Abstract
Neuroblastoma is a solid tumor that arises from the developing sympathetic nervous system. Over the past decade, our understanding of this disease has advanced tremendously. The future challenge is to apply the knowledge gained toward developing risk-based therapies and ultimately improving outcome. Here we review the key discoveries in the developmental biology, molecular genetics, and immunology of neuroblastoma, as well as new translational tools to bring these promising scientific advances into the clinic.
Neuroblastoma (NB) is a rare childhood cancer affecting 10.2 per million children under 15 years of age, and the most common malignancy diagnosed before the first birthday1. As a complex and heterogeneous disease,2 many factors, such as age at diagnosis, stage of disease at diagnosis, and the molecular, cellular, and genetic features of the tumor determine whether it will spontaneously regress or metastasize and become refractory to therapy. Over the past decade, major advances in the clinical staging of NB have improved risk stratification3. However, not enough is known about how these disease features relate to its underlying biology and how this can be exploited to improve outcome. Our challenge is to bridge the gap between characterizing the molecular and genetic properties of NB and understanding the precursor cells that give rise to NB, focusing on those features that make the cells susceptible to malignant transformation.
In the past decade the major effort has been focused on discovering somatic mutations in human tumors. Targeting therapy at tumor-specific mutations holds promise of precision and effectiveness in eradicating cancer, while sparing patients the acute and long term toxicities of chemo-radiotherapy. However, genome-wide searches are uncovering striking differences in the prevalence of mutations among tumor types, from very frequent among melanomas to rare among pediatric cancers such as NB4–5. The infrequency of mutations4–6 is a major disappointment for those looking for actionable targets from gene mutations and an increasingly apparent hurdle for others hunting for tumor-specific immunity. In adult cancers like melanoma, the rich epitope landscape7, or mutanome8, has been successfully exploited for T-cell based therapy. But in NB with a small mutanome, the classic immunotherapy model may be difficult to apply. Antibody-based instead of T-cell-based therapy directed at oncofetal differentiation antigens has provided a viable alternative.
Despite this paucity of recurrent somatic mutations, NB is a complex, heterogeneous disease2. As the search for druggable targets continues, a better understanding of the developmental biology of this tumor may offer new insights. Many cellular processes that guide tissue morphogenesis and differentiation have parallel functions in cancer. For example, tumor cells from the same patient can be remarkably heterogeneous and change dramatically during disease progression. This is reminiscent of progenitor cell heterogeneity and unidirectional changes in progenitor competence in developing tissues and organs. As in normal developing cells, tumor cells are sensitive to non–cell autonomous influences and require a precise balance between differentiation and proliferation for growth and homeostasis. Also, like rapidly growing embryonic tissues and organs, tumors are metabolically tuned for biosynthesis and often evade cell death machinery to proliferate massively. Thus, developmental biology and cancer biology are natural partners, though integrating the two fields for therapeutic applications can be daunting.
In this review we will update our current understanding of the neural crest and cellular origins of NB. We will review the normal differentiation and physiology of the sympathetic neurons, highlighting potential actionable targets unique to NB. The clinical success of anti-ganglioside GD2 [G] antibody therapy in the face of an immunosuppressive tumor microenovironment is analyzed. Looking ahead, we propose a comprehensive translational research roadmap that takes advantage of high throughput drug screening, new generations of animal models, and study designs to mimic real clinical settings. We will not discuss modern evolutions of chemotherapy including those in the myeloablative[G] setting, which have been summarized extensively by other investigators9.
Neural crest origin of neuroblastoma
Most NBs are diagnosed in the abdomen, associated with the adrenal gland [G] or sympathetic ganglia [G]1–2. Based on these common sites of primary disease and the cellular and neurochemical features of NBs, it is widely accepted that the cell origin for NB arises from the sympathoadrenal lineage of the neural crest during development (Figure 1)10.
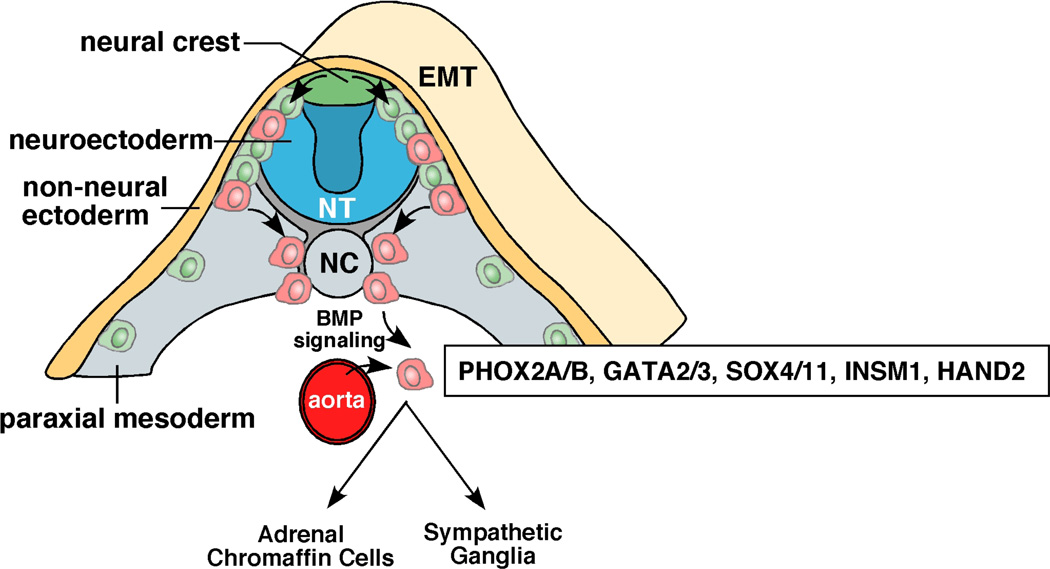
Figure 1. Development of the sympathoadrenal lineage of the neural crest.
As cells of the neural crest (green/red cells) migrate, they undergo epithelial-mesenchymal transition (EMT). A subset of cells (red) migrates toward the dorsal aorta as they commit to the sympathoadrenal lineage. This migration is directed in part by the expression of the chemokine receptor CXCR4 on the migrating neural crest progenitor cells (red) and the expression of the SDF-1 chemoattractant on the dorsal aorta. At the dorsal aorta, the migrating neural crest progenitor cells committed to the sympathoadrenal linege initiate their differentiation program in response to BMP signalling emanating from the dorsal aorta199–200. A series of transcription factors including PHOX2A/B, ASCL1, GATA2/3, SOX4/11, INSM1 and HAND2 are upregulated. Shortly after these transcription factors are induced, neuronal markers are upregulated along with genes that encode enzymes required for catecholamine biosynthesis such as tyrosine hydroxylase (TH) and dopamine beta hydroxylase (DBH). From that point, the cells commit to the adrenal chromaffin lineage[G] or become sympathetic ganglia. Abbreviations: NT, neural tube; NC, notochord; EMT, epithelial to mesenchymal transition.
The neural crest is a remarkable structure that is present only during embryogenesis and gives rise to diverse cell types including peripheral neurons, enteric neurons and glia, melanocytes, Schwann cells, and cells of the craniofacial skeleton and adrenal medulla11. Cells arising in the adrenal medulla are postganglionic neurons that have lost their dendrites and axons. Preganglionic neurons from the central nervous system (CNS) connect directly to adrenal medulla cells and stimulate the release of catecholamines (i.e., epinephrine, norepinephrine, and dopamine). Thus, the adrenal medulla is a ganglion of the sympathetic nervous system. Most NBs arise in the adrenal medulla (65%). The rest is distributed among the chest (20%), neck (5%), and pelvis (5%), a pattern similar to that of normal sympathetic ganglia in the thoracic/lumbar (16%), cervical (3%), and sacral (4%) regions. A small subset of patients presents with bilateral adrenal NB12, suggesting that transformation can be initiated in the neural crest, before the cells migrate. Alternatively, it is possible that patients with bilateral NB had a predisposing genetic lesion and the bilateral tumors result from two independent genetic lesions in the cells of the left and right sympathoadrenal lineage as predicted by Knudson and Strong13. Whole genome sequencing of paired bilateral NB tumors could distinguish between these two possibilities.
Familial neuroblastoma
Familial NB is rare (<2% of all NBs)14. Mutations in some of the signalling pathways (Figure 1) important for the development of the sympathoadrenal lineage are associated with familial genetic syndromes characterized by defects in development and predisposition to NB15. The first predisposition mutation identified in NB was in PHOX2B16–17, a gene encoding a paired homeodomain transcription factor that promotes cell cycle exit and neuronal differentiation18–19, playing a critical role in the development of neural crest-derived autonomic neurons. PHOX2B has two polyalanine repeat sequences. Expression of the second polyalanine repeat is associated with congenital central hypoventilation syndrome (CCHS)[G]20, while non-polyalanine repeat expansion mutations (NPARMs) accompany the NB-HSCR (Hirschsprung disease)[G]-CCHS association. Thus, perturbations in the PHOX2B–regulated differentiation pathway in the sympathoadrenal lineage of the neural crest may contribute to NB tumorigenesis, and subsequent gene expression studies support that hypothesis21. Yet, when NPARMS were introduced into the endogenous Phox2b allele of the mouse, even though most clinical features of HSCR and CCHS were recapitulated, those of NB were not22.
A more common lesion associated with familial NB is in the ALK receptor tyrosine kinase gene14, 23–25 (Figure 2). Its known natural ligands include pleiotrophin and midkine. ALK is expressed in the developing sympathoadrenal lineage of the neural crest26–27, and it may regulate the balance between proliferation and differentiation through multiple cellular pathways, including the mitogen activated protein kinase (MAPK) and Ras-related protein 1 (RAP1) signal transduction pathways28–30. In addition there is evidence to suggest that PHOX2B can directly regulate ALK gene expression31 providing a connection between these two pathways that are mutated in familial NB. Furthermore, ALK signaling through midkine may be important for proliferation of the sympathoadrenal lineage during development32. The ALK-activating mutation F1174L found in some cases of familial NB contributes to NB tumorigenesis in mice33–35.
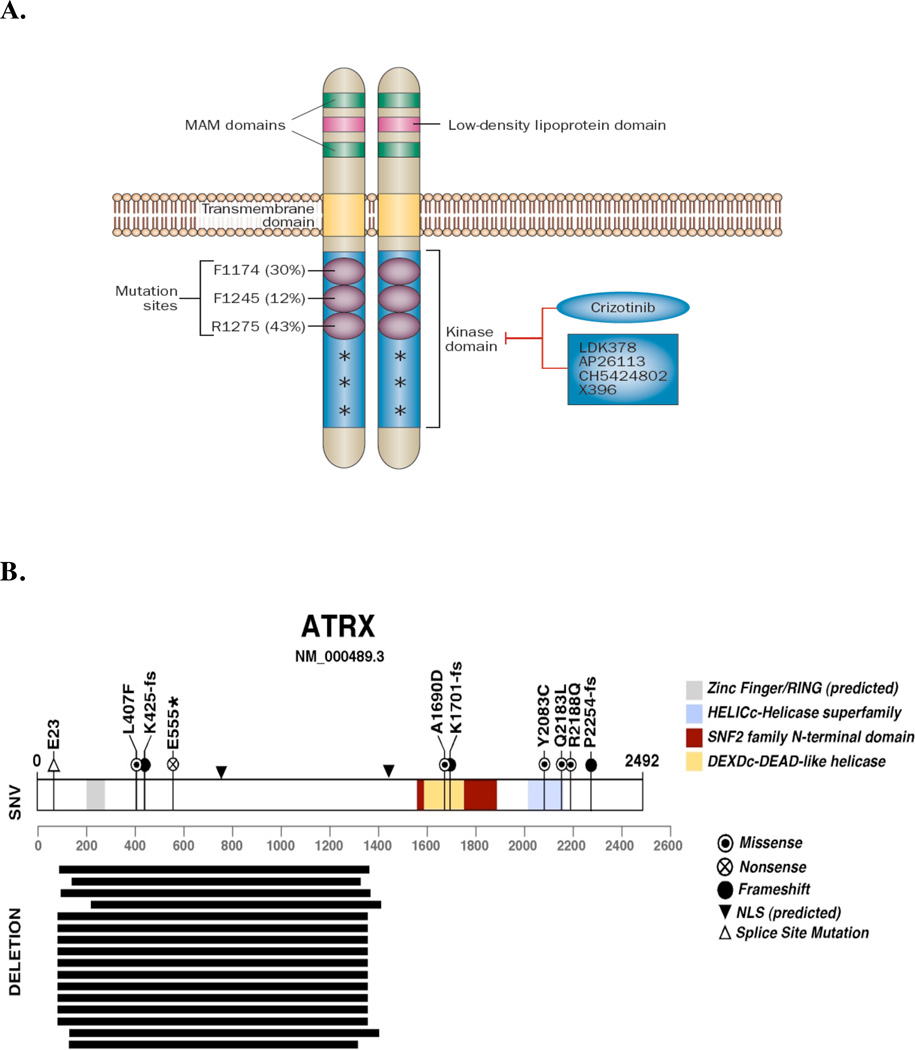
Figure 2. ALK and ATRX mutations in NB.
A | Schematic representation of ALK protein structure and mutations found in NB. The low-density lipoprotein domain, two MAM domains, and the transmembrane and kinase domains of ALK are shown. R1275, F1174 and F1245 are three most common ALK mutations in neuroblastoma; the frequency of these mutations is provided in parenthesis. Other low frequency mutations are denoted with an asterisk. The tyrosine kinase inhibitor crizotinib is in clinical trials and other second-generation ALK inhibitors are in development. (Reproduced with permission from Carpenter et al45).
B | In a recent WGS study of stage-4 NBs from different age groups, ATRX mutations were identified, including in-frame deletions, missense, nonsense and frame-shift single-nucleotide variations. Mutations in ATRX were significantly associated with age at diagnosis. Mutations in ATRX were mutually exclusive of MYCN amplification and were associated with alternative lengthening of telomeres (ALT). Importantly, previous studies have shown that ALT in NB is mutually exclusive of MYCN amplification201. More common among older patients (Group 4, 1), ALT is associated with chronic disease and poor survival. The 5-year OS for patients with ALT-positive NB is 0%; that of patients with ALT-negative is 52%201ATRX mutations may contribute to ALT in NB cells and are associated with poor OS among older patients. (Modified with permission from Cheung et al52).
Ongoing genome-wide association studies (GWAS) have identified additional pathways that contribute to NB initiation and/or progression. Several predisposing single nucleotide polymorphisms (SNPs) have been identified: LINC00340 and LOC729177 (FLJ44180), BARD136, LMO137, DUSP12, HSD17B12, DDX4-IL31RA38, HACE139, and LIN28B39–40. The BARD1 SNP effect has been confirmed in African-American children41, while BARD1β isoform42 and LIN2843 are oncogenic in NB.
Sporadic neuroblastoma
While mutations of PHOX2B and the GWAS predisposing loci are relatively rare in sporadic NB, approximately 6–10% of NBs carry somatic ALK activating mutations, and an additional 3–4% carry high leve ALK gene amplifications4, 14, 23–25. These findings in familial and sporadic NB suggest that ALK is a major oncogenic driver in NB, and activating ALK mutations or amplifications are associated with lethal disease.33, 44 ALK is a natural target for molecular therapy in preclinical studies and clinical trials for NB45.
However, the most common focal genetic lesion in sporadic NB is the amplification of MYCN (≥ 10 copies for diploid genome or >4 fold signal relative to chromosome 2), which occurs in approximately 22% of tumors and is associated with poor outcome2. MYCN regulates the proliferation, growth, differentiation, and survival of cells in the developing CNS. It is expressed in the developing neural crest, and several signalling pathways regulate its expression (e.g., Hh and Wnt)46. Ectopic expression of MYCN in the sympathoadrenal lineage, under the regulation of the tyrosine hydroxylase promoter, is sufficient to drive NB tumorigenesis in zebrafish47 and in the mouse48. Although MYCN is a major oncogenic driver in NB and it has been extensively studied for nearly 3 decades49, there are currently no clinical trials targeting the MYCN protein directly in NB because of the difficulties inherent to developing molecular targeted therapies to transcription factors. However, recent efforts have focused on targeting signaling pathways that are deregulated as a result of elevated MYCN activity such as aurora kinase A50 and bromodomain/extra-terminal (BET) family of proteins51.
ATRX mutations are among the most common lesions in sporadic NB. ATRX encodes a SWI/SNF chromatin-remodelling ATP-dependent helicase (Figure 2)5, 52 and ATRX mutations are associated with X-linked mental retardation (XLMR) and α-thalassemia, suggesting that ATRX functions in various developmental processes. However, little is known about how ATRX contributes to the development or differentiation of the sympathoadrenal lineage. Children with XLMR do not have an increased incidence of NB, suggesting that ATRX mutations alone are not sufficient to promote tumorigenesis. However, there is an important association between ATRX mutations and age at diagnosis of NB52. Very young children (<18 months of age) with stage 4 disease tend to have a better prognosis than their older counterparts, and no ATRX mutations have been identified in this age group. ATRX mutations occur in 17% of children aged 18 months to 12 years with stage 4 disease, and in 44% of patients older than 12 years who uniformly have a very poor prognosis. The relationship between age at diagnosis and ATRX mutations is statistically significant52, but analysis of the prognostic significance of ATRX mutations will require a much larger study. To date, ATRX mutations have not been identified in tumors with MYCN amplification5, 52.
The relationship of ATRX mutation and abnormal telomere highlights the importance of telomere content in NB. Cancer cells must maintain telomeres for survival53. One mechanism of maintaining telomeres in NB is through increased expression of telomerase. Telomerase, composed of an RNA template (hTR) and a catalytic subunit (hTERT), elongates telomeric repeats at chromosomal ends53. Telomerase activity is strongly associated with hTERT and hTR expression54. High telomerase activity is found in 30% of NB at diagnosis and predicts reduced event-free survival[G] and overall survival (OS) in multivariate analyses55–56. However, telomere length does not necessarily correlate with telomerase activity57. In some tumor cells, a homologous recombination based mechanism of telomere maintenance and elongation is activated. This alternative lengthening of telomere (ALT) pathway is often identified by the presence of longer telomeres and large ultrabright telomere signal in tumor cells using telomere fluorescence in situ hybridization. Most of the tumors with ATRX mutations have evidence of ALT and it is possible that this is a direct effect of defects of histone H3.3 deposition at telomeres. ATRX loss in somatic cell hybrids segregate with ALT activation58, but whether ATRX functions in epigenetic processes that are important for sympathoadrenal lineage development or NB differentiation remains unknown. While both males and females were found to have ATRX mutations in the original analyses, additional studies will be required to determine if there is any gender bias for ALT mediated through ATRX mutations in NB. ATRX mutations have not yet been modelled in the mouse, and no molecular therapies have yet targeted this pathway.
By whole-genome sequencing (WGS), recurrent genetic lesions have been reported in the Rac/Rho pathway4–5 as well as chromatin-remodelling genes ARID1A and ARRID1B59. Additional studies will be required to elucidate the role of these genes in NB initiation and/or progression and the prognostic significance of disruptions in these pathways. Taken together, these genomic studies have identified few recurrent genetic lesions in druggable pathways. The major new challenge we face is to further elucidate the underlying biology of the tumors to identify deregulated developmental, epigenetic or metabolic pathways that can be exploited therapeutically for patients with high-risk or recurrent NB.
Neuronal differentiation in neuroblastoma
Analyses of familial NB, polymorphisms by GWAS, and recurrent somatic mutations by WGS have greatly enriched our understanding of NB, and in some cases, identified valuable targets for therapy. Another bridge is the unique physiology associated with the neuronal differentiation of the sympathoadrenal lineage. A valuable connection between cellular differentiation and tumorigenesis has come from the Shimada histology-grading system for NB, where the degree of differentiation, the Schwannian stromal content[G], and mitotic-karyorrhexis index[G] help stratify patients into risk groups60. The seminal observation that neuronal differentiation is driven by retinoids in vitro61 has led to isotretinoin (13-cis-retinoic acid) becoming the standard of care for high-risk NB62. Another connection between neuronal differentiation and tumourigenesis is highlighted by the identification of GD2 on neuronal stem cells63, the neuroblastic but not the glial lineage64, and on NB tumors65–66, which can provide further insight into using oncofoetal differentiation antigens as targets for antibody-based therapy (see below).
Another important clue about the relationship between normal differentiation and NB origins came from secreted catecholamine metabolites in patients’ urine. Catecholamines are found in the cells of the adrenal medulla and paraspinal ganglia67, and NB cells often have dense core vesicles (DCVs) wherein catecholamines are stored. Most catecholamine metabolism occurs in these cells as a result of leakage from the DCVs67–68. Meta-iodobenzylguanidine (MIBG), an analogue of norepinephrine, is readily taken up by NB cells and a fraction of it is stored in DCVs69. Thus, radioiodinated MIBG is used for diagnostic (123I-MIBG) and therapeutic (131I-MIBG) purposes70.
The metabolism of catecholamines in the adrenal medulla differs from that in the sympathetic ganglia. In sympathetic neurons 90% of the dopamine is converted to norepinephrine by dopamine β-hydroxylase and stored in DCVs67. The remaining dopamine is oxidized by monoamine oxidase (MAO) to DOPAL, a toxic catecholaldehyde. DOPAL is rapidly detoxified to DOPAC by aldehyde dehydrogenase. When norepinephrine or epinephrine is leaked from DCVs into cytoplasm, it gets metabolized by MAO to DOPEGAL, another toxic aldehyde. In contrast, the adrenal medulla cells express catechol-O-methyltransferase (COMT) in addition to MAO. Here, the norepinephrine or epinephrine gets metabolized by COMT to metanephrines, or by MAO to DOPEGAL, unless it is actively transported back into the DCVs by vesicular monoamine transporters (VAMTs). While there are some differences in the metabolism of catecholamines in adrenal medulla and sympathetic ganglia, both cell types rely upon the storage of catecholamines in DCVs by VAMTs.
VAMTs belong to an evolutionarily conserved family of genes that includes transporter proteins involved in multidrug resistance (MDR) in cancer cells71. The driving force for VAMTs is a hydrogen electrochemical gradient produced by an ATP-dependent vesicular proton pump67; any change in ATP production or intracellular pH can tilt this delicate balance against the tumor cell and may provide novel therapeutic approaches for NB. For example, ouabain is a Na,K-ATPase inhibitor that lowers intracellular pH and perturbs the electrochemical gradient that sequesters catecholamines in DCVs, thereby reducing the viability of NB cells72. These observations highlight how a deeper understanding of NB’s unique cellular physiology and metabolism can lead to new therapeutics that are not directly related to any particular genetic lesion.
Chromosomal instability and tumor heterogeneity
A patient’s age and stage of disease at diagnosis and the presence of MYCN amplification in NB cells are the three strongest determinants of clinical outcome3. These determinants, combined with the loss of chromosome 11q, histologic properties, and ploidy, are now the foundations of risk-group stratification for patients with NB. Not surprisingly, NB is much more heterogeneous when examined at the genetic level. As with other cancers, nongenetic heterogeneities (e.g., epigenetic or differentiation state) also influence risk74–75. However, how heterogeneity evolves with treatment and disease progression remains unknown.
In general, low risk, intermediate risk and stage 4s NBs (Table 1) have numerical chromosomal gains while high risk NBs have intrachromosomal rearrangements1. The incidence of these chromosomal aberrations increases with age at diagnosis and is strongly prognostic of outcome5, 52, 56. Together with oncogene amplifications, these large-scale genomic alterations may lead to deregulation of messenger RNAs, micro RNAs and other non-coding RNAs that interfere with apoptosis, differentiation, and immune surveillance76–78. In short, connecting clinical behavior to these complex molecular/genetic profiles is an ongoing challenge.
Table 1.
Survival from NB depends on MYCN status, age and stage of disease at diagnosis
| Prognostic Category |
MYCN amplification |
Stage* at Dx |
Age at Dx |
Treatment** |
5-year OS** (%) |
|---|---|---|---|---|---|
| Low Risk | No | 4s | <12 m | Supportive Care |
>90 |
| No | Locoregional | <12 y | Surgery ± Rx |
>90 | |
|
Intermediate Risk |
No | 4 | <18 m | Surgery + Rx |
>90 |
| High Risk | Yes | Locoregional | <12y | Intensive Rx + XRT + Surgery + SCT + CRA |
53 |
| Yes | 4 | <18 m | Intensive Rx + XRT + Surgery + SCT + CRA |
29 | |
| No | 4 | ≥18 m, <12 y |
Intensive Rx + XRT + Surgery + SCT + CRA |
31 | |
| No | 4 | ≥12 y | Intensive Rx + XRT + Surgery + SCT + CRA |
<10## |
Stage 4 176 = metastatic disease; Stage 4s = special metastatic pattern in infants with resectable primary tumors and distant spread to liver and skin, plus minimal bone marrow involvement. Abbreviations: CRA, 13-cis-retinoic acid; Dx, diagnosis; OS, overall survival; Rx, chemotherapy; SCT, myeloablative therapy with autologous stem cell rescue; XRT, radiation therapy to primary tumor and resistant metastatic sites.
OS was extracted from a large international retrospective (1990–2002) analysis, before the era of immunotherapy , when treatment was not uniform.
Genomic instability drives human genome evolution, both in healthy and disease states, and is common in many forms of cancer79. Hyperdiploidy reflects chromosome-segregation failure during mitosis80. Telomere dysfunction, DNA-repair defects, and chromothripsis are possible mechanisms of genome instability. Defects in the p53 pathway, loss of genes mapping to 1p and 11q, or gains of genes mapping to 1q and 17q may also play a role56.
DNA index in the hyperdiploid[G] range, typical for low risk NB, confers favorable outcome81. However, we do not know whether hyperdiploidy is simply a genomic biomarker or whether some mechanism links ploidy, NB-cell differentiation, and outcome. Patients whose high risk tumors harbor either large segmental chromosomal lesions fare much worse82. Established markers of poor prognosis include losses of 1p83 and 11q84 and gain of 17q85; losses of 3p, 4p, 9p, and 14q and gain of 1q, 2p, 7q, and 11p have also been implicated86. The loss of 11q is associated with an older age at diagnosis, absence of MYCN amplification and more chromosomal breaks. Because breakpoints occur on multiple chromosomes, they probably reflect an underlying defect in DNA maintenance or repair82 or elevated levels of double-stranded DNA breaks as a result of the unique cellular physiology of NB.
Chromosome instability is a dynamic process that cannot be accurately measured at a single time point. It is important to distinguish between dynamic processes that reflect the continuous accumulation of genetic lesions and more acute genomic events such as multiple chromosome trisomies in hyperdiploid NB or chromothripsis87, the acute shattering of genomic regions. Chromothripsis, which occurs in 2% to 3% of human cancers, may result from the uncoupling of DNA replication between individual chromosomes in micronuclei and those in the nucleus. If a chromosome in the micronucleus is still replicating its DNA when the cell enters mitosis, a massive break can occur that is limited to that chromosome. In a WGS study of NB, the investigators identified structural variants consistent with chromothripsis in 18% of high-risk NB5. In a separate study of 40 stage 4 NBs, there was only one tumor with evidence of chromothripsis52. Thus, additional studies are required to elucidate the frequency and significance of chromothripsis in NB.
Immunology and Immunotherapy
Because NBs can spontaneously regress, de novo anti-tumor immunity in patients seems logical. However, an active adaptive immunity against NB has been difficult to demonstrate especially in high-risk patients. This is not unexpected, given the exceptionally large tumor bulk (both primary and metastatic) and their rapid proliferation that overwhelm the immature immune system in a child. Beyond the paucity of somatic mutations making NB poorly immunogenic, this tumor has developed a sophisticated immunosuppressive microenvironment to ensure that no effective T-cell immunity can develop or accomplish its functions.
NB escapes the immune system
NB cells evade T-cells and natural killer (NK) cells by down-regulating human leukocyte antigen (HLA)88–89 and adhesion molecules90–91. They express or release proteins to inhibit92–94,95–96, and to kill, T-cells and NK cells97. They even recruit tissue macrophages to disable these lymphocytes98. NB cells carry on their cell surface high levels of ganglioside and sialic acid-containing sugars and proteins65, which are important for migration, adhesion and metastasis99. These highly negatively-charged carbohydrate epitopes are poorly immunogenic100 and sometimes even immunosuppressive101. Natural antibodies against NB are rare, with the possible exception of IgM102. Natural anti-ganglioside antibodies are even rarer, thereby allowing NB to survive in circulation despite their lack of complement decay accelerating factor (CD55)[G]103. In addition, NB can exploit protectin (CD59)[G] to resist complement-mediated lysis104. While NB downregulate HLA to escape T-cells, ironically they can also re-express HLA to resist NK cell mediated antibody-dependent cell mediated cytotoxicity (NK-ADCC)[G] when treated with MAbs105–106. This duplicity highlights the plasticity of NB as treatment pressures are applied. Clinically, NB evades the immune system by escaping to sanctuaries such as the CNS, which is not accessible to circulating antibodies. In fact, the increasing frequency of isolated relapses in the CNS, soft tissues (e.g., lymph nodes), and bone (despite having the marrow space in remission) signals a new curative challenge in the era of MAb therapy106.
NB vaccines to stimulate T-cell mediated immunity
Given these escape mechanisms and the complexity of the T-cell mediated immunity107, constructing an effective T-cell vaccine is daunting. NB antigens recognizable by cytotoxic T lymphocytes (CTLs)108 exist; they include cancer-testes antigens (MAGE and NY-ESO-1109), MYCN110, and survivin89. However, despite compelling evidence that T-cell vaccines using these antigens can be effective in syngeneic mouse models111–112, clinical success has been limited113. Dendritic cells as T-cell vaccines are still early in clinical testing114. Until the patient’s weak T-cell immunity following high-dose chemotherapy can be recharged, strategies to circumvent T-cell blockades (e.g., anti-CTLA4 and anti–PD-1115) may be ineffective.
Anti-GD2 MAb
The discovery that high-risk patients with NB can be maintained in continual remission with anti-GD2-specific MAb therapy was unexpected and is rapidly becoming the standard of care after 2 decades of research (Figure 3). GD2 belongs to a unique class of T-cell independent carbohydrate antigens with high density66, membrane proximity, homogeneity within and across NBs, and rare occurrence of antigen loss116. As an oncofetal differentiation antigen, it is expressed during fetal development and in mature neurons, pain fibers, and skin cells117. Two intravenous (iv) anti-GD2 IgG antibodies have been tested in the clinic, chimeric 14.18 (ch14.18) and mouse 3F8 (Table 2). Ch14.18 combined with iv interleukin-2 (IL-2) and iv granulocyte-macrophage colony-stimulating factor (GM-CSF), and oral 13-cis-retinoic acid (CRA), was proven efficacious in a randomized trial118. 3F8 plus subcutaneous GM-CSF and oral CRA without IL-2 in a single arm study showed a 5-year PFS of 62% and OS of 81% among patients with high-risk stage 4 NB treated in first remission106; although without a randomized placebo control, the efficacy cannot be certain. Because of the pain side effects, the anti-GD2 MAb dose is limited, and efficacy has only been observed to date in patients with minimal residual disease (MRD), while rarely seen in patients with bulky NB. Persistent MRD early during immunotherapy was highly predictive of ultimate treatment failure106. Granulocyte-mediated ADCC[G]119 and NK-ADCC105 are important effector mechanisms.
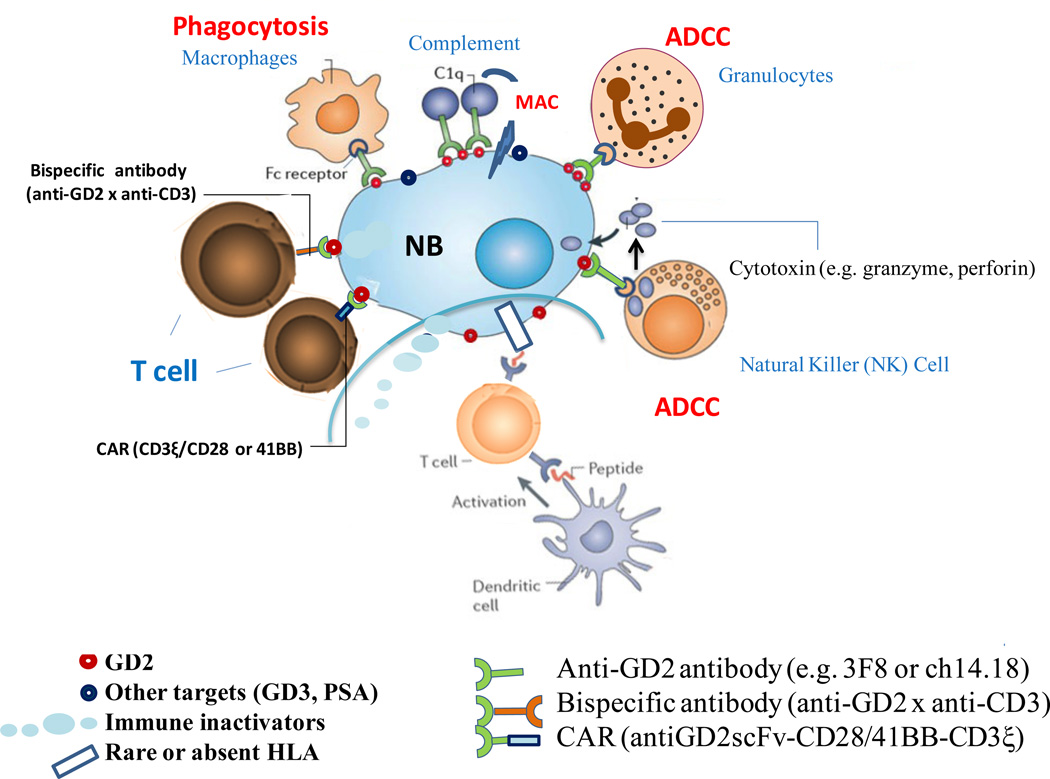
Figure 3. Immunotherapy of Neuroblastoma.
NB evades T-cells by downregulating or losing HLA expression, thereby interfering with the afferent arm (priming through dendritic cells), homing of T-cells to NB, and the CTL effector phase of adaptive immunity. Soluble inhibitors of immune response (e.g. FasL, gangliosides) are constantly released into the tumor stroma to impair cellular immunity. In addition, NB recruits pro-tumor macrophages and silences NK cells. Myeloid suppressor cells and T-reg can also suppress immunity. The paucity of mutations in NB compared to adult cancers like melanoma, the immaturity of the immune system in young patients, their massive disease and the intensive chemotherapy all combine to make NB poorly immunogenic for T-cells. Carbohydrate differentiation antigens (e.g. GD2, GD3 and polysialic acid (PSA)), all of which being classically T-independent antigens, offered alternative targets for antibody-based therapies. In the presence of monoclonal antibodies (e.g. 3F8 or ch14.18) specific for GD2, NB loses their defense and becomes highly susceptible to (1) NK (natural killer) cell mediated antibody-dependent cell mediated cytotoxicity (ADCC), (2) granulocyte mediated ADCC, (3) complement mediated cytotoxicity by binding to C1q thereby activating the complement cascade, delivering membrane attack complex (MAC) to tumor cell membrane, and (4) monocyte-macrophage mediated cytotoxicity. Even polyclonal T-cells can be retargeted to kill NB through MAbs in the form of chimeric antigen receptors (CAR) or bispecific antibodies (anti-GD2 x anti-CD3). CARs are anti-tumor single chain Fv fragments (scFv) genetically fused through a transmembrane domain to T-cell activating motifs (CD3 ξ and CD28/41BB) and transfected into killer lymphocytes. (Modified with permission from Scott et al202.)
Table 2.
Hallmarks of High Risk Neuroblastoma
| Hallmarks79 | Druggable Targets or Cells# |
Potential Therapeutic Inhibitors or Activators |
Developmental Status in NB |
|---|---|---|---|
| Proliferative signaling | 26S proteasome178 | Bortezomib* | Phase I |
| Akt179 | MK2206, Perifosine | Phase I | |
| ALK45 | Crizotinib | Phase II | |
| Aurora Kinase A50 | MLN8237180 | Phase I | |
| Cdk4/6181 | LEE011 | Phase I | |
| IGF-I/IGF-II182 | MEDI-573, m708.5 | Preclinical | |
| IGF-1R183 | Mab R1507, IMC-A12 | Phase I | |
| MYCN (BET)51 | (+)-JQ1 | Preclinical | |
| PI3K/mTOR179 | Pl103/Rapamycin | Phase I | |
| TrkA/TrkB184 | Lestaurtinib (CEP-701) | Phase I | |
| Evading growth suppressors |
Bcl-2185 | ABT-737 | Preclinical |
| Bmi-1186 | Vorinostat | Phase I | |
| MDM2-p53186 | Nutlin | Preclinical | |
| Resisting cell death | Methylation (CASP8, RASSF1A, DcR1, DcR2, DR4, DR5)74 |
Decitabine | Phase I |
| HDAC187 | Vorinostat | Phase I | |
| Enabling replicative immortality |
ATRX (ALT)58 | CHK1 inhibitor (LY2606368) | Preclinical |
| Telomerase56 | Imetelstat | Phase I | |
| Inducing angiogenesis | VEGF, HIF-1α, HIF-2α188 | MAb (Bevacizumab) | Phase II |
| Activating invasion and metastasis |
c-Met/HGF189 | Crizotinib | Phase I |
| EMT/Twist1186 | Vorinostat | Phase I | |
| MMP2, MMP9190 | AZD1236 | Preclinical | |
| Rho/Rac5 | Y27632 | Preclinical | |
| Genome instability | ATRX?, MYCN, Telomerase |
-- | -- |
| Tumor promoting inflammation |
TAM191 | IL15 (NKT)98 | Preclinical |
| Evasion of immune destruction |
B7-H3192 | MAb (8H9)157 | Phase I/II |
| CD59104 | rILYd4193 | Preclinical | |
| Granulocytes/macrophages | GM-CSF119 | Phase I/II/III | |
| HLA | IFN-gamma194 | Phase I | |
| KIR105 | MAb (anti-KIR2DL1/2/3)140 | Preclinical | |
| NK cells/ Granulocytes |
MAb (ch14.18, 3F8, hu14.18- K322A, hu3F8)118,152,154 |
Phase I/II/III | |
| NK/NKT/T-cells | IL-15138 | Prelinical | |
| IL-2118 | Phase I/II/III | ||
| T-cells | CAR (14G2a, 5F11, hu3F8, CE7)159–160 |
Preclinical/ Phase I |
|
| MAb (Bispecific 3F8 or bispecific hu3F8)165 |
Preclinical | ||
| Energy metabolism | GLUT1 | 3-Bromopyruvate, 3-BrOP195 | Preclinical |
Though being assigned under a single hallmark, a specific target could be responsible for multiple hallmarks of high-risk NB
Bold face in blue cell = FDA approved agents for non-NB indications.
A better understanding of the immunology of anti-GD2 MAb therapy should help explain its success and offer future directions. Anti-GD2 MAb does much more than passively attaching tumor cells to NK cells; they rescue NK cells from being suppressed or inhibited by NB. Normally, inhibitory receptors on NK cells (e.g., killer cell immunoglobulin-like receptors [KIRs]) [G] efficiently brake the network of synergizing activating receptors120 until FcγRIII (CD16)-mediated signaling releases that brake. Since KIRs and HLA (the cognate KIR ligands) genes are polymorphic and segregate independently; some individuals (up to 60%) have KIRs on their NK cells with no corresponding HLA ligands during maturation121–122. These cells with missing inhibitory KIR ligands are “uneducated” and hyporeactive until their CD16 is activated during ADCC. On the other hand, “educated” NK cells are restrained by HLA which can be upregulated by cytokines released during ADCC. This restraint by HLA explains why patients with “missing inhibitory KIR ligands” have better outcome following anti-GD2 MAb therapy105–106, 121–122.
Like NK cells, myeloid effectors are also awakened by anti-GD2 MAb. Granulocyte-ADCC of NB is unique among cancers and is clinically important106, 123. It does not depend on oxidative intermediates124, requires azurophil (primary) granule exocytosis123, and is enhanced by GM-CSF123, 125. Among FcγRs, FcγRIIA (CD32) is the receptor for ADCC on granulocytes123, 126 and its affinity for MAb correlates with patient outcome122, 127. Besides FcγR, complement receptors CR3 and CR4 on granulocytes are critical adhesion molecules for this type of ADCC123, 126, 128. One of their natural ligands is membrane-bound C3bi, a breakdown product of C3, resulting from complement activation on NB cells128. When activated, CR3 acquires the conformational neoepitope CBRM1/5129, and this is correlated with better patient survival119, especially in the presence of high-dose GM-CSF administered subcutaneously106. β-glucan binds and activates CR3, and when administered orally, enhances antibody therapy of neuroblastoma130. Tumor-associated macrophages (TAMs) represent another class of myeloid effector gaining prominence in NB research131. Macrophage migration inhibitory factor regulates NB growth, angiogenesis, and metastasis132 and is associated with dedifferentiation in MYCN amplified tumors133. Depending on the microenvironment, TAMs can become polarized into type 1 antitumor or type 2 pro-tumor phenotypes134. In the presence of anti-GD2 MAb, ADCC can turn pro-tumor M-CSF-activated macrophages in vitro into efficient antitumor “killing machines”135.
By activating ADCC to kill NB, anti-GD2 MAb is most efficient when effector cell populations and functions are amplified by cytokines. ADCC requires leukocytes, which are typically depleted following induction chemotherapy or autologous stem cell transplants. Since NK cells and granulocytes are effectors for ADCC, the cytokines IL-2 and GM-CSF are obvious candidates for combination with MAb. IL-2, which activates NK cells, natural killer T (NKT) cells, T-cells, and the undesirable regulatory T-cells (Treg)[G]136, has a modest anti-NB effect as a single agent137. Unlike GM-CSF, IL-2 is associated with substantial toxicity (e.g., 23% of patients experienced capillary leak118). Similar to IL-2, IL-15 activates NK, NKT, and CD8+ T-cells138. However, it does not cause capillary leak, activation-induced cell death, or increased Treg activation in non-human primate studies139. Another type of anti-NB lymphocytes called NKT cells140 can infiltrate NB, kill TAMs96, and they are associated with superior patient survival96, 141. However, for NKT cells to survive the hypoxic NB environment, IL-15 is essential98. Immunocytokines derived from humanized 14.18 (hu14.18-IL-2142 or hu14.18-GM-CSF123) are genetic fusion proteins of anti-GD2 antibody and cytokines targeted to tumor sites as immune stimulants when applied systemically143 or locally144. Despite compelling data in syngeneic mouse models145, intravenous hu14.18-IL-2 produced bone marrow remission only if NB was minimal143, and no benefit was seen for soft-tissue tumors143, 146. The clinical advantage of hu14.18-IL-2 over ch14.18 plus IL-2 awaits confirmation because grades 3/4 capillary leak with abnormal liver functions have been seen in both118, 143. These considerations provide further rationale to consider IL-15 as an alternative to IL-2 for combination with MAb in NB.
Even though MAb therapy is often regarded as passive immunotherapy, the induction of a host anti-tumor or anti-idiotype[G] network following MAb therapy may be important for long term tumor control147. Human anti-mouse antibody response (HAMA), an indirect measure of the host anti-idiotype network, was consistently correlated with long term survival106, 147. Because of these observations, anti-idiotypic vaccines such as mouse IgG1 antibody 1A7 specific for ch14.18148 and rat IgG1 antibody A1G4 specific for 3F8 seem logical149. The GD2 peptide mimotope150 and its DNA vaccine151 can induce serum antibodies and protective anti-GD2 IgG responses in mice. However, in contrast to whole-antigen vaccines, single-epitope vaccines will probably be limited by their narrow target coverage. The whole GD2 antigen has also been conjugated to keyhole limpet hemocyanin (KLH) to overcome the poor immunogenicity of carbohydrates100.
Besides its utility as a target to direct leukocyte-mediated killing, GD2 is also ideal for tumor-selective delivery of radioisotopes, liposomes, or nanoparticles70. However, the pain side effect106, 118, thought to be a consequence of complement activation152, is a major limitation of anti-GD2 MAb therapy. Mutating the Fc region to reduce complement activation152 and using blocking antibodies153 have decreased but not eliminated pain. Humanized 3F8 (hu3F8)154 has low immunogenicity, and deglycosylating hu3F8 can further decrease complement activation and possibly its pain side effects. For immunoconjugates that do not require Fc-effector functions, a pain-free targeting agent is ideal. Other potential NB cell surface targets include GD3, ALK155, polysialic acid108, L1CAM156 and B7-H3157. B7-H3–specific MAb 8H9 has been used successfully for compartmental radioimmunotherapy of NB metastasis to the CNS158.
Adoptive T-cell therapy
The new paradigm of including T-lymphocytes in MAb therapy of NB is taking shape. Although autologous tumor-reactive T-cells are rare in patients with NB and fail to home to tumor sites89, genetically redirected T-cells (e.g., using chimeric antigen receptors [CAR] hold great promise159–160. CAR connects a single-chain variable fragment (scFv) (anti-GD2) with a T-cell intracellular-signaling domain. When they were virally transfected into activated T-cells (ATC), clinical benefit was seen in NB160. CARs built with anti–L1CAM (MAb CE7) have been tested for NB, although the persistence of these ATCs was short-lived (≤42 days)159. To enhance their survival, Epstein-Barr virus–specific CTLs (instead of polyclonal ATC), which are life-long from continuing antigenic challenge, have been successfully used160. These dual-specific CTLs (anti–Epstein-Barr virus through the natural T-cell receptor and anti-GD2 through the CAR) can persist and control tumors for years160. Additional possible genetic modifications include luring T-cells with chemokine receptors141, 161 and reducing Treg interference by turning up the Akt pathway162. Similar strategies of adoptive cytotherapy with NK cells and NKT cells are being explored following their expansion ex vivo using engineered IL-15163 or IL-21164.
The future of NB immunotherapy
The current immunotherapy strategy uses anti-GD2 MAb to direct the traffic of FcR-bearing NK cells and granulocytes, stimulating them through the FcR CD16 and CD32, respectively. By activating through CD3, T-cell engaging bispecific antibody (anti-GD2 x anti-CD3) will exploit T-cells that do not carry FcR165, a compartment of underused effectors with potentials for long term anti-tumor immunity. Since the Fc function is not required for these bispecific MAb and therefore dispensable, the pain side effects of anti-GD2 antibodies could potentially be eliminated. A rational integration of anti-tumor MAb, effector cells, and cytokines to induce tumor cell death, while combining with small molecules to prevent death evasion, can be tested in the appropriate mouse models to guide ongoing and future clinical trials.
Translational Model
Genetic and epigenetic aberrations carried by high-risk NB are continually exploited by these tumors to survive the selective pressures of competing nutrients, hypoxia, immune surveillance and cytotoxic therapy. The latter two prescriptions have successfully been applied to NB, with glimpses of cure but not for all patients. With cytotoxic therapy, increasing dose and dose intensity have been explored166–167, and could be further maximized with better supportive care. Small molecules that target specific genetic and epigenetic aberrations are rapidly transitioning into the clinic for NB (see Table 2). ALK is an exemplary tumor target for NB therapy45, ahead of other promising gene candidates (Figure 2).
Translational and clinical research of pediatric cancers is fundamentally different from that of adult cancers, because there are relatively few patients, and clinical trials are often carried out by cooperative groups. Also, drug metabolism, acute toxicities, and late effects vary greatly between children and adults. Most pediatric preclinical cancer studies use cell lines or flank xenografts in immunocompromised mice168. For immunologic analysis, mouse effectors are poor human surrogates for testing monoclonal antibodies (MAbs)169; humanized mice (Table 3), i.e., those grafted with human immune cells, should be more appropriate170–171. Many research groups incorporate genetically engineered mouse models172, yet few use comprehensive preclinical studies to directly guide clinical trials173. For example, most studies are short-term (2–3 weeks), using drug doses and schedules that are not clinically relevant. They often fail to incorporate combination chemotherapy, and do not have an appropriate benchmark (e.g., current standard of care). Most preclinical studies also lack statistical design, appropriate randomization of animals to treatment regimens, or a mechanism to “blind” researchers to treatments received by study cohorts. An ideal translational research “roadmap” (Box 2) should engage a multidisciplinary team comprising clinical researchers, laboratory-based scientists, chemists, pharmacologists, and biostatisticians.
Table 3.
Animal models of neuroblastoma
| Model | Type | Gene; Lineage | Reference |
|---|---|---|---|
| TH-MYCN | transgenic | human MYCN; sympathoadrenal lineage | Weiss et al. (1997)48 |
| TH-ALKF1174L;TH-MYCN | transgenic | human ALKF1174; sympathoadrenal lineage | Berry et al. (2012)33 |
| ALKF1174L | conditional transgenic | human ALKF1174; sympathoadrenal lineage | Lukas et al. (2012) |
| Lin28 | conditional transgenic | mouse Lin28b; sympathoadrenal lineage | Molenaar et al. (2012)43 |
| TH-MYCN;Caspase 8 | conditional knockout | mouse caspase 8; sympathoadrenal lineage | Teitz et al. (in revision) |
| Xenografts in immune-deficient mice |
orthotopic/xenograft | human orthotopic xenografts cell lines and primary tumors |
Chanthery et al. (2012)196 Teitz et al. (2011) 197 |
| Xenografts in NOD/SCID/IL2rγnull or BALB/c-rag2KO/γcnull |
humanized mice | human orthotopic xenografts cell lines and primary tumors |
Shultz et al.(2007)170 Pek et al.(2011)146 |
| Syngeneic | A/J inbred mouse | mouse neuroblastoma (NXS2, Neuro-2a) |
Huebener et al.(2009)198 Jing et al.(2011)112 |
| MYCN; ALKF1174L | transgenic zebrafish | human MYCN and ALKF1174L dβh promoter |
Zhu et al. (2012)47 |
[Box 2]. Translational research roadmap.
Step 1 Identify key clinical challenges
For NB, this may include patients with high-risk disease and particular emphasis on those with refractory disease. It may also include patients who have very good prognosis, where translational research may reduce treatment intensity and treatment-associated toxicities.
Step 2 Identify druggable targets and pathways
Besides specific kinases (e.g., ALK) or enzymes (e.g., drug transporters), entire pathways and cellular programs (e.g., ALT) should be considered. In some cases, biomarkers (e.g., GD2) can be used for molecular-targeted therapeutics, even if the marker is not directly related to perturbations in cellular pathways required for tumorigenesis.
Step 3 Well-designed preclinical testing
For orphan diseases, only the most promising new agents should move forward, and every available resource should be used to compare/contrast that agent with the standard of care. A comprehensive preclinical trial should consider genetic mouse models and orthotopic xenografts of human primary tumors to establish the relative efficacy of competing treatment options. The choice of mouse models (Table 3) is crucial, especially when immunologics are being tested, human immune cells are required, and the tumor microenvironment is part of the pathway being tested. Ideally, mice should undergo the same diagnostic testing (magnetic resonance imaging, ultrasound, positron emission tomography) and functional assessments (blood counts and chemistries, urine catecholamine monitoring) that patients receive. Minimal residual disease measurement using blood samples is another potentially informative endpoint.
With the exception of ALK, genomic characterization of NB has provided few leads for druggable pathways that can be directly moved into clinical trials. We propose that a multipronged approach will be required to improve outcomes for NB patients over the next decade. As presented here, neuroendocrine differentiation of NBs can be exploited in two ways. First, epigenetic processes are important for coordinating proliferation and differentiation during development. In other pediatric cancers with features of neuronal differentiation such as retinoblastoma, epigenetic profiling provided important new insights into tumor initiation and progression and led to the identification of novel therapeutic approaches174. Therefore, epigenetic profiling of NBs may lead to the identification of differentiation or cancer pathway that can be targeted in the clinic. Second, it may be possible to exploit the unique features of NB differentiation with respect to catecholamine biosynthesis, transport and metabolism by using therapeutics to force intracellular accumulation of toxic catechoaldehydes or other perturbations. Another approach is to identify novel therapeutics using an unbiased high-throughput drug screening as recently used for pediatric brain tumors175. For such approaches, it is essential to have validated cell-based screening assays that faithfully recapitulate the molecular, cellular and genetic heterogeneity of NB found in patients. While small molecules used in single-agent targeted strategies will provide proof of principle, their curative potential in NB will likely require exploiting multiple pathways, and careful integration with active death induction through either chemoradiotherapy or immunotherapy.
Overall conclusion
We have witnessed tremendous advances in our understanding of the genetics and biology of NB that will greatly improve the precision in the stratification of this complex heterogeneous disease. There have also been remarkable advances in our understanding of how NB tumor cells evade the immune system, and some of the most promising therapeutic approaches for NB now involve immunotherapy. With the ability to further refine the classification of patients accurately into risk groups3, the challenge we face in the next decade is to identify the most effective and least toxic therapy for these patient subgroups. The focus must now shift toward multidisciplinary translational research teams using validated preclinical models to select the most promising combinations of molecular targeted therapies, broad-spectrum chemotherapy and immunotherapy to cure patients who currently have a poor prognosis and reduce therapy for those with more favorable outcomes. Emphasis on targeted therapies and reduction of cytotoxic treatments are the overarching goals – to cure NB while sparing young children adverse treatment-related late effects.
[Box 1]. AT A GLANCE.
Neuroblastoma (NB) is a heterogeneous disease. Fifty percent of NBs arise in young children, carry whole-chromosomal gains with relatively few somatic mutations, and are highly curable with either surgery alone or surgery and low-dose chemotherapy. The other 50% of NBs are usually metastatic, and most are diagnosed after 18 months of age, with half of the tumors carrying MYCN amplification and ALK, or ATRX mutations. Other somatic mutations accumulate with increasing age at diagnosis. Neural crest cells and NB share common pathways and genes, including PHOX2B, MYCN, ATRX, and ALK, which offer therapeutic targets.
A predictive profile of genetic predisposition to NB is emerging via genome-wide association and whole-genome sequencing analyses. However, there is general paucity of somatic mutations in contrast to adult cancers.
The unique biology of catecholamine transport has been successfully exploited to provide the tumor-specific neurotransmitter analogue MIBG for diagnosis and anti-NB therapy. This advance exemplifies how understanding unique tumor metabolism can lead to new therapeutics that is not directly related to specific genetic lesions.
Chromosomal aberration is common in NB; numerical whole chromosomal gains are typically found in low-risk tumors, while segmental chromosomal gains or losses and somatic mutations are associated with high-risk disease.
Epigenetic regulation and miRNA control are other prognostic and therapeutic directions likely to uncover new markers and targets for NB.
NB can evade T-cells and NK cells while exploiting inflammatory macrophages to enhance its survival. Monoclonal antibodies, cytokines, and multifunctional antibodies could potentially reactivate antitumor activity in these cells.
Anti-GD2 antibody, when combined with GM-CSF with or without IL-2, is one of the most successful and important strategies for the curative approach to NB. Both myeloid effectors and NK cells and their cell surface activating or inhibitory receptors play crucial roles in the clinical response.
Acknowledgements
We want to thank Dr. Irene Cheung, Dr. Brian Kushner, and Dr. Shakeel Modak for their critical review of this manuscript. This work was supported in part by NCI-CA161978, DOD-PR111043 and NCI-CA154754 (NKC), as well as NCI-CA21765, NIH-EY014867, NIH-EY018599, NCI-CA168875, the American Lebanese Syrian Associated Charities, and the Howard Hughes Medical Institute Early Career Scientist Award (MAD).
Glossary Terms
- GD2
Disialoganglioside expressed on tumors of neuroectodermal origin, including human neuroblastoma, melanoma, small cell lung cancer and many sarcomas, with highly restricted expression on normal tissues (cerebellum and peripheral nerves). Two monoclonal antibody families specific for the oligoxsaccharide epitope of GD2 have been tested extensively in patients, mouse IgG3 antibody 3F8 and its humanized version (hu3F8), and mouse IgG2a antibody 14G2a, and its chimeric (ch14.18) or humanized (hu14.18) forms
- Myeloablative therapy
Bone marrow ablation owing to the loss of haematopoietic stem cells following high-dose radiation or chemotherapy
- Adrenal gland
Endocrine organ responsible for making stress hormones, aldosterone and androgens
- Sympathetic ganglia
Masses of nerve cells that are part of a network controlling autonomic/”fight-or-flight“responses
- Hirschsprung disease
A cogenital disease where large intestines lack innervation
- Congenital Central Hypoventilation Syndrome (CCHS)
A congenital brain stem disease where autonomic control of breathing is defective causing sleep apnea
- Alternative Lengthening of Telomeres (ALT)
A recombination-based mechanism that allows telomere length maintenance in the absence of telomerase activity
- Schwannian stromal content
Glial cells in the surrounding stroma, interspersed among neuroblastoma cells, usually abundant in tumors that are more differentiated
- Mitotic-karyorrhexis index
A measure of the frequency of cells in mitosis with karyorrhexis (nuclear fragmentation associated with cell death)
- Transcribed ultraconserved regions (T-UCR)
T-UCRs are non-coding RNAs transcribed from 481 ultraconserved regions (UCRs) defined as being at least 200 bp in length and 100%
- conserved between the human
mouse and rat genomes
- Hyperdiploid
Having more than the diploid number of chromosomes, where the DNA index is >1.15
- Event-free survival
A measure of time being continually alive without a life-threatening adverse event
- Complement decay accelerating factor (CD55)
A 70 kDa membrane protein that prevents the assembly of the C3bBb complex (C3-convertase of the alternative pathway of complement activation), thereby blocking formation of the membrane attack complex (MAC)
- Protectin (CD59)
A membrane protein that inhibits MAC by binding C5b678 and preventing C9 from binding and polymerizing
- NK cell mediated antibody-dependent cytotoxicity (NK-ADCC)
Killing of tumor cells by natural killer cells whose Fc receptor adheres to the antibody attached to the target
- Granulocyte mediated antibody-dependent cytotoxicity (granulocyte-ADCC)
Killing of tumor cells by granulocytes whose Fc receptor adheres to the antibody attached to the target
- Killer cell immunoglobulin-like receptors (KIRs)
Highly polymorphic NK cell surface proteins that interact with MHC class I molecules, and most KIRs mediate NK inhibition instead of activation
- Regulatory T-cells (Treg)
A T-cell subtype that releases suppressive cytokines and serves to silence immune responses
- Anti-idiotype
An antibody binding specifically to an epitope in the variable region of another antibody
- when the epitope is the actual antigen-binding site
an anti-idiotypic antibody can mimic the de novo antigen
- QS21 adjuvant
A soluble triterpene glucoside saponin discovered in the extract from the Soap bark tree (Quillaja saponaria) that stimulates humoral and cell-mediated immunity
- Chromaffin cells
Neuroendocrine cells in the adrenal medulla that receive sympathetic input and release catecholamine neurotransmitters to the systemic circulation
Footnotes
Conflicts of Interest
MSKCC has a patent application on hu3F8 and NKC was named as one of the inventors. MSKCC has licensed the patent on beta-glucan to Biotec Pharmacon, and the patent on antibody 8H9 to United Therapeutics, and NKC was named as one of the inventors for both agents. Clinical trials of hu3F8 are funded by the NCI (NKC) and the DOD (NKC).
References
- 1.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362:2202–2211. doi: 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 3.Cohn SL, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:289–297. doi: 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pugh TJ, et al. The genetic landscape of high-risk neuroblastoma. Nat Genet. 2013 doi: 10.1038/ng.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Molenaar JJ, et al. Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature. 2012;483:589–593. doi: 10.1038/nature10910. [DOI] [PubMed] [Google Scholar]
- 6.Shukla N, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res. 2012;18:748–757. doi: 10.1158/1078-0432.CCR-11-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Segal NH, et al. Epitope landscape in breast and colorectal cancer. Cancer research. 2008;68:889–892. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G, Zitvogel L. Can the exome and the immunome converge on the design of efficient cancer vaccines? OncoImmunology. 2012;1:579–580. doi: 10.4161/onci.20730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grupp SA, Asgharzadeh S, Yanik GA. Neuroblastoma: issues in transplantation. Biol Blood Marrow Transplant. 2012;18:S92–S100. doi: 10.1016/j.bbmt.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson DJ, Carnahan JF, Michelsohn A, Patterson PH. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J Neurosci. 1991;11:3507–3519. doi: 10.1523/JNEUROSCI.11-11-03507.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Dourin NM, Kalcheim G. The Neural Crest. Cambridge University Press; 1999. [Google Scholar]
- 12.Pages PM, et al. Bilateral adrenal neuroblastoma. Pediatric blood & cancer. 2009;52:196–202. doi: 10.1002/pbc.21765. [DOI] [PubMed] [Google Scholar]
- 13.Knudson AG, Jr, Strong LC. Mutation and cancer: neuroblastoma and pheochromocytoma. American journal of human genetics. 1972;24:514–532. [PMC free article] [PubMed] [Google Scholar]
- 14.Mosse YP, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455:930–935. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maris JM, et al. Evidence for a hereditary neuroblastoma predisposition locus at chromosome 16p12-13. Cancer research. 2002;62:6651–6658. [PubMed] [Google Scholar]
- 16.Mosse YP, et al. Germline PHOX2B mutation in hereditary neuroblastoma. American journal of human genetics. 2004;75:727–730. doi: 10.1086/424530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trochet D, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma. American journal of human genetics. 2004;74:761–764. doi: 10.1086/383253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trochet D, et al. Molecular consequences of PHOX2B missense, frameshift and alanine expansion mutations leading to autonomic dysfunction. Human molecular genetics. 2005;14:3697–3708. doi: 10.1093/hmg/ddi401. [DOI] [PubMed] [Google Scholar]
- 19.Raabe EH, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27:469–476. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- 20.Stovroff M, Dykes F, Teague WG. The complete spectrum of neurocristopathy in an infant with congenital hypoventilation, Hirschsprung’s disease, and neuroblastoma. Journal of pediatric surgery. 1995;30:1218–1221. doi: 10.1016/0022-3468(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 21.Wilzen A, et al. The Phox2 pathway is differentially expressed in neuroblastoma tumors, but no mutations were found in the candidate tumor suppressor gene PHOX2A. International journal of oncology. 2009;34:697–705. doi: 10.3892/ijo_00000196. [DOI] [PubMed] [Google Scholar]
- 22.Nagashimada M, et al. Autonomic neurocristopathy-associated mutations in PHOX2B dysregulate Sox10 expression. The Journal of clinical investigation. 2012;122:3145–3158. doi: 10.1172/JCI63401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455:967–970. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, et al. Oncogenic mutations of ALK kinase in neuroblastoma. Nature. 2008;455:971–974. doi: 10.1038/nature07399. [DOI] [PubMed] [Google Scholar]
- 25.George RE, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975–978. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwahara T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14:439–449. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 27.Degoutin J, Brunet-de Carvalho N, Cifuentes-Diaz C, Vigny M. ALK (Anaplastic Lymphoma Kinase) expression in DRG neurons and its involvement in neuron-Schwann cells interaction. The European journal of neuroscience. 2009;29:275–286. doi: 10.1111/j.1460-9568.2008.06593.x. [DOI] [PubMed] [Google Scholar]
- 28.Souttou B, Carvalho NB, Raulais D, Vigny M. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. The Journal of biological chemistry. 2001;276:9526–9531. doi: 10.1074/jbc.M007333200. [DOI] [PubMed] [Google Scholar]
- 29.Motegi A, Fujimoto J, Kotani M, Sakuraba H, Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. Journal of cell science. 2004;117:3319–3329. doi: 10.1242/jcs.01183. [DOI] [PubMed] [Google Scholar]
- 30.Schonherr C, Yang HL, Vigny M, Palmer RH, Hallberg B. Anaplastic lymphoma kinase activates the small GTPase Rap1 via the Rap1-specific GEF C3G in both neuroblastoma and PC12 cells. Oncogene. 2010;29:2817–2830. doi: 10.1038/onc.2010.27. [DOI] [PubMed] [Google Scholar]
- 31.Bachetti T, et al. PHOX2B–mediated regulation of ALK expression: in vitro identification of a functional relationship between two genes involved in neuroblastoma. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reiff T, et al. Midkine and Alk signaling in sympathetic neuron proliferation and neuroblastoma predisposition. Development. 2011;138:4699–4708. doi: 10.1242/dev.072157. [DOI] [PubMed] [Google Scholar]
- 33.Berry T, et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma. Cancer Cell. 2012;22:117–130. doi: 10.1016/j.ccr.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heukamp LC, et al. Targeted expression of mutated ALK induces neuroblastoma in transgenic mice. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3003967. 141ra91. [DOI] [PubMed] [Google Scholar]
- 35.Schulte JH, et al. MYCN and ALKF1174L are sufficient to drive neuroblastoma development from neural crest progenitor cells. Oncogene. 2012 doi: 10.1038/onc.2012.106. [DOI] [PubMed] [Google Scholar]
- 36.Capasso M, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41:718–723. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang K, et al. Integrative genomics identifies LMO1 as a neuroblastoma oncogene. Nature. 2011;469:216–220. doi: 10.1038/nature09609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen le B, et al. Phenotype restricted genome-wide association study using a gene-centric approach identifies three low-risk neuroblastoma susceptibility Loci. PLoS Genet. 2011;7:e1002026. doi: 10.1371/journal.pgen.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diskin SJ, et al. Common variation at 6q16 within HACE1 and LIN28B influences susceptibility to neuroblastoma. Nat Genet. 2012 doi: 10.1038/ng.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maris JM, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358:2585–2593. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latorre V, et al. Replication of neuroblastoma SNP association at the BARD1 locus in African-Americans. Cancer Epidemiol Biomarkers Prev. 2012;21:658–663. doi: 10.1158/1055-9965.EPI-11-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosse KR, et al. Common variation at BARD1 results in the expression of an oncogenic isoform that influences neuroblastoma susceptibility and oncogenicity. Cancer Res. 2012;72:2068–2078. doi: 10.1158/0008-5472.CAN-11-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molenaar JJ, et al. LIN28B induces neuroblastoma and enhances MYCN levels via let-7 suppression. Nat Genet. 2012 doi: 10.1038/ng.2436. [DOI] [PubMed] [Google Scholar]
- 44.Schulte JH, et al. High ALK Receptor Tyrosine Kinase Expression Supersedes ALK Mutation as a Determining Factor of an Unfavorable Phenotype in Primary Neuroblastoma. Clin Cancer Res. 2011;17:5082–5092. doi: 10.1158/1078-0432.CCR-10-2809. [DOI] [PubMed] [Google Scholar]
- 45.Carpenter EL, Mosse YP. Targeting ALK in neuroblastoma--preclinical and clinical advancements Nature reviews. Clinical oncology. 2012;9:391–399. doi: 10.1038/nrclinonc.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimmer MR, Weiss WA. Childhood tumors of the nervous system as disorders of normal development. Current opinion in pediatrics. 2006;18:634–638. doi: 10.1097/MOP.0b013e32801080fe. [DOI] [PubMed] [Google Scholar]
- 47.Zhu S, et al. Activated ALK collaborates with MYCN in neuroblastoma pathogenesis. Cancer Cell. 2012;21:362–373. doi: 10.1016/j.ccr.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. Targeted expression of MYCN causes neuroblastoma in transgenic mice. Embo J. 1997;16:2985–2995. doi: 10.1093/emboj/16.11.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science. 1984;224:1121–1124. doi: 10.1126/science.6719137. [DOI] [PubMed] [Google Scholar]
- 50.Faisal A, et al. The aurora kinase inhibitor CCT137690 downregulates MYCN and sensitizes MYCN-amplified neuroblastoma in vivo. Mol Cancer Ther. 2011;10:2115–2123. doi: 10.1158/1535-7163.MCT-11-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Puissant A, et al. Targeting MYCN in Neuroblastoma by BET Bromodomain Inhibition. Cancer Discov. 2013 doi: 10.1158/2159-8290.CD-12-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheung NK, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma. JAMA : the journal of the American Medical Association. 2012;307:1062–1071. doi: 10.1001/jama.2012.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blackburn EH. Telomeres and telomerase: the means to the end (Nobel lecture) Angew Chem Int Ed Engl. 2010;49:7405–7421. doi: 10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- 54.Poremba C, et al. Telomerase activity and telomerase subunits gene expression patterns in neuroblastoma: a molecular and immunohistochemical study establishing prognostic tools for fresh-frozen and paraffin-embedded tissues. J Clin Oncol. 2000;18:2582–2592. doi: 10.1200/JCO.2000.18.13.2582. [DOI] [PubMed] [Google Scholar]
- 55.Hiyama E, et al. Correlating telomerase activity levels with human neuroblastoma outcomes. Nat Med. 1995;1:249–255. doi: 10.1038/nm0395-249. [DOI] [PubMed] [Google Scholar]
- 56.Coco S, et al. Age-dependent accumulation of genomic aberrations and deregulation of cell cycle and telomerase genes in metastatic neuroblastoma. Int J Cancer. 2012;131:1591–1600. doi: 10.1002/ijc.27432. [DOI] [PubMed] [Google Scholar]
- 57.Onitake Y, et al. Telomere biology in neuroblastoma: telomere binding proteins and alternative lengthening of telomeres. Journal of pediatric surgery. 2009;44:2258–2266. doi: 10.1016/j.jpedsurg.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 58.Bower K, et al. Loss of Wild-Type ATRX Expression in Somatic Cell Hybrids Segregates with Activation of Alternative Lengthening of Telomeres. PLoS One. 2012;7:e50062. doi: 10.1371/journal.pone.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sausen M, et al. Integrated genomic analyses identify ARID1A and ARID1B alterations in the childhood cancer neuroblastoma. Nat Genet. 2012;45:12–17. doi: 10.1038/ng.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shimada H, et al. The International Neuroblastoma Pathology Classification (the Shimada system) Cancer. 1999;86:364–372. [PubMed] [Google Scholar]
- 61.Sidell N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J Natl Cancer Inst. 1982;68:589–596. [PubMed] [Google Scholar]
- 62.Matthay KK, et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:1007–1013. doi: 10.1200/JCO.2007.13.8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yanagisawa M, Yoshimura S, Yu RK. Expression of GD2 and GD3 gangliosides in human embryonic neural stem cells. ASN Neuro. 2011;3 doi: 10.1042/AN20110006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Acosta S, et al. Identification of tumoral glial precursor cells in neuroblastoma. Cancer Lett. 2011;312:73–81. doi: 10.1016/j.canlet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 65.Shochat SJ, Abt AB, Schengrund CL. VCN-releasable sialic acid and gangliosides in human neuroblastomas. Journal of pediatric surgery. 1977;12:413–418. doi: 10.1016/0022-3468(77)90019-7. [DOI] [PubMed] [Google Scholar]
- 66.Schulz G, et al. Detection of ganglioside GD2 in tumor tissues and sera of neuroblastoma patients. Cancer research. 1984;44:5914–5920. [PubMed] [Google Scholar]
- 67.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacological reviews. 2004;56:331–349. doi: 10.1124/pr.56.3.1. [DOI] [PubMed] [Google Scholar]
- 68.Eisenhofer G, Kopin IJ, Goldstein DS. Leaky catecholamine stores: undue waste or a stress response coping mechanism? Annals of the New York Academy of Sciences. 2004;1018:224–230. doi: 10.1196/annals.1296.027. [DOI] [PubMed] [Google Scholar]
- 69.Sisson JC, Yanik GA. Theranostics: evolution of the radiopharmaceutical meta-iodobenzylguanidine in endocrine tumors. Semin Nucl Med. 2012;42:171–184. doi: 10.1053/j.semnuclmed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 70.Matthay KK, George RE, Yu AL. Promising therapeutic targets in neuroblastoma. Clin Cancer Res. 2012;18:2740–2753. doi: 10.1158/1078-0432.CCR-11-1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiological reviews. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 72.Hiyoshi H, et al. Quiescence and gammaH2AX in neuroblastoma are regulated by ouabain/Na,K-ATPase. British Journal of Cancer. 2012;106:1807–1815. doi: 10.1038/bjc.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norris MD, et al. Expression of the gene for multidrug-resistance-associated protein and outcome in patients with neuroblastoma. N Engl J Med. 1996;334:231–238. doi: 10.1056/NEJM199601253340405. [DOI] [PubMed] [Google Scholar]
- 74.Decock A, Ongenaert M, Vandesompele J, Speleman F. Neuroblastoma epigenetics: from candidate gene approaches to genome-wide screenings. Epigenetics. 2011;6:962–970. doi: 10.4161/epi.6.8.16516. [DOI] [PubMed] [Google Scholar]
- 75.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12:323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 76.Stallings RL, Foley NH, Bryan K, Buckley PG, Bray I. Therapeutic targeting of miRNAs in neuroblastoma. Expert Opin Ther Targets. 2010;14:951–962. doi: 10.1517/14728222.2010.510136. [DOI] [PubMed] [Google Scholar]
- 77.Speleman F, De Preter K, Vandesompele J. Neuroblastoma genetics and phenotype: a tale of heterogeneity. Semin Cancer Biol. 2011;21:238–244. doi: 10.1016/j.semcancer.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 78.Mestdagh P, et al. An integrative genomics screen uncovers ncRNA T-UCR functions in neuroblastoma tumours. Oncogene. 2010;29:3583–3592. doi: 10.1038/onc.2010.106. [DOI] [PubMed] [Google Scholar]
- 79.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 80.Benard J, et al. MYCN-non-amplified metastatic neuroblastoma with good prognosis and spontaneous regression: a molecular portrait of stage 4S. Mol Oncol. 2008;2:261–271. doi: 10.1016/j.molonc.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Look AT, Hayes FA, Nitschke R al., e. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984;311:231–235. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- 82.Schleiermacher G, et al. Accumulation of segmental alterations determines progression in neuroblastoma. J Clin Oncol. 2010;28:3122–3130. doi: 10.1200/JCO.2009.26.7955. [DOI] [PubMed] [Google Scholar]
- 83.Caron H, et al. Allelic loss of chromosome 1p as a predictor of unfavorable outcome in patients with neuroblastoma. N Engl J Med. 1996;334:225–230. doi: 10.1056/NEJM199601253340404. [DOI] [PubMed] [Google Scholar]
- 84.Attiyeh EF, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 85.Bown N, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med. 1999;340:1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 86.Vandesompele J, et al. Unequivocal delineation of clinicogenetic subgroups and development of a new model for improved outcome prediction in neuroblastoma. J Clin Oncol. 2005;23:2280–2299. doi: 10.1200/JCO.2005.06.104. [DOI] [PubMed] [Google Scholar]
- 87.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raffaghello L, et al. Mechanisms of immune evasion of human neuroblastoma. Cancer Lett. 2005;228:155–161. doi: 10.1016/j.canlet.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 89.Coughlin CM, et al. Immunosurveillance and survivin-specific T-cell immunity in children with high-risk neuroblastoma. J Clin Oncol. 2006;24:5725–5734. doi: 10.1200/JCO.2005.05.3314. [DOI] [PubMed] [Google Scholar]
- 90.Favrot MC, et al. Expression of leucocyte adhesion molecules on 66 clinical neuroblastoma specimens. Int J Cancer. 1991;48:502–510. doi: 10.1002/ijc.2910480405. [DOI] [PubMed] [Google Scholar]
- 91.Foreman NK, Rill DR, Coustan-Smith E, Douglass EC, Brenner MK. Mechanisms of selective killing of neuroblastoma cells by natural killer cells and lymphokine activated killer cells. Potential for residual disease eradication. Br J Cancer. 1993;67:933–938. doi: 10.1038/bjc.1993.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Castriconi R, et al. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci U S A. 2004;101:12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Raffaghello L, et al. Downregulation and/or release of NKG2D ligands as immune evasion strategy of human neuroblastoma. Neoplasia. 2004;6:558–568. doi: 10.1593/neo.04316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morandi F, et al. Bone marrow-infiltrating human neuroblastoma cells express high levels of calprotectin and HLA-G proteins. PLoS One. 2012;7:e29922. doi: 10.1371/journal.pone.0029922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Asgharzadeh S, et al. Prognostic significance of gene expression profiles of metastatic neuroblastomas lacking MYCN gene amplification. J Natl Cancer Inst. 2006;98:1193–1203. doi: 10.1093/jnci/djj330. [DOI] [PubMed] [Google Scholar]
- 96.Song L, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–1536. doi: 10.1172/JCI37869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shurin GV, Gerein V, Lotze MT, Barksdale EM., Jr Apoptosis induced in T cells by human neuroblastoma cells: role of Fas ligand. Nat Immun. 1998;16:263–274. doi: 10.1159/000069452. [DOI] [PubMed] [Google Scholar]
- 98.Liu D, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest. 2012;122:2221–2233. doi: 10.1172/JCI59535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dong L, Liu Y, Colberg-Poley AM, Kaucic K, Ladisch S. Induction of GM1a/GD1b synthase triggers complex ganglioside expression and alters neuroblastoma cell behavior; a new tumor cell model of ganglioside function. Glycoconj J. 2011;28:137–147. doi: 10.1007/s10719-011-9330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ragupathi G, et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin Cancer Res. 2003;9:5214–5220. [PubMed] [Google Scholar]
- 101.Shurin GV, et al. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 2001;61:363–369. [PubMed] [Google Scholar]
- 102.Ollert MW, et al. Normal human serum contains a natural IgM antibody cytotoxic for human neuroblastoma cells. Proc Natl Acad Sci U S A. 1996;93:4498–4503. doi: 10.1073/pnas.93.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheung NK, et al. Decay-accelerating factor protects human tumor cells from complement-mediated cytotoxicity in vitro. J Clin Invest. 1988;81:1122–1128. doi: 10.1172/JCI113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen S, Caragine T, Cheung NK, Tomlinson S. CD59 expressed on a tumor cell surface modulates decay-accelerating factor expression and enhances tumor growth in a rat model of human neuroblastoma. Cancer Res. 2000;60:3013–3018. [PubMed] [Google Scholar]
- 105.Tarek N, et al. Unlicensed NK cells target neuroblastoma following anti-GD2 antibody treatment. The Journal of clinical investigation. 2012;122:3260–3270. doi: 10.1172/JCI62749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cheung NK, et al. Murine Anti-GD2 Monoclonal Antibody 3F8 Combined With Granulocyte-Macrophage Colony-Stimulating Factor and 13-Cis-Retinoic Acid in High-Risk Patients With Stage 4 Neuroblastoma in First Remission. J Clin Oncol. 2012;30:3264–3270. doi: 10.1200/JCO.2011.41.3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cheever MA, et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323–5337. doi: 10.1158/1078-0432.CCR-09-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bao L, Dunham K, Lucas K. MAGE-A1, MAGE-A3, and NY-ESO-1 can be upregulated on neuroblastoma cells to facilitate cytotoxic T lymphocyte-mediated tumor cell killing. Cancer Immunol Immunother. 2011;60:1299–1307. doi: 10.1007/s00262-011-1037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sarkar AK, Nuchtern JG. Lysis of MYCN-amplified neuroblastoma cells by MYCN peptide-specific cytotoxic T lymphocytes. Cancer Res. 2000;60:1908–1913. [PubMed] [Google Scholar]
- 111.Fest S, et al. Survivin minigene DNA vaccination is effective against neuroblastoma. Int J Cancer. 2009;125:104–114. doi: 10.1002/ijc.24291. [DOI] [PubMed] [Google Scholar]
- 112.Jing W, Yan X, Hallett WH, Gershan JA, Johnson BD. Depletion of CD25(+) T cells from hematopoietic stem cell grafts increases posttransplantation vaccine-induced immunity to neuroblastoma. Blood. 2011;117:6952–6962. doi: 10.1182/blood-2010-12-326108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Louis CU, Brenner MK. Cellular immunotherapy for neuroblastoma: a review of current vaccine and adoptive T cell therapeutics. Curr Pharm Des. 2009;15:424–429. doi: 10.2174/138161209787315765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Caruso DA, et al. Results of a Phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with Stage 4 neuroblastoma. Cancer. 2005;103:1280–1291. doi: 10.1002/cncr.20911. [DOI] [PubMed] [Google Scholar]
- 115.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kramer K, et al. Disialoganglioside G(D2) loss following monoclonal antibody therapy is rare in neuroblastoma. Clin Cancer Res. 1998;4:2135–2139. [PubMed] [Google Scholar]
- 117.Lammie G, Cheung N, Gerald W, Rosenblum M, Cordoncardo C. Ganglioside gd(2) expression in the human nervous-system and in neuroblastomas - an immunohistochemical study. International journal of oncology. 1993;3:909–915. doi: 10.3892/ijo.3.5.909. [DOI] [PubMed] [Google Scholar]
- 118.Yu AL, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheung IY, Hsu K, Cheung NK. Activation of Peripheral-Blood Granulocytes Is Strongly Correlated With Patient Outcome After Immunotherapy With Anti-GD2 Monoclonal Antibody and Granulocyte-Macrophage Colony-Stimulating Factor. J Clin Oncol. 2012;30:426–432. doi: 10.1200/JCO.2011.37.6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leung W. Use of NK cell activity in cure by transplant. Br J Haematol. 2011;155:14–29. doi: 10.1111/j.1365-2141.2011.08823.x. [DOI] [PubMed] [Google Scholar]
- 121.Venstrom JM, et al. KIR and HLA genotypes are associated with disease progression and survival following autologous hematopoietic stem cell transplantation for high-risk neuroblastoma. Clin Cancer Res. 2009;15:7330–7334. doi: 10.1158/1078-0432.CCR-09-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Delgado DC, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Metelitsa LS, et al. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood. 2002;99:4166–4173. doi: 10.1182/blood.v99.11.4166. [DOI] [PubMed] [Google Scholar]
- 124.Kushner BH, Cheung NK. Clinically effective monoclonal antibody 3F8 mediates nonoxidative lysis of human neuroectodermal tumor cells by polymorphonuclear leukocytes. Cancer Res. 1991;51:4865–4870. [PubMed] [Google Scholar]
- 125.Kushner BH, Cheung NK. GM-CSF enhances 3F8 monoclonal antibody-dependent cellular cytotoxicity against human melanoma and neuroblastoma. Blood. 1989;73:1936–1941. [PubMed] [Google Scholar]
- 126.Kushner BH, Cheung NK. Absolute requirement of CD11/CD18 adhesion molecules, FcRII and the phosphatidylinositol-linked FcRIII for monoclonal antibody-mediated neutrophil antihuman tumor cytotoxicity. Blood. 1992;79:1484–1490. [PubMed] [Google Scholar]
- 127.Cheung NK, et al. FCGR2A polymorphism is correlated with clinical outcome after immunotherapy of neuroblastoma with anti-GD2 antibody and granulocyte macrophage colony-stimulating factor. J Clin Oncol. 2006;24:2885–2890. doi: 10.1200/JCO.2005.04.6011. [DOI] [PubMed] [Google Scholar]
- 128.Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42:61–74. doi: 10.1016/s0162-3109(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 129.Diamond MS, Springer TA. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cheung NK, Modak S. Oral (1-->3),(1-->4)-beta-D-glucan synergizes with antiganglioside GD2 monoclonal antibody 3F8 in the therapy of neuroblastoma. Clin Cancer Res. 2002;8:1217–1223. [PubMed] [Google Scholar]
- 131.Asgharzadeh S, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. J Clin Oncol. 2012;30:3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ren Y, et al. Inhibition of tumor growth and metastasis in vitro and in vivo by targeting macrophage migration inhibitory factor in human neuroblastoma. Oncogene. 2006;25:3501–3508. doi: 10.1038/sj.onc.1209395. [DOI] [PubMed] [Google Scholar]
- 133.Ren Y, et al. Upregulation of macrophage migration inhibitory factor contributes to induced N-Myc expression by the activation of ERK signaling pathway and increased expression of interleukin-8 and VEGF in neuroblastoma. Oncogene. 2004;23:4146–4154. doi: 10.1038/sj.onc.1207490. [DOI] [PubMed] [Google Scholar]
- 134.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 135.Munn DH, Cheung NK. Antibody-dependent antitumor cytotoxicity by human monocytes cultured with recombinant macrophage colony-stimulating factor Induction of efficient antibody-mediated antitumor cytotoxicity not detected by isotope release assays. J Exp Med. 1989;170:511–526. doi: 10.1084/jem.170.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Petrella T, et al. Single-agent interleukin-2 in the treatment of metastatic melanoma: a systematic review. Cancer Treat Rev. 2007;33:484–496. doi: 10.1016/j.ctrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 137.Ladenstein R, et al. Dose finding study for the use of subcutaneous recombinant interleukin-2 to augment natural killer cell numbers in an outpatient setting for stage 4 neuroblastoma after megatherapy and autologous stem-cell reinfusion. J Clin Oncol. 2011;29:441–448. doi: 10.1200/JCO.2009.23.5465. [DOI] [PubMed] [Google Scholar]
- 138.Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci. 2012;33:35–41. doi: 10.1016/j.tips.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berger C, et al. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114:2417–2426. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12:239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Metelitsa LS, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–1221. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Neal ZC, et al. Enhanced activity of hu14.18-IL2 immunocytokine against murine NXS2 neuroblastoma when combined with interleukin 2 therapy. Clin Cancer Res. 2004;10:4839–4847. doi: 10.1158/1078-0432.CCR-03-0799. [DOI] [PubMed] [Google Scholar]
- 143.Shusterman S, et al. Antitumor activity of hu14.18-IL2 in patients with relapsed/refractory neuroblastoma: a Children’s Oncology Group (COG) phase II study. J Clin Oncol. 2010;28:4969–4975. doi: 10.1200/JCO.2009.27.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yang RK, et al. Intratumoral hu14.18-IL-2 (IC) Induces Local and Systemic Antitumor Effects That Involve Both Activated T and NK Cells As Well As Enhanced IC Retention. J Immunol. 2012;189:2656–2664. doi: 10.4049/jimmunol.1200934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Becker JC, Varki N, Gillies SD, Furukawa K, Reisfeld RA. Long-lived and transferable tumor immunity in mice after targeted interleukin-2 therapy. J Clin Invest. 1996;98:2801–2804. doi: 10.1172/JCI119107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Albertini MR, et al. Phase II trial of hu14.18-IL2 for patients with metastatic melanoma. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cheung NK, Guo HF, Heller G, Cheung IY. Induction of Ab3 and Ab3’ antibody was associated with long-term survival after anti-G(D2) antibody therapy of stage 4 neuroblastoma. Clin Cancer Res. 2000;6:2653–2660. [PubMed] [Google Scholar]
- 148.Foon KA, et al. Clinical and immune responses in advanced melanoma patients immunized with an anti-idiotype antibody mimicking disialoganglioside GD2. J Clin Oncol. 2000;18:376–384. doi: 10.1200/JCO.2000.18.2.376. [DOI] [PubMed] [Google Scholar]
- 149.Cheung NK, Canete A, Cheung IY, Ye JN, Liu C. Disialoganglioside GD2 anti-idiotypic monoclonal antibodies. Int J Cancer. 1993;54:499–505. doi: 10.1002/ijc.2910540324. [DOI] [PubMed] [Google Scholar]
- 150.Bleeke M, et al. Systematic amino acid substitutions improved efficiency of GD2-peptide mimotope vaccination against neuroblastoma. Eur J Cancer. 2009;45:2915–2921. doi: 10.1016/j.ejca.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 151.Bolesta E, et al. DNA vaccine expressing the mimotope of GD2 ganglioside induces protective GD2 cross-reactive antibody responses. Cancer Res. 2005;65:3410–3418. doi: 10.1158/0008-5472.CAN-04-2164. [DOI] [PubMed] [Google Scholar]
- 152.Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Curr Cancer Drug Targets. 10:200–209. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kushner BH, Kramer K, Modak S, Cheung NK. Successful multifold dose escalation of anti-GD2 monoclonal antibody 3F8 in patients with neuroblastoma: a phase I study. J Clin Oncol. 2011;29:1168–1174. doi: 10.1200/JCO.2010.28.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cheung NK, Guo H, Hu J, Tassev DV, Cheung IY. Humanizing murine IgG3 anti-GD2 antibody m3F8 substantially improves antibody-dependent cell-mediated cytotoxicity while retaining targeting in vivo. OncoImmunology. 2012;1:477–486. doi: 10.4161/onci.19864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carpenter EL, et al. Antibody targeting of anaplastic lymphoma kinase induces cytotoxicity of human neuroblastoma. Oncogene. 2012 doi: 10.1038/onc.2011.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Novak-Hofer I. The L1 cell adhesion molecule as a target for radioimmunotherapy. Cancer Biother Radiopharm. 2007;22:175–184. doi: 10.1089/cbr.2007.342. [DOI] [PubMed] [Google Scholar]
- 157.Modak S, Kramer K, Gultekin SH, Guo HF, Cheung NK. Monoclonal antibody 8H9 targets a novel cell surface antigen expressed by a wide spectrum of human solid tumors. Cancer Res. 2001;61:4048–4054. [PubMed] [Google Scholar]
- 158.Kramer K, et al. Compartmental intrathecal radioimmunotherapy: results for treatment for metastatic CNS neuroblastoma. J Neurooncol. 2010;97:409–418. doi: 10.1007/s11060-009-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Park JR, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther. 2007;15:825–833. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 160.Pule MA, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–1270. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Craddock JA, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33:780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sun J, et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol Ther. 2010;18:2006–2017. doi: 10.1038/mt.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cho D, et al. Cytotoxicity of activated natural killer cells against pediatric solid tumors. Clin Cancer Res. 2010;16:3901–3909. doi: 10.1158/1078-0432.CCR-10-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Denman CJ, et al. Membrane-bound IL-21 promotes sustained ex vivo proliferation of human natural killer cells. PLoS One. 2012;7:e30264. doi: 10.1371/journal.pone.0030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yankelevich M, et al. Anti-CD3 x anti-GD2 bispecific antibody redirects T-cell cytolytic activity to neuroblastoma targets. Pediatr Blood Cancer. 2012 doi: 10.1002/pbc.24237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heller G, Cheung NK. Dose-intensity analysis and randomized trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1991;9:1715–1716. doi: 10.1200/JCO.1991.9.9.1715. [DOI] [PubMed] [Google Scholar]
- 167.Pearson AD, et al. High-dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncol. 2008;9:247–256. doi: 10.1016/S1470-2045(08)70069-X. [DOI] [PubMed] [Google Scholar]
- 168.Whiteford CC, et al. Credentialing preclinical pediatric xenograft models using gene expression and tissue microarray analysis. Cancer research. 2007;67:32–40. doi: 10.1158/0008-5472.CAN-06-0610. [DOI] [PubMed] [Google Scholar]
- 169.Bergman I, Basse PH, Barmada MA, Griffin JA, Cheung NK. Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol Immunother. 2000;49:259–266. doi: 10.1007/s002620000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 171.Pek EA, Chan T, Reid S, Ashkar AA. Characterization and IL-15 dependence of NK cells in humanized mice. Immunobiology. 2011;216:218–224. doi: 10.1016/j.imbio.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 172.Chesler L, Weiss WA. Genetically engineered murine models--contribution to our understanding of the genetics, molecular pathology and therapeutic targeting of neuroblastoma. Semin Cancer Biol. 2011;21:245–255. doi: 10.1016/j.semcancer.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Quigley D, Balmain A. Systems genetics analysis of cancer susceptibility: from mouse models to humans Nature reviews. Genetics. 2009;10:651–657. doi: 10.1038/nrg2617. [DOI] [PubMed] [Google Scholar]
- 174.Zhang J, et al. A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature. 2012;481:329–334. doi: 10.1038/nature10733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Atkinson JM, et al. An integrated in vitro and in vivo high-throughput screen identifies treatment leads for ependymoma. Cancer Cell. 2011;20:384–399. doi: 10.1016/j.ccr.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Brodeur GM, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:1466–1477. doi: 10.1200/JCO.1993.11.8.1466. [DOI] [PubMed] [Google Scholar]
- 177.Conte M, et al. Neuroblastoma in adolescents: the Italian experience. Cancer. 2006;106:1409–1417. doi: 10.1002/cncr.21751. [DOI] [PubMed] [Google Scholar]
- 178.Brignole C, et al. Effect of bortezomib on human neuroblastoma cell growth, apoptosis, and angiogenesis. J Natl Cancer Inst. 2006;98:1142–1157. doi: 10.1093/jnci/djj309. [DOI] [PubMed] [Google Scholar]
- 179.Fulda S. The PI3K/Akt/mTOR pathway as therapeutic target in neuroblastoma. Curr Cancer Drug Targets. 2009;9:729–737. doi: 10.2174/156800909789271521. [DOI] [PubMed] [Google Scholar]
- 180.Carol H, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68:1291–1304. doi: 10.1007/s00280-011-1618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Molenaar JJ, et al. Cyclin D1 and CDK4 activity contribute to the undifferentiated phenotype in neuroblastoma. Cancer research. 2008;68:2599–2609. doi: 10.1158/0008-5472.CAN-07-5032. [DOI] [PubMed] [Google Scholar]
- 182.Yee D, et al. Insulin-like growth factor I expression by tumors of neuroectodermal origin with the t(11;22) chromosomal translocation A potential autocrine growth factor. The Journal of clinical investigation. 1990;86:1806–1814. doi: 10.1172/JCI114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Tanno B, et al. Down-regulation of insulin-like growth factor I receptor activity by NVP-AEW541 has an antitumor effect on neuroblastoma cells in vitro and in vivo. Clin Cancer Res. 2006;12:6772–6780. doi: 10.1158/1078-0432.CCR-06-1479. [DOI] [PubMed] [Google Scholar]
- 184.Brodeur GM, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15:3244–3250. doi: 10.1158/1078-0432.CCR-08-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Goldsmith KC, et al. Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma. Cancer research. 2012;72:2565–2577. doi: 10.1158/0008-5472.CAN-11-3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Petroni M, Veschi V, Gulino A, Giannini G. Molecular mechanisms of MYCN-dependent apoptosis and the MDM2-p53 pathway: an Achille’s heel to be exploited for the therapy of MYCN-amplified neuroblastoma. Front Oncol. 2012;2:141. doi: 10.3389/fonc.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Coffey DC, et al. The histone deacetylase inhibitor, CBHA, inhibits growth of human neuroblastoma xenografts in vivo, alone and synergistically with all-trans retinoic acid. Cancer research. 2001;61:3591–3594. [PubMed] [Google Scholar]
- 188.Pietras A, Johnsson AS, Pahlman S. The HIF-2alpha-driven pseudo-hypoxic phenotype in tumor aggressiveness, differentiation, and vascularization. Curr Top Microbiol Immunol. 2010;345:1–20. doi: 10.1007/82_2010_72. [DOI] [PubMed] [Google Scholar]
- 189.Crosswell HE, et al. PHA665752, a small-molecule inhibitor of c-Met, inhibits hepatocyte growth factor-stimulated migration and proliferation of c-Met-positive neuroblastoma cells. BMC Cancer. 2009;9:411. doi: 10.1186/1471-2407-9-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sugiura Y, Shimada H, Seeger RC, Laug WE, DeClerck YA. Matrix metalloproteinases-2 and −9 are expressed in human neuroblastoma: contribution of stromal cells to their production and correlation with metastasis. Cancer research. 1998;58:2209–2216. [PubMed] [Google Scholar]
- 191.Asgharzadeh S, et al. Clinical significance of tumor-associated inflammatory cells in metastatic neuroblastoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:3525–3532. doi: 10.1200/JCO.2011.40.9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Xu H, Cheung IY, Guo HF, Cheung NK. MicroRNA miR-29 modulates expression of immunoinhibitory molecule B7-H3: potential implications for immune based therapy of human solid tumors. Cancer Res. 2009;69:6275–6281. doi: 10.1158/0008-5472.CAN-08-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ge X, et al. rILYd4, a human CD59 inhibitor, enhances complement-dependent cytotoxicity of ofatumumab against rituximab-resistant B-cell lymphoma cells and chronic lymphocytic leukemia. Clin Cancer Res. 2011;17:6702–6711. doi: 10.1158/1078-0432.CCR-11-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reid GS, et al. Interferon-gamma-dependent infiltration of human T cells into neuroblastoma tumors in vivo. Clin Cancer Res. 2009;15:6602–6608. doi: 10.1158/1078-0432.CCR-09-0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Levy AG, et al. The combination of the novel glycolysis inhibitor 3-BrOP and rapamycin is effective against neuroblastoma. Invest New Drugs. 2012;30:191–199. doi: 10.1007/s10637-010-9551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Chanthery YH, et al. Paracrine signaling through MYCN enhances tumor-vascular interactions in neuroblastoma. Science translational medicine. 2012;4 doi: 10.1126/scitranslmed.3002977. 115ra3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Teitz T, et al. Preclinical models for neuroblastoma: establishing a baseline for treatment. PLoS One. 2011;6:e19133. doi: 10.1371/journal.pone.0019133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Huebener N, et al. Xenogeneic immunization with human tyrosine hydroxylase DNA vaccines suppresses growth of established neuroblastoma. Mol Cancer Ther. 2009;8:2392–2401. doi: 10.1158/1535-7163.MCT-09-0107. [DOI] [PubMed] [Google Scholar]
- 199.Huber K, et al. Persistent expression of BMP-4 in embryonic chick adrenal cortical cells and its role in chromaffin cell development. Neural development. 2008;3:28. doi: 10.1186/1749-8104-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Betters E, Liu Y, Kjaeldgaard A, Sundstrom E, Garcia-Castro MI. Analysis of early human neural crest development. Developmental biology. 2010;344:578–592. doi: 10.1016/j.ydbio.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lau L, Dagg R, Haber M, Murray J, Reddel RR. Alternative Lengthening of Telomers (ALT) in neuroblastoma tumors. Advances in Neuroblastoma Research. 2012;97:OR36. [Google Scholar]
- 202.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]