo with LPS. Microglia isolated from ADX and sham rats showed similar responses to ex vivo LPS stimulation at both time points (Fig. 5G&H); microglia isolated during the light phase had potentiated cytokine responses compared to microglia isolated during the dark phase (main effect of ZT on IL1 and TNF: F3,26 = 13.63 and F3,26 = 29.63, p < 0.0001). These results indicate the circadian responses to LPS stimulation in microglia do not depend on glucocorticoid concentrations directly prior to or at the time of immunostimulation.
Discussion
The circadian system is an integral part of homeostatic mechanisms in mammals. Daily variations in inflammatory challenges have likely led to temporal regulation of immune functions (Curtis et al., 2014). Here, we demonstrate that there is circadian regulation of inflammatory processes in the CNS. Microglia possess circadian clock mechanisms and display rhythmic fluctuations in basal inflammatory gene expression as well as inflammatory potential. Time-of-day differences in microglia priming appear functionally relevant as they are reflected in circadian differences in sickness response.
Previous work indicates that susceptibility to endotoxic shock varies throughout the day (Halberg et al., 1960, Marpegan et al., 2009, Spengler et al., 2012). However, temporal regulation of a transient immune stimulation had not been characterized. Therefore, we evaluated circadian differences in sickness behavior in rats in response to a sub-septic dose of LPS. Rats injected with LPS during the light phase reduced social exploration, a behavior commonly used to evaluate behavioral sickness. In contrast, rats injected with LPS during the dark phase displayed no change in social exploratory behavior 3 h post-LPS. Dark phase stimulated rats spent a similar amount of time engaged in social investigation as did rats that received a saline injection. Furthermore, rats injected with LPS during the light phase lost more body mass than did rats injected during the dark phase. These results indicate that an endotoxin challenge can elicit distinct sickness responses depending on the time of administration. It is possible that temporal differences in sickness response are an adaptive mechanism to conserve energy at times of high-energy demand. Indeed, animals are most likely to encounter infection and injury during the active phase in fight/flight emergencies. As mounting an immune response is energetically costly, it is potentially adaptive to delay immune responses until after the fight/flight emergency is over during the inactive phase of the circadian cycle.
Temporal changes in sickness behavior were reflected in hippocampal cytokine expression. The hippocampal pro-inflammatory cytokine response was markedly attenuated in rats injected with LPS during the dark as compared to the light phase. Indeed, there was no increase in TNFα mRNA in dark phase injected rats. Furthermore, IL1β was only elevated 3 h post-injection in rats that received LPS during the light phase. IL6 mRNA expression was elevated in both light and dark phase injected rats; however, the response was abrogated in rats injected during the dark phase. The dose of LPS used in this study (100 ug/kg) typically elicits a robust innate immune response. Thus, the fact that dark phase LPS injection failed to upregulate TNFα and IL1β 3 h following LPS administration is noteworthy. Additionally, mRNA expression of the anti-inflammatory cytokine IL-10 was elevated in rats injected during the dark compared to the light phase, suggesting that the inflammatory potential may be altered. The diminished inflammatory response in rats injected with LPS during the dark phase agrees with previous findings that suggest temporal variations in induction of pro- and anti-inflammatory cytokines underlie circadian differences in susceptibility to endotoxic shock (Hrushesky et al., 1994, Liu et al., 2006, Spengler et al., 2012). mRNA expression of several additional inflammatory genes including TLR4, NLRP3, and MHCII was also evaluated in an attempt to identify a pathway through which cytokine responses were altered. While there were no differences in TLR4 or NLRP3 mRNA expression, MHCII expression was elevated in the hippocampus irrespective of LPS injection. This suggests that there may be time-of-day differences in hippocampal microglial activation.
Circadian differences in hippocampal cytokines after an in vivo challenge with LPS may be mediated by cell-specific oscillations within microglia. Our results demonstrate that microglia possess circadian clock mechanisms. Furthermore, microglia display rhythmic expression of several pro-inflammatory cytokines including IL1β, TNFα, IL6, and IL1R1. Circadian clock genes may directly mediate these temporal fluctuations in inflammatory gene expression as translational regulation is a major output of the circadian system (Schibler, 2007), with approximately 10% of the mammalian transcriptome under circadian control (Panda et al., 2002, Eckel-Mahan et al., 2012). Of note, microglia period and cytokine gene expression showed similar rhythmicity, indicating that a common mediator may regulate them. One candidate is the circadian protein CLOCK, which regulates NF-kB-mediated transcription (Spengler et al., 2012) and activates transcription of the period genes (Gekakis et al., 1998). Here we evaluated I B mRNA expression in microglia as an indication of NF- B activation, but there were no temporal differences. However, as CLOCK increases NF-κB transcription by complexing with its p65 subunit (Spengler et al., 2012), changes in I B mRNA expression (a negative regulator of NF-kB) would not necessarily be expected.
In addition to displaying rhythms in inflammatory cytokines, microglia appear differentially primed for inflammatory challenge throughout the day. Hippocampal microglia isolated during the light phase of the day showed elevated cytokine mRNA induction in response to ex vivo LPS stimulation. This finding suggests that the strength of pro-inflammatory cytokine production in the hippocampus in response to inflammatory challenge may be determined by the circadian phase of the microglia clock.
Glucocorticoids have both pro- and anti-inflammatory effects on microglia (Frank et al., 2013). In addition to suppressing ongoing CNS inflammation, glucocorticoids can prime innate immune responses to subsequent pro-inflammatory challenge (Frank et al., 2010b). Diurnal differences in neuroinflammatory response are routinely attributed to the anti-inflammatory effects of corticosterone rather than intrinsic circadian mechanisms (Nguyen et al., 1998, Johnson et al., 2003), although this has rarely been tested. Therefore, we explored whether inflammatory rhythms in microglia were a direct effect of corticosterone. To test this hypothesis, rats were adrenalectomized (ADX) to remove endogenous glucocorticoids and glucocorticoid replacement terminated 24 h prior to microglia isolation during the middle of the light or middle of the dark phase. Microglia from ADX rats had a similar pattern in clock and inflammatory gene expression as rats that underwent a sham surgery, indicating that these rhythms persist in the absence of glucocorticoids. One limitation to this experiment is that isolating microglia only 24 h after corticosterone removal does not eliminate possible priming effects of glucocorticoids (Frank et al., 2014). Furthermore, the fact that circadian rhythms are apparent in the absence of glucocorticoids does not preclude the possibility that glucocorticoids influence circadian rhythms. The master circadian pacemaker synchronizes peripheral clocks through neural and hormonal outputs of the hypothalamic pituitary adrenal axis and the autonomic nervous system (Kalsbeek et al., 2012). Thus, we also evaluated whether glucocorticoids are an entraining signal for microglia rhythms. Per1 expression was induced by corticosterone in isolated microglia, suggesting that glucocorticoids may entrain microglial rhythms. Per1 induction was greater in microglia isolated during the dark phase indicating there may be temporal differences in glucocorticoids receptor expression. These results agree with previous findings indicating that stress (Meerlo et al., 2002) and exogenous glucocorticoid administration (Balsalobre et al., 2000, Sujino et al., 2012) can phase shift peripheral circadian clocks. Glucocorticoids likely shift circadian clock gene expression through a GRE in the promoter region of Per1 (Conway-Campbell et al., 2010). Although these experiments focused on the role of glucocorticoids in mediating inflammatory rhythms in microglia, there are alternative pathways through which the circadian system may influence diurnal rhythms in immune cells. For example, circadian rhythms in noradrenergic sympathetic nervous system innervation of the spleen drive oscillation in natural killer cell function (Logan et al., 2011). It is possible that the sympathetic nervous system is also involved in circadian regulation of neuroinflammation as β-adrenergic signaling can regulate trafficking of myeloid cells to the CNS (Wohleb et al., 2011).
We specifically focused on hippocampal microglia (as opposed to whole brain microglia) in these experiments for two main reasons. First, it is unclear whether circadian clocks are predominantly dependent on cell type or brain region. For example, it is possible that all the different cell types in the hippocampus have synchronized oscillations. Alternatively, it may be that all microglia within the brain oscillate in tandem, with brain region exerting little influence. The fact that the phase of circadian oscillations in microglia parallels rhythms described in macrophages supports the latter idea. Similar rhythms in clock expression may indicate that microglia and macrophages have similar entraining mechanisms that do not depend on the tissue of origin. Second, we chose to evaluate hippocampal microglia because the hippocampus has a high concentration of microglia and expresses a high density of cytokine receptors (Parnet et al., 1994), and thus appears more sensitive to immune stimulation. In addition, the hippocampus plays an important role in mediating the behavioral sickness response following inflammatory challenge (Dantzer et al., 2008).
Circadian disruption is associated with several inflammatory disorders including cancer, metabolic syndrome, and depression (Evans and Davidson, 2013). One important question that is not addressed in this study is whether disruptions in local rhythms in immune cells may underlie some of these negative consequences of circadian disruption. For example, aging is characterized by both elevations in inflammatory responses (including in microglia) and diminished strength of circadian rhythms (Gibson et al., 2009, Frank et al., 2010a). Future work should explore whether local rhythms in microglia are disrupted in aging, potentially leaving cells in the more “primed” phase of the circadian cycle.
Taken together, these findings indicate that the timing of an immune system challenge profoundly impacts the neuroinflammatory response. We show that there are circadian differences in sickness behavior in rats and that these behavioral fluctuations are reflected in temporal changes in hippocampal cytokine and microglial responses. Microglia show dramatic differences in immune activation throughout the day, with the expected robust response to immunostimulation during the animals resting phase but virtually no pro-inflammatory cytokine response during the active phase. We demonstrate that rhythms in microglia are entrained by, but oscillate in the absence of, glucocorticoids. These results suggest that time-of-day is an important factor to consider when planning immunotherapies and procedures that can induce neuroinflammation (Weil et al., 2009).
Supplementary Material
Highlights.
* There are temporal differences in sickness behavior in rats.
* Changes in sickness behavior are reflected in hippocampal cytokine and microglial responses.
* Microglia show dramatic differences in immune activation throughout the day, with peak activation during the light phase.
* Microglia rhythms are entrained by, but oscillate independent of, glucocorticoids
* These results suggest time-of-day should be considered when planning procedures that induce neuroinflammation.
Figure 6. Time-of-day differences in microglia inflammatory potential are not mediated by glucocorticoids.
Hippocampal microglia were isolated at ZT6 or ZT16 from rats that underwent either an ADX or sham surgery (n=4). Microglia were plated with 0, 10, or 100 ng of LPS for 3 h and mRNA expression of (A) IL1 and (B) TNF were evaluated. mRNA concentrations are expressed relative to β-actin and presented as mean ± SEM. *p<0.05 between ZT6 and ZT16.
Acknowledgements
We thank Robert Spencer for helpful discussion and John D'Angelo for excellent animal care. This research was supported by NIH grant MH096224-01 to S.F.M and 1F32AG048672-01 to L.K.F.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: The authors declare no competing financial interests. All authors concur with the submission of this manuscript and none of the data have been previously reported or are under consideration for publication elsewhere.
References
- Arjona A, Sarkar DK. Evidence supporting a circadian control of natural killer cell function. Brain Behav Immun. 2006;20:469–476. doi: 10.1016/j.bbi.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science. 2000;289:2344–2347. doi: 10.1126/science.289.5488.2344. [DOI] [PubMed] [Google Scholar]
- Bjarnason GA, Jordan RC, Wood PA, Li Q, Lincoln DW, Sothern RB, Hrushesky WJ, Ben-David Y. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. The American journal of pathology. 2001;158:1793–1801. doi: 10.1016/S0002-9440(10)64135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin DB, James FO, Wu A, Cho-Park PF, Xiong H, Sun ZS. Circadian clock genes oscillate in human peripheral blood mononuclear cells. Blood. 2003;102:4143–4145. doi: 10.1182/blood-2003-03-0779. [DOI] [PubMed] [Google Scholar]
- Bollinger T, Leutz A, Leliavski A, Skrum L, Kovac J, Bonacina L, Benedict C, Lange T, Westermann J, Oster H, Solbach W. Circadian clocks in mouse and human CD4+ T cells. PLoS One. 2011;6:e29801. doi: 10.1371/journal.pone.0029801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, Besing RC, Menaker M, Gewirtz AT, Davidson AJ. Dysregulation of inflammatory responses by chronic circadian disruption. J Immunol. 2010;185:5796–5805. doi: 10.4049/jimmunol.1001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway-Campbell BL, Sarabdjitsingh RA, McKenna MA, Pooley JR, Kershaw YM, Meijer OC, De Kloet ER, Lightman SL. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the clock gene period 1 in rat hippocampus. Journal of neuroendocrinology. 2010;22:1093–1100. doi: 10.1111/j.1365-2826.2010.02051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O'Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Patel VR, Mohney RP, Vignola KS, Baldi P, Sassone-Corsi P. Coordination of the transcriptome and metabolome by the circadian clock. Proc Natl Acad Sci U S A. 2012;109:5541–5546. doi: 10.1073/pnas.1118726109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JA, Davidson AJ. Health consequences of circadian disruption in humans and animal models. Progress in molecular biology and translational science. 2013;119:283–323. doi: 10.1016/B978-0-12-396971-2.00010-5. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. Mice exposed to dim light at night exaggerate inflammatory responses to lipopolysaccharide. Brain Behav Immun. 2013;34:159–163. doi: 10.1016/j.bbi.2013.08.011. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Nelson RJ. The Effects of Light at Night on Circadian Clocks and Metabolism. Endocrine reviews. 2014:er20131051. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- Frank MG, Barrientos RM, Watkins LR, Maier SF. Aging sensitizes rapidly isolated hippocampal microglia to LPS ex vivo. Journal of neuroimmunology. 2010a;226:181–184. doi: 10.1016/j.jneuroim.2010.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Miguel ZD, Watkins LR, Maier SF. Prior exposure to glucocorticoids sensitizes the neuroinflammatory and peripheral inflammatory responses to E. coli lipopolysaccharide. Brain Behav Immun. 2010b;24:19–30. doi: 10.1016/j.bbi.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain Behav Immun. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain Behav Immun. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MG, Wieseler-Frank JL, Watkins LR, Maier SF. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. Journal of neuroscience methods. 2006;151:121–130. doi: 10.1016/j.jneumeth.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Gekakis N, Staknis D, Nguyen HB, Davis FC, Wilsbacher LD, King DP, Takahashi JS, Weitz CJ. Role of the CLOCK protein in the mammalian circadian mechanism. Science. 1998;280:1564–1569. doi: 10.1126/science.280.5369.1564. [DOI] [PubMed] [Google Scholar]
- Gibson EM, Williams WP, 3rd, Kriegsfeld LJ. Aging in the circadian system: considerations for health, disease prevention and longevity. Experimental gerontology. 2009;44:51–56. doi: 10.1016/j.exger.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg F, Johnson EA, Brown BW, Bittner JJ. Susceptibility rhythm to E. coli endotoxin and bioassay. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1960;103:142–144. doi: 10.3181/00379727-103-25439. [DOI] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, Tezuka M. Characterization of the molecular clock in mouse peritoneal macrophages. Biol Pharm Bull. 2007;30:621–626. doi: 10.1248/bpb.30.621. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Koyanagi S, Kusunose N, Okada R, Wu Z, Tozaki-Saitoh H, Ukai K, Kohsaka S, Inoue K, Ohdo S, Nakanishi H. The intrinsic microglial molecular clock controls synaptic strength via the circadian expression of cathepsin S. Scientific reports. 2013;3:2744. doi: 10.1038/srep02744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrushesky WJ, Langevin T, Kim YJ, Wood PA. Circadian dynamics of tumor necrosis factor alpha (cachectin) lethality. The Journal of experimental medicine. 1994;180:1059–1065. doi: 10.1084/jem.180.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hut RA, Beersma DG. Evolution of time-keeping mechanisms: early emergence and adaptation to photoperiod. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2011;366:2141–2154. doi: 10.1098/rstb.2010.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O'Connor KA, Hansen MK, Watkins LR, Maier SF. Effects of prior stress on LPS-induced cytokine and sickness responses. Am J Physiol Regul Integr Comp Physiol. 2003;284:R422–432. doi: 10.1152/ajpregu.00230.2002. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, Endert E, Buijs RM, Fliers E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Molecular and cellular endocrinology. 2012;349:20–29. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MT, Cho H, Lesch HP, Gosselin D, Heinz S, Tanaka-Oishi Y, Benner C, Kaikkonen MU, Kim AS, Kosaka M, Lee CY, Watt A, Grossman TR, Rosenfeld MG, Evans RM, Glass CK. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch KF, Weitz CJ. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, Sun ZS. The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infection and immunity. 2006;74:4750–4756. doi: 10.1128/IAI.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Arjona A, Sarkar DK. Role of sympathetic nervous system in the entrainment of circadian natural-killer cell function. Brain Behav Immun. 2011;25:101–109. doi: 10.1016/j.bbi.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marpegan L, Leone MJ, Katz ME, Sobrero PM, Bekinstein TA, Golombek DA. Diurnal variation in endotoxin-induced mortality in mice: correlation with proinflammatory factors. Chronobiol Int. 2009;26:1430–1442. doi: 10.3109/07420520903408358. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, Turek FW. The effects of social defeat and other stressors on the expression of circadian rhythms. Stress. 2002;5:15–22. doi: 10.1080/102538902900012323. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Nakazato R, Takarada T, Yamamoto T, Hotta S, Hinoi E, Yoneda Y. Selective upregulation of Per1 mRNA expression by ATP through activation of P2X7 purinergic receptors expressed in microglial cells. Journal of pharmacological sciences. 2011;116:350–361. doi: 10.1254/jphs.11069fp. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. J Neurosci. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan EK, Anderson ST, Moynagh PN, Coogan AN. Long-lasting effects of sepsis on circadian rhythms in the mouse. PLoS One. 2012;7:e47087. doi: 10.1371/journal.pone.0047087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Parnet P, Amindari S, Wu C, Brunke-Reese D, Goujon E, Weyhenmeyer JA, Dantzer R, Kelley KW. Expression of type I and type II interleukin-1 receptors in mouse brain. Brain Res Mol Brain Res. 1994;27:63–70. doi: 10.1016/0169-328x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Saijo K, Glass CK. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- Schibler U. The daily timing of gene expression and physiology in mammals. Dialogues in clinical neuroscience. 2007;9:257–272. doi: 10.31887/DCNS.2007.9.3/uschibler. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain Behav Immun. 2012a;26:407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012b;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, Gitlin, Artemicheva NM, Deluca KA, Gudkov AV, Antoch MP. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci U S A. 2012;109:E2457–2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujino M, Furukawa K, Koinuma S, Fujioka A, Nagano M, Iigo M, Shigeyoshi Y. Differential entrainment of peripheral clocks in the rat by glucocorticoid and feeding. Endocrinology. 2012;153:2277–2286. doi: 10.1210/en.2011-1794. [DOI] [PubMed] [Google Scholar]
- Sumova A, Jac M, Sladek M, Sauman I, Illnerova H. Clock gene daily profiles and their phase relationship in the rat suprachiasmatic nucleus are affected by photoperiod. J Biol Rhythms. 2003;18:134–144. doi: 10.1177/0748730403251801. [DOI] [PubMed] [Google Scholar]
- Takata M, Burioka N, Ohdo S, Takane H, Terazono H, Miyata M, Sako T, Suyama H, Fukuoka Y, Tomita K, Shimizu E. Daily expression of mRNAs for the mammalian Clock genes Per2 and clock in mouse suprachiasmatic nuclei and liver and human peripheral blood mononuclear cells. Japanese journal of pharmacology. 2002;90:263–269. doi: 10.1254/jjp.90.263. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Karelina K, Su AJ, Barker JM, Norman GJ, Zhang N, Devries AC, Nelson RJ. Time-of-day determines neuronal damage and mortality after cardiac arrest. Neurobiol Dis. 2009;36:352–360. doi: 10.1016/j.nbd.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb ES, Hanke ML, Corona AW, Powell ND, Stiner LM, Bailey MT, Nelson RJ, Godbout JP, Sheridan JF. beta-Adrenergic receptor antagonism prevents anxiety-like behavior and microglial reactivity induced by repeated social defeat. J Neurosci. 2011;31:6277–6288. doi: 10.1523/JNEUROSCI.0450-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.