Abstract
Background
Gut damage resulting in microbial translocation (MT) is considered a major cause of immune activation (IA) in HIV infection, but data in children are limited, particularly in the absence of antiretroviral therapy.
Methods
Sixty perinatally HIV-infected, antiretroviral therapy–naive children, aged 2–12 years, were evaluated for plasma levels of lipopolysaccharide, DNA sequences encoding bacterial ribosomal 16 second (16S) RNA (16S rDNA) and soluble CD14 concurrently with markers of CD4 and CD8 T-cell IA and immune exhaustion (IE), CD4 counts, and plasma viral load. At study entry, participants were classified into immune categories (ICs): IC1 (CD4% > 25), IC2 (CD4% 15–25), and IC3 (CD4% < 15). Age-matched HIV-uninfected children served as controls. Data were evaluated at study entry and at 12 months.
Results
Levels of MT, IA, and IE were increased in patients as compared with controls, were highest in patients in IC3 group, and did not change over 12 months. MT products lipopolysaccharide and 16S rDNA correlated with each other and each correlated with plasma viral load, soluble CD14, and T-cell IA and IE. There was a correlation of IA with IE. CD4 counts and percentage were inversely correlated with MT products and underlying CD4 activation.
Conclusions
In a natural history cohort of HIV-infected children not on therapy, MT was more pronounced in the most severely immunocompromised patients and was associated with IA. Strategies to reduce MT may help to reduce IA and prevent CD4 depletion.
Keywords: microbial translocation, HIV-infected children, LPS, 16S rDNA
INTRODUCTION
Microbial translocation (MT) refers to leakage of gut-derived microbes and microbial products into the systemic circulation in the absence of bacteremia and is a characteristic feature of chronic HIV infection. The underlying mechanism of increased permeability of the mucosal barrier is attributed to initial massive viral replication and rapid depletion of gut CD4+ T cells, including Th17 cells, enterocyte damage, and breakdown of the follicle-associated epithelium tight junctions in the gut epithelial cells, which collectively may facilitate the passage of microbes or microbial products into the circulation.1,2 Persistent MT is well documented in adults with chronic HIV infection3–6 and has been proposed to be a major cause of immune activation (IA) and disease progression,7,8 including poor CD4 T-cell reconstitution.3,5,9–11 However, the relationship of MT in IA is controversial,12,13 and there are gaps in knowledge of extent of gut damage and MT in antiretroviral therapy (ART)–naive, HIV-infected children. In particular, 16 second (16S) rDNA as a measure of MT has not been evaluated in such children.
To quantify MT, the most frequently used analyte in plasma is lipopolysaccharide (LPS), a component of gram-negative bacterial cell wall. 16S rDNA, the conserved sequence of bacterial DNA fragment common to most bacteria and LPS core antigen, is also a quantitative indicator of MT.14 Markers of IA include among others increased cell surface co-expression of molecules HLA-DR and CD38 on T cells15,16 and elevated levels of plasma soluble CD14 (sCD14).5 The expression of programmed death receptor 1 (PD-1) is a marker of immune exhaustion (IE)17 and is often associated with T-cell activation.18 It has been suggested that microbial products, which leak into the circulation, can drive IA by stimulating immune cells directly via pattern recognition receptors, such as toll-like receptor 4, with shedding of CD14 from the surface of activated macrophages. Plasma levels of sCD14 are commonly used as indirect measures of MT.11,19,20 In pediatric populations, other factors, such as developmental immaturity of the gut21 and nature of the gut microbiome,22 may also influence gut permeability and contribute to MT.
In the absence of ART, about 20% of perinatally HIV-infected children develop a rapidly progressive disease with high mortality, and children who survive beyond 2 years of age usually have a slower disease progression; information about MT in such children is limited.23–25 Some studies indicate that even after potent ART and suppression of viral replication to undetectable levels, plasma LPS levels remain elevated in HIV-infected children24 and adults26 as compared with uninfected persons. In HIV-infected children, however, levels of plasma LPS did not correlate with T-cell IA.12,25 To better understand the relationship of viral replication with MT and IA in the absence of ART, we analyzed MT, viral load (VL), and markers of IA in a cohort of 60 treatment-naive, HIV-infected children and studied their relationship with immunologic and clinical disease status. IE marker PD-1 was examined in a subset of these patients.
MATERIALS AND METHODS
Characteristics of the Patient Population
The study population of perinatally HIV-infected children were enrolled in the study during 2006–2007 and followed for 48 weeks in the immunology clinic at Tuberculosis Research Center in Chennai, India. The study enrolled 60 children of whom 47 completed the study. Characteristics of the study population are summarized in Table S1 (see Supplemental Digital Content, http://links.lww.com/QAI/A500). None of the patients were on ART for the duration of the present study. The participants (28 men, 32 women) ranged in age from 9 months to 13 years (median 7 years) and had a body mass index of 6.4–24.1 (median 14.4). At entry, CD4 counts ranged between 5% and 44% (median 23%) and plasma HIV-RNA between 3.65 and 5.87 log10 copies per milliliter (median 4.96). Patients were classified into immune categories (ICs) 1, IC2, and IC3 based on the Centers for Disease Control and Prevention classification27: IC1 (CD4% > 25), IC2 (CD4% 15–25), and IC3 (CD4% < 15). Eight age-matched uninfected children were recruited from the same geographical area and served as controls. Cellular assays for immunologic analyses, including flow cytometry assays for T-cell IA and IE, were performed on site.28 Plasma samples were collected during study visits, stored at −80°C, and shipped to the University of Miami in dry ice after approval from the Government of India's Health Ministry Screening Committee for MT studies.
Plasma LPS Assay
Ten microliters of plasma was diluted 1:10 in endotoxin-free water and heat inactivated at 85°C for 15 minutes to inactivate inhibitory plasma proteins. LPS levels were measured in plasma by the use of the limulus amebocyte lysate chromogenic endpoint assay (Lonza Group Ltd, Allendale, NJ) according to the manufacturer's recommendations and calculated in relation to an Escherichia coli endotoxin standard provided with the assay, after background subtraction.5 Results of LPS were recorded as picograms per milliliter.
16S rDNA Quantitation in Plasma
DNA was extracted from 200 μL of plasma by use of the DNeasy Blood and Tissue Kit (Qiagen Inc, CA). DNA purity and concentration were determined by nanodrop spectrophotometer (Thermo Scientific, DE). DNA was amplified in a reaction mixture consisting of 2 μL of 10× polymerase chain reaction (PCR) buffer, 3.5 mmol/L MgCl2, 0.2 mmol/L dNTPs, 0.5 μmol/L forward (8F: 5’-AGT TTG ATC CTG GCT CAG-3’) and reverse (515R: 5’-GWA TTA CCG CGG CKG CTG-3’) primers, 0.32 μmol/L probe (338P: 5’-FAM-GCT GCC TCC CGT AGG AGT-BHQ1–3’), 0.75 U of Taq polymerase, and equal amount of DNA. A negative control (not template control) was used each time to ensure there were no false-positive reactions. The reaction conditions for amplification of DNA were 95°C for 5 minutes, followed by 45 cycles at 95°C for 15 seconds and at 60°C for 1 minute. Real-time fluorescence detection was used with the ABI PRISM 7700 sequence detector (Perkin Elmer Applied Bio-systems, CA) to quantify the bacterial 16S rDNA level in plasma. Real-time PCR was performed in duplicates for each standard dilution and sample, and mean CT value of the duplicate PCRs was determined and used for the calculations. A standard curve was created from serial dilutions of plasmid DNA containing known copy numbers of the template. Copy numbers of the samples were calculated from the standard curve by interpolation.3 Results were expressed as 16S rDNA copy number per microliter plasma.
Plasma sCD14 Analysis
Monocytes and macrophages express membrane CD14 and secrete sCD14 upon activation. Measurement of plasma sCD14 provides evidence for direct chronic LPS stimulation of monocytes and macrophages in vivo. Plasma levels of sCD14 were quantified by Human sCD14 Immunoassay (R&D Systems, Minneapolis, MN). Ten microliters of plasma was diluted 200-fold by the addition of 1990 μL calibrator diluent and assayed in duplicate. Results of sCD14 were expressed in nanograms per milliliter.
Analysis of T-cell Activation and IE
Expression of CD38 and HLA-DR was used as a marker for IA and expression of PDI-1 for IE. One hundred micro-liters of fresh whole blood per tube was incubated for 30 minutes with antibodies to different cell surface markers (CD3, CD8, CD38, HLA-DR, and PD-1) in dark at room temperature. After incubation, red blood cells were lysed with FACS lysing solution (BD Biosciences, San Jose, CA) for 10 minutes. Cells were then washed with wash buffer (2% fetal bovine serum and 0.02% sodium azide in phosphate buffer saline). The stained cells were suspended in equal volumes of wash buffer and 1% paraformaldehyde solution. After staining, the cells were acquired on a BD FACSCalibur (BD Biosciences). All data were analyzed using FlowJo software (version 4.6.2, Tree Star Inc, Ashland, OR). The gating for all other markers was based on isotype controls. In this cohort, testing for PD-1 was introduced later and was examined in a subset of 18 patients.
Statistical Analyses
Differences in the variables between baseline and 12 months were analyzed using the Spearman signed rank test. Planned comparisons between healthy controls and each HIV-infected group were performed by Mann–Whitney U test. Relationship between 2 variables was done using the Spearman correlation and linear regression. Data for LPS and 16S rDNA were analyzed in relation to CD4 counts, VL, T-cell activation markers, and sCD14. GraphPad prism (version 5.0) was used to plot graphs.
RESULTS
HIV-Infected Children Have Increased Gut MT and Generalized IA
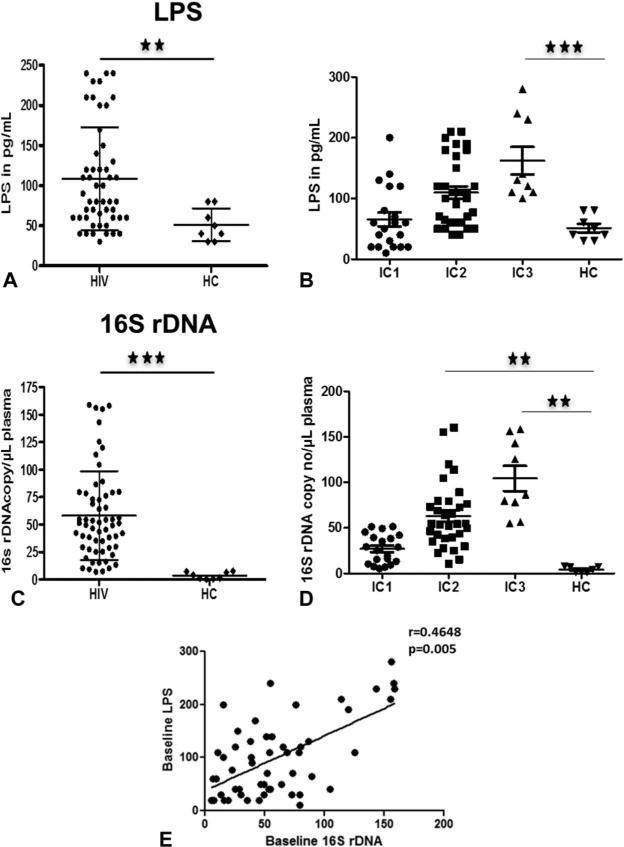
Plasma MT, measured by plasma LPS and 16S rDNA, was significantly higher in patients compared with healthy children (Figs. 1A, C). Analysis was also performed based on the CD4%-based ICs. Plasma MT was highest in IC3 group (Figs. 1B, D) compared with the other 2 groups and with HC. Interestingly, LPS and 16S rDNA of children in IC1 group did not differ from HC. We also noted that both these markers correlated with each other at entry (Fig. 1E).
FIGURE 1.
Plasma levels of microbial products are elevated in HIV-infected children. Baseline plasma LPS (A and B) and 16S rDNA (C and D) levels in total and IC groups. Correlation between LPS and 16S rDNA in total patients (E). Comparisons of median clinical values between different groups of subjects were performed by Mann–Whitney U test. Correlations between 2 variables were done using the Spearman correlation and linear regression. Asterisks indicate the levels of significance. *P < 0.05, **P < 0.01, ***P < 0.001.
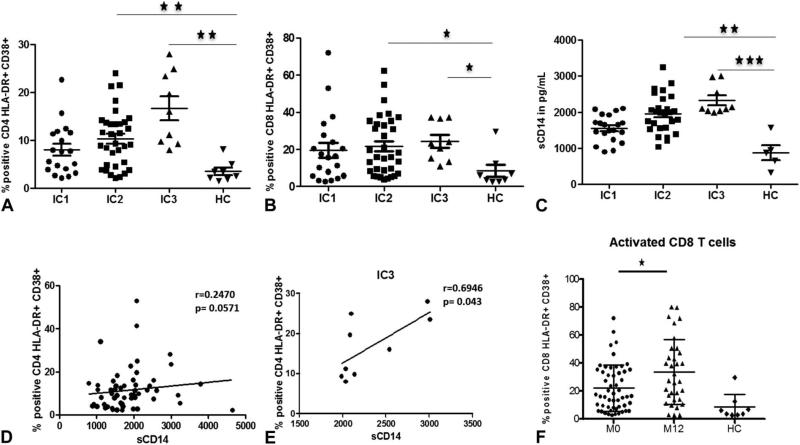
IA markers HLA-DR and CD38 were higher in patients, with a mean frequency of 12% in CD4 and 23% in CD8 T cells as compared with 4% and 6%, respectively, in controls (data not shown), and IA markers in T cells of patients in IC3 and IC2 groups were independently higher than HC (Figs. 2A, B). Monocyte activation, measured by sCD14, was also higher in the full cohort (data not shown). Similar to T-cell activation, monocyte activation was also higher in patients in IC3 and IC2 groups compared with HC (Fig. 2C). T-cell activation correlated with monocyte activation in patients at baseline (Fig. 2D) and in IC3 group (Fig. 2E), indicating that generalized IA is maximal in the most immunocompromised patients. CD8 T-cell activation was higher at the 12-month follow-up visit as compared with entry values (Fig. 2F), but there was no increase in CD4 T-cell activation.
FIGURE 2.
HIV-infected children show generalized IA. CD4 T-cell (A), CD8 T-cell (B), and monocyte activation (C) in IC groups compared with healthy controls. Correlation of CD4 T-cell activation (D) and CD8 T-cell activation (E) with monocyte activation in total patients. CD8 T-cell activation at baseline (M0) and at 12 months follow-up (M12), compared with HC (F). Comparisons of median clinical values between different groups of subjects were performed by Mann–Whitney U test. Correlations between 2 variables were done using the Spearman correlation and linear regression. Asterisks indicate the levels of significance: *P < 0.05, **P < 0.01, ***P < 0.001.
Correlation of MT With VL, IA, and CD4%
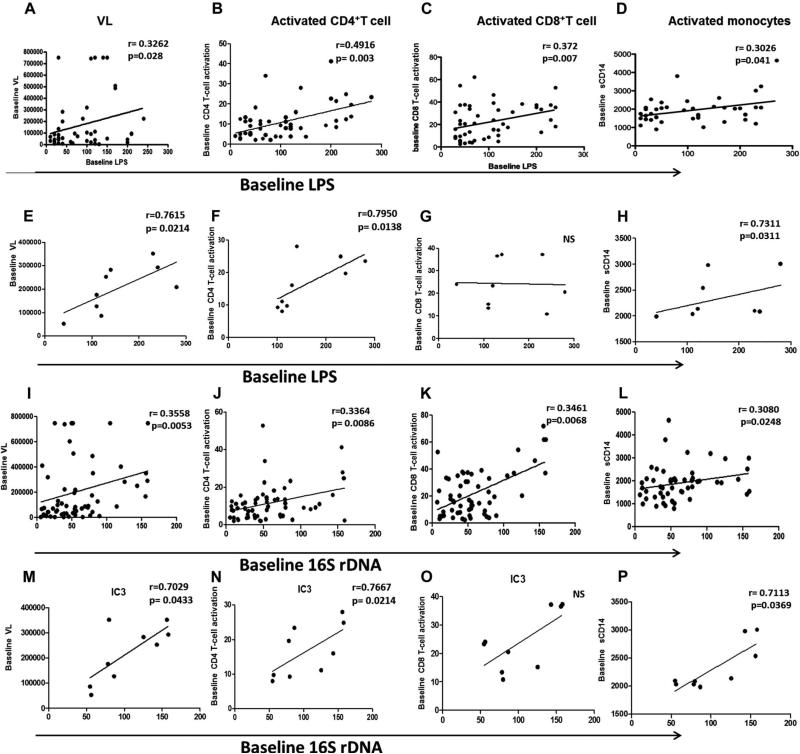
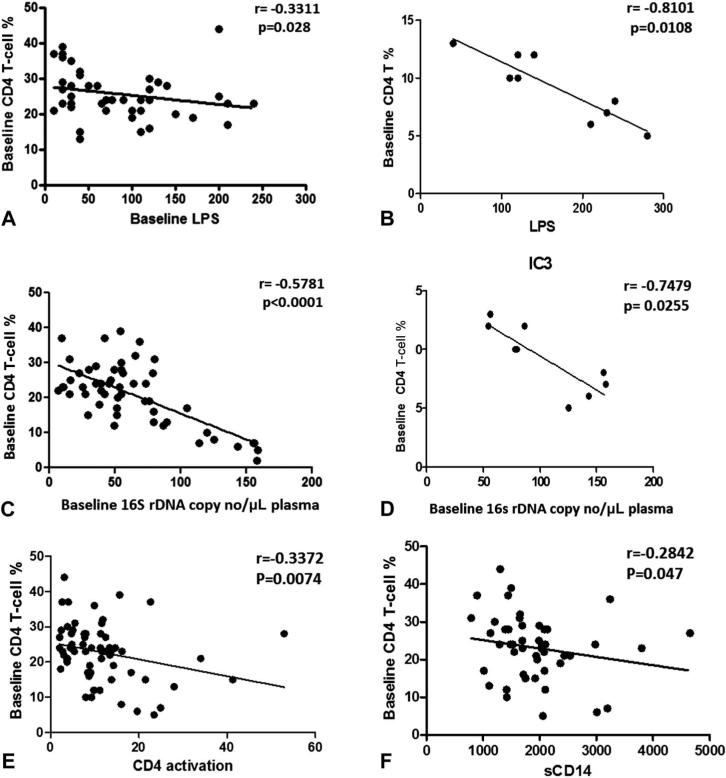
To better understand the clinical importance of LPS and 16S rDNA, we investigated their relationship with viroimmunologic markers of HIV disease, including CD4 T-cell count, plasma VL, and IA. As shown in Figure 3, LPS correlated positively with VL, T-cell (CD4 and CD8) activation (Figs. 3A–C), and monocyte activation (Fig. 3D). Similar correlations were observed with 16S rDNA (Figs. 3I–L). These correlative patterns were also observed in patients in IC3 group for LPS (Figs. 3E–H) and 16S rDNA (Figs. 3M–P). VL did not differ among the IC groups at baseline (data not shown) and was correlated with MT (Figs. 3A, E, I, M), CD8 T-cell activation, and sCD14 in the patient cohort (data not shown). For patients in the IC3 group, in addition to these correlations, VL was also correlated with CD4 T-cell activation. MT parameters showed a negative correlation with CD4% both in total cohort and in IC3 group (Figs. 4A–D). Besides showing a negative correlation with MT products, CD4% also showed a negative correlation with CD4 IA and sCD14 (Figs. 4E, F), implying the association of immune deficiency with MT and IA.
FIGURE 3.
Correlation of MT with IA. Correlation of baseline plasma LPS with VL (A), CD4 T-cell activation (B), CD8 T-cell activation (C), and monocyte activation (D) in total patients and IC groups (E–H). Correlation of baseline plasma 16S rDNA with VL (I), CD4 T-cell activation (J), CD8 T-cell activation (K), and monocyte activation (L) in total patients and IC groups (M–P). Correlations between 2 variables were done using the Spearman correlation and linear regression.
FIGURE 4.
Relationship of CD4% with MT and IA. Correlation of CD4 T-cell percent with LPS (A) and 16S rDNA (C) in total patients and IC3 group (B and D). Correlation of CD4 T-cell percent with CD8 T-cell activation (E) and monocyte activation (F) in total patients. Correlations between 2 variables were done using the Spear-man correlation and linear regression.
T-Cell Exhaustion Correlates With T-Cell IA and MT
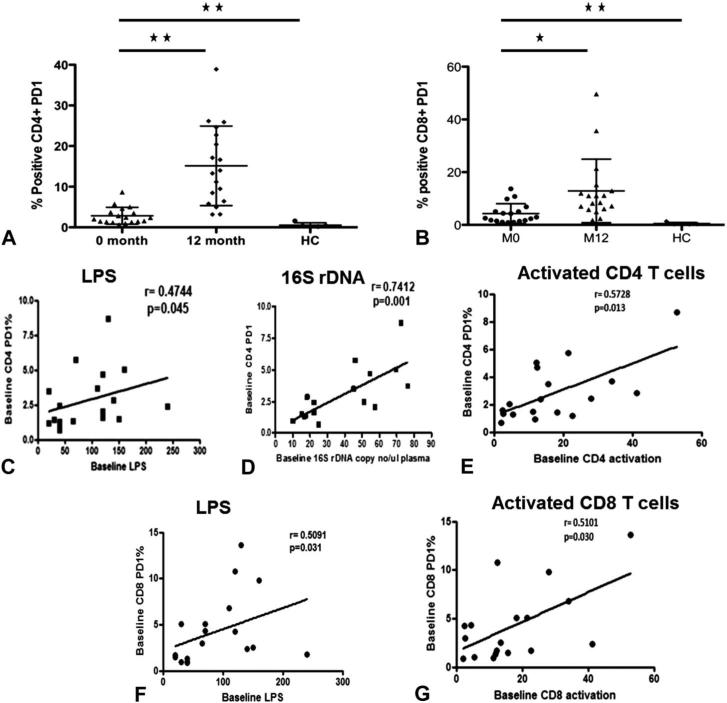
PD-1 is a marker that has been shown to be associated with both IA and IE. We investigated PD1 expression only in a subset of 18 patients. PD1 expression levels on CD4 and CD8 T cells were higher in patients at baseline compared with controls and increased further at the 12-month follow-up visit (Figs. 5A, B). At study entry, frequency of PD-1+ CD4 T cells correlated with LPS levels (Fig. 5C), 16S rDNA (Fig. 5D), and activated CD4 T cells (Fig. 5E). Frequency of PD1+ CD8 T cells correlated with LPS (Fig. 5F) and frequency of activated CD8 T cells (Fig. 5G).
FIGURE 5.
Relationship of IE and IA and MT. Expression of PD-1 on CD4 (A) and CD8 T (B) cells at baseline compared with follow-up and healthy controls. Correlation between frequency of PD1-expressing CD4 T cells with LPS (C), 16S rDNA (D), and activated CD4 T cells (E). Correlation between frequency of PD1-expressing CD8 T cells and LPS (F) and activated CD8 T cells (G). Asterisks indicate the levels of significance: *P < 0.05, **P < 0.01, ***P < 0.001. Correlations between 2 variables were performed using the Spearman correlation and linear regression.
MT and IA in Relation to Disease Progression During 12 months of Follow-Up
To gain insight into disease progression in the absence of ART, we determined the MT and immunologic markers in the 47 HIV+ children who completed 12 months of follow-up. CD4% and absolute counts remained unchanged as did plasma levels of LPS, bacterial DNA, and sCD14 (see Figure S1 A-D, Supplemental Digital Content, http://links.lww.com/QAI/A500). CD4 T-cell and monocyte IA also remained unchanged at 12 months after entry (see Figure S1 E-F, Supplemental Digital Content, http://links.lww.com/QAI/A500). There was a small but significant increase in CD8 T-cell activation (Fig. 2F) and IE (Fig. 5B) as described above, indicating that the immunologic profile was not completely stable. Clinically, this subgroup of children did not experience serious infections in this time span.
DISCUSSION
This study examined MT in a group of perinatally HIV-infected children from the Indian subcontinent who were not on ART. Although perinatal HIV infection is associated with a more rapid disease progression than in adults,29,30 most children in our cohort were in a state of chronic infection, having survived the initial critical period associated with rapid disease progression.31,32 MT is a characteristic feature of chronic HIV infection in adults that can persist with variable severity despite ART.3–5,9,12 In contrast, relatively less is known about MT in children,23–25 especially in the absence of ART, and there are even less data from resource-limited countries where there may be additional nutritional and infectious challenges. In the study population investigated, there was clear evidence of ongoing gut MT that was greater than in uninfected healthy controls as evidenced by increased levels of LPS and the universally conserved microbial 16S ribosomal RNA gene sequences in plasma. Increased plasma LPS levels have been demonstrated within 3–4 months after simian immunodeficiency virus (SIV) inoculation in rhesus macaques.33 This is the first study to provide evidence of MT in treatment-naive, HIV-infected children in a resource-limited setting and to demonstrate the association of MT with IA and disease progression in this background.
There is evidence in adults that failure of immune reconstitution after ART can be associated with persistent MT and ongoing IA.9 Our study in children shows that in the absence of ART, the more severely immunocompromised children in the IC3 group had the highest MT, with elevated levels of both plasma LPS and 16S rDNA, whereas in patients in IC1 group, measures of MT were comparable with healthy controls. This is the first report of 16S rDNA in HIV-infected, treatment-naive pediatric population in a resource-limited setting. Earlier, we also reported that plasma 16S rDNA was elevated compared with healthy volunteers in a treatment-experienced HIV+ pediatric US cohort, but the values were far lower than those observed here.24 A recent study in ART-treated, HIV-infected children in the United Kingdom34 also found low levels of 16S rDNA in low frequency, and gut-associated bacterial species were identified by sequencing the bacterial DNA. In broad range quantitative 16S rDNA PCR assays, there is a possibility of DNA contamination, exogenous or endogenous, for which only cloning or sequencing can confirm gut bacteria. In the present study, the correlation of the 16S rDNA with LPS and its absence in healthy children and in experimental negative controls all point to the observed 16S rDNA as being a true indicator of MT. Future studies that determine the bacterial species in the 16S rDNA in different regional settings are warranted.
In agreement with previous reports in adults,3,9 plasma LPS and 16S rDNA levels in our cohort were correlated with each other and also with plasma VL. These associations seen in the population as a whole were also evident in patients in IC3 group. During the initial stages of chronic HIV infection, LPS levels have been reported to be predictive of disease progression, independently of immunovirological markers, that is, absolute CD4 cell count and VL.35 LPS values in this cohort of HIV-infected children were similar to a US cohort of a similar age range.12 The direct correlation of both LPS and 16S rDNA with VL in our study is not unexpected as the children were not on ART. In patients on ART, however, the IA that persists despite apparent virological control has been attributed to ongoing MT because of delayed gut healing, rather than viral replication.36,37 However, other studies have shown that viral replication is evident in the gut even in the absence of detectable plasma viremia in patients on potent ART.38–40 Thus, it is possible that viral replication is contributing to MT to some degree, even in situations when plasma VL is undetectable by conventional laboratory assays that would not detect low-level viral replication in tissue sites.
Although many factors have been suggested to contribute to IA during chronic HIV/SIV infection, MT is considered to be a major cause for it.6,41–43 The microbial products can stimulate immune cells directly via pattern recognition receptors, such as toll-like receptors. As activated T cells have a relatively short half-life, the persistent IA in aviremic long-term ART-treated patients has been attributed to ongoing antigenic stimulation from MT.6 MT is also associated with IA during the chronic phase of SIV infection in rhesus macaques,44 whereas chronic SIV infection of sooty mangabeys does not cause damage to the intestinal barrier or result in MT and does not cause IA. The relationship of gut MT with IA and disease progression, however, remains controversial. In our study, MT was correlated with generalized IA as evidenced by HLA-DR+ CD38+ expressing T cells, circulating levels of sCD14 (a marker of monocyte activation), and increased IE (PD-1). The correlation between MT and activated T-cell phenotype points toward polyclonal T-cell activation, contributed by MT either directly or indirectly via cytokines and chemokines. The levels of neither of the MT products (LPS and 16S rDNA) showed a change during the 12-month follow-up in the absence of ART. CD4 IA also remained unchanged, but CD8 IA increased along with the frequency of PD-1+ T cells. These data affirm that MT is evident even in immunologically stable children although it is worse in association with CD4 immune deficiency, suggesting a potentially deleterious effect of ongoing MT on disease progression. MT and IA have been associated with disease progression even in long-term nonprogresssor adults.45
Not only LPS levels but sCD14 levels were also increased in the children at baseline, indicating monocyte activation. Increased sCD14 has been reported in chronically infected individuals4; this observation is not unexpected as LPS is known to be a direct stimulant of the immune system in vivo. In agreement with this reasoning, LPS and 16S rDNA correlated with both T-cell and monocyte activation but only in association with severe immunosuppression of IC3. In contrast to reported absence of association between the level of sCD14 and CD8+ T-cell activation in adults, we found a correlation of sCD14 with both CD4 and CD8 activation in our pediatric population. A strong inverse correlation of sCD14 with CD4+ T cells and a direct correlation of sCD14 with VL have been previously reported.5,46 We found an inverse correlation of sCD14 with CD4% but did not find any correlation with VL (data not shown). T-cell IA was correlated with monocyte activation at baseline, suggesting that there is generalized IA. In contrast to our results and of others from the United States, sCD14 levels were not altered in an Ugandan cohort among groups with different disease courses in a longitudinal study,47 which needs further investigation. Even though sCD14 is one of the important markers for the evaluation of MT, it cannot be considered as a specific marker for MT because sCD14 levels are also elevated in other infections (RSV, dengue virus, mycobacteria) and inflammatory conditions.48,49
In the present study, we found significantly higher expression of the exhaustion marker PD-1 in T cells of HIV-infected children compared with age-matched controls, and its expression was directly related with IA. IE is a component of aberrant IA in chronic HIV-1 infection, which is associated with ongoing viral replication. We and others have reported previously that PD-1 and IA are closely linked in HIV-infected adults.18,50 Further supporting the link between IA and IE is the fact that PD-1 is expressed by activated T cells, but not naive T cells.51 PD-1 expression was further increased at the 12-month follow-up. As PD-1 expression was also directly associated with both LPS and 16S rDNA, these data suggest that MT not only is associated with IA but also contributes to IE.
In conclusion, in this group of clinically stable HIV-infected children, disease severity was manifested by ongoing MT, IA, and IE. As the comparison group of age-matched healthy controls was exposed to similar environmental conditions, the observed effects are most likely attributable to HIV infection. MT was clearly evident in HIV-infected children, including those with mild–moderate immune deficiency; thus, strategies aimed to minimize MT would be useful with the aim to decrease IA and slow the decline of CD4 T cells. The children in this study were not “eligible” for treatment in accordance with the guidelines of India's National AIDS Control Organization at the time this study was conducted. With change in World Health Organization guidelines, more children will have the benefit of ART earlier in life that could have an impact on MT and IA by preventing, reducing, or reversing MT. Factors related to differences in gut microbiota in children and adults along with additional developmental factors may play an important role in MT in children.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr Daniel Douek at the Vaccine Research Center at National Institutes of Health who provided the plasmid DNA containing known copy numbers and sequence of the primers and probe for the PCR. They also thank the HIV2 and the HIV-1+ children who participated in the study, their families, and their providers.
Supported by a grant (R03-HD052154-01) from the Indo-US Program for Maternal and Child Health, sponsored by The Eunice Kennedy Shriver National Institute of Child Health and Human Development, USA, and Indian Council of Medical Research, India.
Footnotes
The authors have no conflicts of interest to disclose.
Presented in part (paper #126) at CROI-2011, February 27 to March 2, 2011, Boston, MA.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Kotler DP, Reka S, Clayton F. Intestinal mucosal inflammation associated with human immunodeficiency virus infection. Dig Dis Sci. 1993;38:1119–1127. doi: 10.1007/BF01295730. [DOI] [PubMed] [Google Scholar]
- 2.Heise C, Miller CJ, Lackner A, et al. Primary acute simian immunodeficiency virus infection of intestinal lymphoid tissue is associated with gastrointestinal dysfunction. J Infect Dis. 1994;169:1116–1120. doi: 10.1093/infdis/169.5.1116. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ancuta P, Kamat A, Kunstman KJ, et al. Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 6.Cassol E, Malfeld S, Mahasha P, et al. Persistent microbial translocation and immune activation in HIV-1-infected South Africans receiving combination antiretroviral therapy. J Infect Dis. 202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 7.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JL, Taylor JM, Manna B, et al. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. AIDS. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Marchetti G, Bellistri GM, Borghi E, et al. Microbial translocation is associated with sustained failure in CD4+ T-cell reconstitution in HIV-infected patients on long-term highly active antiretroviral therapy. AIDS. 2008;22:2035–2038. doi: 10.1097/QAD.0b013e3283112d29. [DOI] [PubMed] [Google Scholar]
- 10.Rajasuriar R, Booth D, Solomon A, et al. Biological determinants of immune reconstitution in HIV-infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis. 202:1254–1264. doi: 10.1086/656369. [DOI] [PubMed] [Google Scholar]
- 11.Mavigner M, Cazabat M, Dubois M, et al. Altered CD4+ T cell homing to the gut impairs mucosal immune reconstitution in treated HIV-infected individuals. J Clin Invest. 122:62–69. doi: 10.1172/JCI59011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallet MA, Rodriguez CA, Yin L, et al. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS. 24:1281–1290. doi: 10.1097/QAD.0b013e328339e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papasavvas E, Pistilli M, Reynolds G, et al. Delayed loss of control of plasma lipopolysaccharide levels after therapy interruption in chronically HIV-1-infected patients. AIDS. 2009;23:369–375. doi: 10.1097/QAD.0b013e32831e9c76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kramski M, Gaeguta AJ, Lichtfuss GF, et al. Novel sensitive real-time PCR for quantification of bacterial 16S rRNA genes in plasma of HIV-infected patients as a marker for microbial translocation. J Clin Microbiol. 49:3691–3693. doi: 10.1128/JCM.01018-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kestens L, Vanham G, Gigase P, et al. Expression of activation antigens, HLA-DR and CD38, on CD8 lymphocytes during HIV-1 infection. AIDS. 1992;6:793–797. doi: 10.1097/00002030-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Levacher M, Hulstaert F, Tallet S, et al. The significance of activation markers on CD8 lymphocytes in human immunodeficiency syndrome: staging and prognostic value. Clin Exp Immunol. 1992;90:376–382. doi: 10.1111/j.1365-2249.1992.tb05854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 18.Sachdeva M, Fischl MA, Pahwa R, et al. Immune exhaustion occurs concomitantly with immune activation and decrease in regulatory T cells in viremic chronically HIV-1-infected patients. J Acquir Immune Defic Syndr. 54:447–454. doi: 10.1097/QAI.0b013e3181e0c7d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson KV. Toll signaling pathways in the innate immune response. Curr Opin Immunol. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- 20.Marchetti G, Tincati C, Silvestri G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin Microbiol Rev. 26:2–18. doi: 10.1128/CMR.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kourtis AP, Ibegbu CC, Wiener J, et al. Role of intestinal mucosal integrity in HIV Transmission to infants through Breast-feeding: the BAN study. J Infect Dis. doi: 10.1093/infdis/jit221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherman MP. New concepts of microbial translocation in the neonatal intestine: mechanisms and prevention. Clin Perinatol. 37:565–579. doi: 10.1016/j.clp.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papasavvas E, Azzoni L, Foulkes A, et al. Increased microbial translocation in ≤180 days old perinatally human immunodeficiency virus-positive infants as compared with human immunodeficiency virus-exposed uninfected infants of similar age. Pediatr Infect Dis J. 30:877–882. doi: 10.1097/INF.0b013e31821d141e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pilakka-Kanthikeel S, Huang S, Fenton T, et al. Increased gut microbial translocation in HIV-infected children persists in virologic responders and virologic failures after antiretroviral therapy. Pediatr Infect Dis J. 31:583–591. doi: 10.1097/INF.0b013e31824da0f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anselmi A, Vendrame D, Rampon O, et al. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin Exp Immunol. 2007;150:442–450. doi: 10.1111/j.1365-2249.2007.03526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baroncelli S, Galluzzo CM, Pirillo MF, et al. Microbial translocation is associated with residual viral replication in HAART-treated HIV+ subjects with <50copies/ml HIV-1 RNA. J Clin Virol. 2009;46:367–370. doi: 10.1016/j.jcv.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 27.CDC WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. 2007.
- 28.Selvaraj A, Kanthikeel SP, Pk B, et al. Defective dendritic cell response to toll like receptor 7/8 agonists in perinatally HIV infected children. Pathog Dis. doi: 10.1111/2049-632X.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luzuriaga K, Sullivan JL. Pediatric HIV-1 infection: advances and remaining challenges. AIDS Rev. 2002;4:21–26. [PubMed] [Google Scholar]
- 30.de Martino M, Tovo PA, Galli L, et al. Prognostic significance of immunologic changes in 675 infants perinatally exposed to human immunodeficiency virus. The Italian Register for Human Immunodeficiency Virus Infection in Children. J Pediatr. 1991;119:702–709. doi: 10.1016/s0022-3476(05)80283-5. [DOI] [PubMed] [Google Scholar]
- 31.Hainline C, Taliep R, Sorour G, et al. Early Antiretroviral Therapy reduces the incidence of otorrhea in a randomized study of early and deferred antiretroviral therapy: evidence from the Children with HIV Early Antiretroviral Therapy (CHER) Study. BMC Res Notes. :448. doi: 10.1186/1756-0500-4-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 26:1685–1690. doi: 10.1097/QAD.0b013e328355d0ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leinert C, Stahl-Hennig C, Ecker A, et al. Microbial translocation in simian immunodeficiency virus (SIV)-infected rhesus monkeys (Macaca mulatta). J Med Primatol. 39:243–251. doi: 10.1111/j.1600-0684.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 34.Fitzgerald F, Harris K, Doyle R, et al. Evidence that microbial translocation occurs in HIV-infected children in the United Kingdom. AIDS Res Hum Retroviruses. 2013:29. doi: 10.1089/aid.2013.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marchetti G, Cozzi-Lepri A, Merlini E, et al. Microbial translocation predicts disease progression of HIV-infected antiretroviral-naive patients with high CD4+ cell count. AIDS. 25:1385–1394. doi: 10.1097/QAD.0b013e3283471d10. [DOI] [PubMed] [Google Scholar]
- 36.Lopez M, Soriano V, Peris-Pertusa A, et al. Elite controllers display higher activation on central memory CD8 T cells than HIV patients successfully on HAART. AIDS Res Hum Retroviruses. 27:157–165. doi: 10.1089/aid.2010.0107. [DOI] [PubMed] [Google Scholar]
- 37.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guadalupe M, Reay E, Sankaran S, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poles MA, Boscardin WJ, Elliott J, et al. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43:65–68. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 40.Brenchley JM, Schacker TW, Ruff LE, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenchley JM, Douek DC. The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS. 2008;3:356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 44.Estes JD, Harris LD, Klatt NR, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salgado M, Rallon NI, Rodes B, et al. Long-term non-progressors display a greater number of Th17 cells than HIV-infected typical progressors. Clin Immunol. 139:110–114. doi: 10.1016/j.clim.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 46.Vesterbacka J, Nowak P, Barqasho B, et al. Kinetics of microbial translocation markers in patients on efavirenz or lopinavir/r based antiretroviral therapy. PLoS One. 8:e55038. doi: 10.1371/journal.pone.0055038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Redd AD, Dabitao D, Bream JH, et al. Microbial translocation, the innate cytokine response, and HIV-1 disease progression in Africa. Proc Natl Acad Sci U S A. 2009;106:6718–6723. doi: 10.1073/pnas.0901983106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anas A, van der Poll T, de Vos AF. Role of CD14 in lung inflammation and infection. Crit Care. 14:209. doi: 10.1186/cc8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayaslioglu E, Kalpaklioglu F, Kavut AB, et al. The role of CD14 gene promoter polymorphism in tuberculosis susceptibility. J Microbiol Immunol Infect. doi: 10.1016/j.jmii.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Sauce D, Almeida JR, Larsen M, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 51.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8:765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.