Abstract
Purpose
Survivorship care plans (SCP) are recommended for all cancer patients and could be especially useful to survivors 65 years and over (“older”). This study examined receipt of SCPs among older breast cancer survivors and whether SCPs were associated with improved patient-reported outcomes.
Methods
Three hundred and twenty-eight older women diagnosed with invasive, nonmetastatic breast cancer between 2007–2011 were recruited from 78 cooperative-group sites. Participants completed telephone interviews at baseline and 1-year posttreatment. Regression analyses examined SCP receipt (yes/no) and functioning (EORTC-QLQ-C30), cancer worry, and experiences of survivorship care (care coordination, knowledge).
Results
Only 35 % of women received SCPs. For each 1-year increase in age, there was a 5 % lower odds of receiving an SCP (odds ratio (OR)=0.94, 95 % confidence interval (CI) 0.91–0.98, p=0.007). Besides age, no other factor predicted SCPs. SCP receipt was associated with greater knowledge and understanding of requisite follow-up care (p<0.05); however, functioning was not significantly different among those with vs. without SCPs.
Conclusions
Receipt of care plans was limited. SCPs improved understanding of breast cancer follow-up care among older survivors, but did not impact functioning one year post-treatment.
Implications for Cancer Survivors
To impact functioning and salient needs of the growing cohort of older survivors, survivorship care plans likely should be tailored to geriatric-specific issues. To improve functioning, SCP content should expand to include exercise, nutrition, polypharmacy, social support and management of symptom burden from cancer, and other comorbid conditions. To improve follow-up care for cancer survivors, SCPs should delineate shared care roles between oncology and primary care in managing recurrence surveillance, screening, and cancer sequelae.
Keywords: Survivorship care plan, Breast cancer, Cancer survivors, Older adults, Cancer survivorship and aging
Introduction
Women 65 years and older (“older”) constitute 55 % of the three million U.S. breast cancer survivors, and will account for a greater absolute number and proportion of survivors with “the graying of America” [1, 2]. This older survivor population often has age-related declines in functioning and reserve, increasing levels of comorbid illness, and diminished social and economic resources [3–6].
These forces of aging can pose unique challenges for survivorship care, including the need to monitor adjuvant hormonal therapy, manage multiple symptoms and medications, and coordinate care delivery by multiple physicians [7–10]. Survivorship care can be further complicated if older patients are confused about their cancer treatment history [11], recommended follow-up care [8, 12], or how to manage their multiple illnesses [13]. Older patients may also misattribute modifiable symptoms to “normal aging” or believe that their symptoms are not treatable, leading to under-reporting during follow-up visits [14–17].
To improve post-treatment cancer care, the Institute of Medicine recommends providing survivorship care plans, and this recommendation was formally incorporated into oncology practice guidelines in 2007 [18–23]. The expectation was that care plans would enhance survivorship experiences and translate into improved functioning and survival [18, 20, 24, 25]. While dissemination has been slow [26–28], emerging data suggests that care plans may decrease cancer worry [29], enhance understanding of care coordination [30, 31], increase confidence in communicating with providers [31], and improve adherence to late effects surveillance [32]. However, there is little data regarding the effectiveness of care plans for improving functioning [33], and no data on their use in older populations, a group where functioning is an especially important outcome [34].
To fill this gap, we analyzed data from a national prospective cohort of older breast cancer patients to evaluate the use and impact of survivorship care plans on patient-reported outcomes one-year postactive treatment. After describing correlates of care plan receipt, we test the hypothesis that older women who received plans (vs. not) would report better experiences of survivorship care, controlling for covariates. Further, we postulated that older women with care plans would report better functioning and less worry than women without plans, controlling for age and prediagnosis functioning. These associations were also examined among different subgroups based on age, education, and social support. Finally, we conducted exploratory analyses to assess whether survivors’ experiences of care were associated with functioning. Results of this study are intended to contribute to the growing literature on survivorship care plans, evaluate their benefits among older breast cancer survivors, and guide future interventions to refine care plans for the growing older survivorship population.
Methods
Study design and data collection
This report is a secondary analysis of data from a larger longitudinal cohort study examining chemotherapy patterns and outcomes among older women at 78 hospitals/practices affiliated with Cancer and Leukemia Group B (CALGB) protocol #369901, presently part of the Alliance for Clinical Trials in Oncology [35, 36]. The protocol met HIPAA standards and was approved by CALGB, NCI, and institutional review boards at all sites. Clinical research associates (CRAs) ascertained patients, confirmed eligibility, and upon physician approval, obtained consent. Registration was managed by the CALGB Statistical Center. Oncologists completed a one-time mail survey, and clinical data were obtained via medical chart abstraction. Participants in the cohort were assessed via structured telephone interviews at baseline, 6 months, 12-months postbaseline, 24-months postbaseline, and then annually for up to 7 years. This secondary analysis focused on outcomes at 24-months, as this follow-up timepoint roughly corresponds to one year after active treatment for breast cancer. Active treatment for breast cancer comprised surgery, chemotherapy, and radiation, but excluded hormonal therapy.
Setting and population
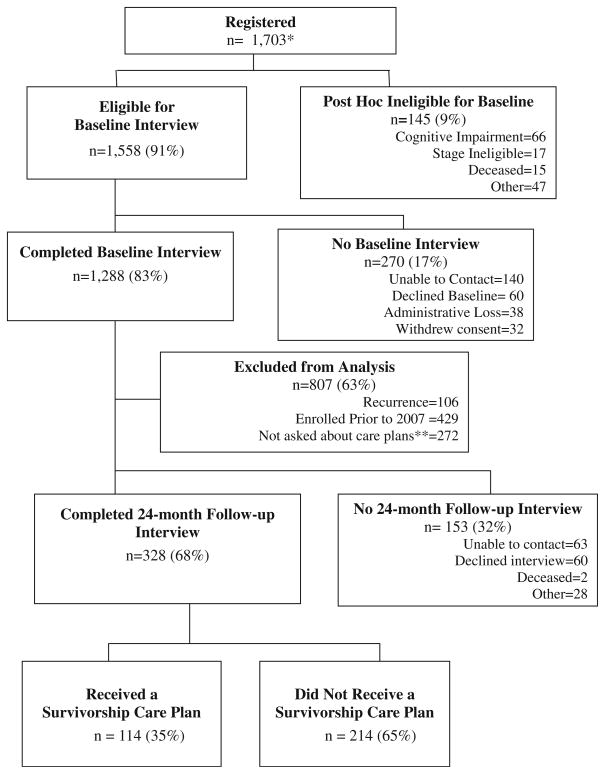
Participants were enrolled between January 2004 and April 2011. To enroll in the study, one must have been (a) female with newly diagnosed with invasive nonmetastatic breast cancer (tumors ≥1 cm, stage I–III), b) at least 65 years old at the time of breast cancer diagnosis, (c) English- or Spanish-speaking, and (d) within 20 weeks of their last definitive surgery. Among the 1,703 women registered, 91 % were eligible to complete the baseline interview (Fig. 1). One hundred and forty-five women were ineligible for baseline interviews due to failing the cognitive screen [37], dying prior to interview, or being found stage ineligible. The larger cohort sample included 1,558 participants, 1,288 of whom completed a baseline interview. Among these 1,288 participants, women were excluded from this secondary analysis due to the following: study enrollment before 2007 when care plans were first recommended [19] and enrollment in late 2008 before questions regarding care planning were added to interviews (n= 701). Additionally, we excluded 106 women who experienced breast cancer recurrence between baseline and 24-month follow-up due to re-initiation of active breast cancer treatment.
Fig. 1.
Consort diagram for sample selection of older breast cancer patients and survivorship care planning. * a total of 1,703 participants registered to the study. This is a correction from a 2012 publication indicating 1,704 participants [36]. A duplicate entry for one participant was deleted. ** 272 participants were missing a response to the item regarding receipt of a survivorship care plan because this item was only added to surveys mid-study in late 2008
From this target sample, 328 women (68 %) completed 24-month interviews (one year postactive treatment), thus forming the final analytic sample for this secondary analysis. There were no differences in sociodemographic or clinical factors between the final analytic sample and the 153 women excluded due to missing data. Additionally, the 328 women in the analytic sample were similar to the overall cohort, except for having earlier stage (p=0.04, due to the exclusion of recurrences), and having received treatment in a community setting (vs. comprehensive cancer center, p=0.004). These two factors were not controlled for as covariates in multivariate analyses, given that “site of treatment” did not affect the results when included in the model and “earlier stage” was anticipated given our deliberate exclusion of women with recurrence from the final analytic sample.
Measures
Measures were guided by the care planning research paradigm described by Parry and colleagues [33]. After examining correlates of receipt of a survivorship care plan (yes/no), this variable was the primary factor used to assess associations with study outcomes. Women were asked if they “were ever given a written breast cancer care plan by the doctors you have seen when you finished your treatment? This plan might include a summary of your breast cancer and all the treatments you got, and suggestions for what things you or your doctors should do in the next year.”
Outcomes
We examined the association of care plans with two patient-reported outcomes: measures of survivorship care experiences (patient-oncologist communication, care coordination, self-efficacy in communicating with physicians, and knowledge of survivorship care) and functioning (physical, emotional, and role). Cancer worry was a secondary outcome.
Communication with oncologists was adapted from the five-item scale from the Primary Care Assessment Survey (PCAS), and assesses thoroughness of oncologists’ questions about health concerns and clarity of instructions regarding when to seek additional care [38]. Scores range from 0–100 (Cronbach’s=0.97), but given that observed scores were skewed toward excellent communication, scores were categorized into “high” vs. “low” based on the sample median.
Self-efficacy in communicating with physicians (communication self-efficacy) was measured by the Perceived Efficacy in Patient-Physician Interactions scale [39]. This 10-item scale assesses patients’ confidence in effectively communicating their needs and health concerns to providers, with scores ranging from 10–50 (Cronbach’s=0.90). Scores were categorized into “high” vs. “low” based on the sample median since there were ceiling effects.
Perception of care coordination was assessed using one item from the Picker adult in-patient questionnaire [40, 41] adapted for cancer survivors. The item asked the survivor to rate the degree to which “doctors coordinated her care so that each physician knows what s/he is responsible for” where responses ranged from “very coordinated” to “very uncoordinated” on a 5-point Likert scale. Based on prior research, responses were grouped into “very coordinated” vs. all other responses [26, 42, 43].
Understanding of breast cancer survivorship care was assessed by a single 4-point Likert-scaled item and dichotomized as “excellent” vs. “less than excellent” [26, 42, 43]. One Picker [40, 44] item was adapted for survivors in order to assess having knowledge of the next steps in cancer follow-up care (“always” vs. “less than always”) [26, 42, 43].
Functional outcomes were assessed by the European Organization for Research and Treatment questionnaire (QLQ-C30), initially developed to assess functioning and quality of life outcomes in cancer populations [45–49]. Three QLQ-C30 subscales, physical functioning (Cronbach’s=0.62), emotional functioning (Cronbach’s=0.76), and role functioning (Cronbach’s=0.87), were used; higher scores represent better functioning [45–49].
Cancer worry was assessed by four items from the Cancer Rehabilitation Evaluation System (CARES) (Cronbach’s= 0.77) [50]. Lower scores represent less worry.
Controlling variables
Socio-demographic, clinical, and physician measures were included in these analyses as correlates of receiving a care plan and as potential controlling variables for assessment of outcomes. Socio-demographic factors include age, race, marital status, type of health insurance, education, and year of enrollment. Clinical factors include stage, estrogen receptor status, time since diagnosis, and treatment (surgery, chemotherapy); data on radiotherapy were not collected. Comorbid illness was assessed by the 16-item Physical Health scale of the Older Americans Resources and Services Multidimensional Functional Assessment [51]. Pre-cancer functioning were measured using the physical (Cronbach’s=0.94, PCS) and mental (Cronbach’s=0.97, MCS) component summary scores from Medical Outcomes Survey SF-12; prior role function was measured using a single item [52, 53]. Oncologist factors included gender, years since medical school graduation, practice setting, and patient volume for breast cancer and for patients 65 and older (high vs. low based on sample median).
Statistical analysis
We used t tests and X2 tests to determine differences between women who received care plans versus those who did not with respect to socio-demographic, clinical, and physician factors. Next, univariate and logistic regression analyses tested for associations of survivorship care planning and individual measures of experiences of survivorship care. Variables related at p≤0.05 in univariate analysis to both survivorship care plans and experiences of care were considered for inclusion in the regression model. Backward elimination was then used to select the final model.
Separate linear regression models were used to examine associations between care plans and the three functional outcomes and cancer worry, controlling for age and pre-cancer functioning, a priori covariates. Additionally, in exploratory analyses, we used linear regression models to examine relationships between care plans and functioning among subgroups based on age, education, and social support. Additional exploratory analyses of the associations between functioning and experiences of survivorship care followed similar methodology. The latter were examined to suggest potential pathways of the effect of survivorship care plans. Hosmer-Lemeshow (H-L) and R2 statistics were used to assess model fit for logistic and linear regressions, respectively.
Since this was an unplanned analysis, we also estimated post hoc power to detect a relationship between survivorship care plans and our primary outcome—functioning. Given the sample size, the study had more than 80 % power (two-sided, p=0.05) to detect a clinically meaningful difference of 8–10 points on all three functional outcomes [54], representing approximately 1 standard deviation on the EORTC [45–49], between women who received a care plan vs. those who did not. Power to detect a smaller effect (4-point difference or a 0.5 standard deviation) was lower at 34, 63, and 39 % for physical, emotional, and role function scales, respectively. We did not consider correlation of care plans within site since results were largely null; correction for intra-class correlations should only have further decreased significance and power. Data were analyzed with SAS v.9.2 software (SAS Institute Inc., Cary, NC). Results analyzed were available in the study database as of November 6, 2013.
Results
The older survivors included in this analysis were, on average, 72.8 years old (Table 1). Most women had ER-positive, node negative breast cancers, and 39.8 % had received adjuvant chemotherapy. Women reported physical (mean=51.3± 7.9SD) and emotional (mean=56.7±5.4SD) function prior to cancer diagnosis, comparable to population norms (mean=50±10SD).
Table 1.
Characteristics of older breast cancer survivors by self-reported receipt of a survivorship care plan
| Total samplea, b
|
Survivorship care planb
|
||||||
|---|---|---|---|---|---|---|---|
|
N=328
|
YES, N=114
|
NO, N=214
|
|||||
| No. | % | No. | % | No. | % | p-value | |
| Age (65–88 years) mean±SD | 72.8±5.8 | 71.6±5.5 | 73.4±5.8 | 0.006 | |||
| 65–69 | 116 | 35 | 47 | 41 | 69 | 59 | |
| 70–74 | 86 | 26 | 34 | 40 | 52 | 60 | |
| 75–79 | 72 | 22 | 21 | 29 | 51 | 71 | |
| 80 or older | 54 | 17 | 12 | 22 | 42 | 78 | |
| Marital status | |||||||
| Married | 191 | 58 | 76 | 40 | 115 | 60 | 0.02 |
| Not married | 137 | 42 | 38 | 28 | 99 | 72 | |
| Racec | |||||||
| Hispanic/Latina | 4 | 1 | 1 | 25 | 3 | 75 | 0.12c |
| AI/AN | 5 | 2 | 1 | 20 | 4 | 80 | |
| A/PI | 2 | <1 | 2 | 100 | – | – | |
| AA/B | 20 | 6 | 11 | 55 | 9 | 45 | |
| C/W | 296 | 90 | 99 | 33 | 197 | 67 | |
| More than one race | 1 | <1 | – | – | 1 | 100 | |
| Education | |||||||
| 12 years or less | 128 | 39 | 41 | 32 | 87 | 68 | 0.62 |
| More than 12 years | 200 | 61 | 73 | 36 | 127 | 64 | |
| Insurance | |||||||
| Medicare only | 29 | 8 | 11 | 38 | 18 | 62 | 0.93 |
| Medicare+Medicaid | 34 | 10 | 12 | 35 | 22 | 65 | |
| Medicare+private | 265 | 82 | 91 | 34 | 174 | 66 | |
| HMO | |||||||
| Yes | 53 | 16 | 24 | 45 | 29 | 55 | 0.08 |
| No | 275 | 84 | 90 | 33 | 185 | 67 | |
| Cancer stage | |||||||
| I | 160 | 49 | 58 | 36 | 102 | 64 | 0.81 |
| IIA | 108 | 33 | 37 | 34 | 71 | 66 | |
| IIB–III | 60 | 18 | 19 | 32 | 41 | 68 | |
| Surgery type | |||||||
| Breast conservation | 210 | 64 | 74 | 35 | 136 | 65 | 0.85 |
| Mastectomy | 117 | 36 | 40 | 34 | 77 | 66 | |
| Estrogen receptor status | |||||||
| Positive | 280 | 88 | 94 | 34 | 186 | 66 | 0.36 |
| Negative | 47 | 12 | 19 | 40 | 28 | 60 | |
| Chemotherapy | |||||||
| No | 197 | 60 | 68 | 35 | 129 | 65 | 0.87 |
| Yes | 130 | 40 | 46 | 35 | 84 | 65 | |
| Time since cancer diagnosis (mean±SD) | 24.1±4.1 | 24.1±4.3 | 24.0±3.8 | 0.79 | |||
| Comorbid illness (mean±SD) | 2.8±1.6 | 2.7±1.6 | 2.9±1.6 | 0.40 | |||
| Physical function, pre-cancerd (mean±SD) | 51.3±7.9 | 51.6±7.3 | 51.2±8.2 | 0.71 | |||
| Mental function, pre-cancere (mean±SD) | 56.7±5.4 | 57.0±5.1 | 56.6±5.6 | 0.47 | |||
| Setting of cancer care | |||||||
| NCI Cancer Center | 74 | 23 | 24 | 32 | 50 | 68 | 0.63 |
| Community | 254 | 77 | 90 | 35 | 164 | 65 | |
| Oncologist gender | |||||||
| Male | 192 | 62 | 70 | 36 | 122 | 64 | 0.24 |
| Female | 117 | 38 | 35 | 30 | 82 | 70 | |
| Years since graduation from medical schoolf (mean±SD) | 20.9±9.0 | 20.7±9.9 | 20.9±8.5 | 0.82 | |||
| % Patients 65 and older (mean±SD) | 44.4±12.5 % | 44.8±13 % | 44.1±12 % | 0.68 | |||
| Patient volume breast cancer | |||||||
| Less than 50 % | 162 | 56 | 55 | 34 | 107 | 66 | 0.95 |
| 50 % or more | 128 | 44 | 43 | 34 | 85 | 66 | |
| Seen by a medical oncologist | |||||||
| Yes | 304 | 93 | 104 | 34 | 200 | 66 | 0.46 |
| No | 24 | 7 | 10 | 42 | 14 | 58 | |
| Year of study enrollmentg | |||||||
| 2007 | 50 | 15 | 10 | 20 | 40 | 80 | 0.06 |
| 2008 | 110 | 33 | 42 | 38 | 68 | 62 | |
| 2009 | 99 | 30 | 37 | 37 | 62 | 63 | |
| 2010/2011 | 69 | 21 | 25 | 36 | 44 | 64 | |
No. number of participants, SD standard deviation, for p-values, where italics indicates p < .05, AI/AN American Indian/Alaskan Native, A/PI Asian/Pacific Islander, AA/B African American/Black, C/W Caucasian/non-Hispanic White, HMO health maintenance organization, NCI National Cancer Institute
Some numbers may not add to total, since some variables have missing data. All variables are missing <5 %, except physician-reported patient volumes (12–16 % missing)
We have used column percentages to describe the distribution of each characteristic variable and row percentages to describe the distribution of survivorship care plan for each level of the characteristic variables
Race was collapsed into two categories for univariate analyses, White (n=296) vs. non-White(n=32)
Pre-cancer physical function assessed by the SF-12 Physical Component Summary. Scores range from 0–100, where higher PCS scores represent better function; the population average score is 50 with a standard deviation of 10 [52, 53]
Pre-cancer mental function assessed by the SF-12 Mental Component Summary. Scores range from 0–100, where higher MCS scores represent better mental function; the population average score is 50 with a standard deviation of 10 [52, 53]
Years since medical school graduation=(survey completion date)–(physician-reported graduation date)
Use of care plans increased from 20 to 37 % in the study period (p value for trend=0.10), but overall, only an average of 35 % of older women reported ever receiving a plan (Table 1). Women who received plans were younger than those who did not (p=0.006) and more likely to be married (p=0.02). Receipt of a care plan was not related to any other socio-demographic, clinical, or physician-related factors, including receipt of chemotherapy. Only age remained significantly related to care plans in multivariate analyses, where the odds of receiving a survivorship care plan decreased by 5 % for each one-year increase in age (OR=0.94, 95 % CI 0.91–0.98, p=0.007).
Women who received a care plan tended to report better experiences of survivorship care than those who did not receive plans (Table 2). Specifically, those who received care plans (vs. not) had higher adjusted odds of reporting an excellent (vs. less) understanding of follow-up care (aOR 1.73, 95 % CI 1.08–2.9, p=0.02) and greater knowledge of next steps in cancer care (aOR 1.72, 95 % CI 1.03–2.9, p=0.04), controlling for covariates. Women who received care plans tended to also report higher (vs. lower) self-efficacy in communicating with their oncologists, compared to women without care plans (aOR 1.42, 95 % CI 0.92–2.35, p=0.11). Cancer worry and physical, social, and role functioning did not significantly differ among older women with vs. without care plans at one-year post-treatment (Table 3). No association was found between care plan receipt and functioning in any subgroups (e.g., based on age, education, social support; data not shown).
Table 2.
Relationships between receipt of survivorship care plan and experiences of survivorship care among older women with breast cancer
| Overall sample | Survivorship care plan
|
Odds ratios, 95 % confidence intervals and p-values
|
|||||
|---|---|---|---|---|---|---|---|
|
N=328 % |
YES, n=114 % |
NO, n=214 % |
Unadjusted OR(CI) | p | Adjusted OR(CI)ab | p | |
| Patient-oncologist communication | |||||||
| Low communication | 50 | 44 | 53 | Referent | 0.12 | Referent | 0.16 |
| High communication | 50 | 56 | 47 | 1.45 (0.90–2.35) | 1.42 (0.87–2.30) | ||
| Communication self-efficacy | |||||||
| Low self-efficacy | 50 | 44 | 53 | Referent | 0.11 | Referent | 0.11 |
| High self-efficacy | 50 | 56 | 47 | 1.45 (0.91–2.31) | 1.42 (0.92–2.35) | ||
| Care coordination | |||||||
| Less than very coordinated | 32 | 27 | 35 | Referent | 0.13 | Referent | 0.23 |
| Very coordinated | 68 | 73 | 65 | 1.48(0.8–2.47) | 1.38(0.82–2.32) | ||
| Understanding of breast cancer survivorship care | |||||||
| Less than excellent | 60 | 50 | 65 | Referent | 0.01 | Referent | 0.02 |
| Excellent understanding | 40 | 50 | 35 | 1.84 (1.15–2.9) | 1.73 (1.08–2.9) | ||
| Knowledge of next step in your cancer follow-up care | |||||||
| Less than always | 36 | 28 | 41 | Referent | 0.02 | Referent | 0.04 |
| Always know | 64 | 72 | 59 | 1.86 (1.12–3.1) | 1.72 (1.03–2.9) | ||
N number of participants, OR odds ratio, CI confidence interval, for p-values, where italics indicates p < .05
Adjusted models control for age as covariate
The p values from the Hosmer-Lemeshow goodness of fit tests for the logistic regression models used to generate the adjusted results ranged from 0.23–0.68, supporting the fit of those models
Table 3.
Associations between survivorship care plan, functioning and cancer worry among older breast cancer survivors 1-year postactive treatment
| Overall sample | Survivorship care plan
|
Mean differences (MD) care plan vs. no care plan
|
|||||
|---|---|---|---|---|---|---|---|
| N=328 Mean±SD |
YES=114 Mean±SD |
NO=214 Mean±SD |
Unadjusted MD (95 % CI) |
p-value | Adjusted ab MD (95 % CI) |
p-valueb | |
| Physical functionc | 80.3±22.5 | 82.8±21.3 | 79.0±23.0 | 3.9 (−1.5, 9.2) | 0.16 | 2.7 (−2.3, 7.6) | 0.29 |
| Role functionc | 89.8±20.5 | 91.4±16.14 | 89.0±22.5 | 2.4 (−2.5, 7.3) | 0.33 | 0.8(−3.7, 5.3) | 0.72 |
| Emotion functionc | 88.8±15.1 | 90.5±15.2 | 87.9±15.0 | 2.6 (−1.0, 6.2) | 0.15 | 2.2(−1.4, 5.9) | 0.22 |
| Cancer worryd | 1.4±0.6 | 1.3±0.5 | 1.4±0.7 | −0.1(−0.2, 0.1) | 0.25 | −0.1(−0.2, 0.1) | 0.21 |
MD mean differences, SD standard deviation, CI confidence interval, P p-value
Adjusted in a linear regression for age as well as pre-cancer functioning (a priori control variable)
The R-squared values ranged from .02 (worry) to .18 (physical function). Pre-cancer function was measured by the physical and mental function summary component scores of the MOS SF-12 [52, 53]. Thus, QLQ-C30 Physical Function was adjusted for age and physical component summary score(PCS, SF-12); QLQ-C30 Emotion Function was adjusted for age and the emotional component summary score(MCS, SF-12); QLQ-C30 Role Function was adjusted for age and one item on the SF-12 of pre-cancer role function. Cancer Worry was adjusted for age and emotional component summary score(MCS, SF-12)
Physical, role, and emotion function subscales of the Quality of Life Questionnaire from the European Organisation for Research and Treatment of Cancer (EORTC-QLQ-C30). Scores range from 0–100, where higher scores indicate better functioning in the specific functional domain [45–49, 54]
Higher scores indicate greater worry on the Worry Subscale of the CARES [50]
Finally, we conducted separate linear regression analyses to explore potential relationships between functioning and experiences of survivorship care. Each functional domain was significantly associated with two or more care factors. For instance, mean physical function among women reporting low communication with their oncologists (76.6±1.9SD) was nearly 7 points below that of women with higher communication (83.2±1.9SD), p=0.02 (data not shown), where an 8-point difference is considered clinically meaningful.
Discussion
This is the first report of the correlates of survivorship care plan receipt and relationships between care plans and functional outcomes in older breast cancer patients. Only about one-third of older women reported receipt of a care plan and rates decreased with advancing age. While care plans were associated with better survivorship care experiences, they were not related to outcomes of importance to older indiviuals one year after active treatment, including physical, emotional, and role functioning, nor did they diminish worry about cancer.
Very few older women in our sample received a survivorship care plan. This result is similar to that seen nationally [19, 26–28]. Forsythe noted that only 20 % of oncologists reported always providing care plans [26]. In our older cohort, rates rose from 20 % in 2007 to about 37 % by 2011. Low rates of care plans in an older patient population is of specific concern since this is a group where coordination of care, recognition of symptoms related to late effects of cancer care, and distinguishing these symptoms from those attributable to other comorbid illnesses are especially important in improving functioning [34, 55]. Notably, women who underwent chemotherapy were no more likely to receive care plans, despite their heightened risk of heart failure and other treatment-related late effects [56].
The original development of survivorship care plan recommendations was motivated by concerns that many cancer patients reported confusion regarding their follow-up care [8, 11, 12]. Care coordination and communication have recently been reemphasized as important goals of care plans [33]. Our results indicate that care plans have realized the goal of enhanced understanding of cancer survivorship care in an older patient population. In younger populations, care plans have improved survivors’ knowledge of requisite surveillance [32], the provider responsible for their follow-up care [30], and awareness of the need for survivorship care [32]. If confirmed, the trends toward relationships between experiences of survivorship care and functioning in our exploratory analysis suggest that patient-oncologist communication about function may be one pathway whereby care plans could potentially affect outcomes important to older survivors. Increased knowledge of recommended cancer follow-up care could also enhance adherence to long-term hormonal therapy regimens, surveillance for symptoms of recurrence, and screening for new primary cancers. While we did not have data to measure these outcomes, evaluation of the ability of care plans to impact these additional care components should be a high priority.
Our finding that care plans did not affect the functioning of older women is not surprising since most plans focus on treatment summaries and recommended surveillance [26–28], but do not include instructions directed specifically at improving functioning [57]. This null result is also consistent with two prior randomized controlled trials [29, 30]. For instance, Grunfeld and colleagues found that a care plan intervention for younger breast cancer survivors did not improve physical functioning, mental well-being, or cancer-related distress 12-months postintervention [30, 58]. Hershman and colleagues replicated these null results in a small trial of survivors predominately under age 65 [29]. The intervention in these negative studies was a single, brief nurse-led session reviewing the care plan. Juxtaposed against the null results are findings that cancer patients are more satisfied with information received during treatment than in survivorship [59, 60] and that patients like survivorship care plans but view them as too technical, incomplete, and somewhat limited in scope regarding recommendations for health promotion and prevention [8, 61, 62]. Taken together, these results suggest that a priority for future research is to create and test geriatric-centered care plans specifically targeting late effects, comorbidities, and other symptoms that affect the functioning of older adults [8, 34, 52, 57, 63]. Plans may also need to include explicit guidance on methods to maintain and enhance functioning, such as being physically active and communicating symptoms to providers.
Strengths of this study include the unique focus on a large sample of older breast cancer survivors. However, there are several caveats that should be considered in evaluating our results. First, we did not have data on the delivery mode and content of survivorship care plans. A related concern is that we relied on self-report of care plan receipt, and as such, misclassification was possible. If non-systematic, this could have biased toward the observed null result. However, we have no data to determine if misclassification of care plans existed. Next, our sample was relatively healthy with limited variability in baseline functioning, so that our measures may not have been sensitive enough to capture small differences [45, 46]. However, we did have power to detect minimally clinically meaningful changes in function [54]. Another limitation of this analysis is that our sample included a significant proportion of well-educated survivors treated in cooperative group settings, which limits generalizability. Additionally, it is difficult to estimate the impact of loss to follow-up in the subsample. However, the women in the analysis were similar to those enrolled but lost to follow-up, decreasing the probability of systematic biases affecting results.
Overall, the results of this study suggest that while care plans may be having the intended positive effect on experiences of survivorship care for older breast cancer patients, the promise of benefits in terms of functioning has yet to be realized. However, care plans continue to be promoted to improve the quality of survivorship care in the absence of a strong empirical evidence base [20, 21, 33] and will soon be required for accreditation by the American College of Surgeons Commission on Cancer [20]. Given the projected dramatic increases in the number of older cancer survivors, additional research developing and testing geriatric-specific survivorship care plans is urgently needed to provide the knowledge base to meet the health needs and maximize functioning of this growing population.
Acknowledgments
This research was supported by grants from the National Cancer Institute (NCI), including U10-CA 84131 (JSM), R01-CA127617 (JSM). This work was also supported in part by NCI grants K07-CA166342 (LAF) and K05 CA096940 (JSM). This research was also supported by the Clinical and Molecular Epidemiology(now NTSR) and Biostatistics and Bioinformatics Shared Resources at Lombardi Comprehensive Cancer Center under NCI grant P30CA51008 (Weiner). The Cancer and Leukemia Group B (CALGB) protocol #369901 was supported, in part, by NCI grant CA31946 to the Cancer and Leukemia Group B (Monica Bernagnoli, MD, Chairman) and grant CA33601 to the CALGB Statistical Center (Stephen George, PhD). The content of this manuscript is solely the responsibility of the authors and does not represent official views of the NCI at the National Institutes of Health or CALGB. Cancer and Leukemia Group B is presently part of the Alliance for Clinical Trials in Oncology. The research was also funded, in part, by a grant to support patient accrual from Amgen Pharmaceuticals to the CALGB Foundation.
Footnotes
Conflicts of interest Claudine Isaacs received honoraria from AstraZeneca, Genentech. All other authors have no conflict of interest.
Author Contributions Conception and design: Leigh Anne Faul, Jeanne S. Mandelblatt
Collection and assembly of data: Jeanne S. Mandelblatt, Claudine Isaacs, Michelle Tallarico, Hyman B. Muss, Eric Winer, Harvey J. Cohen, Clifford Hudis
Data analysis and interpretation: Leigh Anne Faul, Jeanne S. Mandelblatt, Gheorghe Luta, Jonathan Clapp, Vanessa B. Sheppard, Claudine Isaacs
Manuscript writing: All authors
Final approval of manuscript: All authors
This work was conducted while Dr. Barry was in the Department of Biostatistics and Bioinformatics, Duke University Medical Center, and Alliance/Cancer and Leukemia Group B Statistical Center, Durham, NC
Contributor Information
Leigh Anne Faul, Email: laf56@georgetown.edu, Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Gheorghe Luta, Department of Biostatistics, Bioinformatics and Biomathematics, Georgetown University Medical Center and Georgetown-Lombardi Comprehensive Cancer Center, Cancer Prevention and Control, Washington, DC, USA.
Vanessa Sheppard, Department of Oncology, Georgetown University Medical Center and Breast Cancer Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Claudine Isaacs, Departments of Medicine and Oncology, Georgetown University Medical Center and Breast Cancer Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Harvey J. Cohen, Department of Medicine and Center for the Study of Aging and Human Development, Duke University, Durham, NC, USA
Hyman B. Muss, Department of Medicine, University of North Carolina, Chapel Hill, NC, USA
Rachel Yung, Department of Medical Oncology, Dana-Farber Cancer Institute Harvard Medical School, Boston, MA, USA.
Jonathan D. Clapp, Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA
Eric Winer, Department of Medical Oncology, Dana-Farber Cancer Institute Harvard Medical School, Boston, MA, USA.
Clifford Hudis, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Michelle Tallarico, Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
Julhy Wang, Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA.
William T. Barry, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Jeanne S. Mandelblatt, Department of Oncology, Georgetown University Medical Center and Cancer Prevention and Control Program, Georgetown-Lombardi Comprehensive Cancer Center, Washington, DC, USA
References
- 1.National Cancer Institute Office of Cancer Survivorship. [Accessed 30 Apr 2014];Estimated US cancer prevelance: Who are cancer survivors in the US? 2008 http://www.dccps.nci.nih.gov/ocs/prevalence/index.html.
- 2.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer survivors: a booming population. Cancer Epidemiol Biomarkers Prev. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanchate AD, Clough-Gorr KM, Ash AS, Thwin SS, Silliman RA. Longitudinal patterns in survival, comorbidity, healthcare utilization and quality of care among older women following breast cancer diagnosis. J Gen Intern Med. 2010;25:1045–50. doi: 10.1007/s11606-010-1407-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clough-Gorr KM, Stuck AE, Thwin SS, Silliman RA. Older breast cancer survivors: geriatric assessment domains are associated with poor tolerance of treatment adverse effects and predict mortality over 7 years of follow-up. J Clin Oncol. 2010;28:380–6. doi: 10.1200/JCO.2009.23.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, et al. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29:1458–64. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yabroff KR, Lawrence W, Clauser S, Davis W, Martin ML. Burden of illness in cancer survivors: findings from a population-based national sample. J Natl Cancer Inst. 2004;96:1322–30. doi: 10.1093/jnci/djh255. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BK, Howe HL, Ries LAG, Thun MJ, Rosenberg HM, Yancik R, et al. Annual report to the nation on the status of cancer, 1973–1999, featuring implications of age and aging on U.S. cancer burden. Cancer. 2002;94:2766–92. doi: 10.1002/cncr.10593. [DOI] [PubMed] [Google Scholar]
- 8.Royak-Schaler R, Gardner LD, Shardell M, Zhan M, Passmore SR, Gadalla SM, et al. Evidence-based care for breast cancer survivors: communicating the Institute of Medicine Guidelines in medical practice. Patient Educ Couns. 2009;77:413–20. doi: 10.1016/j.pec.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 9.Earle CC, Burstein H, Winer, Weeks JC. Quality of non-breast cancer health maintenance among elderly breast cancer survivors. J Clin Oncol. 2003;21:1447–51. doi: 10.1200/JCO.2003.03.060. [DOI] [PubMed] [Google Scholar]
- 10.Cheung W, Neville B, Earle CC. Associations among cancer survivorship discussions, patient and physician expectations, and receipt of follow-up care. J Clin Oncol. 2010;28:2577–83. doi: 10.1200/JCO.2009.26.4549. [DOI] [PubMed] [Google Scholar]
- 11.Nissen MJ, Tsai ML, Blaes AH, Swenson KK. Breast and colorectal cancer survivors’ knowledge about their diagnosis and treatment. J Cancer Surviv. 2010;6:20–32. doi: 10.1007/s11764-011-0189-3. [DOI] [PubMed] [Google Scholar]
- 12.Forsythe LP, Alfano CM, Leach CR, Ganz PA, Stefanek ME, Rowland JH. Who provides psychosocial follow-up care for post-treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol. 2012;30:2897–905. doi: 10.1200/JCO.2011.39.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: implications for cancer care. J Cancer Surviv. 2008;2:179–89. doi: 10.1007/s11764-008-0055-0. [DOI] [PubMed] [Google Scholar]
- 14.Yeom HE. Symptoms, aging-stereotyped beliefs, and health-promoting behaviors of older women with and without osteoarthritis. Geriatr Nurs. 2013;34:307–13. doi: 10.1016/j.gerinurse.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Sarkisian CA, Hays RD, Berry SH, Mangione CM. Expectations regarding aging among older adults and physicians who care for older adults. Med Care. 2001;39:1025–36. doi: 10.1097/00005650-200109000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Oladimeji O, Farris KB, Urmie JG, Doucette WR. Symptomatology, attribution to medicines, and symptom reporting among Medicare enrollees. Res Social Adm Pharm. 2009;5:225–33. doi: 10.1016/j.sapharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Sarkisian CA, Hays RD, Mangione CM. Do older adults expect to age successfully? The association between expectations regarding aging and beliefs regarding healthcare seeking among older adults. J Am Geriatr Soc. 2002;50:1837–43. doi: 10.1046/j.1532-5415.2002.50513.x. [DOI] [PubMed] [Google Scholar]
- 18.Hewitt M, Greefield S, Stovall E. From cancer patient to cancer survivor: lost in transition. Washington, DC: National Acadamies Press; 2005. [Google Scholar]
- 19.National Coalition for Cancer Survivorship. Institute of Medicine and National Cancer Policy Forum, Implementing Cancer Survivorship Care Planning(workshop summary) Washington: National Academies Press; 2007. [Google Scholar]
- 20.American College of Surgeons(ACoS) Commission on Cancer. [Accessed 30 Apr 2014];Cancer Program Standards 2012: Ensuring patient-centered care. 2012 http://www.facs.org/cancer/coc/programstandards.
- 21.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology. [Accessed 30 Apr 2014];Survivorship Guidelines v1. 2013 http://www.nccn.org/survivorship.
- 22.LIVESTRONG. [Accessed 30 Apr 2014];LIVESTRONG Care Plan. 2012 http://www.livestrongcareplan.org.
- 23.American Society of Clinical Oncology(ASCO) [Accessed 30 Apr 2014];ASCO Breast Cancer Treatment Plan. 2009 http://www.ASCO.org/ASCO/QualityCareGuidelines/QualityMeasurementImprovement/ChemotherapyTreatmentPlanandSummary/Resources.
- 24.Ganz PA, Hahn EE. Implementing a survivorship care plan for patients with breast cancer. J Clin Oncol. 2008;26:759–67. doi: 10.1200/JCO.2007.14.2851. [DOI] [PubMed] [Google Scholar]
- 25.Siegel RD, Clauser SB, Lynn JM. National Collaborative to Improve Oncology Practice: The National Cancer Institute Community Cancer Centers Program Quality Oncology Practice Initiative Experience. J Oncol Pract. 2009;5:276–81. doi: 10.1200/JOP.091050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Forsythe LP, Parry C, Alfano CM, Kent EE, Leach CR, Haggstrom DA, et al. Use of survivorship care plans in the United States: associations with survivorship care. J Natl Cancer Inst. 2013;105:1579–87. doi: 10.1093/jnci/djt258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stricker CT, Jacobs LA, Risendal B, Jones A, Panzer S, Ganz PA. Survivorship care planning after the Institute of Medicine recommendations: how are we faring? J Cancer Surviv. 2011;5:358–70. doi: 10.1007/s11764-011-0196-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sabatino SA, Thompson TD, Smith JL, Rowland JH, Forsythe LP, Pollack L, et al. Receipt of cancer treatment summaries and follow-up instructions among adult cancer survivors: results from a national survey. J Cancer Surviv. 2013;7:32–43. doi: 10.1007/s11764-012-0242-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized controlled trial of a clinic-based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Res Treat. 2013;138:795–806. doi: 10.1007/s10549-013-2486-1. [DOI] [PubMed] [Google Scholar]
- 30.Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, et al. Evaluating survivorship care plans: results of a randomized, clinical trial of patients with breast cancer. J Clin Oncol. 2011;29:4755–62. doi: 10.1200/JCO.2011.36.8373. [DOI] [PubMed] [Google Scholar]
- 31.Faul LA, Rivers B, Shibata D, Townsend I, Meredith K, Cabrera P. Survivorship care planning in colorectal cancer: feedback from survivors and providers. J Psychosoc Oncol. 2012;30:198–216. doi: 10.1080/07347332.2011.651260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oeffinger KC, Hudson MM, Mertens AC, Smith SM, Mitby PA, Eshelman-Kent DA, et al. Increasing rates of breast cancer and cardiac surveillance among high-risk survivors of childhood Hodgkin lymphoma following a mailed, one-page survivorship care plan. Pediatr Blood Cancer. 2011;56:818–24. doi: 10.1002/pbc.22696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parry C, Kent EE, Forsythe LP, Alfano CM, Rowland JH. Can’t see the forest for the care plan: a call to revisit the context of care planning. J Clin Oncol. 2013;31:2651–3. doi: 10.1200/JCO.2012.48.4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cohen HJ. Functional assessment and the cancer survivor: something old, something new. J Natl Cancer Inst. 2010;102:1450–1. doi: 10.1093/jnci/djq365. [DOI] [PubMed] [Google Scholar]
- 35.Mandelblatt JS, Sheppard VB, Hurria A, Kimmick G, Isaacs C, Taylor KL, et al. Breast cancer adjuvant chemotherapy decisions in older women: the role of patient preference and interactions with physicians. J Clin Oncol. 2010;28:3146–53. doi: 10.1200/JCO.2009.24.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mandelblatt JS, Faul LA, Luta G, Makgoeng SB, Isaacs C, Taylor K, et al. Patient and physician decision styles and breast cancer chemotherapy use in older women: Cancer and Leukemia Group B protocol 369901. J Clin Oncol. 2012;30:2609–14. doi: 10.1200/JCO.2011.40.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 38.Safran DG, Kosinski M, Tarlov AR, Rogers WH, Taira DH, Lieberman N, et al. The primary care assessment survey: tests of data quality and measurement performance. Med Care. 1998;36:728–39. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Maly RC, Frank JC, Marshall GN, DiMatteo MR, Reuben DB. Perceived efficacy in patient-physician interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46:889–94. doi: 10.1111/j.1532-5415.1998.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 40.Cleary PD, Edgman-Levitan S, Roberts M, Moloney TW, McMullen W, Walker JD, et al. Patients evaluate their hospital care: a national survey. Health Aff(Millwood) 1991;10:254–67. doi: 10.1377/hlthaff.10.4.254. [DOI] [PubMed] [Google Scholar]
- 41.Gerteis M, Edgman-Levitan S, Daley J, Delblanco T. Through the patient’s eyes. San Franscisco: Jossey-Bass; 1993. [Google Scholar]
- 42.Arora NK, Reeve BB, Hays RD, Clauser SB, Oakley-Girvan I. Assessment of quality of cancer-related follow-up care from the cancer survivor’s perspective. J Clin Oncol. 2011;29:1280–9. doi: 10.1200/JCO.2010.32.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teno JM, Lima JC, Lyons KD. Cancer patient assessment and reports of excellence: reliability and validity of advanced cancer patient perceptions of the quality of care. J Clin Oncol. 2009;27:1621–6. doi: 10.1200/JCO.2008.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coulter A, Cleary PD. Patients’ experiences with hospital care in five countries. Health Aff(Millwood) 2001;20:244–52. doi: 10.1377/hlthaff.20.3.244. [DOI] [PubMed] [Google Scholar]
- 45.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 46.Sprangers MA, Groenvold M, Arraras JI, Franklin J, et al. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–68. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 47.Scott N, Fayers P, Aaronson N, Bottomley A, de Graeff A, Groenvold M, et al. EORTC Quality of Life Group. [Accessed 30 April 2014];EORTC QLQ-C30 Reference Values manual. 2008 http://www.groups.eortc.be/qol/sites/reference_values_manual2008.
- 48.Hjermstad M, Fossa S, Bjordal K, Kaasa S. Test-retest of the European Organization for Research and Treatment of Cancer Core Quality of Life Questionnaire. J Clin Oncol. 1995;13:1249–54. doi: 10.1200/JCO.1995.13.5.1249. [DOI] [PubMed] [Google Scholar]
- 49.Hjermstad M, Fayers P, Bjordal K, Kaasa S. Using reference data on quality of life: the importance of adjusting for age and gender, exemplified by the EORTC QLQ-C30 (+3) J Clin Oncol. 1998;34:1381–9. doi: 10.1016/s0959-8049(98)00136-1. [DOI] [PubMed] [Google Scholar]
- 50.Ganz PA, Schag CA, Lee JJ, Sim MS The CARES. A generic measure of health-related quality of life for patients with cancer. Qual Life Res. 1992;1:19–29. doi: 10.1007/BF00435432. [DOI] [PubMed] [Google Scholar]
- 51.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–34. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 52.Ware J, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 53.Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: Results from the IQOLA Project. J Clin Epidemiol. 1998;51:1171–8. doi: 10.1016/s0895-4356(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 54.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–44. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 55.Cohen HJ, Lan L, Archer L, Kornblith AB. Impact of age, comorbidity and symptoms on physical function in long-term breast cancer survivors (CALGB 70803) J Geriatr Oncol. 2012;3:82–9. doi: 10.1016/j.jgo.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chavez-Macgregor M, Zhang N, Buchholz TA, Zhang Y, Niu J, Elting L, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–8. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cohen HJ. Cancer survivorship and aging: a double whammy. Lancet Oncol. 2006;7:882–3. doi: 10.1016/S1470-2045(06)70913-5. [DOI] [PubMed] [Google Scholar]
- 58.Coyle D, Grunfeld E, Coyle K, Pond G, Julian J, Levine M. Cost effectiveness of a survivorship care plan for breast cancer survivors. J Oncol Pract. 2014;10:e86–92. doi: 10.1200/JOP.2013.001142. [DOI] [PubMed] [Google Scholar]
- 59.Griggs J, Sorbero M, Mallinger J, Quinn M, Waterman M, Brooks B, et al. Vitality, mental health and satisfaction with information after breast cancer. Patient Educ Couns. 2007;66:58–66. doi: 10.1016/j.pec.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 60.Mao J, Bowman M, Stricker CT, DeMichele A, Jacobs L, Chan D, et al. Delivery of survivorship care by primary care physicians: the perspective of breast cancer patients. J Clin Oncol. 2009;27:933–8. doi: 10.1200/JCO.2008.18.0679. [DOI] [PubMed] [Google Scholar]
- 61.Kantsniper M, McDonald E, Geller G, Shockney L, Snyder C, Wolff A. Transitioning to breast cancer survivorship: perspectives of patients, cancer specialists, and primary care providers. J Gen Intern Med. 2009;24 (suppl 2):S459–66. doi: 10.1007/s11606-009-1000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burg M, Lopez E, Dailey A, Keller M, Pendergrast B. The potential of survivorship care plans in primary care follow-up of minority breast cancer patients. J Gen Intern Med. 2009;24 (suppl 2):S467–71. doi: 10.1007/s11606-009-1012-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: recommendations from an IOM report. JAMA. 2013;310:1795–6. doi: 10.1001/jama.2013.280416. [DOI] [PubMed] [Google Scholar]