SUMMARY
During development and evolution, the morphology of ectodermal organs can be modulated so that an organism can adapt to different environments. We have proposed that morphoregulation can be achieved by simply tilting the balance of molecular activity. We test the principles by analyzing the effects of partial downregulation of Bmp signaling in oral and dental epithelia of the keratin 14-Noggin transgenic mouse. We observed a wide spectrum of tooth phenotypes. The dental formula changed from 1.0.0.3/1.0.0.3 to 1.0.0.2(1)/1.0.0.0. All mandibular and M3 maxillary molars were selectively lost because of the developmental block at the early bud stage. First and second maxillary molars were reduced in size, exhibited altered crown patterns, and failed to form multiple roots. In these mice, incisors were not transformed into molars. Histogenesis and differentiation of ameloblasts and odontoblasts in molars and incisors were abnormal. Lack of enamel caused misocclusion of incisors, leading to deformation and enlargement in size. Therefore, subtle differences in the level, distribution, and timing of signaling molecules can have major morphoregulatory consequences. Modulation of Bmp signaling exemplifies morphoregulation hypothesis: simple alteration of key signaling pathways can be used to transform a prototypical conical-shaped tooth into one with complex morphology. The involvement of related pathways and the implication of morphoregulation in tooth evolution are discussed.
INTRODUCTION
The formation of ectodermal organs depends on a series of epithelial–mesenchymal interactions mediated by signaling pathways. Some components of these pathways are shared as evidenced by ectodermal dysplasia syndromes in which hair, teeth, sweat glands, or sometime lungs, all become defective by the mutation of a single gene (Slavkin et al. 1998; Pispa and Thesleff 2003; Ohazama and Sharpe 2004). We have suggested earlier that different ectodermal organs are variations sharing a common theme (Chuong 1998). The sharing of major signaling pathways (e.g., Shh, Bmp, Fgf, Notch, Wnt pathways, etc.) among the organogenesis of hairs, feathers, teeth, mammary glands, etc., attests to the concept of these common themes. Yet how the variations are generated and regulated are mostly unknown. We surmise the variation is based on autonomous regional specificity (e.g., Hox codes) and nonautonomous morphoregulators (e.g., activity of secreted signaling molecules). Identification of the molecular basis of these variations is ongoing and in this study we focus on the role of morphoregulation.
In contrast to proposing novel molecular pathways, the concept of morphoregulation postulates that diverse organ phenotypes, in development or evolution, can be achieved through physiological modulations of existing morphogenesis-related pathways (Edelman 1992). Although originally proposed for adhesion molecules as mediators, the concept of morphoregulation later expanded to cover signaling molecules that work upstream of adhesion molecules (Plikus et al. 2004). Direct ablation of a fundamental signaling pathway is likely to be lethal. However, nature has devised a strategy using a series of antagonists expressed in a temporal–spatial specific way to fine tune pathway activity and to generate a spectrum of moderate modifications of organ morphology. We have recently used keratin (K14)-Noggin mice to demonstrate the basics of this concept (Plikus et al. 2004). We showed that modulation of Bmp activity indeed leads to various morphoregulation changes in multiple ectodermal organs including increased numbers of hair filaments, reduced size of claws, enlarged size of external genitals, and the conversion of sweat glands in foot pads and Meimobian glands in eyelids into hairs.
To further analyze the principles of ectodermal organ morphoregulation at different hierarchical morphogenetic levels, we chose one organ to analyze the consequences of tuning down, but not shutting off, Bmp pathway activity at different stages of organogenesis. Teeth were chosen for the following reasons: (i) They show hierarchical levels of morphological complexity resulting from successive stages of morphogenesis (Thesleff and Mikkola 2002; Tucker and Sharpe 2004). As teeth in different parts of the oral cavity develop with different temporal schedules, there is a good chance that different teeth will be affected by decreased Bmps activity at different developmental stages. (ii) In mammals, there are regionally specific tooth phenotypes (Sharpe 1995). It has been reported that the mouse incisor can be re-specified to become a molar in explant cultures (Tucker et al. 1998) and it would be very interesting to test if this phenomenon happens in vivo in our K14-Noggin mice. (iii) Teeth have an excellent fossil record, and it now is possible to study the roles of genes in development experimentally, and then match the resulting patterns to dental diversity caused by natural selection by studying fossils and extant animals. Teeth fulfill all these criteria more than hairs, feathers, and glands (Yu et al. 2002; Wu et al. 2004), and therefore have begun to be analyzed from this perspective (Kangas et al. 2004). (iv) Structural defects in teeth occur often in various human dental diseases. The versatile phenotype of teeth in K14-Noggin mice may be useful as an experimental model for pathological studies.
During vertebrate evolution, teeth have been lost and gained. As reptiles evolved into birds and mammals, teeth met very different fates. Although most Mesozoic birds had teeth (Hou et al. 2003, 2004), teeth were lost in the Cenozoic era. In the mammalian lineage, the generally conical-shaped reptilian teeth became more complex, and mammalian dentition underwent remarkable morphological and functional diversification, showing great variations of number, size, and shape (Line 2003). In general, four classes of teeth are arranged from the distal to the proximal snout, incisors, canines, premolars, and molars, with the number expressed in sequence as the dental formula. The primitive placental mammals have a dental formula of 3.1.4.3./3.1.4.3. Some rodents, mice, for example, have the dental formula, 1.0.0.3/1.0.0.3, with only one incisor, no canines, no premolars, and three molars. Homologous teeth can greatly range in size among different mammalian species. Incisors in many species such as humans are relatively small. In contrast, incisors in rodents can reach a large size in relative proportions (Tummers and Thesleff 2003). The shape of teeth within the same class can also vary. Molars and premolars generally have a more complicated crown pattern than canines and incisors, and are well understood from a functional perspective and are usually related to specific molar functions such as shearing, crushing, and grinding of food (Hiiemae 2000). Although there are functional reasons for the expansion of mammalian teeth diversity, the gain, and loss of teeth, the ways to achieve that remain mostly unknown. This is particularly intriguing as quite different morphologies can occur in closely related clades (e.g., the aforementioned mouse and vole), whereas similar changes (e.g., loss of teeth in baleen whales, pangolins, and anteaters) occur independently in different clades. On a larger scale, these phenomena suggest that the tooth morphogenetic pathway is in a quasi-stable equilibrium in individual species and that it is sensitive to molecular tuning, resulting in phenotypic plasticity.
Many growth factors (Fgf8, Egf, Tgfb1, Bmp2, Bmp4, etc.) and transcription factors (Msx1, Msx2, Pax9, Lef1, etc.) were shown to play key roles during various phases of odontogenesis (reviewed in Thesleff and Sharpe 1997; Scarel-Caminaga et al. 2002; Thesleff and Mikkola 2002; Tucker and Sharpe 2004). It is believed that diversification of the dentition is achieved through the modulation of activities and timing of these pathways, as well as differences in requirements by various tooth primordia (Thesleff and Sharpe 1997; Jernvall and Thesleff 2000). Among the multiple signaling pathways known to regulate tooth development, the Bmp pathway stands out for its importance (Bei and Maas 1998, 2000; Reddi 1998; Zhang et al. 2000; Miyazono et al. 2001). Various Bmps are expressed throughout odontogenesis. They exhibit a complex expression pattern (Aberg et al. 1997; Yamashiro et al. 2003). Overall, Bmp2, Bmp4, and Bmp7 expression patterns largely overlap. Bmp3 and Bmp5 show a rather distinct and restricted distribution in tooth compartments. Most interestingly, inhibition of Bmps signaling with noggin changes tooth identity from incisor to molar. Prior to odontogenesis embryonic day 9 (E9)–(E10) Bmp4 inhibits Barx1 expression in the presumptive incisor mesenchyme. In contrast, Barx1 expression is stimulated by Fgf8 in the presumptive molar mesenchyme. If Bmps activity is experimentally downregulated by exposing the E9–E10 mandibular arch to exogenous noggin, incisors transform into molars (Tucker et al. 1998). This compelling experiment has prompted us to look further into how normal Bmp4 signaling may regulate epithelial–mesenchymal interactions and specify the fate and shape of tooth primordia.
In this study we use noggin as a tool to tune down the activity of the Bmp pathway. By overexpressing noggin in the oral and dental epithelium, we expected to disturb the otherwise balanced activity of Bmp pathway in odontogenesis. As noggin is secreted, mis-expressed noggin should have a nonautonomous effect on both dental epithelium and dental mesenchyme, blocking Bmp signaling in epithelial–mesenchymal interactions. Indeed we found a broad spectrum of changes in different aspects of odontogenesis. We describe these changes in detail and discuss them in the context of tooth development, diseases, and in the broader context of the morphological evolution of ectodermal organs.
MATERIALS AND METHODS
Production and genotyping of transgenic mice
Mice were generated in the Norris Cancer Center transgenic mouse facility at the University of Southern California as previously described (Fig. 1, A–C adopted from Plikus et al. 2004). All phenotypic features of K14-Noggin mice showed high penetrance. All animals were treated under humane conditions following protocols approved by the University of Southern California IACUC.
Fig. 1.
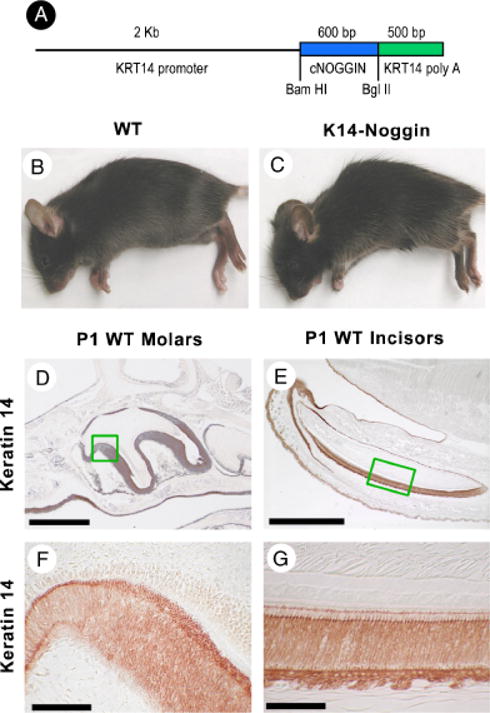
Production of K14-Noggin mouse. K14 Noggin construct used to generate transgenic mouse. The size of inset used and restriction enzyme are indicated (A). Appearance of control C57BL/6J (B) and mutant K14-Noggin 2.5-month-old mice (C). Examples of K14 immunostaining is shown in newborn molars (D, F) and incisors (E, G) which are strongly K14 positive. Section plane: sagittal (A–H). Scale bars: 100 μm (E); 50 μm (D); 10 μm (F, G).
Histological and immunochemical staining
Tissues were collected and fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and sectioned at 5–6 μm. When necessary, specimens were additionally decalcified after fixation. Standard H&E staining was performed for basic histological analysis. Immunostaining was performed using the Ventana Discovery™ automated immunostaining module (Ventana Medical Systems, Tucson, AZ, USA). Primary antibodies used were rabbit anti-PCNA (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and rabbit anti-K14 (1:400, Berkeley Antibody Company, Richmond, CA, USA). The DAB (Ventana Medical Systems) or HistoStain (Zymed Laboratories, San Francisco, CA, USA) detection kits were used for color development.
In situ hybridization
Mouse tissues from various ages were used for in situ hybridization. Tissues for in situ samples were fixed and dehydrated in DEPC-treated solutions according to a standard protocol. To detect mRNA expression, the tissue was hybridized with the appropriate digoxigenin-labeled probe. Signals were detected using an anti-digoxigenin antibody coupled to alkaline phosphatase. Some tissue samples were processed using the Ventana Discovery™ automated in situ hybridization instrument (Ventana Medical Systems).
Scanning electron microscopy (SEM) analysis
Tissues were prepared according to the standard SEM protocol. Briefly, samples were fixed in 2.5% glutaraldehyde in 0.1 M sodium cacodylate, dehydrated, and critical point dried from ethanol. Samples were coated with gold in a sputter coat chamber. SEM was performed in the Doheny Eye Institute Core Facility at the University of Southern California.
Ground sections analysis
After fixation and dehydration, tissues were embedded in Eponate 12 (Ted Pella Inc., Redding, CA, USA). Curing was done at 60°C for 48 h. The resin-embedded specimens were ground all the way to the middle of the teeth. The surface was then polished using a fine sharpening stone.
RESULTS
K14 promoter activities in oral cavity
Basal K5 and K14 are first detected in E9.5 in mice. β-galactosidase driven by human KRT5 promoter was seen in the first branchial arch as early as E10.5 (Byrne et al. 1994). We examined K14 immunohistochemistry in the oral epithelium at E13 (not shown). In the incisor bud, K14 was expressed in the proximo-labial part of the bud, but was absent from the distal–lingual part. Similarly in the E13 molar, K14 was predominantly expressed in the proximal part of the bud. As tooth development progressed, levels of K14 expression in molars increased but remained low in the E15.5 incisor (not shown). Expression of K14 stayed high in the oral epithelium. At postnatal day 1 (P1), K14 expression was high throughout the oral epithelium, the ameloblast cell layer, and the stratum intermedium both in molars (Fig. 1, D and F) and incisors (Fig. 1, E and G). K14 in preameloblast and ameloblast was also detected by immunochemistry (Tabata et al. 1996). Expression levels of endogenous K14 in the K14-Noggin mice were similar to those of the WT teeth (not shown).
The number of K14-Noggin molars is reduced
Both genotypically and phenotypically two groups of K14-Noggin mice could be identified: low-transgenic (TG) copy number and high-TG copy number animals (Plikus et al. 2004). High-TG copy number K14-Noggin mice exhibited overall more dramatic pathological changes in various skin appendages (Fig. 1C). Therefore, in our present study we analyzed the dental phenotype in high-TG copy number (HCN) K14-Noggin mice only.
Mandibular molars
The most dramatic finding is that K14-Noggin mice lack all mandibular molars. K14-Noggin mice show consistent changes of the dental formula to 1.0.0.2(1)/1.0.0.0 (Fig. 2, A–H). All three pairs of mandibular molars were absent in all (n = 24) animals studied (Table 1). On histology, thickened and invaginated oral epithelium was present in the adult transgenic animals (Fig. 2H). There were no indications of teeth or teeth-like structures.
Fig. 2.
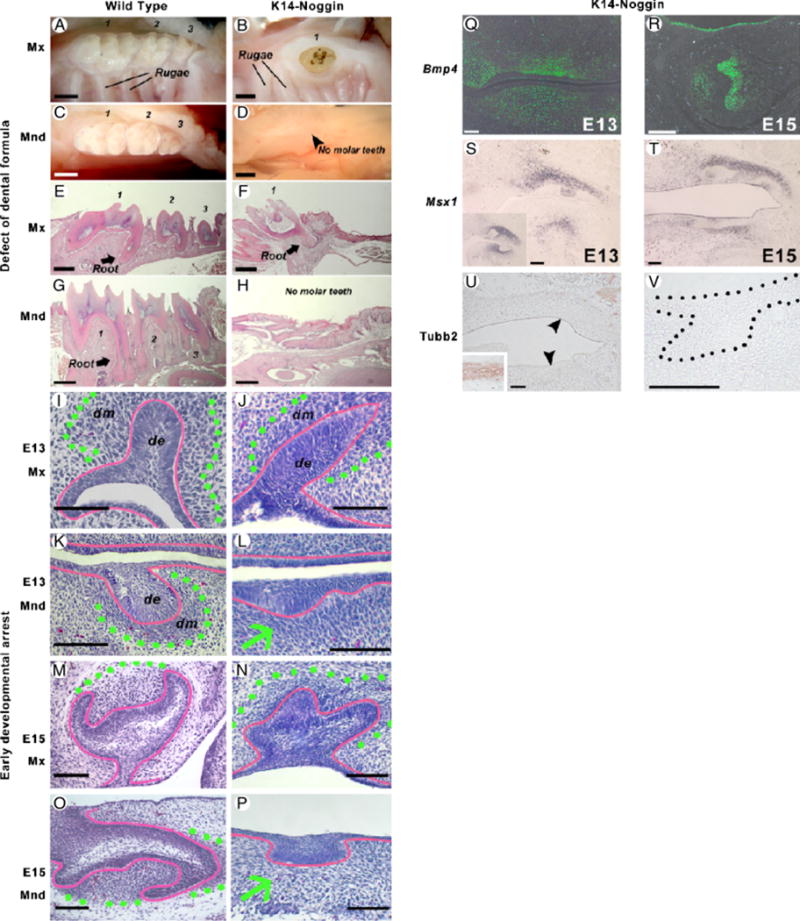
Early arrest of K14-Noggin molar development. (A–D) WT mice have three maxillary (A) and three mandibular molars (C). Most of the K14-Noggin mice have only two or rarely one maxillary molar (B). None of the K14-Noggin mice have mandibular molars (D). (E–H) WT mice have three maxillary (E) and three mandibular molars (G) with a well-developed crown and roots. K14-Noggin mice have a reduced number of maxillary molars (F) and no mandibular molars. Instead, there is thickened oral epithelium, often resembling residual molar lamina (H). (I–L) Developing molars in the E13 WT (I, K) and K14-Noggin (J, L) mouse embryos. In WT mice, tooth buds have subjacent mesenchymal condensations (I, K). In K14-Noggin mice, the maxillary molar bud has a distinct mesenchymal condensation (outlined with green dotted line). However, the mandibular molar bud (L) lacks a mesenchymal condensation. Epithelium is outlined with red. (M–P) E15 molars in the WT (M, O) and K14-Noggin (N, P) mouse embryo. Unlike maxillary molars (N), K14-Noggin mandibular molars fail to develop further (P). Epithelium resembles initial tooth lamina (red). There is no mesenchymal condensation beneath it (green arrow). (Q, R) Bmp4 expression in the mesenchyme of the developing K14-Noggin teeth at E13 (Q) and E15 (R). (S, T) Msx1 expression pattern during early odontogenesis in K14-Noggin mice. In WT mice at E13, Msx1 is distinctly expressed in the dental mesenchyme, but not in the epithelium (S, inset). In E13 K14-Noggin mice, Msx1 is expressed in the dental mesenchyme of the maxillary molars (S). Msx1 expression is seen in the mandible, but not directly underneath the oral epithelium (S). At E15, when K14-Noggin mandibular molars show developmental arrest, Msx1 is expressed in the maxillary dental mesenchyme, as well as in the mesenchyme of the arrested mandibular early bud (T). (U, V) Absence of the neuro-specific differentiation of the dental mesenchyme in both maxillary and developmentally arrested mandibular tooth buds (V), as judged by the Tubb3 expression. Fibers of the trigeminal nerve are strongly positive for Tubb3 (see inset on U). Section plane: sagittal (E–H, J, L, N, P, Q–V), frontal (I, K M, O). Scale bars: 1 mm (A–H), 100 μm (I–V). de, dental epithelium; dm, dental mesenchyme.
Table 1.
Frequency of molar presence in K14-Noggin mice
| Maxilla
|
Mandible
|
|||
|---|---|---|---|---|
| Left | Right | Left | Right | |
| M1 | 24/24 | 24/24 | 0/24 | 0/24 |
| M2 | 23/24 | 23/24 | 0/24 | 0/24 |
| M3 | 1/24 | 1/24 | 0/24 | 0/24 |
Twenty-four mice were used for analyses.
M1, first maxillary molar; M2, second maxillary molar; M3, third maxillary molar; K14, keratin 14.
Maxillary molars
The number of maxillary molars is also reduced in K14-Noggin mice. The third maxillary molars (M3) were absent with very high penetrance (>95%, Table 1). In two out of 24 mice, the second molars (M2) were missing, one on the left side and the other on the right side (Fig. 2B, Table 1). The first molars (M1) were always present in all (n = 24) animals included in this study (Table 1).
Developmental changes
To further determine the stage where the developmental block occurs, we studied morphogenesis of both maxillary and mandibular M1 molars of the K14-Noggin mice. At E13.5 M1 molars of the WT mice were in the bud stage (Scarel-Caminaga et al. 2002). Oral epithelium formed bud-like downgrowth surrounded by mesenchymal condensation (Fig. 2, I and K; Maas and Bei 1997). K14-Noggin maxillary M1 molars showed bud stage morphology (Fig. 2J). In contrast, mandibular molars were blocked at the early bud stage showing only a thickened dental epithelium, which did not seem to invaginate. There were only slight increases in the cellular density of the underlying mesenchyme (Fig. 2L).
In WT mice, molars entered cap stage at E14.5 and further progressed to the bell stage at E16.5 (Fig. 2, M and O). At E15, K14-Noggin maxillary M1 molars appeared to be at the early cap stage. Epithelial tooth buds seemed to fold, but did not form a distinctive cap-like structure surrounding the mesenchymal papilla (Fig. 2N). Transition from the bud to cap stage was delayed in maxillary molars. Mandibular molars did not progress in their development any further. They remained early bud-like in appearance, exhibiting thickened dental epithelium with little or no mesenchymal condensation beneath it (Fig. 2P).
To ensure that the effect seen is because of noggin activity, the expression of Bmp4 was confirmed by in situ hybridization (Fig. 2, Q and R). No differences were found between the WT and K14-Noggin mice. In a similar manner, the expression of Msx1 was examined as it has been suggested to mediate epithelial–mesenchymal interactions during tooth induction (Chen et al. 1996). We found Msx1 to be expressed in the mesenchymal component of the E13 WT tooth buds (Fig. 2S, inset). In E13 K14-Noggin embryos, Msx1 expression was seen in both maxillary and mandibular incisor buds (not shown) and throughout the maxillary molar mesenchyme. In the mandible, Msx1 showed mesenchymal expression, yet not directly under the dental epithelium (Fig. 2S). By E15, Msx1 was strongly expressed in the dental mesenchyme of maxillary molars and only weakly in the mesenchyme adjacent to the mandibular dental placode (Fig. 2T). The results suggest that Msx1 expression during the early development of K14-Noggin molars remains largely normal.
Another marker examined was tubulin, β3 (Tubb3). In Msx1-deficient mice dental mesenchyme differentiated abnormally and was reported to express the neuronal marker Tubb3 (Han et al. 2003). In E15 K14-Noggin mice, neither of the dental mesenchyme from the maxillary molars and mandibular laminae expressed Tubb3 (Fig. 2, U and V). As a control, the trigeminal nerve was strongly Tubb3 positive (Fig. 2U, inset). These results suggest that the developmental inhibition caused by noggin could be an event occurring later than Msx1-related blockage. Alternatively a non-Msx1-mediated pathway is used.
We then examined whether rates and distribution of apoptosis are altered in these mutants. E15 tooth buds from both WT and K14-Noggin were TUNEL negative, suggesting the absence of apoptosis (not shown). At the same time, other areas of the embryos were TUNEL positive (not shown). No TUNEL-positive cells were found within the K14-Noggin mandibular lamina structure at E15 (not shown).
Defects in K14-Noggin maxillary molars include abnormal crown/root patterning and enamel/dentine differentiation
Crown size/pattern
Compared with the WT, K14-Noggin maxillary molars were significantly smaller (Fig. 3, J vs. K). There was a reduction in the size of the crown base and low proliferation rates. Miniaturization was obvious both at P21, when K14-Noggin molars started to erupt (Fig. 3, A vs. B), and in adulthood. Overall morphology of the crown was changed as well (Fig. 3, G vs. H). Widths of both K14-Noggin M1 and M2 molars at the neck level were about 53% less than that in WT (Table 2).
Fig. 3.
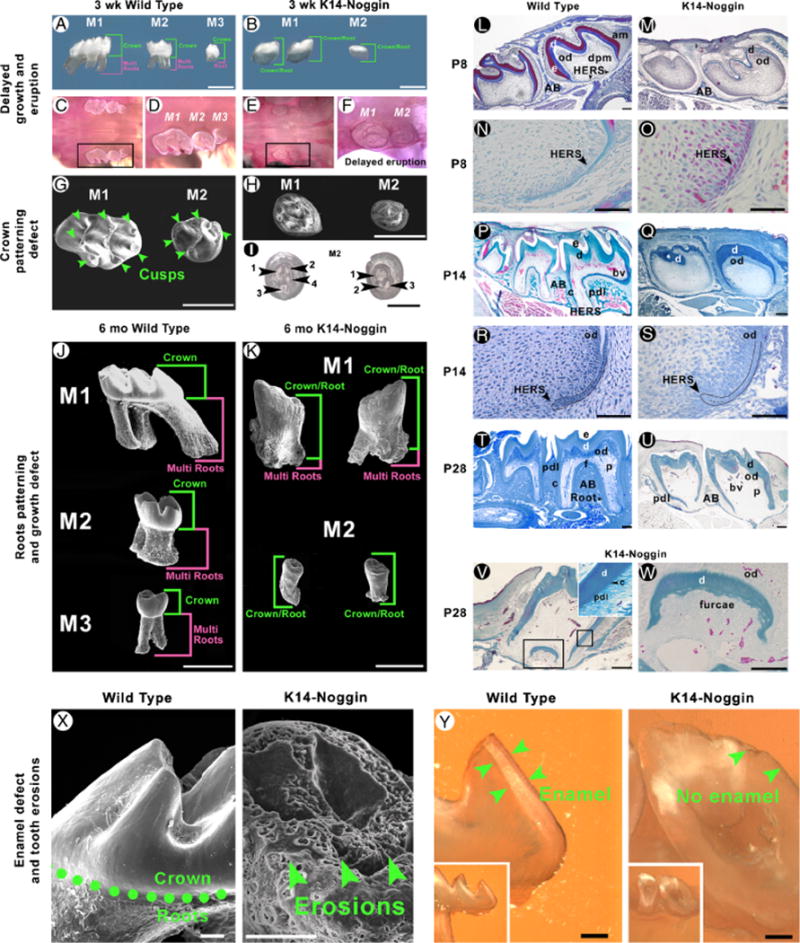
Growth, eruption, and patterning and differentiation defects in K14-Noggin molars. (A, B) Comparative morphology of the maxillary molars in the P21 WT (A) and K14-Noggin mice (B). WT molars have a well-developed crown. M1 and M2 WT molars have well-developed multiple roots. Unlike WT teeth, maxillary M1 and M2 molars in K14-Noggin mice are smaller, have no clear crown to root separation, and do not form multiple roots. Postnatal development of the K14-Noggin maxillary molars is largely retarded. (C–F) Delayed eruption of molars in K14-Noggin mice. Although P21 M1 and M2 WT molars have fully erupted (C, D), both maxillary molars in P21 K14-Noggin mice remain at the early stages of eruption, with the crown mostly seated deep within the alveolae (E, F). (G–I) Abnormal crown pattering in K14-Noggin maxillary molars. P21 WT maxillary molars have a well-defined cusp pattern, with seven cusps in M1 and five cusps in the M2 molar (G). P21 K14-Noggin M1 and M2 maxillary molars have a severely abnormal crown pattern with small cusp-like prominences (H). Position and number of the intercusps are inconsistent in P21 M2 K14-Noggin molars (inverted view, I). (J, K) K14-Noggin molars have multiple root defects. WT molars have multiple well-developed roots (J). K14-Noggin molars have either two short, misconfigured roots (M1), or only one, very short root (M2), and multiple, irregular, grape-like growths on their surface (K). In WT molars, root length dominates over crown length (J). In K14-Noggin molars, the crown/root to multiple root ratio is changed and the crown/root length dominates over multiple root length (K). (L–S) Comparative morphology of P8 (L–O), P14 (P–S) WT, and K14-Noggin maxillary molars. Both in WT and K14-Noggin molars HERS is clearly seen at P8 (N, O). At P14 HERS in K14-Noggin molars has largely normal morphology (S vs. R). Although roots start to form at or before P8, furcae does not form until much later in K14-Noggin molars. (T–W) Formation of small furcae in P28 K14-Noggin molars. In M1 K14-Noggin molars furcae is first seen at P28 (V). Furcae is delineated by a layer of disorganized, mineralized dentin (W). Periodontal apparatus forms with periodontal ligament connected to the cementum (V, inset). Not all K14-Noggin molars form furcae and multiple roots (U vs. T). (X, Y) Crown surface defect in K14-Noggin molars. Unlike in WT molars, the crown surface of the K14-Noggin adult molars is uneven and highly eroded (X). On ground sections, WT molars show a distinct layer of enamel (Y, green arrows), whereas no enamel is seen in K14-Noggin molars (Y). Section plane: sagittal (L–W, Y). Scale bars: 1 mm (A, B, G, H, J, K); 0.5 mm (I); 200 μm (L, M, P, Q, T, U); 100 μm (V, X, Y), 50 μm (N, O, R, S, W). AB, aleveolar bone; am, ameloblasts; bv, blood vessels; c, cementum; d, dentin; dpm, dental papillae mesenchyme; e, enamel; f, furcae; HERS, Hertwig’s epithelial root sheath; od, odontoblasts; p, pulp; pdl, periodontal ligament.
Table 2.
Morphological characterization of WT and K14-Noggin molars
| WT M1 | K14-Noggin M1 |
WT M2 | K14-Noggin M2 |
|
|---|---|---|---|---|
| Neck width (%) | 100 | 46 | 100 | 47 |
| Crown/multi-roots ratio | 0.53 | 1.8 | 0.85 | 2.1 |
M1, first maxillary molar; M2, second maxillary molar; K14, keratin 14.
Reduction in the crown size was generally associated with low rates of proliferation. Although normally the growing area of the cervical loop is highly Pcna positive (Fig. 7G), it was virtually devoid of proliferating cells in P1 K14-Noggin molars (Fig. 7H). Other areas of the crown, such as ameloblasts and stratum intermedium of the intercusps also showed low proliferation rates in K14-Noggin teeth (Fig. 7, L vs. K). On the other hand, elevated proliferation was seen in the epithelium and preodontoblasts of the presumptive cusps in P1 K14-Noggin molars (Fig. 7, J vs. I). Proliferation rates in the epidermis and developing hair follicles of the P1 WT and K14-Noggin mice were virtually the same (Fig. 7, B vs. A).
Fig. 7.
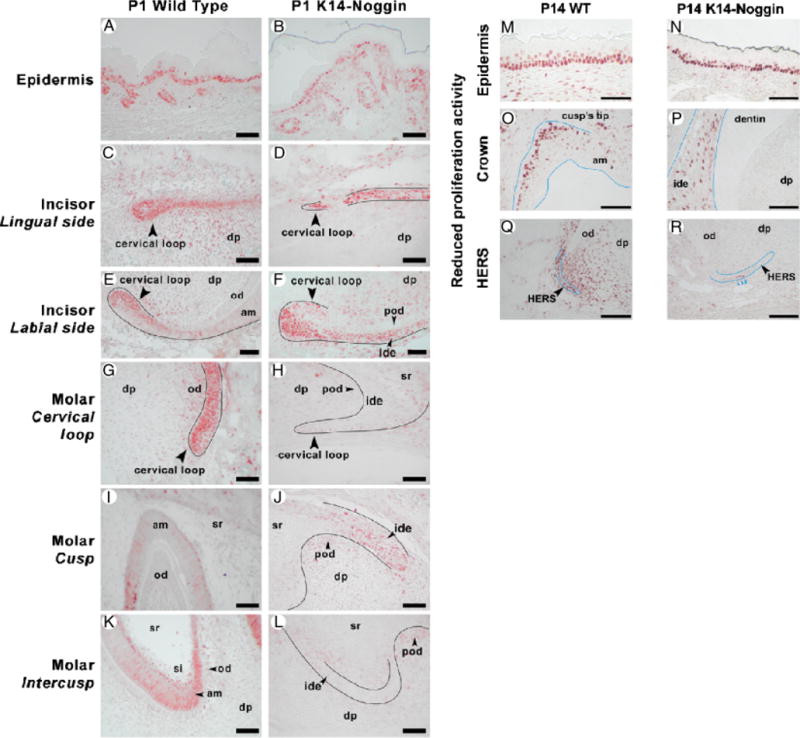
Proliferation defect in K14-Noggin teeth. (A, B) Similar rates of proliferation in the epidermis and hair follicles of P1 WT (A) and K14-Noggin (B) mice. (C–F) Proliferation pattern in P1 WT (C, E) and K14-Noggin (D, F) incisors. Proliferation rates are comparable on the lingual side of the cervical loop of both WT (C) and K14-Noggin (D) incisors. However, the proliferation zone on the labial side of the K14-Noggin cervical loop is significantly expanded distally (F vs. E). (G–L) Proliferation pattern in P1 WT (G, I, K) and K14-Noggin (H, J, L) molars. K14-Noggin molars show greatly reduced rates of proliferation, especially in the cervical loop (H vs. G) and intercusp area (L vs. K). (M–R) Reduced and nonlocalized proliferation activity in P14 K14-Noggin molars. WT molars show a localized zone of proliferation at the tip of the cusps (O) and extensive proliferation activity within HERS and the surrounding dental mesenchyme (Q). In contrast, K14-Noggin molars have reduced, de-centralized proliferation activity within the dental epithelium of the crown (P), largely reduced proliferation in HERS, and virtually no proliferation in the surrounding dental mesenchyme (R). Contrarily, the oral epithelium, adjacent to teeth, shows comparable proliferation activity both in WT (M) and K14-Noggin mice (N). Section plane: sagittal (A–R). Scale bars: 50 μm (A–R). am, ameloblasts; dp, dental papilla; ide, inner dental epithelium; od, odontoblasts; pod, preodontoblasts; si, stratum intermedium, sr, stellate reticulum.
We found the crown pattern in maxillary molars to be altered. Normal crowns form discrete cusp and intercusp regions. In adult mice, the maxillary M1 and M2 molar have seven and five cusps, respectively (Fig. 3G). K14-Noggin molars appeared to develop blunt cusp morphology (Fig. 3B). However, progressive deterioration prevented accurate assessment of crown patterning in adult mutants (Fig. 2B). The crown pattern was studied at P21 instead. At P21, K14-Noggin molars were just starting to erupt and were not affected by mechanical wearing and caries (Fig. 3, E and F). On SEM, individual cusps were clearly visible in P21 WT molars (Fig. 3G). In contrast, no distinct cusps could be identified in K14-Noggin mice. Instead, small, partially fused prominences formed (Fig. 3H). We also compared several P21 M2 molars and found the number and position of intercusps to be inconsistent even among molars from the same animal (Fig. 3I). The eruption of K14-Noggin molars was significantly delayed. At P21, both K14-Noggin maxillary molars were just starting to erupt (Fig. 3, E and F). At the same time, all three maxillary molars were fully erupted in WT mice (Fig. 3, C and D).
Root size/pattern
At P8, WT maxillary molars showed formation and downward migration of the Hertwig’s epithelial root sheath (HERS) at the apical end of the molars at which time the shape of the roots starts to appear (Fig. 3, L and N). In contrast, P8 K14-Noggin maxillary molars although having formed the HERS structure, showed no evidence of root formation (Fig. 3, M and O). By P14, the roots of both M1 and M2 WT maxillary molars were formed and were covered by a layer of cementum. The periodontal ligament (PDL) connecting the cementum and the alveolar bone was clearly seen (Fig. 3, P and R). At P14, K14-Noggin molars progressed little and the only sign of root formation was the presence of HERS (Fig. 3, Q and S). However, the forming roots of the K14-Noggin molars had largely reduced rates of proliferation, both within the HERS and dental mesenchyme (Fig. 7, R vs. Q). At the same time, rates of proliferation were comparable in the oral epithelium adjacent to molars (Fig. 7, N vs. M). There was still no evidence of furcae formation that signifies the splitting of one root into multiple roots (Fig. 3Q).
At P21, WT maxillary M1 molars had well-formed roots, with the root length nearly equal to the length of the crown. M2 molars had formed distinct roots, but they were shorter than the crown. M3 molars had initiated root development (Fig. 3A). Both maxillary M1 and M2 K14-Noggin molars were lacking any signs of root bifurcation (Fig. 3B). At P28, the WT molars were in the process of erupting with the enamel exposed to the oral cavity. There was a clear cementoenamel junction (CEJ) demarcating the ending of the crown and the beginning of the roots, which had completed their development. The periodontal apparatus was in place (Fig. 3T). In the K14-Noggin molars, there was no CEJ and no clear separation between the crown and the roots (Fig. 3U); however, there was a layer of cementum and PDL present. Some M1 K14-Noggin molars formed a very small furcae, which indicates the formation of the different roots (Fig. 3, V and W). Hence, we called this structure a crown/root (Fig. 3K).
Although all WT maxillary molars had three distinct roots (Fig. 3J), mutant M1 maxillary molars showed some signs of root bifurcation (Fig. 3K), whereas M2 molars did not form multiple roots and, in essence, consisted of crown/roots. In addition, irregular, bud-like outgrowths were observed over the surface of mutant molars (Fig. 3K). Crown/root to multiple root proportions were reversed in K14-Noggin molars (Fig. 3, J vs. K). In adult M1 and M2 WT molars, the crown to multiple root ratio was 0.53 and 0.85, respectively. This ratio became 1.8 in M1 adult K14-Noggin molars (Table 2).
Enamel/dentin differentiation
WT molars are white, smooth, and shiny. K14-Noggin molars were dull, gray, and showed numerous, widespread caries-like lesions, suggesting a poorly mineralized enamel structure (Fig. 2, B vs. A). On SEM, adult mutant molars showed multiple macro- and microscopic sites of dental decay (Fig. 3X). Ground sections showed they did not have an enamel layer (Fig. 3Y).
At E18, M1 WT molars entered the late bell stage and soon started ameloblast and odontoblast differentiation. K14-Noggin maxillary M1 molars appeared to be still at the early bell stage at P1. The epithelial–mesenchymal interface of the P1 WT molars was highly structured. From the epithelial side there was a layer of polarized ameloblasts interfacing at the basal end by the stratum intermedium. From the mesenchymal side there was a layer of dentin-producing polarized odontoblasts (Fig. 4A). In contrast, the epithelial– mesenchymal interface of the K14-Noggin molars at P1 did not show a similar specialization. There were no polarized ameloblasts but rather multiple layers of disorganized preameloblasts. There were no signs of odontoblasts on the periphery of the dental papillae. There were no enamel or dentin depositions (Fig. 4B).
Fig. 4.
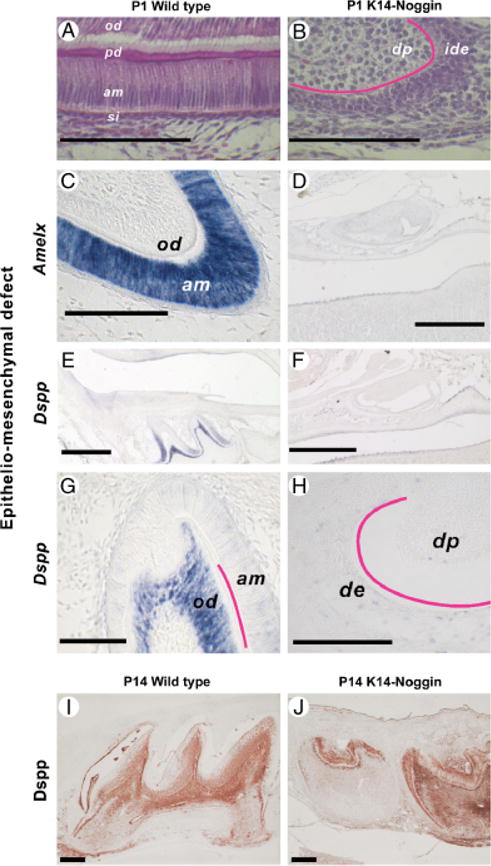
Epithelio-mesenchymal defect in K14-Noggin molars. (A– D) Crown surface defect in K14-Noggin molars. Unlike in WT molars (A), the crown surface of the K14-Noggin adult molars is uneven and highly eroded (B). On ground sections, WT molars show a distinct layer of enamel (C, green arrows), whereas no enamel is seen in K14-Noggin molars (D). (A, B) Epithelium– mesenchyme interface of the P1 WT (A) and K14-Noggin (B) M1 molars. Unlike WT, K14-Noggin molars stay largely undifferentiated. (C–J) Absence of ameloblast-specific and delayed expression of odontoblast-specific markers. In P1 WT teeth, Amelx is strongly expressed in the ameloblasts, especially in the cusp (C). Dspp has strong expression in the preodontoblasts and odontoblasts, as well as in the preameloblasts of the intercusp (E, G). In P1 K14-Noggin molars, both Amelx (D) and Dspp (F, H) are largely absent. K14-Noggin molars gain Dspp expression later. Strong Dspp expression is seen at P14 (I vs. J). Scale bars: 500 μm (D–F); 200 μm (I, J); 100 μm (A–C, G, H). Section plane: sagittal (A–J). am, ameloblasts; de, dental epithelium; dp, dental papilla; ide, inner dental epithelium; od, odontoblasts; pd, predentine; si, stratum intermedium.
To further determine the developmental status of the K14-Noggin molars, we studied the expression of differentiation markers Amelx (amelogenin) and dentin sialophosphoprotein (Dspp). Expression of Amelx in ameloblasts marks the onset of the secretory stage and was present at P1 in WT teeth (Fig. 4C). In contrast, P1 molars in K14-Noggin mice did not express Amelx (Fig. 4D). Dspp expression starts at the late bell (differentiation) stage and was present in P1 WT teeth in the preodontoblasts/odontoblasts. Consistent with previous reports, some Dspp expression was seen in the preameloblasts (Fig. 4, E and G; Begue-Kirn et al. 1998). In K14-Noggin P1 molars Dspp expression was largely absent (Fig. 4, F and H). However, dentin-like material was seen in K14-Noggin molars starting from P8 (Fig. 3I). These deposits of dentin-like material were associated with the late expression of the odontoblasts-specific Dspp markers, as seen at P14 (Fig. 4, J vs. I).
Defects in K14-Noggin incisors happen predominantly during late morphogenetic events
WT mice have one pair of incisors in the upper and lower jaw with a shiny, semi-transparent, yellowish surface (Fig. 5, A and I). In K14-Noggin mice all incisors were always present, but were thick, wide, blunt ended, and misaligned with a dull white surface (Fig. 5, B and J–L). They deteriorated and developed marked unilateral erosions because of constant tooth-wear between upper and lower pairs. These changes started early and became more severe with age. Mandible incisors grew very long and became needle sharp (Fig. 5L). The surface of K14-Noggin incisors was rough and defective (Fig. 5, E and F vs. C and D). It showed both macroscopic signs of deterioration in the form of deep, parallel ridges (Fig. 5E), and microscopic irregularities in the form of multiple bud-like formations (Fig. 5F). On the ground sections, the labial side of WT incisors displayed a clear, thick layer of enamel (Fig. 5G). In contrast, K14-Noggin incisors do not have any enamel (Fig. 5H).
Fig. 5.
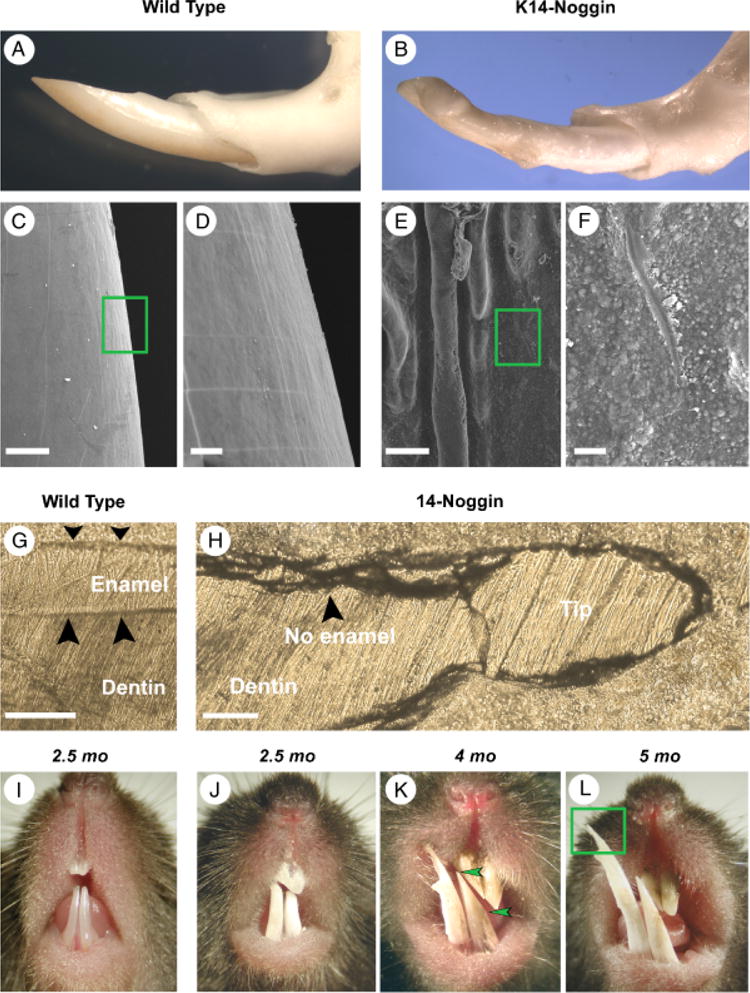
Growth abnormalities of the K14-Noggin incisors caused by the loss of enamel. (A, B) Comparative gross morphology of the mandibular incisors in the adult WT (A) and K14-Noggin mice (B). K14-Noggin incisors are thick, wide, blunt ended, and misaligned. (C–F) On SEM, the surface of the K14-Noggin incisors is rough and defective (E, F). It shows both macroscopic signs of deterioration in the form of deep, parallel ridges (E) and microscopic irregularities in the form of multiple bud-like formations (F). The surface of WT incisors is smooth (C, D). (G, H) On ground sections, WT incisors display a clear, thick layer of enamel (G). In contrast, K14-Noggin incisors do not have any enamel layer present (H). (I–L) Progressive changes of the incisors in K14-Noggin mice. Unlike WT incisors (I), K14-Noggin incisors are a dull white and deteriorate because of constant rubbing against each other (J–L). These changes start early in life and with age become more severe. The bottom incisors grew very long and became needle sharp (L). Section plane: sagittal (G, H). Scale bars: 100 μm (C, E, G, H); 20 μm (D, F).
Early developmental stages of the K14-Noggin incisors seemed to be unaffected. Similar to the WT incisors, at E13, K14-Noggin incisors were at the bud stage and by E15 further progressed into the cap stage (not shown). Developmental defects became apparent at later differentiation and secretion stages. At P1, WT incisors showed clear specialization of the dental epithelium into the ameloblasts and stratum intermedium. Peripheral dental papilla differentiated into odontoblasts. Sandwiched layers of enamel and dentin were seen in between ameloblasts and odontoblasts (Fig. 6A). From the labial side of the growing end, actively proliferating cells were confined to the small area of the cervical loop (Fig. 7E). Differentiation of the P1 K14-Noggin incisors was delayed. Retarded differentiation was associated with a markedly expanded zone of proliferation on the labial side of the cervical loop (Fig. 7, F vs. E). K14-Noggin incisors formed irregular layers of preameloblasts and preodontoblasts (Fig. 6B). Preameloblasts lacked clear parallel cell alignment, did not express Amelx (Fig. 6, E and F vs. C and D), and did not deposit enamel. Preodontoblasts looked poorly differentiated and at that stage did not express Dspp (Fig. 6, I and J vs. G and H), but seemed to deposit predentin-like material (Fig. 6B). The Epithelia–mesenchymal border had an irregular, wavy appearance throughout the K14-Noggin incisors (Fig. 6B, arrowheads). It is notable, however, that K14-Noggin P1 incisors were generally more differentiated than P1 molars (Fig. 4, F vs. 6B).
Fig. 6.

Differentiation defect in K14-Noggin incisors. (A, B) Epithelium–mesenchyme interface of the P1 WT (A) and K14-Noggin (B) M1 incisors from the labial side. Unlike WT incisors, K14-Noggin incisors remain less differentiated. They do not form a distinct layer of polarized ameloblasts and there is no dentin or enamel deposition. Epithelium–mesenchyme interface is uneven and wavy. (C–J) Absence of the tooth-specific differentiation markers in the early postnatal (P1) K14-Noggin incisors. Amelx is strongly expressed in the ameloblasts of the WT incisors (C, D). In WT P1 molars Dspp has distinct expression (G). Dspp is expressed in the preodontoblasts and odontoblasts, as well as in the preameloblasts of the proximal labial side (H). In K14-Noggin P1 incisors, both Amelx (E, F) and Dspp (I, J) are largely absent. (K, L) Epithelium–mesenchyme interface of the P14 WT (K) and K14-Noggin (L) M1 incisors from the labial side. K14-Noggin incisors remain poorly differentiated. Epithelium–mesenchyme interface is uneven and wavy. Section plane: sagittal (A–L). Scale bars: 100 μm (A–L). am, ameloblasts; dp, dermal papilla; od, odontoblasts; pam, preameloblasts; pod, preodontoblasts; pd, predentine; si, stratum intermedium.
DISCUSSION
The tooth phenotypes in K14-Noggin mice are summarized in Table 3.
Table 3.
K14-Noggin dental phenotypes are summarized
| Teeth affected in K14-Noggin mouse
|
||||
|---|---|---|---|---|
| Developmental stages | M1–M3 mandibular molars | M3 (M2) maxillary molars | M1, M2 maxillary molars | Incisors |
| Induction | ||||
| Initiation stage | X | X | ||
|
| ||||
| Morphogenesis | X | X | ||
| Bud stage | ||||
| Cap stage | ||||
| Bell stage | ||||
| Crown patterning | ||||
| Root formation | ||||
| Differentiation | ||||
|
| ||||
| Crown secretory stage | X | X | ||
| Roots secretory stage | X | |||
M1, first maxillary molar; M2, second maxillary molar; M3, third maxillary molar; K14, keratin 14.
Bmp signaling is required for the growth of molars and crown morphogenesis
Bmp signaling is critical for molar progression through the early bud stage. At E13, the K14-Noggin mandibular molar lamina does not progress to form a distinct bud, and only a dental lamina-like epithelial thickening remains. Although at E13 there is some increase in the dental mesenchymal density beneath the lamina, it disappears by E15. Despite the morphological abnormalities in dental mesenchyme, we are surprised to find that K14-Noggin mice have largely normal Msx1 expression. We also found mesenchymal Bmp4 expression in both maxillary and mandibular K14-Noggin molar regions at E13. During early molar development Bmp4 and Msx1 were shown to cooperate closely (Chen et al. 1996). It is generally believed that epithelial Bmp4 signals through Msx1 in the mesenchyme to induce its mesenchymal expression of Bmp4, as supported by the fact that Msx1-deficient mice express epithelial but not mesenchymal Bmp4. Tooth development in these mutants does not progress past the bud stage (Chen et al. 1996; Bei and Maas 1998). We suggest that K14-Noggin mandibular molars are developmentally blocked during mesenchymal Bmp4 signaling back to the dental epithelium, when the mis-expressed noggin abolishes this signaling cascade.
During normal odontogenesis, molecular signals from primary enamel knots determine the size of the tooth crown by coordinating dental epithelia proliferation and folding, as well as the position of secondary enamel knots. The location of secondary enamel knots defines normal crown patterning in multi-cusp molars (Jernvall et al. 1994). Crown defects in K14-Noggin mice are similar to, but more severe than in K14-Follistatin mice (Wang et al. 2004a). In follistatin-deficient mice, lack of activin/Bmps antagonist results in reduced inner dental epithelium proliferation, irregular folding, and shallow, unpolarized cusps (Wang et al. 2004a). Bmps might also control the distance between adjacent secondary enamel knots, thus regulating the positioning of the cusps (Jernvall and Thesleff 2000). Here we observe that K14-Noggin produces a disturbed distribution of growth centers in incisors and molars. In the K14-Noggin molar crown, proliferation is abnormally low in the epithelium and mesenchyme within the cervical loop, leading to miniaturized maxillary molars. K14-Noggin molars develop very small cusp-like prominences that are partially fused without clear intercusps. Paradoxically, the K14-Noggin incisor produces an abnormal but more diffuse distribution of proliferation. Therefore, there are region-specific responses to the same stimuli.
Bmp signaling is required for roots patterning and growth
A recent report showed Bmp2, Bmp4, and Msx2 in the HERS cells of P10 mice by in situ hybridization (Yamamoto et al. 2004). It appears that proliferation of HERS in the K14-Noggin mice is reduced resulting in delayed root formation. Even more pronounced is the defect in root patterning. The formation of the furcae that serves to delineate the different forming roots is largely delayed in M1 molars and never occurs in M2 molars. M1 K14-Noggin molars showing a small furcae, form what appear to be two small rudimentary roots, whereas their wild-type counterparts form three distinct roots. The combined root defect and absence of a well-delineated CEJ result in an indistinct crown–root border.
Growth factors such as Tgfb1 and its receptors (Gao et al. 1998, 1999), Bmp2, Bmp3, and Bmp7 (Thomadakis et al. 1999), have been found in cementoblasts, PDL, and alveolar bone. However, until now no direct functional role in root formation has been demonstrated for these factors. The involvement of Dlx3 in root development is supported by the phenotype expressed by patients with tricho-dento-osseous (TDO) syndrome. The K14-Noggin dental phenotype has some resemblance to TDO in that the maxillary molars have an altered crown-to-root ratio and very short malformed roots (taurodontism). A nonsense mutation in the DLX3 gene was identified in a family with TDO syndrome (Price et al. 1998). Among defects in hair, bone, and enamel, patients with TDO syndrome also present root defects. It is interesting that Bmp2 has been shown to regulate transcription of Dlx3 in keratinocytes (Park and Morasso 2002). These results are consistent with the notion that Bmps are required for root formation.
Bmp signaling is essential for the histogenesis of enamel and the dentin layer
Our results suggest that Bmp signaling controls the maturation of the epithelial–mesenchymal interfaces in developing teeth. It appears that, after the morphogenesis phase, Bmps are critical for instructing the inner dental epithelium to stratify and form ameloblasts during the differentiation phase. In K14-Noggin mice, the epithelial–mesenchymal border is disorganized and the inner dental epithelium fails to polarize and stratify. Ectopic noggin prevents normal differentiation of the ameloblast lineage. The predentin layer contains several secreted growth factors that stimulate the differentiation of ameloblasts and secretion of amelogenin. Bmps, such as Bmp2, are known to be transcribed in odontoblasts (Aberg et al. 1997), and can induce ameloblast differentiation (Coin et al. 1999). Odontoblast-derived Bmps are believed to stimulate ameloblast differentiation through the induction of p21 and Ambn (ameloblastin; Wang et al. 2004b). Additionally, Bmps are present in tumors that form enamel, dentin, cementum, or bone (Gao et al. 1997). Here we provide supportive in vivo evidence that Bmps are indeed required for ameloblast differentiation. Follistatin was shown to act as the main inhibitor of the Bmps-driven ameloblast differentiation in the lingual dental epithelium of mice incisors (Wang et al. 2004b). Asymmetric expression of follistatin accounts for the presence of the enamel on the labial side only. Overexpression of noggin throughout the dental epithelium of the K14-Noggin incisors wipes out the asymmetry of Bmp pathway activity, and results in incisors free of enamel on both the lingual and labial sides.
Signaling along the epithelial–mesenchymal interface is reciprocal. Ameloblasts in turn secrete growth factors that stimulate osteogenesis and cementogenesis in the adjacent tissues. Enamel extracts were shown to contain an osteoinductive ability, which is reduced when preincubated with Bmp2/Bmp4 antibody or noggin (Iwata et al. 2002). K14-Noggin teeth fail to develop odontoblasts in a timely fashion. Although layers of dentin-like material eventually form, they are deposited irregularly and are functionally defective. The dentin phenotype could be interpreted either as a direct effect of noggin or indirectly via the lack of proper differentiation of the ameloblast layer. Whatever the mechanism, the results show the essential importance of Bmps in the differentiation of the enamel and dentine layers.
Differential effects of Bmp signaling along the dental axis
We are somewhat disappointed to find the absence of the incisor to molar conversion in K14-Noggin mice (Tucker et al. 1998). It is possible that this is the result of timing and strength of the KRT14 promoter activity. The KRT14 promoter may not be active or active enough in the oral epithelium over the presumptive incisor region at E9–E10 (Byrne et al. 1994). In fact, unlike in molars, the KRT14 promoter has low activity in incisors even at the cap stage (E15). Incisors show a dramatic increase in KRT14 activity at later developmental stages (P1). Indeed, late developmental defects dominate in K14-Noggin incisors.
Still we observed region-specific responses to noggin by teeth in different molar forming regions. These differences cannot be simply explained by the temporal difference of tooth development, and may imply some fundamental differences in their development or evolutionary basis. More work is obviously required, but these results are consistent with the hypothesis that mandibular teeth have different signaling pathway requirements during their development (Thomas et al. 1997). By tuning the levels of Bmps activity, our results suggest that maxillary and mandibular molars have different levels of requirements for Bmp signaling and that mandibular molars show higher dependency on Bmp signaling in the early bud stage. Maxillary molars, albeit abnormal, proceeded through the early bud stage with reduced level of Bmps activity, whereas mandibular molar primordia did not form at all. However, maxillary molars still require some Bmps activity, as complete abolishment of Bmp signaling in Bmpr1a-deficient mice blocks the development of all teeth (Andl et al. 2004).
Among the maxillary molars, we observed inhibition of odontogenesis more on the distal, but less on the mesial end of the dental axis. The M3 almost always fail to develop and the M2 are sometimes missing. We propose three possible mechanisms for this phenomenon. First, the M3 develops later than the first two (M1 goes through bud stage at E13, M2 at E16, and M3 only upon birth), and it is possible that by the newborn stage, KRT14 promoter activity in the dental epithelia has risen high enough to completely block development of the tooth bud. Our K14 immunostaining data support this hypothesis. Second, early Bmp signaling deficiency may reduce the size of the committed molar field and there is simply not enough cellular mass left to form the third molar (Wang et al. 2004a). The more provocative third possibility is that either epithelial or mesenchymal cells forming the maxillary M3 molars are intrinsically different from those forming M1 and M2 molars, and may have similarly high Bmp signaling dependencies like the mandibular molars. Indirect evidence suggests that neural crest cells along the jaw axis may be intrinsically different (Ruch 1995; Sharpe 1995). Neural crest cells originate at different time points from topologically different areas. Maxillary molar crest cells appear to derive from the anterior midbrain at the five-somite stage and posterior midbrain at the six-somite stage (Imai et al. 1996; Köntges and Lumsden 1996). Mandibular molar crest cells are predominantly derived from the posterior midbrain and partially from the anterior hindbrain at the 5/6-somite stage. We speculate that distal maxillary mesenchyme may behave similar to the mandibular dental mesenchyme.
Indeed different dental regions have different competences based on their distinct intracellular molecular compositions. Barx1 is expressed in molar mesenchyme and determines molar phenotype. Isl1 (homeobox gene; synonym: Islet 1) is expressed in incisor epithelium and determines incisor phenotype (Mitsiadis et al. 2003). For the maxilla/mandible, it is shown that in response to the epithelial Fgf8, maxillary mesenchyme respond with Dlx2 expression only, and mandibular mesenchyme respond with both Dlx2 and Dlx5 expressions. This was used as evidence for the existence of internal differences of maxillary and mandibular mesenchyme, probably because of differences in their origin from the neural crest (Ferguson et al. 2000).
Comparison with other mouse tooth mutants and human dental diseases
Genetically engineered mice involving the Bmp pathway have been generated. Mice with a deletion of Bmpr1a in their epithelium driven by the KRT14 promoter lack all teeth upon birth. Development of both molars and incisors is arrested at the bud stage (Andl et al. 2004). As teeth are arrested at early stages of development, there is no opportunity to evaluate the role(s) of Bmp signaling in later events of histogenesis and differentiation. On the other hand, no dental phenotype was documented for Bmp5 (Kingsley et al. 1992) and Bmp7 (Dudley and Robertson 1997) loss-of-function mutations. This could be because of the redundancy of Bmp ligands; multiple, if not all Bmps have to be inactivated to disturb normal odontogenesis. Mice deficient for noggin or Chrd (another Bmps antagonist; synonym: chordin) also do not demonstrate significant dental phenotypes (Stottmann et al. 2001). Under physiological conditions noggin is apparently not expressed during early dental development; however, follistatin and perhaps ectodin (ectodermal Bmp inhibitor; aka: Sostdc1, sclerostin domain containing 1) antagonize Bmps signaling in developing teeth (Laurikkala et al. 2003).
In the teeth of other genetically engineered or mutated mice, there are several interesting findings related to our observation (Fig. 8C). All teeth in Lef1- (Kratochwil et al. 1996), Pitx2- (Szeto 1999), Pax9- (Peters et al. 1998), Msx1/Msx2-(Bei and Maas 1998), and Gli2/Gli3- (Hardcastle et al. 1998) deficient mice are also completely absent, implying a block in the earlier inductive stage. These molecules would not be ideal molecular tools for fine tuning tooth morphology in the context of morphoregulation. There are also selective losses of tooth types. In activin mutant mice, incisors and mandibular molars fail to develop beyond the bud stage, whereas their maxillary molars are unaffected (Ferguson et al. 1998). Mice overexpressing follistatin (Tgfb inhibitor) under the KRT14 promoter lack both maxillary and mandibular third molars, have a disturbed cusp pattern, and show premature wearing of enamel (Wang et al. 2004a). Dlx1/Dlx2-deficient mice do not form maxillary molars, but their incisors and mandibular molars are normal. It was shown that the mutated ectomesenchyme underlying the maxillary molar regions loses its odontogenic potential and forms chondrocytes instead (Qiu et al. 1997; Thomas et al. 1997). These results further corroborate the hypothesis that there are both qualitative and quantitative differences among different teeth.
Fig. 8.
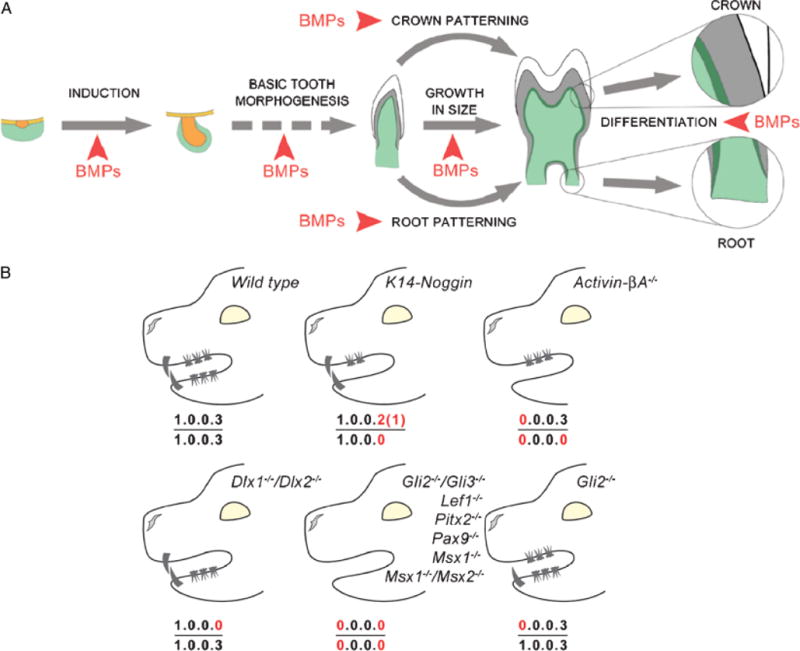
Summary of the multiple dental defects caused by the disruption of the Bmp pathway in the oral epithelium. (A) Summary diagram of the tooth developmental events affected by the reduced strength of Bmp signaling in K14-Noggin mice. (B) Comparison of the dental formulas between WT, K14-Noggin, and other known mutant mice with reduction in teeth number.
In various dental diseases, humans also suffer from malformation abnormal differentiation and loss of teeth. Humans have the dental formula of 2.1.2.3/2.1.2.3. Agenesis of one or several teeth (hypodontia) is a common congenital defect (Vastardis 2000). Less prevalent is oligodontia, when six or more permanent teeth are missing. Mutations in transcription factor genes MSX1 and PAX9 were found responsible for the nonsyndromic forms of oligodontia (Vastardis et al. 1996; Stockton et al. 2000; Nieminen et al. 2001; Lammi et al. 2003). Syndromic oligodontias are often associated with multi-organ syndromes, and like hypodontia, they are believed to have heterogeneous and mostly nonestablished genetic backgrounds. In addition to tooth agenesis, congenital dental pathology also enlists defects in dentin differentiation (e.g., dentinogenesis imperfecta type II; Zhang et al. 2001), enamel differentiation (e.g., amelogenesis imperfecta; Lagerstrom et al. 1991), root formation (e.g., hypoplasia of teeth roots; Lind 1972), teeth eruption (Stoelinga et al. 1976), etc. There are also nongenetic human dental diseases involving defects in the enamel, dentine, and cementum. The diverse dental phenotypes in K14-Noggin mice may become a useful experimental model to study the progression, consequences, side effects, and management of these defects in vivo.
Evo-Devo implications of teeth and other ectodermal organs
Among ectodermal organs, teeth are the most common elements found in the fossil record. A large body of literature on vertebrate paleontology describes evolutionary changes in tooth morphology (Crompton 1995; Hiiemae 2000). Although these changes are well understood from a functional perspective, the developmental mechanisms behind these changes are mostly unknown.
The evolutionary origin of molar shape and dentition patterns was previously summarized (Crompton 1971). In archetypical reptiles, the teeth have one single elevation (or a prototypic cusp), which is triangular in shape with a cylindrical base. There is not much regional diversity in the oral cavity. Most Mesozoic birds have teeth along their entire jaw (e.g., Archeopteryx; Feduccia 2001), or only in the distal beak (e.g., Longirostravis; Hou et al. 2004). The morphology of their teeth is of the prototypical conical shape without any signs of regional diversity. Modern and Cenozoic birds as well as some Mesozoic birds (e.g., Confucisornis) lack teeth completely (Hou et al. 2003). As mammal-like reptiles evolved, their dentitions became more complex (Crompton 1995; Hiiemae 2000). From the prototypic dental formula of 3.1.4.3./ 3.1.4.3., loss of teeth is a common theme in mammalian evolution, whereas addition is rare. In some cases, teeth are lost completely (e.g., baleen whales), or all teeth of one jaw are lost (e.g., upper jaw of the spermwhales). More frequently, a whole class of teeth is missing, such as the loss of the upper incisors in deer (Cervidae), or of the premolars in mice. Changes in crown patterning are associated with different modes of food processing. Examples were described above in the mice and voles (Tummers and Thesleff 2003). Loss of regional specificities can result in similarity of all teeth along the tooth row (e.g., armadillos, Dasypodidae; dolphins, Delphinidae). In different animals, there are different proportions and arrangements of hard tissues (enamel, dentin, and cementum), because of their different material properties (Martin et al. 2003). In grass-eating herbivores (e.g., horses, antelope), enamel forms high-crowned molars, with valleys filled in with dentin and cementum for grinding. On the other hand, enamel is present in scanty quantities on elephant tusks, which wear off soon, and teeth are reduced to dentin pegs. With new understanding in tooth formation, we can learn more on how these diversities can be generated by variations in the induction (gain or loss of number), morphogenesis (patterning), or differentiation (ratio of hard tissues) stages. This Bmp pathway study provides some clues that can be verified in extant animals (Fig. 8, A and B).
Some efforts have been made to understand the Evo-Devo of teeth. Tooth loss has happened frequently and independently during evolution. Developmentally, how was this achieved? Are there multiple ways to lose teeth? To see if teeth can be rescued in avians, Kollar recombined chicken oral mucosa and mouse dental mesenchyme (Kollar and Fisher 1980). Chicken dental lamina can progress to the bud stage when Bmps/Fgfs are added to the organ culture. Chicken oral epithelium is competent to form tooth-like appendage follicles when it is adjacent to feather forming mesenchyme (Chen et al. 2000) or mouse cephalic crest cells (Mitsiadis et al. 2003). Another tooth region is the diastema between incisors and molars in mice. It seems to be achieved through the localized sequestration of Shh by Gas1 in the diastema mesenchyme, causing ablation of the Shh signaling necessary for tooth development (Cobourne et al. 2004). Thus, loss of teeth can be achieved through the modulation of the activity of several different signaling pathways, and noggin-induced tooth loss may also have been used.
Another molecular pathway that behaves like a morphoregulator is the ectodysplastin (Eda) pathway (Srivastava et al. 1997; Tucker et al. 2004). It is interesting that molar phenotype of the K14-Noggin mice is somewhat similar to that of Tabby mutant mice. Tabby mice carry a spontaneous mutation in the Eda (anhidrotic ectodermal dysplasia) gene and show multiple developmental defects in the ectodermally derived organs, such as hairs, sweat glands, and teeth. Tabby molars are small. Some cusps are missing; others are either fused or too closely positioned to each other. In Tabby molars, the primary enamel knot is small, which results in the smaller crown base and ultimately in the fusion of the secondary enamel knots. Additionally, growth of the cervical loop epithelium in Tabby molars is slow. This results in slow formation of the tooth crown base (Pispa et al. 1999; Jernvall and Thesleff 2000). With the increased level of Eda in the tooth-forming region, Eda activity imposes a moderate yet persistent effect on the number of teeth, cusps, and crown complexity (Kangas et al. 2004). Together with its roles on other ectodermal appendages (Pispa and Thesleff 2003), the Eda pathway can be considered as another morphoregulatory pathway.
Our understanding in the Evo-Devo of ectodermal organs has just begun (Chuong 1998; Pispa and Thesleff 2003). Interpreting the transgenic morphology in the context of the fossil record and analyzing the molecular basis of tooth diversity in extant mammals beyond the mouse will add to our appreciation of the fundamental mechanisms of epithelial appendage morphogenesis. Here we demonstrated that the Bmp pathway is one of the major morphoregulatory pathways. We showed that tooth characteristics can be sculptured by tuning Bmps activity at different stages of tooth morphogenesis. The repetitive use of the Bmp pathway is consistent with the concept of co-opting an existing molecular pathway for evolutionary novelty in a different hierarchical level to reach new complexity (True and Carroll 2002; Prum and Dyck 2003). The study of the morphoregulatory principles in ectodermal organs will help our long-term objectives of how to guide epithelial stem cells to form organs we desire.
Acknowledgments
This work is supported by grants from NIH (C. M. C.) AR42177, AR47364, by grant DE012346 (M. Zeichner-David), and by a grant from the NSF (J. G. M. Thewissen) NSF-EAR 0207370. M. P. is supported by the USC Graduate School Oakley Fellowship. We thank Dr. Ting-Xin Jiang, Dr. Randall B. Widelitz, and Dr. Wen Pin Wang for their help.
References
- Aberg T, Wozney J, Thesleff I. Expression patterns of bone morphogenetic proteins (bmps) in the developing mouse tooth suggest poles in morphogenesis and cell differentiation. Dev Dyn. 1997;210:383–396. doi: 10.1002/(SICI)1097-0177(199712)210:4<383::AID-AJA3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Andl T, et al. Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development. 2004;131:2257–2268. doi: 10.1242/dev.01125. [DOI] [PubMed] [Google Scholar]
- Begue-Kirn C, Krebsbach PH, Bartlett JD, Butler WT. Dentin sialoprotein, dentin phosphoprotein, enamelysin and ameloblastin: tooth-specific molecules that are distinctively expressed during murine dental differentiation. Eur J Oral Sci. 1998;106:963–970. doi: 10.1046/j.0909-8836.1998.eos106510.x. [DOI] [PubMed] [Google Scholar]
- Bei M, Kratochwil K, Maas RL. BMP4 rescues a non-cell-autonomous function of Msx1 in tooth development. Development. 2000;127:4711–4718. doi: 10.1242/dev.127.21.4711. [DOI] [PubMed] [Google Scholar]
- Bei M, Maas R. FGFs and BMP4 induce both Msx1-independent and Msx1-dependent signaling pathways in early tooth development. Development. 1998;125:4325–4333. doi: 10.1242/dev.125.21.4325. [DOI] [PubMed] [Google Scholar]
- Byrne C, Tainsky M, Fuchs E. Programming gene expression in developing epidermis. Development. 1994;120:2369–2383. doi: 10.1242/dev.120.9.2369. [DOI] [PubMed] [Google Scholar]
- Chen Y, Bei M, Woo I, Satokata I, Maas R. Msx1 controls inductive signaling in mammalian tooth morphogenesis. Development. 1996;122:3035–3044. doi: 10.1242/dev.122.10.3035. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. Conservation of early odontogenic signaling pathways in Aves. Proc Natl Acad Sci USA. 2000;97:10044–10049. doi: 10.1073/pnas.160245097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin: 1998. [Google Scholar]
- Cobourne MT, Miletich I, Sharpe PT. Restriction of sonic hedgehog signalling during early tooth development. Development. 2004;131:2875–2885. doi: 10.1242/dev.01163. [DOI] [PubMed] [Google Scholar]
- Coin R, Haikel Y, Ruch JV. Effects of apatite, transforming growth factor beta-1, bone morphogenetic protein-2 and interleukin-7 on ameloblast differentiation in vitro. Eur J Oral Sci. 1999;107:487–495. doi: 10.1046/j.0909-8836.1999.eos107611.x. [DOI] [PubMed] [Google Scholar]
- Crompton A. The origin of the tribosphenic molar. In: Kermack D, Kermack K, editors. Early Mammals. Academic Press; New York: 1971. [Google Scholar]
- Crompton A. Masticatory function in non-mammalian cynodonts and early mammals. In: Thomason J, editor. Functional Morphology in Vertebrate Paleontology. Cambridge University Press; Cambridge: 1995. pp. 55–74. [Google Scholar]
- Dudley AT, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Morphoregulation. Dev Dyn. 1992;193:2–10. doi: 10.1002/aja.1001930103. [DOI] [PubMed] [Google Scholar]
- Feduccia A. Fossils and avian evolution. Nature. 2001;414:507–508. doi: 10.1038/35107144. [DOI] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Christensen L, Lau AL, Matzuk MM, Sharpe PT. Activin is an essential early mesenchymal signal in tooth development that is required for patterning of the murine dentition. Genes Dev. 1998;12:2636–2649. doi: 10.1101/gad.12.16.2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson CA, Tucker AS, Sharpe PT. Temporospatial cell interactions regulating mandibular and maxillary arch patterning. Development. 2000;127:403–412. doi: 10.1242/dev.127.2.403. [DOI] [PubMed] [Google Scholar]
- Gao J, Symons AL, Bartold PM. Expression of transforming growth factor-beta 1 (TGF-beta1) in the developing periodontium of rats. J Dent Res. 1998;77:1708–1716. doi: 10.1177/00220345980770090701. [DOI] [PubMed] [Google Scholar]
- Gao J, Symons AL, Bartold PM. Expression of transforming growth factor-beta receptors types II and III within various cells in the rat periodontium. J Periodontal Res. 1999;34:113–122. doi: 10.1111/j.1600-0765.1999.tb02230.x. [DOI] [PubMed] [Google Scholar]
- Gao YH, Yang LJ, Yamaguchi A. Immunohistochemical demonstration of bone morphogenetic protein in odontogenic tumors. J Oral Pathol Med. 1997;26:273–277. doi: 10.1111/j.1600-0714.1997.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Han J, Ito Y, Yeo JY, Sucov HM, Maas R, Chai Y. Cranial neural crest-derived mesenchymal proliferation is regulated by Msx1-mediated p19(INK4d) expression during odontogenesis. Dev Biol. 2003;261:183–196. doi: 10.1016/s0012-1606(03)00300-2. [DOI] [PubMed] [Google Scholar]
- Hardcastle Z, Mo R, Hui CC, Sharpe PT. The Shh signalling pathway in tooth development: defects in Gli2 and Gli3 mutants. Development. 1998;125:2803–2811. doi: 10.1242/dev.125.15.2803. [DOI] [PubMed] [Google Scholar]
- Hiiemae KM. Feeding Form, Function, and Evolution in Tetrapod Vertebrates. Academic Press; New York: 2000. pp. 411–448. [Google Scholar]
- Hou L, Chiappe LM, Zhang F, Chuong CM. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften. 2004;91:22–25. doi: 10.1007/s00114-003-0489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou LH, Chuong CM, Yang A, Zeng XL, Hou JF. Fossil Birds of China. Yunnan Science and Technology; China: 2003. [Google Scholar]
- Imai H, Osumi-Yamashita N, Ninomiya Y, Eto K. Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev Biol. 1996;176:151–165. doi: 10.1006/dbio.1996.9985. [DOI] [PubMed] [Google Scholar]
- Iwata T, Morotome Y, Tanabe T, Fukae M, Ishikawa I, Oida S. Noggin blocks osteoinductive activity of porcine enamel extracts. J Dent Res. 2002;81:387–391. doi: 10.1177/0810387. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Kettunen P, Karavanova I, Martin LB, Thesleff I. Evidence for the role of the enamel knot as a control center in mammalian tooth cusp formation: non-dividing cells express growth stimulating Fgf-4 gene. Int J Dev Biol. 1994;38:463–469. [PubMed] [Google Scholar]
- Jernvall J, Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19–29. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- Kangas AT, Evans AR, Thesleff I, Jernvall J. Nonindependence of mammalian dental characters. Nature. 2004;432:211–214. doi: 10.1038/nature02927. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, et al. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Kollar EJ, Fisher C. Tooth induction in chick epithelium: expression of quiescent genes for enamel synthesis. Science. 1980;207:993–995. doi: 10.1126/science.7352302. [DOI] [PubMed] [Google Scholar]
- Köntges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Dull M, Fariñas I, Galceran J, Grosschedl R. Lef1 expression is activated by BMP-4 and regulates inductive tissue interactions in tooth and hair development. Genes Dev. 1996;10:1382–1394. doi: 10.1101/gad.10.11.1382. [DOI] [PubMed] [Google Scholar]
- Lagerstrom M, et al. A deletion in the amelogenin gene (AMG) causes X-linked amelogenesis imperfecta (AIH1) Genomics. 1991;10:971–975. doi: 10.1016/0888-7543(91)90187-j. [DOI] [PubMed] [Google Scholar]
- Lammi L, Halonen K, Pirinen S, Thesleff I, Arte S, Nieminen P. A missense mutation in PAX9 in a family with distinct phenotype of oligodontia. Eur J Hum Genet. 2003;11:866–871. doi: 10.1038/sj.ejhg.5201060. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Kassai Y, Pakkasjarvi L, Thesleff I, Itoh N. Identification of a secreted BMP antagonist, ectodin, integrating BMP, FGF, and SHH signals from the tooth enamel knot. Dev Biol. 2003;264:91–105. doi: 10.1016/j.ydbio.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Lind V. Short root anomaly. Scand J Dent Res. 1972;80:85–93. doi: 10.1111/j.1600-0722.1972.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Line SR. Variation of tooth number in mammalian dentition: connecting genetics, development, and evolution. Evol Dev. 2003;5:295–304. doi: 10.1046/j.1525-142x.2003.03036.x. [DOI] [PubMed] [Google Scholar]
- Maas R, Bei M. The genetic control of early tooth development. Crit Rev Oral Biol Med. 1997;8:4–39. doi: 10.1177/10454411970080010101. [DOI] [PubMed] [Google Scholar]
- Martin LB, Olejniczak AJ, Maas MC. Enamel thickness and microstructure in pitheciin primates, with comments on dietary adaptations of the middle Miocene hominoid Kenyapithecus. J Hum Evol. 2003;45:351–367. doi: 10.1016/j.jhevol.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J. Development of teeth in chick embryos after mouse neural crest transplantations. Proc Natl Acad Sci USA. 2003;100:6541–6545. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J Cell Physiol. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- Nieminen P, et al. Identification of a nonsense mutation in the PAX9 gene in molar oligodontia. Eur J Hum Genet. 2001;9:743–746. doi: 10.1038/sj.ejhg.5200715. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Sharpe PT. TNF signalling in tooth development. Curr Opin Genet Dev. 2004;14:513–519. doi: 10.1016/j.gde.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Park GT, Morasso MI. Bone morphogenetic protein-2 (BMP-2) transactivates Dlx3 through Smad1 and Smad4: alternative mode for Dlx3 induction in mouse keratinocytes. Nucleic Acids Res. 2002;30:515–522. doi: 10.1093/nar/30.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pispa J, et al. Cusp patterning defect in Tabby mouse teeth and its partial rescue by FGF. Dev Biol. 1999;216:521–534. doi: 10.1006/dbio.1999.9514. [DOI] [PubMed] [Google Scholar]
- Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- Plikus M, Wang WP, Liu J, Wang X, Jiang TX, Chuong CM. Morpho-regulation of ectodermal organs: integument pathology and phenotypic variations in K14-Noggin engineered mice through modulation of bone morphogenic protein pathway. Am J Pathol. 2004;164:1099–1114. doi: 10.1016/S0002-9440(10)63197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JA, Wright JT, Kula K, Bowden DW, Hart TC. A common DLX3 gene mutation is responsible for tricho-dento-osseous syndrome in Virginia and North Carolina families. J Med Genet. 1998;35:825–828. doi: 10.1136/jmg.35.10.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prum RO, Dyck J. A hierarchical model of plumage: morphology, development, and evolution. J Exp Zool—Part B Mol Dev Evol. 2003;298:73–90. doi: 10.1002/jez.b.27. [DOI] [PubMed] [Google Scholar]
- Qiu M, et al. Role of the Dlx homeobox genes in proximodistal patterning of the branchial arches: mutations of Dlx-1, Dlx-2, and Dlx-1 and -2 alter morphogenesis of proximal skeletal and soft tissue structures derived from the first and second arches. Dev Biol. 1997;185:165–184. doi: 10.1006/dbio.1997.8556. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Ruch JV. Tooth crown morphogenesis and cytodifferentiations: candid questions and critical comments. Connect Tissue Res. 1995;32:1–8. doi: 10.3109/03008209509013699. [DOI] [PubMed] [Google Scholar]
- Scarel-Caminaga RM, Pasetto S, Silva ER, Peres RCR. Genes and tooth development: reviewing the structure and function of some key players. Braz J Oral Sci. 2002;2:339–347. [Google Scholar]
- Sharpe P. Homeobox genes and orofacial development. Connect Tissue Res. 1995;32:17–25. doi: 10.3109/03008209509013701. [DOI] [PubMed] [Google Scholar]
- Slavkin HC, Shum L, Nuckolls GH. Ectodermal dysplasia: a synthesis between evolutionary, developmental, and molecular biology and human clinical genetics. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Landes Bioscience; Austin: 1998. [Google Scholar]
- Srivastava AK, et al. The Tabby phenotype is caused by mutation in a mouse homologue of the EDA gene that reveals novel mouse and human exons and encodes a protein (ectodysplasin-A) with collagenous domains. Proc Natl Acad Sci USA. 1997;94:13069–13074. doi: 10.1073/pnas.94.24.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockton DW, Das P, Goldenberg M, D’Souza RN, Patel PI. Mutation of PAX9 is associated with oligodontia. Nat Genet. 2000;24:18–19. doi: 10.1038/71634. [DOI] [PubMed] [Google Scholar]
- Stoelinga PJW, de Koomen HA, Davis GB. Multiple non-erupting teeth, maxillo-zygomatical hypoplasia and other congenital defects: an autosomal recessive disorder. Clin Genet. 1976;10:222–225. doi: 10.1111/j.1399-0004.1976.tb00038.x. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Anderson RM, Klingensmith J. The BMP antagonists Chordin and Noggin have essential but redundant roles in mouse mandibular outgrowth. Dev Biol. 2001;240:457–473. doi: 10.1006/dbio.2001.0479. [DOI] [PubMed] [Google Scholar]
- Szeto DP, et al. Role of the Bicoid-related homeodomain factor Pitx1 in specifying hindlimb morphogenesis and pituitary development. Genes Dev. 1999;13:484–494. doi: 10.1101/gad.13.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata MJ, Matsumura T, Liu JG, Wakisaka S, Kurisu K. Expression of cytokeratin 14 in ameloblast-lineage cells of the developing tooth of rat, both in vivo and in vitro. Arch Oral Biol. 1996;41:1019–1027. doi: 10.1016/s0003-9969(96)00087-8. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Mikkola M. The role of growth factors in tooth development. Int Rev Cytol. 2002;217:93–135. doi: 10.1016/s0074-7696(02)17013-6. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Sharpe P. Signalling networks regulating dental development. Mech Dev. 1997;67:111–123. doi: 10.1016/s0925-4773(97)00115-9. [DOI] [PubMed] [Google Scholar]
- Thomadakis G, Ramoshebi LN, Crooks J, Rueger DC, Ripamonti U. Immunolocalization of bone morphogenetic protein-2 and -3 and osteogenic protein-1 during murine tooth root morphogenesis and in other craniofacial structures. Eur J Oral Sci. 1999;107:368–377. doi: 10.1046/j.0909-8836.1999.eos107508.x. [DOI] [PubMed] [Google Scholar]
- Thomas BL, et al. Role of Dlx-1 and Dlx-2 genes in patterning of the murine dentition. Development. 1997;124:4811–4818. doi: 10.1242/dev.124.23.4811. [DOI] [PubMed] [Google Scholar]
- True JR, Carroll SB. Gene co-option in physiological and morphological evolution. Annu Rev Cell Dev Biol. 2002;18:53–80. doi: 10.1146/annurev.cellbio.18.020402.140619. [DOI] [PubMed] [Google Scholar]
- Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Headon DJ, Courtney JM, Overbeek P, Sharpe PT. The activation level of the TNF family receptor, Edar, determines cusp number and tooth number during tooth development. Dev Biol. 2004;268:185–194. doi: 10.1016/j.ydbio.2003.12.019. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282:1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- Tummers M, Thesleff I. Root or crown: a developmental choice orchestrated by the differential regulation of the epithelial stem cell niche in the tooth of two rodent species. Development. 2003;130:1049–1057. doi: 10.1242/dev.00332. [DOI] [PubMed] [Google Scholar]
- Vastardis H. The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofacial Orthop. 2000;117:650–656. [PubMed] [Google Scholar]
- Vastardis H, Karimbux N, Guthua SW, Seidman JG, Seidman CE. A human MSX1 homeodomain missense mutation causes selective tooth agenesis. Nat Genet. 1996;13:417–421. doi: 10.1038/ng0896-417. [DOI] [PubMed] [Google Scholar]
- Wang XP, et al. Modulation of activin/bone morphogenetic protein signaling by follistatin is required for the morphogenesis of mouse molar teeth. Dev Dyn. 2004a;231:98–108. doi: 10.1002/dvdy.20118. [DOI] [PubMed] [Google Scholar]
- Wang XP, Suomalainen M, Jorgez CJ, Matzuk MM, Werner S, Thesleff I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev Cell. 2004b;7:719–730. doi: 10.1016/j.devcel.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Wu P, et al. Evo-Devo of amniote integuments and appendages. Int J Dev Biol. 2004;48:249–270. doi: 10.1387/ijdb.041825pw. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H, Cho SW, Kim EJ, Kim JY, Fujiwara N, Jung HS. Developmental properties of the Hertwig’s epithelial root sheath in mice. J Dent Res. 2004;83:688–692. doi: 10.1177/154405910408300906. [DOI] [PubMed] [Google Scholar]
- Yamashiro T, Tummers M, Thesleff I. Expression of bone morphogenetic proteins and Msx genes during root formation. J Dent Res. 2003;82:172–176. doi: 10.1177/154405910308200305. [DOI] [PubMed] [Google Scholar]
- Yu M, Wu P, Widlitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]
- Zhang Y, et al. A new function of BMP4: dual role for BMP4 in regulation of Sonic hedgehog expression in the mouse tooth germ. Development. 2000;127:1431–1443. doi: 10.1242/dev.127.7.1431. [DOI] [PubMed] [Google Scholar]


