Abstract
Complex skin appendages are built from the epidermal cells through induction, cell fate specification, proliferation, apoptosis, and differentiation, etc. Here we used the TUNEL assay and caspase-3 immuno-localization to examine apoptotic events in different stages of feather morphogenesis. We deduced three modes through which apoptosis may impact morphogenesis. In Mode 1A, apoptosis occurs within the localized growth zone to regulate growth as seen in growing buds. In Mode 1B, morphogen secreting cells are present adjacent to localized growth zones and apoptosis may work as a mechanism to remove such signaling centers. This was seen in marginal / barbule plate interactions. In Mode 2, keratinocytes apoptosed before terminal differentiation and left spaces between branches, as seen in the marginal plate epithelia. In Mode 3A, keratinocytes cornified and flaked off between two epidermal layers, thus helping to free skin appendages as seen in the feather sheath and pulp epithelium. In Mode 3B, keratinized apoptosed epithelial cells became permanent structures as seen in the rachis and barbs. Thus, the mode of death has a major impact on epithelial morphogenesis. We further tested the effects of imbalanced Shh on apoptosis with retroviral mediated transgenic feathers. Shh suppression not only reduces marginal plate apoptosis and caspase-3 expression, but also causes abnormal differentiation of barbule plates. Expression of Patched in the barbules, but not the marginal plates, implied that the previously shown Shh dependent marginal plate apoptosis is mediated through a paracrine mechanism. New Shh over-expression data showed enhanced proliferation and reduced apoptosis in barb ridges. This work complements our recent work on the role of shifting localized growth zones in feather morphogenesis (Chodankar et al., 2003; J. Invest. Dermatol. 120:20), and shows how adding and removing cell masses in temporally and spatially specific ways are coordinated to sculpt skin appendages out from epidermal layers.
Keywords: cell death, keratinization, hair, feather development, Sonic hedgehog (SHH)
There are a variety of skin appendages including hair, nails, sweat glands, and mammary glands in mammals; feathers, scales, claws and beaks in birds. Morphogenesis of skin appendages involves the topographical transformation of the primarily flat epidermis into specialized complex structures for specific functions. The morphogenesis of these varied skin appendages shares common developmental processes including induction, cell fate specification, proliferation, differentiation, epithelial cycling, and intimate interactions with the mesenchyme (Chuong, 1998). The role of stem cells (Lavker and Sun, 2000), signaling molecules (Millar, 2002; Widelitz and Chuong, 2000), cell proliferation (Chodankar et al., 2003), and differentiation (Ahmad et al., 1998; Ma et al., 2003) have been studied. Cell proliferation is required to build up cellular mass. Cell rearrangement is required to form appendage primordia. Differentiation is required to make specialized keratinized structures. How do these elaborate structures separate and release from the rest of the epidermis to become independent functional units?
One of the major shaping forces is apoptosis. Apoptosis is an evolutionarily conserved form of cell death and is an important process for organogenesis. The biochemistry of apoptosis is well characterized (Hengartner, 2000). Apoptosis can be activated by triggering the death receptor pathway or the mitochondrial pathway, termed the extrinsic and intrinsic pathways, respectively. It is characterized by the activation of an evolutionarily conserved proteolytic system involving initiator and effector caspases (reviewed in Strasser et al., 2000) leading to the cleavage of an array of cellular substrates including cytoskeletal elements, transcription factors, and proteins involved in DNA repair, replication, signal transduction, and the cell cycle. The purpose of the current study is not to dissect these well characterized pathways further, but to study the diverse roles and distinct consequences of apoptosis in morphogenesis and organogenesis.
Most apoptosis studies on skin appendages have been performed on hair cycling in mice and humans (Paus, 1999; Soma et al, 1998) and focused on the destruction of the follicle region below the bulge. However, the roles of apoptosis during skin appendage development have not been studied in detail from the viewpoint of morphogenesis. In this study, we examined the apoptotic events by TdT-mediated dUTP nick end label (TUNEL) staining (Gavrieli et al. 1992). This technique enables the identification of apoptotic cells in restricted regions (Stadelmann and Lassmann, 2000). Since caspases mediate apopototic events (Strasser et al., 2000), we also used the expression of effector caspase-3, which is shared by the TNF receptor and mitochondria apoptotic pathways, as an additional marker. In a previous study, we have shown that there are TUNEL positive cells in the developing hair germs and hair canal of embryonic human skin (Chang et al., 2001). Here we sought to use feather morphogenesis, an experiment-accessible and complex skin appendage model, to identify fundamental principles of how apoptotic events are used in different stages of skin appendage morphogenesis.
The feather has become a major model for studying skin appendage biology because of its accessibility to experimentation, distinct morphology and exact temporal sequence of cellular events (Chen and Chuong, 1999; Chuong et al., 2000). Events sequentially unfold along the proximal-distal (more differentiated in the distal) axis of the feather filament shaft, posterior – anterior axis (more differentiated in the anterior) and centrifugal axis within each barb ridge (more differentiated in the periphery), facilitating a comparison of the timing of cellular events. Furthermore, we recently developed a technique to transduce exogenous genes into regenerating feathers (Yu et al., 2002). This allows us to analyze the roles of gene activities on apoptosis with relevance to keratinocyte proliferation and differentiation. Because of the specific high expression of Shh in the marginal plate epithelia, we suggested that Shh might be associated with cell death (Ting–Berreth and Chuong, 1996). Subsequently, we showed that Shh is involved in feather branching (Yu et al, 2002). Here, we use this pathway, as an example, to analyze further the roles of balanced molecular activity in coordinating apoptosis, proliferation, differentiation and hence morphogenesis. From these results, we are able to deduce both the specific locations and the timing of apoptosis, relative to the proliferation and differentiation events can have dramatically different consequences in morphogenesis.
Materials and Methods
Chicken specimens
White Leghorn skin was from embryonic (E9-E18) and newborn chicks. The ulnar side of the alar wing tract was fixed with 4% paraformaldehyde and paraffin-embedded. Six-micrometer sections were prepared for staining. Virally transduced feathers were processed in the same way.
TUNEL staining
TUNEL staining was performed following the recommendations of the manufacturer (In Situ Cell Death Detection, POD Kit (Roche)). Methyl green (DAKO) was used for counterstaining when necessary.
Immunohistochemistry and in situ hybridization
Immunohistochemistry was performed as described in Jiang et al., 1992. Anti-chicken PCNA (clone 424, Clontech, dilution 1:200) and β-catenin (clone 14, BD Transduction, dilution 1:100, Santa Cruz Biotech) antibodies were each applied on samples, and incubated overnight, at 4 °C. Antibodies to caspase-3 are from Santa Cruz Biotech. Color was detected using the LSABII kit (DAKO) or the AEC kit (Vector Laboratories). In situ hybridization was performed as described in Jiang et al., 1998. Other reagents are listed in Chen and Chuong, 2000; Ting-Berreth and Chuong, 1996; Yu et al., 2002; and Chodankar et al., 2003. Some section in situ hybridization was performed using the automated Discovery™ system (Ventana Medical System) with recommended protocols.
Shh suppression and over-expression
For gene perturbation, RCAS-Shh or RCAS-Shh antisense retrovirus was injected into the flight feather follicles (primary remiges) as described in Yu et al., 2002. Briefly, feathers of 2 week old SPF white leghorn chickens are plucked. About 5 μl (106 i.u./ml) of virus containing medium are injected into the follicle and the follicles were allowed to regenerate. Three weeks after injection follicles were removed, examined, and dissected for histological studies. Cyclopamine (kindly provided by Dr. Gaffield), a known Shh pathway inhibitor, was injected to the shafts of flight feathers, which were at the active growing phase (3 weeks after feather plucking). About 5 μl (10 μg/ml) was injected to the tip of the feather shaft using a 27 ½ gauge syringe.
Results
TUNEL staining in developing feather buds and follicles
In growing buds, TUNEL-positive cells were observed in regions of active cell proliferation (Fig. 1B). During follicle formation, TUNEL-positive cells were in areas of active tissue remodeling (Fig. 1C). Once the follicle formed, after E13, TUNEL-positive cells were seen in the follicle sheath (the inner layer of the follicle wall) and the feather sheath (the outer layer of the follicle) (Fig. 1D, D′). At E16, a clear space was created separating the feather follicle and follicle dermal sheath (Fig. 1E, E′). TUNEL-positive cells were present in these terminally differentiated zones. The dermal papilla and other mesenchymal tissues have much less apoptosis than the epidermis.
Figure 1. Distribution of TUNEL+ cells in developing feather buds and follicles.
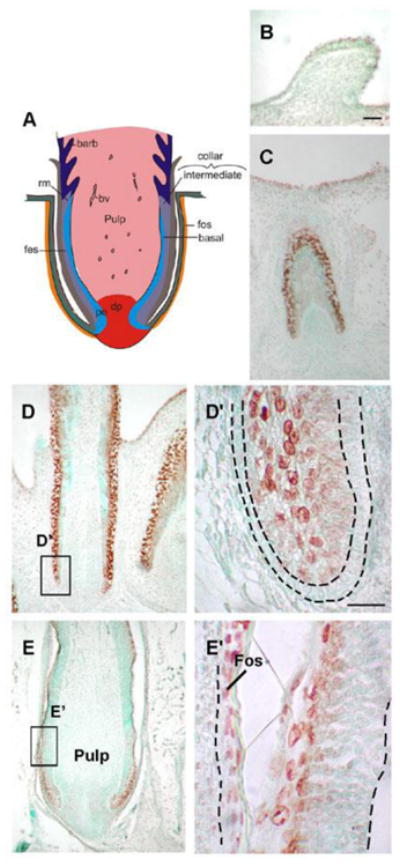
(A) Schematic representation of longitudinal feather follicle sections. There are three epidermis layers in the feather filament: the basal layer, intermediate layer, and feather sheath. The feather filament surrounds the pulp and shows continuity with the invaginated epidermis that has become the feather sheath. (B) E 9 feather buds. (C) A section of E12 invaginating feather follicles. TUNEL+ cells in regions undergo active tissue remodeling. (D) At E15 a longitudinal section of a wing feather, TUNEL+ cells are detected in the feather sheath, higher magnification of the box area is shown in (D′). Strong TUNEL+ cells are detected in the feather sheath and dermal sheath, but the basal layer, demarcated by a black dotted line, lacks TUNEL+ cells. (E) At E16, a space is created between the feather follicle and dermal sheath, higher magnification of the box area is shown in (E′), TUNEL+ cells distribute along the separated and keratinized feather sheath and dermal sheath. A feather now can emerge from its sheath. bv, blood vessels; dp, dermal papilla; Fes, feather sheath; Fos, follicle sheath; rm, ramogenic zone. Scale bars, 100 μm (B-E), 50 μm (D′, E′).
Temporal sequence of apoptosis, cell proliferation, differentiation and molecular expression in feather filament morphogenesis
The next stage is a succession of complex process of “sculpturing” the epidermal layers into multiple hierarchically arranged branches. Since this may not be a familiar subject, we will first summarize the known parts in this paragraph. During feather filament growth, proliferative cells were added to the proximal end of the follicle and there was a proximal – distal maturation gradient (distal ends were produced earlier and therefore were more differentiated; Chodankar et al., 2003). The epidermal collar had a cylindrical configuration, and consisted of a basal layer, intermediate layer, and feather sheath. Surrounding the cylinder is the follicle sheath (Fig. 1A). Distal to the ramogenic zone, the basal layer starts to invaginate and evaginate to form barb ridges (Yu et al., 2002). The basal layer on the lateral side of the barb ridge will form the marginal plate, and the basal layer on the top of the barb ridge will form the “barb ridge growth zone”, also known as the ramogenic zone since they later become the ramus (Lucas and Stettenheim, 1972). Here, we will use the term “ramogenic zone” for the follicle region where ridge formation initiates along the proximal distal axis. The intermediate layer will form the barbule plate and axial plate. The outer layer will form the feather sheath (see Fig. 2A). Cross sections revealed that barb ridges were more mature toward the rachis side (anterior side), thus there was a posterior - anterior maturation gradient. Within a single barb ridge, the growth zone was located at the tip of the barb ridge next to the pulp, therefore there was a centrifugal differentiation gradient (peripheral keratinocytes were more differentiated). The disappearance of marginal plates led to the separation of barbs, and the disappearance of the axial plates led to the separation of proximal and distal barbules. A cylinder of pulp is located at the center of the feather germ. As the barb ridges matured, the inner surfaces of the barb ridge basal epidermis fused around the pulp to form the pulp epithelium. The death of the pulp epithelial cells enabled the cylindrical feather filament to open and form a feather vane. Barb ridges within a single feather filament cross section usually consist of more than one developmental stage.
Figure 2. Temporal and spatial sequence of apoptosis (TUNEL), cell proliferation (PCNA), differentiation (feather keratin), and signaling molecules expression (Shh) during feather filament branching morphogenesis.
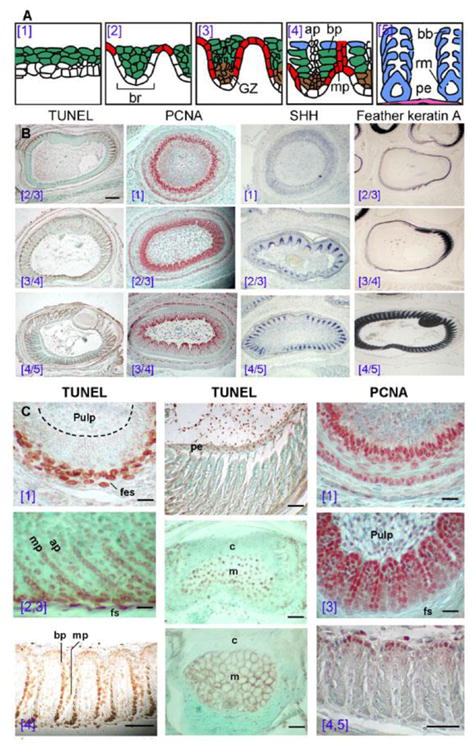
(A) Schematic drawing modified from Chuong and Edelman, 1985 to introduce the 5 stages of the barb ridge forming process (the blue numbers in parentheses). ap, axial plate. bp, barbule plate; bb, barbules. GZ, growth zone of barb ridge and in brown; mp, marginal plate and in red; rm, ramus; pe, pulp epithelium and in purple. Blue, ramus and barbules; green, intermediate layer; red, marginal plate. (B) Apoptosis is determined by TUNEL, proliferation by PCNA, Shh expression by in situ hybridization, and differentiation by feather keratin A transcript expression. E16 wing feather cross sections are used. All expressions were determined in a parallel temporal sequence. Stages of barb formation are indicated by blue brackets. Apoptosis appeared in the feather sheath, marginal plate, and later in the barbule plate and pulp epithelium. PCNA appeared first in the basal layer and barb ridge growth zone. Shh was in the marginal plate epithelium. Keratin A was expressed in the feather sheath, rachis, barbules, and also in the pulp epithelium. (C) Higher magnification views. TUNEL is negative in the basal cells surrounding the pulp and positive in the feather sheath (fes). PCNA stains the basal layer strongly and the intermediate layer weakly. As barb ridges form, TUNEL+ cells appeared in the marginal plates (mp) and axial plates (ap), beginning from cells closest to the feather sheath. At this stage, most of the barb ridge cells were strongly stained by PCNA, including the marginal plate and barbule plate. Later, cell proliferation was limited to the growth zone of the barb ridge, adjacent to the pulp. During later barbule plate differentiation, TUNEL+ cells were also detected in the keratinized barbule plate, beginning from the cells near the feather sheath. In the late barb ridge stage, barbule plates were fully differentiated, and TUNEL staining was present in the pulp epithelium. The rachis was last to undergo apoptosis compared to the barb ridges. TUNEL+ cells were detected in the medulla (m) rather than the cortex (c) region at this stage. These cells died, forming an air filled honeycomb structure. Finally, the pulp membrane became detached and the feather vane opened. Scale bars, 100 μm (panel B), 50 μm (panel C).
To study the progressive differentiation in the feather follicle, we stained sections taken from different heights along the proximal-distal axis for TUNEL to detect apoptosis, PCNA for cell proliferation, and feather keratin A expression for differentiation (Fig. 2B, C). These new data are compared with the published Shh data (Ting-Berreth and Chuong, 1996) for studying their relative expression sequences (Fig. 2B).
TUNEL staining demonstrated that apoptosis began from the rachis, then progressively spread bilaterally (Fig. 2B, C). In each barb ridge, apoptosis began from the periphery (close to the feather sheath) and moved toward the center (pulp). In each barb ridge, TUNEL-positive cells first appeared in the marginal plates and axial plates, but later also appeared in the keratinized barbule plates. The rachis is composed of a cortex and medulla. TUNEL-positive cells were identified in the medulla before the medulla became a hollow structure. Cells within the rachis and barbs underwent terminal differentiation; they expressed feather keratin A and apoptosed. The keratinized structures remained after the cells died. Later, cells in the pulp epithelium also became TUNEL positive.
In the collar epithelium, the basal and suprabasal layers showed strong PCNA staining. At the barb development stage, PCNA was present in the barb ridge keratinocytes. As barb ridges started to differentiate, PCNA became limited to the growth zone located at the tip of the barb ridges next to the pulp (Fig. 2B, C). Keratin A was first weakly expressed in the feather sheath. Later their expression was sequentially enhanced strongly in the rachis, barbule plate, ramus and finally in the pulp epithelium (Fig. 2B). Cytokeratin I was expressed to higher levels in the feather sheath than the barbule plate. Hence, proliferation, differentiation and apoptosis followed a temporal differentiation progression along the proximal – distal, anterior – posterior, and centrifugal axes.
In the collar epithelium, there was no Shh expression. Shh appeared in the basal cells during the time of barb ridge formation (Fig. 2B, 3A). As barb ridge formation entered stage [2], Shh was strongly expressed within the marginal plate. As the barbule plate started to differentiate, the intensity of Shh in the marginal plate gradually decreased. Marginal plate cells became flat, lost Shh expression, and underwent apoptosis. While Shh was expressed in the marginal plate, patched-1, the Shh receptor, was not. Rather it was present in barbule plate cells (Fig. 3B). This suggests that the role of Shh in apoptosis is indirect. Apoptosis of the marginal plate appears to be a paracrine process dependent on interactions with the barbule plates.
Figure 3. Molecular expression during barb ridge formation.
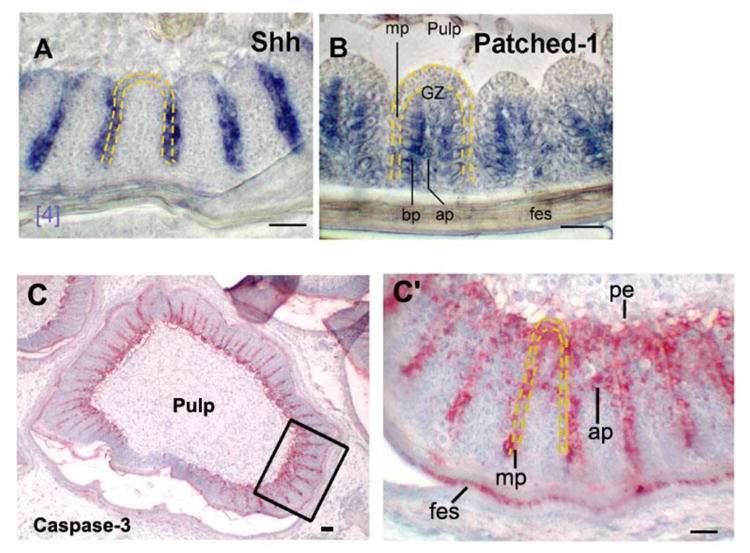
Cross sections of E16 wing feather. (A) In situ hybridization with RNA anti-sense probe to Shh. Shh was expressed higher in the distal than the proximal marginal plate. (B) Patched-1 was expressed in the barbule plate. Ap, axial plate; bp, barbule plate; fes, feather sheath; mp, marginal plate; GZ, growth zone; pe, pulp epithelium. Bar, 50 μm. (C, C′) Immuno-staining of caspase-3. Low and high power views. Rectangular regions in C is shown in C′, Note the presence of caspase-3 staining in the pulp epithelium, marginal plate, feather sheath, and also begins to be seen in the axial plate. Staining in feather sheath will soon disappear as it develops earlier. Scale bars, 100 μm.
Caspase-3 is the effector for both TNF receptor mediated apoptosis and growth factor deprived Bcl2 related apoptosis (Strasser et al., 2000). Here, we use antibodies to locate its distribution during branching morphogenesis. We observe it in the marginal plate, feather sheath, pulp epithelium, and also the axial plate (Fig. 3C, C′). These are the places we observe TUNEL positive cells (Fig. 2C).
Shh pathway is involved in the apoptotic events during barb ridge morphogenesis
We showed that Shh is involved in the apoptosis of marginal plate epithelia in our earlier study by suppression of SHH (Yu et al., 2002). Here, we found that Patched-1 was absent from the marginal plate but present in the barbule plate epithelia. Therefore, the apoptotic effect could not be mediated through an autocrine pathway residing in the marginal plate epithelia, but may result from interactions between the marginal and barbule plate epithelia.
Here we suppressed Shh signaling and further analyze its effects on the homeostasis among apoptosis, proliferation and differentiation in the barb ridge keratinocytes. We inhibited Shh with cyclopamine or by transduction of RCAS-anti-sense Shh (Fig. 4A-E). We found that 1) periodic patterning of barb ridges initiated normally, even though Shh signaling was reduced (Fig. 4 A, B). 2) However, these barb ridges were abnormal. There was reduced cell proliferation (PCNA staining) in the basal cells and presumptive barb ridge growth zone (Fig. 4C). 3) The marginal plates formed abnormally and contained plump cells which did not become TUNEL positive (Fig. 4C, D left). 4) Without a normal marginal plate, part of the presumptive barbule plate cells underwent abnormal differentiation and degeneration. They show patches of TUNEL positive cells (Fig. 4D right). Because the barb ridges formed abnormally, they could not separate from one another and part of the feather vanes became continuous sheets (Fig. 4A, B). Staining with antibodies to caspase-3 showed reduction (Fig. 4E).
Figure 4. Shh influences apoptosis and proliferation during feather morphogenesis.
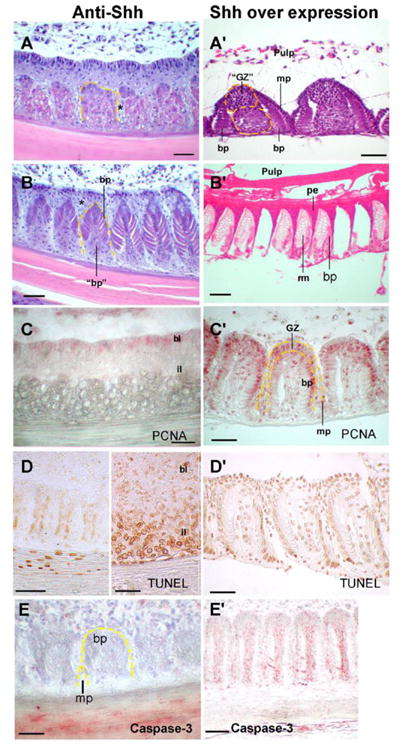
Left column: Anti-Shh was achieved by injection of Shh inhibitor, RCAS antisense Shh or cyclopamine into the plucked wing feather pulp. Part of the cross section of a feather is shown. (A) Early barb ridge keratinocytes show abnormal cell types, but the periodicity still form. Abnormal marginal plate cells can be seen (*). (B) Some barbule plates (bp) differentiate, but some abnormal barbule plate cells (“bp”) start to degenerate. (C) PCNA. Inhibited proliferation in basal layer (bl) and intermediate layers (il) was seen by decreased intensity and number of PCNA + cells. (D) In some, there was reduced apoptosis in the presumptive marginal plate region (left). Apoptosis in the feather sheath can still be seen. In some regions, intermediate layer show the degenerative barbule plate epithelia (“bp” in panel B) underwent massive apoptosis (right). Caspase-3 staining is remarkably reduced (E).
Right column: Shh over expression. (A′) Cross sections showed ectopic Shh expression caused an abnormal, enlarged growth zone (“GZ”) and an abnormal expanded cell column between the two barbule plates (yellow dotted line). (B′) In more mature regions, the expanded growth zones contributed to the enlarged rami formation. The barb septa and pulp epithelium also were expanded. (C′) PCNA showed expanded cell proliferation beyond the growth zone to the peripheral barbule plate epithelia, marginal plate, and the peripheral axial regions that normally do not have proliferating cells at this stage. (D′) Apoptosis was suppressed (not shown) leading to expanded cell mass. (E′) Caspase-3 immuno-staining is mildly reduced but remained in the axial plate and pulp epithelium. Scale bars, 100μm.
We have not reported over-expression of Shh on barb branching morphogenesis before. RCAS-Shh showed the following changes. 1) The periodic patterning of barb ridges was also not influenced (Fig. 4A - B′). 2) There was wider distribution of PCNA staining cells that extend into the barbule plate and marginal plate (Fig. 4C. C′, compare with Fig. 2C, PCNA). 3) The pulp epithelium and marginal plate were more resistant to apoptosis and formed thickened keratinized structures (Fig. 4B, B′, D, D′). 4). As a result, there was increased cell mass in the barb ridge between two rows of barbule plates where the axial plate should be (Fig. 4A′). They later formed hypertrophic rami. Caspase-3 staining showed a reduction in the marginal plate, feather sheath, and pulp epithelium, but remained expressed in the axial plate region (Fig. 4E, E′).
Therefore, Shh suppression or over-expression resulted in aberrant proliferation, apoptosis, and differentiation, and therefore formed abnormal feathers.
Discussion
Skin appendage formation involves induction, morphogenesis, differentiation and cycling stages (Wu-Kuo and Chuong, 2000; Chuong et al., 2000; Paus and Cotsarellis, 1999). In the current study, we demonstrated that different regions of developing feathers are programmed to apoptose at different times relative to proliferation and differentiation. We tried to define three possible modes of outcomes resulting from apoptosis (Fig. 5A). 1) Apoptosis may function to shape and restrict proliferative cells within a localized growth zone. 2) Apoptosis can create spaces and down regulate signaling centers that are only needed transiently. 3) Apoptosis with terminal differentiation may lead to the formation of permanent structures or temporary cornea that can later flake off, depending on the types of keratin deposited. Apoptosis is also important during hair cycling (Paus, 1999), but we will not discuss this here.
Figure 5. Schematic drawing of the modes of apoptosis and the morphogenetic consequences on skin appendage morphogenesis.
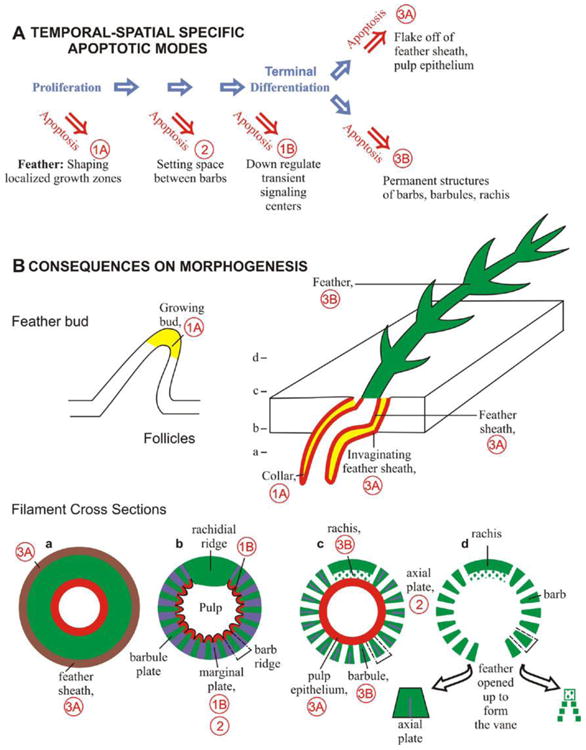
(A) Temporal - spatial modes of apoptosis relative to cell proliferation and differentiation highlighting different morphogenetic consequences. Mode 1. Apoptosis in the proliferative zone. These cells may be involved via apoptosis by themselves (1A) or by signaling others to apoptose or not to apoptose (1B). Mode 2. Apoptosis without keratinization. This leaves spaces important for morphogenesis. Mode 3. Apoptosis with terminal differentiation. Depending on specific types of keratinization, these cells may cornify and flake off to separate epidermal layers (3A) or become permanent keratinized structures (3B). (B) Different modes of apoptosis leave distinct consequences on epithelial morphogenesis. Examples are shown in developing skin appendage primordia, invaginating follicles and branching feathers. Cross sections at different levels of feather filaments from the proximal to the distal end (a to d) are shown in the bottom row. Diagrams a-d are modified from Fig. 238, Lucas and Stettenheim, 1972. Note the dynamic shift of localized apoptotic zones in different stages of skin appendage development, and how these successive apoptotic events sculpt the epidermal cylinder into highly branched structures and set the feathers free to fly. Hence, apoptosis gives life in death.
Apoptosis in the localized proliferative zone (Mode 1, Fig. 5)
We have recently shown that dynamically shifting localized growth zones are instrumental in building the morphology of skin appendages (Chodankar et al. 2003). In general, Wnt gene expression correlates with the distribution of these localized growth zones, and over-expression of Wnt 6 induces an ectopic localized growth zone. However, the specific location of the growth zone varied over time, suggesting the involvement of complex regulation. In light of our current findings, localized growth and localized apoptosis events are likely to work in a complementary fashion to add or remove keratinocytes to shape the morphology of skin appendages. Studies analyzing cell cycle proteins in apoptosis have suggested that apoptosis and the cell cycle may partially share common pathways. There is coordinated regulation of these antagonistic pathways within a cell (King and Cidlowski, 1995, 1998). We named this mode 1A.
Interestingly, during barb ridge morphogenesis, while Shh is in the marginal plate, the Shh receptor, Patched-1, is not present in the marginal plate epithelia, but rather is present in the barbule plate epithelia. Therefore, Shh secreted from the marginal plate cells may have a role in regulating barbule plate cell proliferation and differentiation. The marginal plate may serve as a temporary signaling center during barb ridge formation. The apoptosis of the marginal plate implies the removal of the signaling center when it is no longer needed. Similarly, during chicken limb development, apoptosis takes place and may play a role in shutting off Shh expression in the zone of polarizing activity (Sanz-Ezquerro and Tickle, 2000). We named this mode 1B.
Apoptosis creates space or down regulates the temporary signaling center (Mode 2, Fig. 5)
Morphogenesis of feather filaments is a dazzling event during which complex feather branches are “sculptured” and freed from the rest of the epidermal layers. Marginal plate cells undergo apoptosis without keratinization, and therefore become the spaces between barbs (Fig. 5, Apoptosis mode 2). Developing human hairs in embryonic skin travel long distances within the skin epidermal layer before emerging (Chang et al., 2001). The observed apoptosis in the long hair canals should belong to this category.
Molecules expressed in the marginal plate include lunatic fringe (Chen et al, 2000), NCAM (Chuong and Edelman, 1985), BMP2 (Yu et al, 2002), Shh (Ting-Berreth and Chuong, 1996); etc. RCAS-BMP transduction produced a similar barb ridge phenotype as RCAS-antisense-Shh, suggesting that BMP may be upstream of the Shh signaling pathway in barb ridge formation (Yu et al, 2002). BMP also has been shown to be important for apoptosis in other organs such as hair follicles (Botchkarev, 2003), the limb bud (Guha et al., 2002) and cephalic crest (Graham et al., 1994). TUNEL staining detects apoptotic cells in the marginal plate in both early and late stages of barb ridge formation (Fig. 2C). At early stages the marginal plate cell shape is cuboidal and the cells are actively proliferating. Apoptosis at this stage may regulate cell number and maintain these cells as a single layer to serve as a signaling center with a stable Shh gradient. At later stages when barbule plate cells undergo terminal differentiation, marginal plate cells become flattened and die through apoptosis. In so doing, they create space and set the feather branches free.
During barb ridge formation, several signaling pathways interact to build the pattern. Among them, Shh and BMP may form a pair of signaling modules (Harris et al., 2002). The crosstalk between these pathways remains to be studied. Here we used retroviral transduction experiments to show that Shh is critically important for marginal plate cell fate specification. Previously we showed marginal plates cannot form properly when Shh is inhibited. This leads to abnormal barbule plate differentiation and barb ridge formation, therefore resulting in feathers with webby and abnormal branches. We now show Shh over expressing branches also can not form properly when Shh is in excess. Shh causes active proliferation and increases in cell mass in the growth zone, barbule plates and marginal plates. These cells are more resistant to differentiation and apoptosis. Downstream to this, Shh has been shown to make cells resistant to differentiation and to block p21CIP1/WAF1 – induced growth arrest in the cultured cells (Fan and Khavari, 1999). Our in vivo results are consistent with these results.
Apoptosis in terminally differentiated cells (Mode 3, Fig. 5)
Barbule plate cells acquire keratin expression and undergo apoptosis in parallel with terminal differentiation, thus becoming permanent feather structures. In developing feathers, α- and β- keratins are differentially expressed (Presland et al., 1989). α- keratin (cytokeratin I) is present in the intermediate layer cells and feather sheath. β-keratin (feather keratin A) is present in feather branches, and to a lesser amount in the feather sheath. Thus each structure has different properties based on the different ratios of α- and β-keratin. While a more detailed study requires biochemical characterization of avian keratins, based on the observation we postulate that when α-keratin is dominant, keratinocytes become cornified, similar to what happens in mammalian skin epidermal keratinocytes. This is observed in the feather sheath, follicle sheath, and pulp epithelium after they keratinize. These structures flake off to allow separation of tissue layers (Fig. 5, Apoptosis mode 3A). When β-keratin is dominant, keratinocytes keratinized into permanent structures, similar to what happens in mammalian hair keratin. This is observed in the barbule plate, ramus, and rachis. The physical structure of the keratin scaffold also makes a difference in the property of the feather. For example, medulla cells in the rachis and ramus die to make an air-filled hollow structure which has reduced weight and increased architectural strength (Fig. 5, Apoptosis mode 3B). Thus, through specifically timed and placed apoptotic events, the complex branched designs are cast and feathers are freed from the epidermal layers for use in flight.
The terminal differentiation of epidermal keratinocytes has been regarded as an example of programmed cell death (Fesus et al, 1991; Polakowska et al, 1994). Indeed terminal differentiated cells also show TUNEL positive staining. Cross-talk between differentiation and apoptosis pathways remains elusive. Filaggrin, transglutaminase or ceramide generated in the terminal differentiated cells may trigger the apoptosis pathway (Kuechle et al, 2000; Ishida-Yamamoto et al, 1999; Fesus and Thomazy, 1988; Geilen et al, 1997). Some suggest that terminal differentiation and apoptosis are biochemically distinct processes (Gandarillas et al., 1999). Here we grouped them together, since in this paper we are trying to develop a common theme of how apoptosis is used in sculpting morphogenesis (Fig. 5), not biochemical mechanisms.
In summary, we have tried to learn the specific roles of apoptosis in the morphogenesis of the vertebrate integument using chicken feathers as the research model. We have identified apoptosis as one of the major forces in sculpting the complex skin appendages. However, the question does not end here. If the temporal and spatial controls of apoptosis are so important in morphogenesis, how are they regulated? The complicated molecular expression patterns during barb ridge morphogenesis imply a delicate balance among molecular signaling pathways. Recent work showed that homeobox genes may be involved in designating the apoptotic regions during Drosophila head morphogenesis (Lohmann, et al, 2002). Homeobox genes may also endow regional specificity in skin appendages (Chuong et al., 1993; Chuong, 2003). Thus the building of diverse skin appendages in vertebrates may be explained by the temporal and spatial regulation and balance of cell behaviors including cell proliferation (Chodankar et al., 2003), cell adhesion (Jiang and Chuong, 1992), apoptosis, and differentiation.
Acknowledgments
We thank Dr. Richard Presland for critically reviewing the manuscript. We thank Ms Fiona McCulloch for helping with manuscript preparation. We acknowledge Mr. Bongha Shin for carrying out the early portion of this project. We are grateful to all those who have provided reagents used in this work. This work was supported by grants from the NIAMS (CMC) and NCI (RW) in the US, National Science Council of Taiwan (CHC), and Tzu Chi Medical Center (CHC).
References
- Ahmad W, Faiyaz ul Haque M, Brancolini V, et al. Alopecia universalis associated with a mutation in the human hairless gene. Science. 1998;279:720–724. doi: 10.1126/science.279.5351.720. [DOI] [PubMed] [Google Scholar]
- Chang CH, Yu H, Chuong CM. Spatio-temporal distribution of apoptosis in developing human hair follicles. J Invest Dermatology. :117, 427. [Google Scholar]
- Charrier JB, Lapointe F, Le Douarin NM, Teillet MA. Anti-apoptotic role of Sonic hedgehog protein at thr early stages of nervous system organogenesis. Development. 2001;128:4011–4020. doi: 10.1242/dev.128.20.4011. [DOI] [PubMed] [Google Scholar]
- Chen CW, Chuong CM. Avian integument provides multiple possibilities to analyse different phases of skin appendage morphogenesis. J Investig Dermatol Symp Proc. 1999;4:333–337. doi: 10.1038/sj.jidsp.5640240. [DOI] [PubMed] [Google Scholar]
- Chen CWJ, Chuong CM. Dynamic expression of lunatic fringe during feather morphogenesis: a switch from medial-lateral to anterior-posterior asymmetry. Mech Dev. 2000;91:351–354. doi: 10.1016/s0925-4773(99)00285-3. [DOI] [PubMed] [Google Scholar]
- Chodankar R, Chang CH, Yue Z, Suksaweang S, Burrus L, Chuong CM, Widelitz RB. Localized growth zones shift during skin appendage morphogenesis: association with Wnt / beta-catenin pathway activities. J Invest Dermatol. 2003;120:20–26. doi: 10.1046/j.1523-1747.2003.12008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM. The making of a feather: Homeoproteins, Retinoids and Adhesion molecules. Bioessays. 1993;15:513–521. doi: 10.1002/bies.950150804. [DOI] [PubMed] [Google Scholar]
- Chuong CM. Morphogenesis of epithelial appendages: variation on top of a common theme and implications in regeneration. In: Chuong CM, editor. Molecular Basis of Epithelial Appendage Morphogenesis. Austin, Texas: Landes Bioscience; 1998. pp. 57–74. [Google Scholar]
- Chuong CM. Homeobox genes, fetal wound healing, and skin regional specificity. J Invest Dermatol. 2003;120:9–11. doi: 10.1046/j.1523-1747.2003.00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Chodankar R, Widelitz RB, Jiang TX. Evo-devo of feathers and scales: building complex epithelial appendages. Curr Opin Genet Dev. 2000;10:449–456. doi: 10.1016/s0959-437x(00)00111-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong CM, Edelman GM. Expression of cell adhesion molecules in embryonic induction. I. Morphogenesis of nestling feathers. J Cell Biol. 1985;101:1009–1026. doi: 10.1083/jcb.101.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Khavari PA. Sonic hedgehog opposes epithelial cell cycle arrest. J Cell Biol. 1999;147:71–76. doi: 10.1083/jcb.147.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fesus L, Thomazy V. Searching for the function of tissue transglutaminases: its possible involvement in the biochemical pathway or programmed cell death. Adv Exp Med Biol. 1988;231:119–134. doi: 10.1007/978-1-4684-9042-8_10. [DOI] [PubMed] [Google Scholar]
- Fesus L, Davies PJA, Piacentini M. Apoptosis: molecular mechanisms in programmed cell death. Eur J Cell Biol. 1991;56:170–177. [PubMed] [Google Scholar]
- Gandarillas A, Goldsmith LA, Gschmeissner S, Leigh IM, Watt FM. Evidence that apoptosis and terminal differentiation of epidermal keratinocytes are distinct processes. Exp Dermatol. 1999;8:71–79. doi: 10.1111/j.1600-0625.1999.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geilen CC, Wieber T, Orfanos CE. Ceramide signaling: regulatory role in cell proliferation, differentiation and apoptosis in human epidermis. Arch Dermatol Res. 1997;289:559–566. doi: 10.1007/s004030050240. [DOI] [PubMed] [Google Scholar]
- Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–6. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA. In vivo evidence that BMP signaling is necessary for apoptosis in the mouse limb. Dev Biol. 2002;249:108–20. doi: 10.1006/dbio.2002.0752. [DOI] [PubMed] [Google Scholar]
- Harris MP, Fallon JF, Prum RO. Shh-Bmp2 signaling module and the evolutionary origin and diversification of feathers. J Exp Zool. 2002;294:160–176. doi: 10.1002/jez.10157. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Ishida-Yamamoto A, Tanaka H, Nakane H, Takahashi H, Hashimoto Y, Iizuka H. Programmed cell death in notmal epidermis and loricrin keratoderma. Multiple function of profilaggrin in keratinization. J Invest Dermatol Symp Proc. 1999;4:145–9. doi: 10.1038/sj.jidsp.5640198. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Chuong CM. Mechanism of skin morphogenesis. I. Analysis with antibodies to adhesion molecules tenascin, N-CAM, and integrin. Dev Biol. 1992;150:82–98. doi: 10.1016/0012-1606(92)90009-6. [DOI] [PubMed] [Google Scholar]
- Jiang TX, Stott NS, Widelitz RB, Chuong CM. Current methods in the Study of Avian Skin Appendages. In: Chuong CM, editor. Molecular basis of epithelial appendage morphogenesis. Vol. 1998. Austin, Texas: Landes Bioscience; 1998. pp. 395–408. [Google Scholar]
- King KL, Cidlowski JA. Cell cycle and apoptosis: common pathways to life and death. J Cell Biol. 1995;58:175–180. doi: 10.1002/jcb.240580206. [DOI] [PubMed] [Google Scholar]
- King KL, Cidlowski JA. Cell cycle regulation and apoptosis. Annu Rev Physil. 1998;60:601–617. doi: 10.1146/annurev.physiol.60.1.601. [DOI] [PubMed] [Google Scholar]
- Kuechle MK, Presland RB, Lewis SP, Fleckman P, Dale BA. Inducible expression of filaggrin increases keratinocyte susceptibility to apoptotic cell death. Acll Death Differ. 2000;7:566–573. doi: 10.1038/sj.cdd.4400687. [DOI] [PubMed] [Google Scholar]
- Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann I, McGinnis N, Bodmer M, McGinnis W. The Drosophila Hox gene deformed sculpts head morphology via direct regulation of the apoptosis activator reaper. Cell. 2002;110:457–66. doi: 10.1016/s0092-8674(02)00871-1. [DOI] [PubMed] [Google Scholar]
- Lucas AM, Stettenheim PR. Avian anatomy Integument Agriculture handbook, Agricultural research sciences. Vol. 362. Washington DC; US Department of Agriculture; p. 1972. [Google Scholar]
- Ma L, Liu J, Wu T, Plikus M, Jiang TX, Bi Q, Liu YH, Muller-Rover S, Peters H, Sundberg JP, Maxson R, Maas RL, Chuong CM. ‘Cyclic alopecia’ in Msx2 mutants: defects in hair cycling and hair shaft differentiation. Development. 2003;130:379–389. doi: 10.1242/dev.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Paus R. Principles of hair cycle control. J Dermatol. 1999;25:793–802. doi: 10.1111/j.1346-8138.1998.tb02507.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- Polakowska RR, Piacentini M, Bartlett R, Goldsmith LA, Haake AR. Apoptosis in human skin development: morphogenesis, periderm, and stem cell. Dev Dyn. 1994;199:176–188. doi: 10.1002/aja.1001990303. [DOI] [PubMed] [Google Scholar]
- Presland RB, Gregg K, Molloy PL, Morris CP, Crocker LA, Rogers GE. Avian keratin genes. I. A molecular analysis of the structure and expression of a group of feather keratin genes. J Mol Biol. 1989;209:549–559. doi: 10.1016/0022-2836(89)90593-7. [DOI] [PubMed] [Google Scholar]
- Sanz-Ezquerro JJ, Tickle C. Autoregulation of Shh expression and Shh induction of cell death suggest a mechanism for modulating polarizing activities during chick limb development. Development. 2000;127:4811–4823. doi: 10.1242/dev.127.22.4811. [DOI] [PubMed] [Google Scholar]
- Soma T, Ogo M, Suzuki J, Takahashi T, Hibino T. Analysis of apoptotic cell death in human hair follicles in vivo and in vitro. J Invest Dermatol. 1998;111:948–954. doi: 10.1046/j.1523-1747.1998.00408.x. [DOI] [PubMed] [Google Scholar]
- Stadelmann C, Lassmann H. Detection of apoptosis in tissue sections. Cell Tissue Res. 2000;301:19–31. doi: 10.1007/s004410000203. [DOI] [PubMed] [Google Scholar]
- Strasser A, O'Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- Ting-Berreth SA, Chuong CM. Sonic Hedgehog in feather morphogenesis: induction of mesenchymal condensation and association with cell death. Dev Dyn. 1996;207:157–170. doi: 10.1002/(SICI)1097-0177(199610)207:2<157::AID-AJA4>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA. Bone morphogenetic proteins and their antagonists in skin and hair follicle biology. J Invest Dermatol. 2003;120:36–47. doi: 10.1046/j.1523-1747.2003.12002.x. [DOI] [PubMed] [Google Scholar]
- Widelitz RB, Chuong CM. Early Events in Skin Appendage Formation: Induction of Epithelial Placodes and Condensation of Dermal Mesenchymal Cells. J Invest Dermatol. 2000;4:302–306. doi: 10.1038/sj.jidsp.5640234. [DOI] [PubMed] [Google Scholar]
- Wu-Kuo T, Chuong CM. Developmental biology of hair follicles and other skin appendages. In: Camacho FM, Randall VA, Price VH, editors. Hair and its Disorders: Biology, pathology and management. Dunitz Martin Ltd; 2000. pp. 17–37. [Google Scholar]
- Yu MK, Wu P, Widelitz RB, Chuong CM. The morphogenesis of feathers. Nature. 2002;420:308–312. doi: 10.1038/nature01196. [DOI] [PMC free article] [PubMed] [Google Scholar]


