Abstract
The insula has been identified as a key region involved in interoceptive awareness. Whilst imaging studies have investigated the neural activation patterns in this region involved in intero- and exteroceptive awareness, the underlying biochemical mechanisms still remain unclear.
In order to investigate these, a well-established fMRI task targeting interoceptive awareness (heartbeat counting) and exteroceptive awareness (tone counting) was combined with magnetic resonance spectroscopy (MRS). Controlling for physiological noise, neural activity in the insula during intero- and exteroceptive awareness was confirmed in an independent data sample using the same fMRI design.
Focussing on MRS values from the left insula and combining them with neural activity during intero- and exteroceptive awareness in the same healthy individuals, we demonstrated that GABA concentration in a region highly involved in interoceptive processing is correlated with neural responses to interoceptive stimuli, as opposed to exteroceptive stimuli. In addition, both GABA and interoceptive signal changes in the insula predicted the degree of depressed affect, as measured by the Beck Hopelessness Scale. On the one hand, the association between GABA concentration and neural activity during interoceptive awareness provides novel insight into the biochemical underpinnings of insula function and interoception. On the other, through the additional association of both GABA and neural activity during interoception with depressed affect, these data also bear potentially important implications for psychiatric disorders like depression and anxiety, where GABAergic deficits, altered insula function and abnormal affect coincide.
Keywords: MRS, fMRI, GABA, insula, interoception, depressed affect
1. Introduction
A growing body of research has suggested that the insula cortex integrates the processing of various stimulus types (Craig, 2009). This includes stimuli that originate from both internally and externally to the body. Processing of such stimuli has been located within the insula and has been suggested to underlie our ability to be aware of our internal states (Craig, 2002), termed interoceptive awareness (IA). Imaging studies in humans of the insula have provided details of task-specific subdivisions within the region (Chang et al., 2012; Simmons et al., 2012), highlighting, for example, distinctions between the anterior and posterior insula (Farb et al., 2012). In addition, functional connectivity analyses have been used to identify networks of regions in the brain that have patterns of spontaneous activity that are correlated with that in the insula (Cauda et al., 2011; Deen et al., 2011). What remains unclear, however, is the neurochemistry that underlies task-induced activity in the insula in humans.
Studies regarding regions of the brain other than the insula have revealed links between task-evoked neural responses and a number of different neurotransmitters in humans. Concentrations of GABA (γ-aminobutyric acid) – the main inhibitory transmitter in the brain – in the visual cortex have been shown in a number of studies to be correlated with both the amplitude of the BOLD response to visual stimuli and with the particular dynamic properties of this response (i.e., the latency and width) (Muthukumaraswamy et al., 2009, 2012; Donahue et al., 2010). Similarly, GABA concentrations in the medial prefrontal cortex (mPFC) also correlate with BOLD responses to stimuli (Northoff et al., 2007). At the same time, concentrations of glutamate – the primary excitatory transmitter – as measured in areas of the anterior cingulate have been shown to correlate with task-induced activity changes in multiple other brain regions during different tasks (Duncan et al., 2011; Falkenberg et al., 2012). Taken together, these prior results suggest that GABA and glutamate concentrations may be related to IA-related responses in the insula.
Under a prominent theory of emotions that views them as arising, in part, from bodily states, IA is linked closely to affective experience (Lamm and Singer, 2010). Support for this link comes from a growing body of research, including studies that show an anatomical overlap in the insula between emotional and interoceptive processing (Kelly et al., 2012; Zaki et al., 2012; Terasawa et al., 2013) and work that demonstrates a correlation between quality of emotional experience and bodily awareness (Wiens, 2005; Herbert et al., 2007; Pollatos, Gramann, et al., 2007; Dunn et al., 2010). The link between IA activity in the insula and emotional experience suggests a role for the region in mood disorders, such as major depressive disorder (MDD), that are characterised by negative affect. Such an involvement of the insula in depression is supported by findings of altered functional responses in the region (Liotti et al., 2002; Paulus and Stein, 2010), as well as structural changes (Sprengelmeyer et al., 2011) and deficits in IA (Terhaar et al., 2012). In addition to such changes, MDD is associated with altered GABAergic and glutamatergic function in multiple brain regions (Alcaro et al., 2010; Zhao et al., 2012), whilst drugs acting on these systems have an antidepressant effect (Möhler, 2012; Sanacora et al., 2012). These combined factors suggest that IA in the insula may be related to depressive symptoms of negative affect and that this association is related to glutamate or GABA in the region. This remains to be investigated, however.
Based on these combined strands of IA, insula neurochemistry, and the relation between the insula and depressive symptoms, we examined whether the concentrations of GABA or glutamate and glutamine (Glu + Gln = Glx) can be related to neural activity in the insula during IA and to depressed affect. A well-established paradigm for functional magnetic resonance imaging (fMRI) was used (Wiebking et al., 2010, 2012), that consisted of a target task to induce IA (heartbeat counting), a closely matched control task to induce exteroceptive awareness (EA, tone counting), and fixation periods. In a separate session, measures of GABA and Glx concentrations from a voxel located in the target region, the left insula, were obtained in the same healthy participants using magnetic resonance spectroscopy (MRS). A comparison voxel was placed in the mPFC. It was hypothesised that BOLD responses in the insula would be correlated with GABA concentrations in the same region and that these responses would be further correlated with depressed affect, as measured using the Beck Hopelessness scale (BHS) (Beck et al., 1974).
2. Methods
2.1 Participants
Twenty-eight right-handed healthy participants (10 females, mean age 22.37 years ± 3.77 SD, 18-34 years) underwent fMRI and 27 out of this group participated in MRS scanning (10 females, 22.37 ± 3.85 years, days between scans 3.7 ± 2.7). All participants had a Beck Depression Inventory (Beck et al., 1996) score ≤ 4 and were questioned about psychiatric, neurological, or other diseases. Participants were recruited from the McGill University (Montréal) student body and the local community. The study was approved by the local ethics committee. All participants gave their written informed consent and were financially compensated.
Four participants were excluded due to anatomical abnormalities or motion artefacts (≥ 2 mm), culminating in 24 datasets for fMRI analysis (n = 24 participants, 9 females, 22.71 ± 3.95 years). Quality control of the MRS data (i.e., only results with Cramer-Rao lower bounds (CRLB) ≤ 20% were included in the analyses) resulted in the following analysis groups: GABA/NAA: n = 15 in the insula (5 females, 23.13 ± 4.45 years), n = 9 in the mPFC (4 females, 21.11 ± 2.98 years); Glx/NAA: n = 14 in the insula (4 females, 23.29 ± 4.58 years), n = 18 in the mPFC (9 females, 22.33 ± 3.51 years).
2.2 fMRI task
A well-established fMRI design for investigating intero- and exteroceptive awareness was used in the study, based on a paradigm introduced by Critchley and Pollatos (Critchley et al., 2004; Pollatos, Schandry, et al., 2007; Wiebking et al., 2010). This consists of three independent conditions presented in pseudo-randomised order for 6-10 seconds each. These were: a task for internal awareness (IA); a task for external awareness (EA); and fixation periods.
Immediately before the scan, participants had a practice session. Following a standardized protocol, which included the presentation of predefined task instructions, participants were carefully instructed and familiarized with the task. In order to limit cognitive processes other than intero- or exteroception, simple visual stimuli were used to indicate the task type. In case of IA, a dark coloured heart on a light background was presented and indicated onset and duration of the task. As long as the task type indicator was visible on the screen, participants were asked to silently count their own heartbeat. Any kind of manipulation, such as holding their breath or evaluating their pulse at the radial artery, was not allowed. This was monitored by visual inspection from the control room. Attempts to control the breathing rate were monitored using the breathing belt of the Siemens Physiological Monitoring Unit (PMU). Participants were thoroughly instructed to concentrate on their body and their heartbeat as well as possible. The number of counted heartbeats was reported via a visual scale (3.5 s). Here, participants moved the indicator on the scale to the labelled position that represented the number of beats they counted.
In case of EA, a symbol of a dark coloured musical note - of the same size as the heart symbol - was presented on the same light background. During such tasks individuals silently counted the number of tones played through headphones attached to the scanner. Participants reported the number of counted heartbeats or tones after each trial using a visual analogue scale (3.5 s).
In order to make the difficulty of the IA and the EA tasks comparable, tones were presented at an individually determined volume. To ensure equivalent difficulty of both tasks, participants were instructed to adjust the volume of the tone to the same perception difficulty level as that of counting their own heartbeat. This was done at the beginning of the scan (i.e., with the scanner acquiring images to also account for scanner noise) using right and left button presses, corresponding to increases or decreases in volume. To illustrate, where the heartbeat counting was more difficult, an individual would lower the volume of the external tone in order make that aspect of the task equally difficult to the heartbeat counting. This was explained to the participants before the scan and they also practiced this procedure beforehand.
To control for habituation effects (i.e., counting the own heartbeat becoming easier over time), the same interactive enquiry was presented a second time in the middle of the scan. Here, individuals had another possibility to readjust the volume of the tone and hence to balance the level of difficulty between the internal and external stimuli.
The numbers of counted tones and counted heartbeats, both of which were present continuously, were compared to the actual number of played tones and recorded heartbeats, respectively. The latter was measured using the Siemens PMU. In addition, the presentation frequency of the tones was adapted to correspond to each subject's pulse-rate, which was read off from the online value of the PMU. The onset time of the tones was jittered by 200 ms to avoid habituation.
Fixation periods were indicated by a dark cross (of the same size and colour as the IA and EA symbols) on a light background. Participants were instructed to relax and minimise any cognitive work during these period.
The paradigm was executed on a computer running the software package “Presentation” (Neurobehavioral Systems, http://www.neurobs.com). Visual stimuli were projected via an LCD projector onto a screen visible through a mirror mounted on the headcoil.
2.3 Heartbeat acquisition and analysis
Pulse oximetry data were recorded using the Siemens Physiological Monitoring Unit (PMU). This wireless recording device was clipped to the index finger of the participant. It was aligned to the magnetic field to minimise distortion effects. Participants were instructed to avoid moving their hand. A total of 17 complete pulse-rate data sets were acquired. Due to technical problems such as interrupted recordings of the PMU transmission, seven of these data sets were excluded.
Each condition was presented 36 times in total. The first trial of each condition was excluded due to novelty effects. Similarly, heartbeats at the transition between trials were excluded (defined as being within 300 ms of trail transition). Consequently, the total mean error of 35 IA conditions (total error between recorded number of heartbeats and subjective reports of counted heartbeats per IA condition) and 35 EA conditions (total error between played number of tones and subjective reports of counted tones per EA condition) was calculated per participant and compared using a paired t-test (two-tailed).
2.4 fMRI acquisition and analysis
Functional scans were acquired on a 3-Tesla whole body MRI system (Siemens Trio, Erlangen, Germany), using a body transmit and 32-channel receive headcoil at the MNI (McGill University, Montréal, Canada). The settings were as follows: 47 T2*-weighted echo planar images per volume with BOLD contrast; alignment at 30° off the AC-PC plane in an odd-even interleaved acquisition order; FoV: 205 × 205 mm2; spatial resolution: 3.2 × 3.2 × 3.2 mm3; TE = 25 ms; TR = 2270 ms; flip angle = 90°. Data were recorded in one scanning session containing 580 volumes per participant. A high resolution T1-structural 3D image was also acquired. In addition to record the breathing rate by using the breathing belt of the PMU, pulse oximetry data were recorded using a wireless recording device that was clipped to the index finger of the participant.
The fMRI data from 24 subjects were pre-processed and statistically analysed using the general linear model approach in SPM8 (http://www.fil.ion.ucl.ac.uk) and MATLAB 7.11 (The Mathworks Inc., Natick, MA, USA). All functional images were slice time corrected with reference to the first acquired slice, corrected for motion artefacts by realignment to the mean image, and spatially normalized to the SPM standard T1-template (Ashburner and Friston, 1999). The normalization parameters were generated by warping the coregistered anatomical image to the MNI T1-template and applying these parameters to all functional images. Images were resampled to 2 × 2 × 2 mm3, smoothed with an isotropic 6 mm full-width half-maximum (FWHM) Gaussian kernel. The time-series fMRI data were filtered using a high pass filter (threshold 128 s).
Since structured noise still remains in the fMRI data after traditional steps of pre-processing, an independent component analysis (ICA) was applied to denoise the data and hence improve the sensitivity and specificity of the results. Using Probabilistic Independent Component Analysis (Beckmann and Smith, 2004), which is implemented in the MELODIC toolbox in the FSL Software Library, http://www.fmrib.ox.ac.uk/fsl/) (Smith et al., 2004; Woolrich et al., 2009), a group ICA was performed on the pre-processed fMRI data, which were temporally concatenated across subjects. Two independent raters (CW, NWD) visually inspected 40 components and classified them as noise or signals of interest, according to a detailed description of an operationalized denoising procedure (Kelly et al., 2010). In particular, components were considered as noise when they showed a ring-like pattern in the periphery of the brain and tightly clustered areas in the frontal regions (McKeown et al., 1998), clusters with a location in the white matter/CSF or an association with blood vessels (Sui et al., 2009; Zou et al., 2009), spotted patterns diffusely spread over the brain, and time courses showing a saw-tooth pattern or spikes (McKeown et al., 1998). Components were independently identified as noise and removed from the original fMRI data through linear regression.
IA and EA conditions (i.e., a task for internal awareness (IA); a task for external awareness (EA)), were included in the SPM model as separate events, as were their feedback phases. The statistical model for each subject was created by convolving trial onsets with a canonical haemodynamic response function (Friston et al., 1998). Moreover, the six movement parameters (movement in x, y, and z direction plus rotation on three axes), which were calculated during the realignment step in SPM (as described above), were included in the SPM model as additional nuisance variables, resulting in a total of nine regressors. Regionally specific condition effects were tested by employing linear contrasts for each subject and different conditions. The resulting contrast images were submitted to a second level random-effects analysis. Here, a one-sample t-test was used on images obtained for each subject's volume set and different conditions (e.g., IA > EA).
The Marseille Region of Interest Toolbox software package (MarsBaR, (Brett et al., 2002), http://www.sourceforge.net/projects/marsbar) was used to extract time courses from the insula and mPFC MRS voxels. The maximum of the time course of the estimated event for a single condition (values for IA and EA) was calculated by MarsBaR, divided by the mean signal across the time course of the whole session and multiplied by 100. This value, the percent signal change, represents an individual value for each subject and each condition within a certain ROI. Percent signal changes were controlled for possible outliers and entered into SPSS 17.0 (SPSS inc., Chicago, IL). As described in the MarsBar documentation, mean values are commonly less than 0.1 percent. Signal changes for each condition were extracted from both MRS voxels and correlated with GABA/NAA and BHS scores. The proportion of grey matter in each MRS voxel was calculated using the FSL FAST tool (http://fsl.fmrib.ox.ac.uk/) and included as a control variable in all analysis regarding MRS values.
In order to provide further detail as to where in particular within the MRS voxels correlating signal changes are located, as well as to identify any correlating regions outside of the MRS voxels, the IA-specific contrast (IA vs. implicit baseline) was used in second-level correlation analyses in SPM. Here, the individual biochemical measures for each MRS voxel, as well as scores from the BHS, were entered as regressors, using the proportion of grey matter as regressor of no interest in all calculations.
The anatomical localization of significant results was assessed with reference to the standard stereotactic atlas by superimposition of the SPM maps on a standard brain template provided by MRIcron (Chris Rorden, www.mricro.com). The contrast [IA > EA] was corrected by a false discovery rate (voxel P FDR ≤ 0.02, k > 25). Results of the regression analysis were small volume corrected by the respective MRS voxel. Significant clusters were corrected by familywise error correction (FWE) (Suppl. table 2) using a voxel-wise cut-off of P ≤ 0.005, uncorrected. Reported peaks for the whole brain regression, using GABA/NAA from the left insula as a regressor, were restricted to (cluster P FWE ≤ 0.06) and assigned to the most probable brain area by using the SPM Anatomy Toolbox (Eickhoff et al., 2007) and the WFU pickatlas (Maldjian et al., 2003, 2004).
Functional MRI results were confirmed by using an independent set of data, in line with proposed good practice (Kriegeskorte et al., 2009; Poldrack and Mumford, 2009; Vul et al., 2009). Both MRS voxels were applied to another independent sample of 30 healthy participants. Data were acquired at the Department of Neurology, Otto-von-Guericke University Magdeburg, Germany. Participants in both studies performed the same tasks (IA, EA, Fix), and were instructed by the same researcher (CW). Functional measurements of this independent data set were performed on an identical 3-Tesla whole body MRI system (Siemens Trio, Erlangen, Germany). Although headcoils differed across studies (an 8-channel headcoil compared to a 32-channel headcoil), research has been shown that fMRI results across different imaging sites are very well comparable, even when comparing across different headcoils and scanner machines (see for example Casey et al., 1998; Zou et al., 2005; Gountouna et al., 2010; Kaza et al., 2011). The independent data were acquired with the following settings: thirty-two T2*-weighted echo planar images per volume with BOLD contrast; alignment parallel to the AC- PC plane in an odd–even interleaved acquisition order; FoV: 224 × 224 mm2; spatial resolution: 3.5 × 3.5 × 4 mm3; TE = 30 ms; TR = 2.000 ms; flip angle = 80°. A total of 1.160 volumes were recorded for each of the 30 healthy subjects. These data were processed in the exact same way (including ICA denoising) as the main data set. This was a reanalysis of a data set presented previously (Wiebking et al., 2011).
2.5 MRS acquisition and analysis
Single voxel edited 1H MR spectra were acquired using MEGA-PRESS (Mescher et al., 1998; Marjanska et al., 2007) on a 3-Tesla MRI system (Siemens Trio, Erlangen, Germany) equipped with a 12-channel headcoil at the University of Montréal. Utilizing a high resolution T1-image (MPRAGE; FOV = 205 × 205 mm2; spatial resolution = 1 × 1 × 1 mm3; TE = 3.02 ms; TR = 2000ms; flip angle = 5°), voxels were placed in the left insula and the mPFC. In order to achieve consistent volume of interest (VOI) positioning, placement was done by the same investigator for all subjects according to easily identifiable anatomical landmarks: left insula voxels (23 × 48 × 27 mm3) were aligned with the line of the insula cortex in an anterior-posterior direction with the most anterior edge of the VOI aligned to the anterior limit of the insula; mPFC voxels (48 × 21 × 21 mm3) were placed anterior to the genu of the corpus callosum, parallel to the AC-PC plane.
First- and second-order shim terms were adjusted using FAST(EST)MAP (Gruetter and Tkác, 2000). MRS data were acquired using a MEGA-PRESS sequence (Mescher et al., 1998) with double-banded pulses used to simultaneously suppress water signal and edit the γ–CH2 resonance of GABA at 3 ppm. Additional water suppression using variable power with optimized relaxation delays (VAPOR) and outer volume suppression (OVS) techniques (Tkác et al., 1999) was optimized for the human 3–T system and incorporated prior to MEGA-PRESS. The final spectra were obtained by subtracting the signals from alternate scans with the selective double-banded pulse applied at 4.7 ppm and 7.5 ppm (‘EDIT OFF’) and the selective double-banded pulse applied at 1.9 ppm and 4.7 ppm (‘EDIT ON’). MEGA-PRESS data were acquired in four interleaved blocks of 32 (‘EDIT OFF’, ‘EDIT ON’) scans each with frequency drift correction between each block. Free induction decays (FIDs) were stored separately in memory for individual frequency and phase correction using the tCr signal at 3.03 ppm, as well as correction for residual eddy-current using unsuppressed water signal obtained from the same voxel.
Difference spectra were analyzed with LCModel 6.2-1A (Provencher, 1993, 2001) using the basis set which included an experimentally measured metabolite-nulled macromolecular spectrum (average from 10 participants) and experimentally measured spectra from 100 mM phantoms of NAA, creatine, GABA, Glu, and Gln with pH adjusted to 7.2 and at 37°C. The LCModel fitting was performed over the spectral range from 0.5-4.0 ppm. No baseline correction, zero-filling, or apodization functions were applied to the in vivo data prior to LCModel analysis. The usage of a subtraction spectrum with MEGA-PRESS entails imprecise acquisition of a creatine peak. Hence, metabolite of interest concentrations were used in subsequent steps as a ratio to NAA (see also Stagg, Bachtiar, et al., 2011).
Only results with the Cramer-Rao lower bounds (CRLB) ≤ 20% were included in the analysis. Concentrations with CRLB > 20% were classified as not detected. Individual spectra acquired in the insula voxel are shown in Supplementary figure 2. LCModel metabolite concentrations, CRLB values, and correlation coefficients between Glu/Gln are given in Suppl. table 1A.
3. Results
For the investigation of a relationship between IA neural activity and GABA/NAA in the insula, a well-established fMRI paradigm was applied (Critchley et al., 2004; Wiebking et al., 2010). Task difficulty effects between intero- and exteroceptive awareness (IA and EA) were excluded by showing no differences between the total mean error of IA (mean ± SD: 1.3 ± 0.56) and EA (1.1 ± 0.66) condition (T: 1.1, df: 16, P = 0.3, two-tailed) counts.
Firstly, the whole brain contrast [IA > EA] was overlayed with the MRS insula voxel to illustrate regional specific activity in the insula during IA (Figure 1A). Next, BOLD responses in the insula MRS voxel were explored (Figure 1B). As shown in Figure 1B, IA induced significantly higher positive BOLD responses in the insula when compared to EA (P ≤ 0.001) (Suppl. table 1B). To support these results of higher positive BOLD responses during IA compared to EA, both MRS voxels were also applied to an independent data sample that used the same fMRI paradigm and image preprocessing (Kriegeskorte et al., 2009; Poldrack and Mumford, 2009; Vul et al., 2009). The signal changes of the independent data sample confirmed the differences between conditions in the left insula voxel (Figure 1C & Suppl. table 1B). In contrast, activity patterns in the mPFC MRS voxel (Suppl. figure 1A) showed task induced negative BOLD responses during both conditions (Suppl. figure 1B & Suppl. table 1B). No significant differences between IA and EA were seen in this region, a pattern that was confirmed in the independent data sample (Suppl. figure 1C & Suppl. table 1B).
Figure 1.
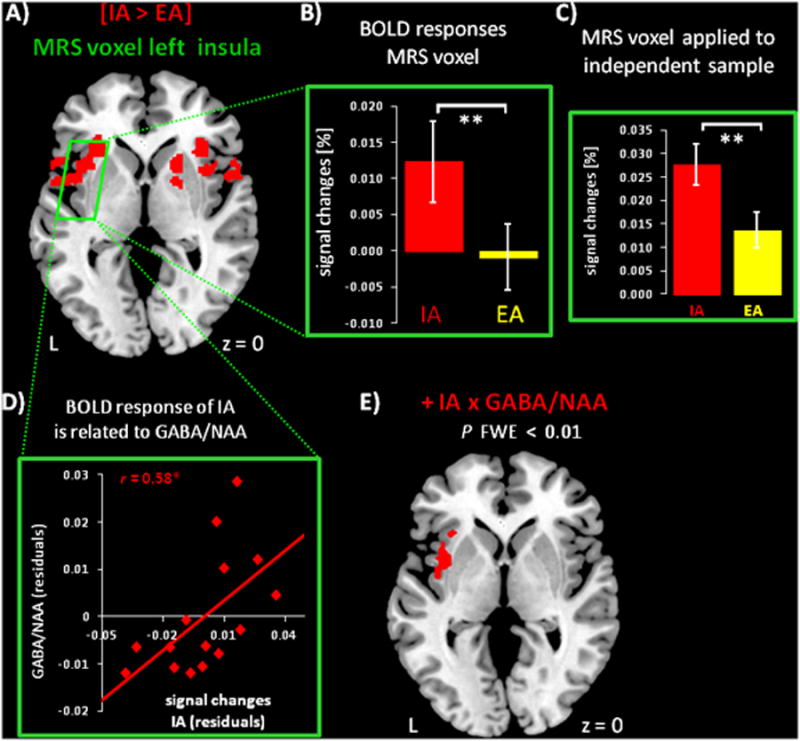
A) Whole brain contrast [IA > EA] (voxel P FDR ≤ 0.02) and placement of the left insula MRS voxel (green).
B) Extracted signal changes from the insula MRS voxel. Bars show BOLD responses (mean ± SEM, n = 15 participants) for IA (red) and EA (yellow) (see also Suppl. table 1B). Paired t-tests (two-tailed) show significant differences between the conditions (** P ≤ 0.001).
C) The region of the insula MRS voxel was applied to an independent data sample (n = 30 healthy participants) that used the same fMRI paradigm (see also Suppl. table 1B). Paired t-tests (two-tailed) between IA (red) and EA (yellow) confirm results seen in B (** P ≤ 0.001). D) The scatter plot shows residuals for the BOLD responses of IA in the MRS insula voxel (r = 0.58, * P ≤ 0.05) as well as GABA/NAA following grey matter correction (see also Table 1A). Signal changes for EA show no association to GABA/NAA.
E) Voxel-wise regressions within the MRS voxel (red, cluster P FWE ≤ 0.01) (see also Suppl. table 2A) identify the anatomical region within the MRS voxel showing a positive relationship between IA and GABA/NAA. The amount of grey matter in the MRS voxel was included as regressor of no interest.
Having demonstrated that both IA and EA induce reliable BOLD responses in the insula, neurochemical correlates of these conditions were investigated. Individual neurotransmitter concentrations (quantified in relation to NAA, see Suppl. table 1A for details) were correlated with IA- and EA-related BOLD responses derived from the MRS voxel. Since the proportion of grey matter in the MRS insula voxel was included as a control variable, correlation graphs show the residuals of signal changes and GABA/NAA. GABA/NAA values showed a significant positive correlation with neural activity during IA in the left insula (Figure 1D, Suppl. table 2A & 2B). No relationship was observed in the insula when comparing GABA/NAA to EA (Table 1), nor when comparing the IA-specific contrast with other MRS values (Glx/NAA, Glu/NAA, Gln/NAA) (see Suppl. table 2A). A positive relationship between GABA/NAA and glutamate was observed in both MRS voxels (as detailed in Table 1). Using the neurotransmitter concentrations of the insula as regressors in the IA-specific contrast, the anatomical location of the positive relation between GABA/NAA and IA within the insula MRS voxel was further specified (Figure 1E). At the same time, voxel-wise regressions in the mPFC MRS voxel revealed an exclusive negative relationship between GABA/NAA and EA (Suppl. figure 1D & Suppl. table 2B). Calculations using the extracted BOLD responses of EA in the mPFC MRS voxel showed no specific relation to GABA/NAA or other biochemical values (Table 1B).
Table 1.
R-values for correlations between MRS metabolites, BHS, and signal changes for IA and EA (controlled for the amount of grey matter in the respective MRS voxels). Subscripted numbers indicate number of participants. Colour code as in corresponding Figure 1/Suppl. figure 1: red = interoceptive awareness (IA); yellow = exteroceptive awareness (EA);
A) Results for the left insula MRS voxel.
B) Results for the mPFC MRS voxel.
| A) insula MRS voxel | ||||||
|---|---|---|---|---|---|---|
| GABA15/NAA | Glx14/NAA | Glu14/NAA | Gln14/NAA | BHS15 | BHS24 | |
| IA | 0.58* | 0.03 | 006 | -0.03 | -0.58* | -0.42* |
| EA | 0.44 | 0.02 | 0.00 | 0.02 | -0.35 | -0.20 |
| GABA15/NAA | / | 0.51(*) | 0.54(*) | 0.23 | -0.62* | -0.62* |
| Glx14/NAA | 0.51(*) | / | 0.92** | 0.72** | -0.14 | -0.14 |
| Glu /NAA | 0.54(*) | 0.92** | / | 0.38 | -0.14 | -0.14 |
| Gln14/NAA | 0.23 | 0.72** | 0.38 | / | -0.08 | -0.08 |
| BHS15 | -0.62* | -0.14 | -0.14 | -0.08 | / | 1** |
| B) mPFC MRS voxel | ||||||
| GABA9/NAA | Glx18/NAA | Glu18/NAA | Gln18/NAA | BHS18 | BHS24 | |
| IA | -0.38 | 0.21 | -0.06 | 0.37 | 0.14 | 0.09 |
| EA | -0.62 | -0.11 | -0.28 | 0.22 | 0.03 | 0.03 |
| GABA9/NAA | / | 0.22 | 0.79* | -0.32 | -0.28 | -0.28 |
| Glx18/NAA | 0.23 | / | 0.74** | 0.47(*) | 0.55* | 0.55* |
| Glu18/NAA | 0.79* | 0.74** | / | -0.25 | 0.29 | 0.29 |
| Gln18/NAA | -0.32 | 0.47(*) | -0.25 | / | 0.41 | 0.41 |
| BHS18 | 0.15 | 0.55* | 0.29 | 0.41 | / | 1** |
P ≤ 0.01,
P ≤ 0.05,
P ≤ 0.1.
Finally, considering a probable involvement of the insula in depressed affect, GABA/NAA was correlated with scores of the Beck Hopelessness Scale (BHS), a behavioural measure for depressed affect. This revealed a negative relationship between BHS and GABA/NAA (Figure 2A & Table 1A). Signal changes of the insula MRS voxel were then correlated with BHS (controlled for the amount of grey matter in the MRS voxel), which showed a significant negative relationship (Figure 2B & Table 1A). EA showed no association to BHS in the insula (Table 1A). Signal changes for IA and EA showed a significant differential association to BHS, as revealed by using a paired t-test for r-values according to Williams (Williams, 1959; Steiger, 1980) (T = -2.46, df = 12, P < 0.05, two-tailed). As described above, the individual measures for BHS were also entered into a second-level correlation analysis in SPM to provide further anatomical detail for MRS voxel correlations (Suppl. table 2B & Figure 2C in purple). Overlaying both IA voxel-wise regressions (i.e., the positive IA × GABA/NAA result (as in Figure 1E) and the negative IA × BHS result) showed a shared region in the middle of the left insula (Figure 2C in white).
Figure 2.
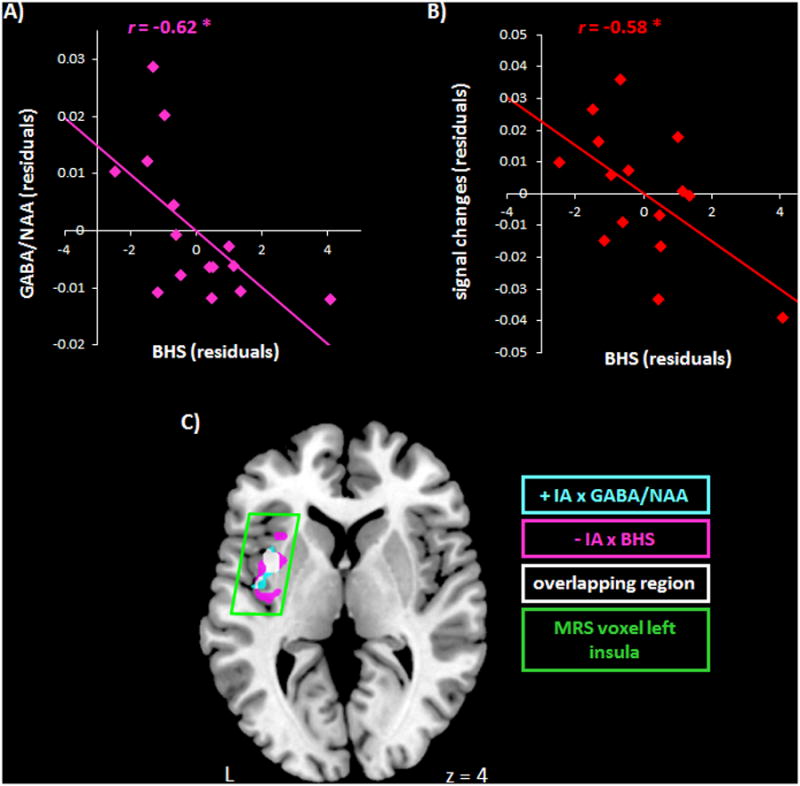
A) The left scatter plot (in purple) shows a negative relationship between residuals for BHS and GABA/NAA in the left insula (r = -0.62, * P ≤ 0.05, n = 15) (see also Table 1A), controlling for grey matter volume.
B) The right scatter plot (in red) shows residuals for the BOLD responses of IA in the MRS insula voxel correlated with BHS (r = 0.58, * P ≤ 0.05) (see also Table 1A). Signal changes for EA show no association to BHS.
C) Voxel-wise regressions show in cyan the positive regression IA × GABA/NAA (as in Figure 1E, cluster P FWE ≤ 0.01), in purple the negative regression IA × BHS (cluster P FWE ≤ 0.001) (Suppl. table 2A), and in white the overlap between these. All regressions included the amount of grey matter as control variable.
The inter-relationship between insula GABA/NAA concentrations, IA related signal changes, and depressed affect is illustrated in Figure 3. In addition, the mPFC showed a positive voxel-wise regression between IA and BHS (Suppl. figure 1E & Suppl. table 2B). Estimated signal changes of the mPFC MRS voxel showed no relation between IA and BHS. Instead, BHS was positively correlated with mPFC Glx/NAA (Table 1B).
Figure 3.
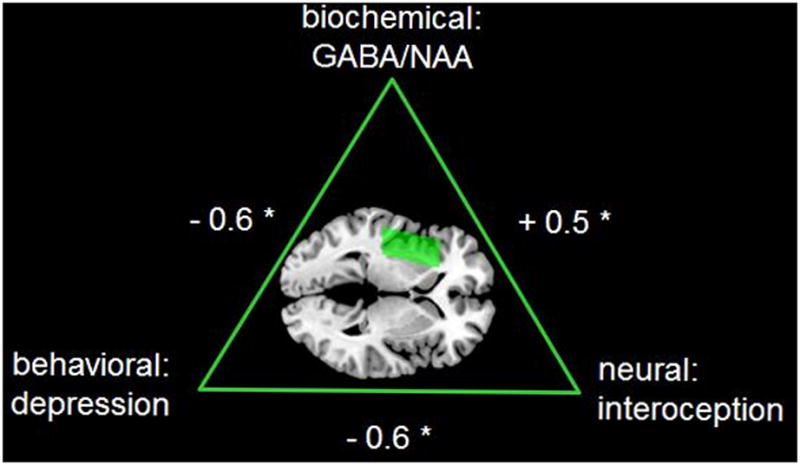
Overview showing the inter-relationship between the neurochemical level by GABA/NAA concentration, the neural level by signal changes for interoception, and the behavioral level of depression as assessed by the Beck Hopelessness Scale.
4. Discussion
The BOLD responses seen in the main MRS voxel in the left insula and the comparison MRS voxel in the mPFC are in accordance with previous studies. In detail, IA induced significantly higher positive BOLD responses in the insula when compared to EA. This is a well-documented neural response (e.g., Critchley et al., 2004; Pollatos, Schandry, et al., 2007; Zaki et al., 2012) and can be confirmed in the present study by another independent data sample, which underlines the reliability of the heartbeat monitoring task used here. In contrast, the mPFC voxel showed task induced negative BOLD responses during both conditions. As part of the default-mode network, this finding conforms with studies investigating effects of external stimuli along this network (Raichle, 2009), as well as with a previous exploratory fMRI study of IA (Wiebking et al., 2011). Again, this finding can be confirmed here by another independent fMRI study that used the same paradigm.
Combining fMRI with biochemical measures derived from MRS imaging, we investigated a possible influence of GABA/NAA (and Glx/NAA) on IA-related activity in the insula. This region is highly involved in these processes and hence likely to be influenced by one of the common inhibitory (GABA) or excitatory (glutamate) neurotransmitters. Taking BOLD responses from the insula MRS voxel, it was shown that IA was positively correlated with GABA/NAA values. This relationship was also confirmed using GABA/NAA values in a voxel-wise regression with IA. Since the insula is not a region of pure internal awareness, but rather a structure that can be seen to link external representations of the outside world with the body's internal state (Farb, Segal, and Anderson 2012), extracting neural signals from the whole insula MRS voxel increases the variation of the signals and leads to a weaker differentiation between them. Yet, results concerning both GABA/NAA and BHS scores in combination with IA reveal a distinct association. This leads to the assumption that future studies, having the possibility of more advanced spectroscopy imaging methods, may investigate subregions of the insula like the anterior parts, which might be expected to show an even stronger association between IA and GABA/NAA as well as BHS. No correlation was seen between GABA/NAA values and EA-related signal changes, nor was there any correlation between IA or EA signal changes and insula glutamatergic measures (i.e., Glx/NAA, Glu/NAA, or Gln/NAA).
In the mPFC, a negative correlation was seen between GABA/NAA values and the EA contrast. No other correlations were seen in this region. This result corresponds well with prior results suggesting a closer link between GABAA receptor availability and EA rather than IA in cortical midline structures (Wiebking et al., 2012). In addition, it is in line with a previously described negative correlation between GABA concentrations and signal changes in the mPFC evoked by an external stimulus (Northoff et al., 2007). Both MRS voxels show a positive relationship between GABA/NAA and glutamate, which is in line with previous findings in the motor cortex (Stagg et al., 2009; Stagg, Bachtiar, et al., 2011; Stagg, Bestmann, et al., 2011).
The positive correlation between GABA/NAA values and insula IA-related signal changes contrasts with the negative correlation seen in studies of the visual cortex (Donahue et al., 2010; Muthukumaraswamy et al., 2012). A tentative potential explanation for this is that the task used here involves the shifting of attention between competing stimuli (the heartbeat and the tone) whereas the tasks used in the visual cortex studies only involve a single stimulus that is switched on and off. With competing stimuli, it has been suggested that GABAergic inhibition is involved in suppressing activity related to the distractor stimulus, promoting target stimulus activity (Sumner et al., 2010). In the present case it can be hypothesized that EA activity may be being suppressed by GABAergic inhibition, promoting IA-related activity. More work is required to test such a hypothesis, however. Also to be noted is the lack of correlations between regional signal changes and glutamatergic measures. This does, however, fit with previous glutamate imaging studies where correlations are mostly seen between glutamate concentrations and signal changes in regions other than that in which glutamate is measured (Duncan et al., 2011; Falkenberg et al., 2012). This suggests that MRS measures of glutamate are more related to inter-regional effects than to signal changes within regions.
Previous studies have suggested that relationships between activity and GABA concentrations may be regionally specific (i.e., that, in general, concentrations in one region are unrelated to activity in another) (Sumner et al., 2010). The current results fit with this view in two respects. Firstly, GABA/NAA values in the left insula and mPFC were not correlated with each other (r = -0.37, P = 0.47, n = 6). Similarly, GABA/NAA concentrations in one region do not correlate with signal changes in the other (insula GABA/NAA vs mPFC: IA − r = -0.14, P = 0.63; EA − r = 0.09, P = 0.74; n = 15; mPFC GABA/NAA vs insula: IA − r = -0.32, P = 0.40; EA − r = -0.41, P = 0.27; n = 9). Secondly, GABA/NAA concentrations were correlated with opposing conditions in the two regions studied – IA in the insula and EA in the mPFC – further suggesting specificity in each region.
The findings of the present study represent evidence for a relationship between GABAergic tone and possible functional activity during IA in the insula. In depressed subjects both of these factors have been shown to be disrupted. On the one hand a GABAergic deficit in depressed patients has been reported (Croarkin et al., 2011; Möhler, 2012), whilst on the other deficits in IA on the neural and behavioural level have been reported (Paulus and Stein, 2010; Grimm et al., 2011; Terhaar et al., 2012). We thus considered a probable involvement of the insula in depressed affect by, firstly, correlating insula GABA/NAA values with scores from the Beck Hopelessness Scale (BHS). This revealed a negative relationship between GABA/NAA and depressed affect. Signal changes in the insula for the IA condition also correlated negatively with BHS, whilst the mPFC showed a positive relationship between BHS and IA. The overall pattern of relationships fits well with the evidence of GABAergic and IA deficits in depression, demonstrating that even in non-clinical subjects, a consistent relationship between the three measured factors can be seen. This interaction between neurochemistry, interoception, and depressive symptoms presents a promising line of research for future studies in depressed patients.
A number of limitations of this study should be noted. Firstly, heartbeat perception was used as the IA task; repetition with different forms of interoception, such as breath monitoring (Farb et al., 2012), should also be investigated to underline the role of GABA/NAA in the insula during interoceptive processing. Secondly, a more direct involvement of the right insula in interoception has been proposed (Craig, 2002, 2009). As the left insula was used in the current study, it may be useful to repeat the experiment using MRS from the right insula to support the current findings and underline the role of the insula in interoception. Along similar lines, the anterior part of the insula has been proposed to be more involved in the processing of internal stimuli (Craig, 2009; Lamm and Singer, 2010; Price and Drevets, 2012); we were, however, only able to acquire MRS data from the whole insula due to technical limitations. With improvements in MRS techniques, future studies might aim to investigate in more detail regional differences in neurochemistry across the insula subregions. Although the same quality threshold was applied to both MRS regions (CRB ≤ 20%), there is also the possibility that the slight difference in size between the insula and mPFC voxels may have influenced the results. Hence, future studies need to apply same sized MRS voxels. In addition, it may be interesting in future studies to acquire MRS data from a comparison voxel in a region such as the dorsolateral prefrontal cortex, since this region is involved in processing of external awareness, which elicits positive BOLD responses (Siegle et al., 2007; Grimm et al., 2008).
Finally, one might criticize that the measurements of fMRI and MRS did not, for logistical reasons, take place on the same day. Although several studies have shown reasonable reliability over time of MRS measures (Geurts et al., 2004; O'Gorman et al., 2011), repetition of the current results with data acquired at the same time is required.
In conclusion, this study demonstrates that GABA/NAA concentration in the left insula is particularly associated with neural activity during interoceptive awareness, as compared to exteroceptive awareness. Moreover, both GABA/NAA and neural activity during interoceptive awareness are related to measures of depressed affect. The findings thus support a triangular relationship between GABA/NAA, depressed affect and interoceptive processing in the left insula. This may have implications for psychiatric disorders, such as depression, in which alterations to all three aspects of this triangle – GABA levels, IA-related activity, and depressed affect – can be observed.
Supplementary Material
Supplementary figure 1: A) Placement of the mPFC MRS voxel.
B) Bars show extracted signal changes from the mPFC MRS voxel. A paired t-test (two-tailed) shows no difference between signal changes for IA (red) and EA (yellow) (mean ± SEM, n = 9 participants) (Suppl. table 1B).
C) The region of the mPFC MRS voxel was applied to an independent data sample (n = 30 healthy participants) that used the same fMRI paradigm (see also Suppl. table 1B). Paired t-tests (two-tailed) between IA (red) and EA (yellow) confirm results seen in B.
D) Voxel-wise regressions within the mPFC MRS voxel show a negative relationship between GABA/NAA and EA (yellow, n = 9, cluster P FWE ≤ 0.02).
E) Voxel-wise regressions within the mPFC MRS voxel show a positive relationship between BHS and IA (red, n = 24, cluster P unc. ≤ 0.08, Suppl. table 1B & Suppl. table 3B).
Supplementary figure 2: Individual spectra of n = 15 participants acquired in the insula MRS voxel using MEGA-PRESS (Mescher et al., 1998; Marjanska et al., 2007).
Supplementary table 1: A) Concentrations of brain metabolites (subscripted numbers indicate number of participants), Cramer-Rao lower bounds (CRLB) and correlation coefficients between Glu/Gln from the MRS voxel in left insula and mPFC (mean ± SD, min/max).
B) Signal changes for IA and EA from the insula and mPFC MRS voxels (mean ± SD). Values between IA and EA are compared by a paired t-test (two-tailed) (* P ≤ 0.001, (*) P ≤ 0.1, / P > 0.1). The size of subject groups is based on number of participants for this study as well as an independent study (n = 30).
Supplementary table 2: Neurochemical values (subscripted numbers indicate number of participants) and BHS scores were used as regressors in SPM analysis. Positive correlations are marked in purple, negative correlations in blue.
A) Results for the insula (small volume corrected for the MRS voxel in the left insula) (cluster *** P FWE ≤ 0.005, ** P FWE ≤ 0.01, * P FWE ≤ 0.05, (*) P FWE ≤ 0.1).
B) Results for the mPFC (small volume corrected for the MRS voxel in the mPFC, P-values as in A).
C) Whole brain regressions using GABA/NAA values from the insula as regressor (masked by a grey matter mask). Reported results are restricted to (cluster P FWE ≤ 0.06). All peaks were assigned to the most probable brain area by using the SPM Anatomy Toolbox (Eickhoff et al., 2007) and the WFU pickatlas (Maldjian et al., 2003, 2004) (** P FWE ≤ 0.01, * P FWE ≤ 0.05, (*) P FWE ≤ 0.1).
Acknowledgments
The authors would like to thank O. Lyttelton and the staff from the MNI as well as from the University of Montréal for their excellent technical support. Thanks also to K. Dedovic and A. Perna for helping with participant recruitment and screening procedures. The authors thank Edward J. Auerbach, Ph.D. (Center for Magnetic Resonance Research, University of Minnesota) for implementing the MEGA-PRESS sequence on Siemens, and Romain Valabregue, Ph.D. (Centre de NeuroImagerie de Recherche, Paris, France) for developing processing tools. MM acknowledges support from Biotechnology Research Center grant P41 RR008079 (NCRR) and P41 EB015894 (NIBIB), and NCC P30 NS057091. GN acknowledges support from the Hope of Depression Research Foundation (HDRF), the Canadian Institutes of Health Research (CIHR) and the EJLB-Michael Smith Foundation (CIHR-EJLB).
References
- Alcaro A, Panksepp J, Witczak J, Hayes DJ, Northoff G. Is subcortical-cortical midline activity in depression mediated by glutamate and GABA? A cross-species translational approach. Neurosci Biobehav Rev. 2010;34:592–605. doi: 10.1016/j.neubiorev.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7:254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Weissman A, Lester D, Trexler L. The measurement of pessimism: the hopelessness scale. J Consult Clin Psychol. 1974;42:861–5. doi: 10.1037/h0037562. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE T Med Imaging. 2004;23:137–52. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabrgue R, Poline JB. Region of interest analysis using an SPM toolbox. Neuroimage; Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2-6, 2002; Sendai, Japan. 2002. pp. 210–217. [Google Scholar]
- Casey BJ, Cohen JD, O'Craven K, Davidson RJ, Irwin W, Nelson CA, Noll DC, Hu X, Lowe MJ, Rosen BR, Truwitt CL, Turski PA. Reproducibility of fMRI results across four institutions using a spatial working memory task. Neuroimage. 1998;8:249–261. doi: 10.1006/nimg.1998.0360. [DOI] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. NeuroImage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the Role of the Insula in Human Cognition: Functional Parcellation and Large-Scale Reverse Inference. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–95. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Levinson AJ, Daskalakis ZJ. Evidence for GABAergic inhibitory deficits in major depressive disorder. Neurosci Biobehav Rev. 2011;35:818–25. doi: 10.1016/j.neubiorev.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey Ka. Cerebral cortex. Vol. 21. New York, N.Y.: 2011. Three systems of insular functional connectivity identified with cluster analysis; pp. 1498–506. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. NeuroImage. 2010;53:392–8. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Enzi B, Wiebking C, Northoff G. Involvement of glutamate in rest-stimulus interaction between perigenual and supragenual anterior cingulate cortex: A combined fMRI-MRS study. Hum Brain Mapp. 2011;32:2172–82. doi: 10.1002/hbm.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn BD, Galton HC, Morgan R, Evans D, Oliver C, Meyer M, Cusack R, Lawrence AD, Dalgleish T. Listening to your heart. How interoception shapes emotion experience and intuitive decision making. Psychol Sci. 2010;21:1835–44. doi: 10.1177/0956797610389191. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Falkenberg LE, Westerhausen R, Specht K, Hugdahl K. Resting-state glutamate level in the anterior cingulate predicts blood-oxygen level-dependent response to cognitive control. Proc Natl Acad Sci U S A. 2012:1–6. doi: 10.1073/pnas.1115628109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farb NaS, Segal ZV, Anderson AK. Cerebral cortex. New York, N.Y.: 2012. Attentional Modulation of Primary Interoceptive and Exteroceptive Cortices; pp. 1–13. 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Fletcher P, Josephs O, Holmes A, Rugg MD, Turner R. Event-related fMRI: characterizing differential responses. Neuroimage. 1998;7:30–40. doi: 10.1006/nimg.1997.0306. [DOI] [PubMed] [Google Scholar]
- Geurts JJG, Barkhof F, Castelijns Ja, Uitdehaag BMJ, Polman CH, Pouwels PJW. Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: metabolite concentrations, quantification precision, and reproducibility. Journal of Magnetic Resonance Imaging. 2004;20:366–71. doi: 10.1002/jmri.20138. [DOI] [PubMed] [Google Scholar]
- Gountouna VE, Job DE, McIntosh AM, Moorhead TW, Lymer GK, Whalley HC, Hall J, Waiter GD, Brennan D, McGonigle DJ, Ahearn TS, Cavanagh J, Condon B, Hadley DM, Marshall I, Murray AD, Steele JD, Wardlaw JM, Lawrie SM. Functional Magnetic Resonance Imaging (fMRI) reproducibility and variance components across visits and scanning sites with a finger tapping task. Neuroimage. 2010;49:552–560. doi: 10.1016/j.neuroimage.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Grimm S, Beck J, Schuepbach D, Hell D, Boesiger P, Bermpohl F, Niehaus L, Boeker H, Northoff G. Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry. 2008;63:369–376. doi: 10.1016/j.biopsych.2007.05.033. [DOI] [PubMed] [Google Scholar]
- Grimm S, Ernst J, Boesiger P, Schuepbach D, Boeker H, Northoff G. Reduced negative BOLD responses in the default-mode network and increased self-focus in depression. World J Biol Psychiatry. 2011;12:627–37. doi: 10.3109/15622975.2010.545145. [DOI] [PubMed] [Google Scholar]
- Gruetter R, Tkác I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43:319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Pollatos O, Schandry R. Interoceptive sensitivity and emotion processing: an EEG study. Int J Psychophysiol. 2007;65:214–27. doi: 10.1016/j.ijpsycho.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Kaza E, Klose U, Lotze M. Comparison of a 32-channel with a 12-channel head coil: are there relevant improvements for functional imaging? J Magn Reson Imaging. 2011;34:173–183. doi: 10.1002/jmri.22614. [DOI] [PubMed] [Google Scholar]
- Kelly C, Toro R, Di Martino A, Cox CL, Bellec P, Castellanos FX, Milham MP. A convergent functional architecture of the insula emerges across imaging modalities. NeuroImage. 2012;61:1129–42. doi: 10.1016/j.neuroimage.2012.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189:233–45. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Singer T. The role of anterior insular cortex in social emotions. Brain Structure & Function. 2010;214:579–91. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- Liotti M, Mayberg HS, McGinnis S, Brannan SL, Jerabek P. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry. 2002;159:1830–1840. doi: 10.1176/appi.ajp.159.11.1830. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Marjanska M, Henry PG, Auerbach EJ, Franc D, Mueller B, Ugurbil K, Lim KO. Reproducibility of In Vivo GABA Quantification in Anterior Cingulate at 3 Tesla. Proc Intl Soc Mag Reson Med. 2007;15 [Google Scholar]
- McKeown MJ, Makeig S, Brown GG, Jung TP, Kindermann SS, Bell AJ, Sejnowski TJ. Analysis of fMRI data by blind separation into independent spatial components. Hum Brain Mapp. 1998;6:160–188. doi: 10.1002/(SICI)1097-0193(1998)6:3<160::AID-HBM5>3.0.CO;2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62:42–53. doi: 10.1016/j.neuropharm.2011.08.040. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumaraswamy SD, Evans CJ, Edden RAE, Wise RG, Singh KD. Individual variability in the shape and amplitude of the BOLD-HRF correlates with endogenous GABAergic inhibition. Hum Brain Mapp. 2012;33:455–65. doi: 10.1002/hbm.21223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Walter M, Schulte RF, Beck J, Dydak U, Henning A, Boeker H, Grimm S, Boesiger P. GABA concentrations in the human anterior cingulate cortex predict negative BOLD responses in fMRI. Nat Neurosci. 2007;10:1515–7. doi: 10.1038/nn2001. [DOI] [PubMed] [Google Scholar]
- O'Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. Journal of Magnetic Resonance Imaging. 2011;33:1262–1267. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Structure & Function. 2010;214:451–63. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Gramann K, Schandry R. Neural systems connecting interoceptive awareness and feelings. Hum Brain Mapp. 2007;28:9–18. doi: 10.1002/hbm.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Res. 2007;1141:178–87. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends Cogn Sci. 2012;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30:672–679. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14:260–4. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Raichle M. A paradigm shift in functional brain imaging. J Neurosci. 2009;29:12729–34. doi: 10.1523/JNEUROSCI.4366-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Avery Ja, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: Insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Human brain mapping. 2012 doi: 10.1002/hbm.22113. 000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. Journal of affective disorders. 2011;133:120–7. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The role of GABA in human motor learning. Current biology : CB. 2011;21:480–4. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O'Shea J, Wylezinska M, Kincses ZT, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bestmann S, Constantinescu aO, Moreno LM, Allman C, Mekle R, Woolrich M, Near J, Johansen-Berg H, Rothwell JC. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. The Journal of physiology. 2011;589:5845–55. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger JH. Tests for comparing elements of a correlation matrix. Psychological Bulletin. 1980;87:245–251. [Google Scholar]
- Sui J, Adali T, Pearlson GD, Calhoun VD. An ICA-based method for the identification of optimal FMRI features and components using combined group-discriminative techniques. NeuroImage. 2009;46:73–86. doi: 10.1016/j.neuroimage.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner P, Edden RaE, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: a neurochemical predictor of motor decision speed. Nature neuroscience. 2010;13:825–7. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Shibata M, Moriguchi Y, Umeda S. Anterior insular cortex mediates bodily sensibility and social anxiety. 2013 doi: 10.1093/scan/nss108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terhaar J, Viola FC, Bär KJ, Debener S. Heartbeat evoked potentials mirror altered body perception in depressed patients. Clin Neurophysiol. 2012;123:1950–7. doi: 10.1016/j.clinph.2012.02.086. [DOI] [PubMed] [Google Scholar]
- Tkác I, Starcuk Z, Choi IY, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–56. doi: 10.1002/(sici)1522-2594(199904)41:4<649::aid-mrm2>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality, and Social Cognition (a.k.a. Voodoo Correlations in Social Neuroscience) Perspectives on Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Bauer A, De Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J Biol Psychiatry. 2010;11:538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wiebking C, De Greck M, Duncan NW, Heinzel A, Tempelmann C, Northoff G. Are emotions associated with activity during rest or interoception? An exploratory fMRI study in healthy subjects. Neuroscience letters. 2011;491:87–92. doi: 10.1016/j.neulet.2011.01.012. [DOI] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Qin P, Hayes DJ, Lyttelton O, Gravel P, Verhaeghe J, Kostikov AP, Schirrmacher R, Reader AJ, Bajbouj M, Northoff G. External awareness and GABA - A multimodal imaging study combining fMRI and [18F]flumazenil-PET. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiens S. Interoception in emotional experience. Current opinion in neurology. 2005;18:442–7. doi: 10.1097/01.wco.0000168079.92106.99. [DOI] [PubMed] [Google Scholar]
- Williams EJ. The comparison of regression variables. Journal of the Royal Statistical Society. 1959;21:396–399. [Google Scholar]
- Woolrich M, Jbabdi S, Patenaude B, Chappell M. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage. 2012;62:493–9. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bao AM, Qi XR, Kamphuis W, Luchetti S, Lou JS, Swaab DF. Gene expression of GABA and glutamate pathway markers in the prefrontal cortex of non-suicidal elderly depressed patients. J Affect Disord. 2012;138:494–502. doi: 10.1016/j.jad.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Zou KH, Greve DN, Wang M, Pieper SD, Warfield SK, White NS, Manandhar S, Brown GG, Vangel MG, Kikinis R, Wells WM., 3rd Reproducibility of functional MR imaging: preliminary results of prospective multi-institutional study performed by Biomedical Informatics Research Network. Radiology. 2005;237:781–789. doi: 10.1148/radiol.2373041630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Q, Wu CW, Stein EA, Zang Y, Yang Y. Static and dynamic characteristics of cerebral blood flow during the resting state. NeuroImage. 2009;48:515–24. doi: 10.1016/j.neuroimage.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: A) Placement of the mPFC MRS voxel.
B) Bars show extracted signal changes from the mPFC MRS voxel. A paired t-test (two-tailed) shows no difference between signal changes for IA (red) and EA (yellow) (mean ± SEM, n = 9 participants) (Suppl. table 1B).
C) The region of the mPFC MRS voxel was applied to an independent data sample (n = 30 healthy participants) that used the same fMRI paradigm (see also Suppl. table 1B). Paired t-tests (two-tailed) between IA (red) and EA (yellow) confirm results seen in B.
D) Voxel-wise regressions within the mPFC MRS voxel show a negative relationship between GABA/NAA and EA (yellow, n = 9, cluster P FWE ≤ 0.02).
E) Voxel-wise regressions within the mPFC MRS voxel show a positive relationship between BHS and IA (red, n = 24, cluster P unc. ≤ 0.08, Suppl. table 1B & Suppl. table 3B).
Supplementary figure 2: Individual spectra of n = 15 participants acquired in the insula MRS voxel using MEGA-PRESS (Mescher et al., 1998; Marjanska et al., 2007).
Supplementary table 1: A) Concentrations of brain metabolites (subscripted numbers indicate number of participants), Cramer-Rao lower bounds (CRLB) and correlation coefficients between Glu/Gln from the MRS voxel in left insula and mPFC (mean ± SD, min/max).
B) Signal changes for IA and EA from the insula and mPFC MRS voxels (mean ± SD). Values between IA and EA are compared by a paired t-test (two-tailed) (* P ≤ 0.001, (*) P ≤ 0.1, / P > 0.1). The size of subject groups is based on number of participants for this study as well as an independent study (n = 30).
Supplementary table 2: Neurochemical values (subscripted numbers indicate number of participants) and BHS scores were used as regressors in SPM analysis. Positive correlations are marked in purple, negative correlations in blue.
A) Results for the insula (small volume corrected for the MRS voxel in the left insula) (cluster *** P FWE ≤ 0.005, ** P FWE ≤ 0.01, * P FWE ≤ 0.05, (*) P FWE ≤ 0.1).
B) Results for the mPFC (small volume corrected for the MRS voxel in the mPFC, P-values as in A).
C) Whole brain regressions using GABA/NAA values from the insula as regressor (masked by a grey matter mask). Reported results are restricted to (cluster P FWE ≤ 0.06). All peaks were assigned to the most probable brain area by using the SPM Anatomy Toolbox (Eickhoff et al., 2007) and the WFU pickatlas (Maldjian et al., 2003, 2004) (** P FWE ≤ 0.01, * P FWE ≤ 0.05, (*) P FWE ≤ 0.1).


