Abstract
Objective
We sought to evaluate body composition in children and young adults with Fontan physiology. Leg lean mass (LM) deficits correlate with diminished exercise capacity in other populations and may contribute to exercise limitations in this cohort.
Methods
This cross-sectional study included whole body dual energy X-ray absorptiometry scans in 50 Fontan participants ≥5 years, and measures of peak oxygen consumption (VO2) in 28. Whole body and leg LM (a measure of skeletal muscle) were converted to sex- and race-specific Z-scores, relative to age and stature, based on 992 healthy reference participants.
Results
Median age was 11.5 (range 5.1–33.5) years at 9.3 (1.1–26.7) years from Fontan. Height Z-scores were lower in Fontan compared with reference participants (−0.47±1.08 vs 0.25±0.93, p<0.0001). Body mass index Z-scores were similar (0.15±0.98 vs 0.35±1.02, p=0.18). LM Z-scores were lower in Fontan compared with reference participants (whole body LM −0.33±0.77 vs 0.00±0.74, p=0.003; leg LM −0.89 ±0.91 vs 0.00±0.89, p<0.0001). LM Z-scores were not associated with age or Fontan characteristics. Leg LM Z-scores were lower in vitamin D deficient versus sufficient Fontan participants (−1.47±0.63 vs −0.71±0.92, p=0.01). Median per cent predicted peak VO2 was 81% (range 13%–113%) and was associated with leg LM Z-scores (r=0.54, p=0.003).
Conclusions
Following Fontan, children and young adults are shorter than their peers and have significant LM deficits. Skeletal muscle deficits were associated with vitamin D deficiency and reduced exercise capacity. Future studies should examine the progression of these deficits to further understand the contribution of peripheral musculature to Fontan exercise capacity.
INTRODUCTION
Over the four decades since its initial description, outcomes following the Fontan operation have improved dramatically. Surgical mortality is now less than 5%,1 and transplant-free survival for the earliest Fontan patients is greater than 80% over a 15–20-year period.2 As survival improves, focus has rightly shifted toward the long-term consequences of this abnormal physiology. The impact of Fontan physiology on growth and body composition (allocation of lean mass and fat mass) has not been addressed in the paediatric Fontan population. Young Fontan patients have multiple risk factors for abnormal body composition including pubertal delay, abnormalities of endocrine growth factors, vitamin D and other nutritional deficiencies, and insufficient physical activity.3
Despite these risk factors, existing paediatric literature is limited to anthropometric studies demonstrating poor longitudinal growth4–6 and low body mass index (BMI) in Fontan patients compared with the general population.6,7 However, specific abnormalities in lean mass and fat mass and the relationship between body composition and functional outcomes have not been characterised in paediatric Fontan patients. Dual energy X-ray absorptiometry (DXA) is a tool that can provide precise whole body and regional measurements of lean and fat mass.8 This technique has been used to investigate abnormalities in body composition in other paediatric chronic diseases9–11 and in adult Fontan patients12 but has not been used to evaluate body composition in children with Fontan physiology.
Paediatric growth, BMI (kg/m2) and body composition data are typically reported as Z-scores. A Z-score, or SD score, represents the number of SDs above or below the expected median value for age and sex. Based on a normal distribution, a Z-score of 0 means that an individual is at the 50th percentile, while a Z-score of −1 or +1 indicates the 16th or 84th percentile, respectively.
The primary objectives of this study were to characterise lean and fat mass Z-scores in children, adolescents and young adults after Fontan palliation compared with healthy reference participants; to identify risk factors for abnormalities of body composition; and to evaluate the association between lean mass deficits and exercise capacity.
METHODS
Study participants
Fontan participants age ≥5 years were prospectively enrolled in a cross-sectional study from July 2011 through October 2013. Subjects were eligible if they had single ventricle physiology and undergone Fontan palliation. Exclusion criteria included: pregnancy; pacemaker, defibrillator or metal hardware that prevented cardiac MRI or DXA; Fontan baffle obstruction or single lung physiology; moderate to severe chronic kidney disease; moderate to severe hepatic impairment; and inability to complete the study procedures due to significant developmental delay. Fontan participants were compared with a previously described cohort of healthy reference participants (ages 5–30 years) from the greater Philadelphia area from whom anthropometric measure as well as measures of body composition were collected.9,13,14 The study protocol was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia.
Anatomic and Fontan characteristics
Pertinent variables including cardiac anatomy, presence of heterotaxy syndrome, diagnosis of a genetic syndrome, date and type of Fontan, presence of a fenestration, and diagnosis of protein losing enteropathy (PLE) were obtained through patient interviews and confirmed in the medical record. Medications and nutritional supplements were reviewed. The most recent echocardiogram, cardiac MRI and cardiopulmonary stress test relative to study enrolment were reviewed.
Anthropometry and pubertal development
Anthropometric measures were obtained in light clothing with shoes removed. Weight (0.1 kg) was measured using a digital scale (Scaltronix, White Plains, New York, USA). Height and sitting height (0.1 cm) were measured using a stadiometer (Holtain, Croswell, Crymych, UK) and used to calculate leg length (leg length=height − sitting height). Pubertal status (Tanner stage) was assessed with a validated self-assessment questionnaire.15
DXA scans
Whole body lean and fat mass (kg) were measured with a Hologic Delphi densiometer (Bedford, Massachusetts, USA) with a fan beam in the array mode (software V.12.4), excluding the head. Measurements were performed with standard supine positioning techniques. Lean mass was calculated as fat-free mass minus bone mineral content. Whole body lean mass may not be a true representation of muscle mass because it also includes organ mass, vasculature, interstitial space and other components; therefore, leg lean mass was used as a measure of skeletal muscle. Calibration was performed daily with a hydroxyapatite phantom and weekly with a whole-body phantom. Coefficients of variation ranged from 1% to 4%.
Laboratory studies
Serum albumin and brain natriuretic peptide were measured using standard techniques. Quantification of circulating 25(OH) vitamin D was performed by tandem mass-spectrometry, as previously described.16 Fontan participants were considered vitamin D deficient if serum level was less than 20 ng/mL.17 Insulin-like growth factor-1 was measured by Esoterix Laboratory Services using a radioimmunoassay. Insulin-like growth factor-1 levels were converted to sex-, age- and Tanner-specific Z-scores using Esoterix laboratory’s reference data. Adult participants were considered to be 18 years old.
Dietary assessment
The 24-h dietary recalls were performed interactively by paediatric registered dieticians. Energy intake was expressed as percentage of expected energy requirement for ‘sedentary’ individuals of a given age and sex. Protein was expressed as a percentage of the recommended dietary allowance based on age and sex.
Non-invasive imaging and measures of VO2
Echocardiograms were performed on a Phillips IE33 machine (Phillips, Andover, Massachusetts, USA) according to our standard imaging protocol. Five or 8 MHz transducers were used according to patient’s size and acoustic windows. Cardiac MRI studies were performed on a 1.5 T Avanto MRI scanner (Siemens Medical Solutions, Erlangen, Germany) with a 6-channel phased-array body coil using our standard imaging protocol. Phase contrast velocity mapping was used to determine the summed caval flow and indexed to body surface area. Cardiopulmonary exercise test were performed on an electronically braked cycle ergometer (Ergometrics 800, Sensor-Medics, Yorba Linda, California, USA) or a 1 min incremental treadmill according to our standard protocol. Metabolic data were obtained throughout the study and for the first 2 min of recovery on a breath-by-breath basis using a metabolic cart (SensorMedics V29, Yorba Linda, California, USA). Oxygen consumption at maximum exertion (peak VO2) and the anaerobic threshold was normalised to the percentage expected for age, gender and body weight.18
Statistical analysis
Analyses were performed using Stata V.12.0 (Stata Corp., College Station, Texas, USA). A p value <0.05 was considered significant and two-sided tests were used throughout. Continuous variables were expressed as means±SD or median (range). Group differences in continuous variables (eg, height, BMI and body composition Z-scores) between Fontan and reference participants were assessed using Student t test as all of these variables demonstrated a normal distribution. Analyses within the Fontan group included correlations between body composition Z-scores and continuous variables assessed by Pearson or Spearman rank correlations (if not normally distributed), and comparisons of Z-scores according to categorical variables such as the presence of PLE or vitamin D deficiency (defined as 25(OH) vitamin D <20 ng/mL consistent with the Institute of Medicine definition).
Growth and body composition variables were converted to Z-scores (SD scores). The 2000 National Center for Health Statistics growth data were used to calculate sex-specific Z-scores for height, weight and BMI relative to age.19 DXA body composition data from the healthy reference participants were used to convert the measures in the Fontan participants to sex- and race-specific Z-scores relative to age using the LMS method.20 Body composition measures are highly correlated with height (r=0.95 and 0.56 for the correlations of height with whole body lean and fat mass, respectively, in the reference participants (p<0.0001 for both)) and Fontan physiology is associated with impaired linear growth. Therefore, the whole body lean and fat mass Z-scores were then adjusted for height Z-score, and the leg lean and fat mass Z-scores were adjusted for leg length Z-score.21
RESULTS
Subject characteristics
Table 1 summarises the demographic and anthropometric characteristics of the 50 Fontan participants. The Fontan cohort was 52% male participants with a median age of 11.5 (range 5.1–33.5) years at 9.3 (1.1–26.7) years from Fontan. Across the population, Fontan participants had lower mean height Z-scores compared with reference participants (−0.47±1.08 vs 0.25 ±0.93, p<0.0001) and Fontan participants exhibited significantly delayed puberty; they were older than reference participants within Tanner stages 2, 3 and 4, adjusted for sex and race (p=0.001). Though height Z-scores were diminished, BMI Z-scores did not differ between the two groups (0.15 ±0.98 vs 0.35±1.02, p=0.18).
Table 1.
Demographic and anthropometric characteristics of the Fontan participants
| Variable | Fontan participants |
|---|---|
| N | 50 |
| Age, median (range) year | 11.5 (5.1–33.5) |
| Interval from Fontan, median (range) year | 9.3 (1.1–26.7) |
| Male, n (%) | 26 (52) |
| Race, n (%) | |
| White | 34 (68) |
| Black | 9 (18) |
| Other | 7 (14) |
| Tanner stage 1–2, n (%) | 27 (54) |
| Height Z-score, mean±SD | −0.47±1.08 |
| Range | −4.30 to 0.95 |
| BMI Z-score, mean±SD | 0.15±0.98 |
| Range | −2.49 to 2.24 |
BMI, body mass index.
The disease-specific characteristics of the Fontan participants are summarised in table 2. Single right ventricular physiology was most common (44%), and the most common cardiac diagnosis was hypoplastic left heart syndrome (36%). Four patients (8%) had heterotaxy syndrome. One patient had Turner syndrome; another had VATER association. Four patients (8%) had been diagnosed with PLE at the time of the study visit (serum albumin 1.7–2.5 g/dL) and two were on systemic corticosteroids. Twelve patients (24%) were vitamin D deficient with levels <20 ng/mL. Black participants were more commonly vitamin D deficient compared with non-black participants (56% vs 17%, p=0.01).
Table 2.
Fontan clinical characteristics
| Variable | Value |
|---|---|
| Anatomy, n (%) | |
| HLHS | 18 (36) |
| Tricuspid atresia | 8 (16) |
| Unbalanced AV canal defect | 8 (16) |
| Other single ventricle variants | 8 (16) |
| DORV | 6 (12) |
| PA/IVS | 2 (4) |
| Heterotaxy syndrome, n (%) | 4 (8) |
| Ventricular morphology, n (%) | |
| RV | 22 (44) |
| LV | 16 (32) |
| Mixed | 12 (24) |
| Type of Fontan, n (%) | |
| Extracardiac conduit | 29 (58) |
| Lateral tunnel | 21 (42) |
| Fenestration, n (%)* | 42 (84) |
| Diagnosis of PLE, n (%) | 4 (8) |
| Dietary intake | |
| % EER | 108 (26–192) |
| % RDA protein | 184 (35–487) |
| Medications, n (%) | |
| Aspirin | 46 (92) |
| ACE inhibitor | 24 (48) |
| Sildenafil | 7 (14) |
| Diuretic | 5 (10) |
| Aldosterone antagonist | 5 (10) |
| Corticosteroids | 2 (4) |
| Supplements, n (%) | |
| Calcium | 15 (30) |
| Vitamin D | 24 (48) |
| Laboratory values | |
| Albumin (g/dL) | 4.6 (1.7–5.3) |
| Intact PTH (pg/mL) | 49 (12.3–236) |
| Vitamin (OH) D (ng/mL) | 30.1 (4.5–64.2) |
| BNP (pg/mL) | 19.5 (10–246.8) |
| IGF-1 Z-score | −0.34 (−2.7 to 3) |
| Echocardiographic assessment, n (%)† | |
| Normal or low normal ventricular function | 35 (80) |
| Absent or mild AV valve regurgitation | 37 (84) |
| Indexed systemic flow on CMR (L/min/m2)‡ | 2.3 (1.9–3.3) |
Results expressed as median (range) or n (%).
At time of initial operation.
N=44.
N=20.
AV, atrioventricular valve; BNP, brain type natriuretic peptide; CMR, cardiac magnetic resonance; DORV, double outlet RV; EER, expected energy requirement; HLHS, hypoplastic left heart syndrome; IGF-1, insulin-like growth factor-1; PA/IVS, pulmonary atresia/intact ventricular septum; PLE, protein losing enteropathy; PTH, parathyroid hormone; RDA, recommended dietary allowance.
Body composition and growth
Whole body lean mass Z-scores were lower in the Fontan participants compared with reference participants (−0.33±0.77 vs 0.00 ±0.74, p=0.003) (figure 1). Whole body fat mass Z-scores did not differ (0.22±0.99 in Fontan participants vs 0.02±0.95 in reference participants, p=0.1). Leg lean mass Z-scores were substantially lower in Fontan participants (−0.89±0.91 vs 0.00±0.89, p<0.0001) compared with reference participants (figure 1). The odds of leg lean mass Z-score less than the 5th percentile were 6.5 times greater in Fontan participants compared with reference participants (OR 6.5, 95% CI 2.9 to 14.5, p<0.0001).
Figure 1.
The box plots represent the Z-score distribution for whole body and leg lean mass in Fontan participants. The horizontal line within each box plot represents the median Z-score (50th percentile) for the Fontan participants; the median Z-score value is provided on the plot. The top and bottom of the boxes represent the 75th and 25th percentiles, and the whiskers represent 2 SDs from the mean Z-score. The black horizontal line at Z=0 represents the Z-score of zero in the reference participants; equivalent to the 50th percentile for age. The p values for comparison of Z-scores between Fontan and reference participants are provided below each plot.
Lean mass Z-scores were not associated with age, delayed puberty, time from Fontan, cardiac anatomy, Fontan characteristics, presence of heterotaxy syndrome, medications, dietary energy or protein intake, insulin-like growth factor-1 Z-scores, brain natriuretic peptide level, qualitative ventricular function or atrioventricular valve regurgitation on echocardiogram, or indexed systemic flow measured on cardiac MRI.
Whole body lean mass tended to be lower in vitamin D deficient participants, although the difference was not significant (−0.65±0.58 vs −0.22±0.80, p=0.09). However, leg lean mass Z-scores were significantly lower in vitamin D deficient Fontan participants compared with those with adequate vitamin D levels (−1.47±0.63 vs −0.71±0.92, p=0.01) (figure 2).
Figure 2.
Leg lean mass Z-scores were worse in Fontan participants with vitamin D deficiency compared with those with adequate vitamin D levels. The box plots represent the Z-score distribution for leg lean mass in Vitamin D sufficient versus deficient Fontan participants. The horizontal line within each box plot represents the median Z-score (50th percentile) for the group; the median Z-score value is provided on the plot. The top and bottom of the boxes represent the 75th and 25th percentiles, and the whiskers represent 2 SDs from the mean Z-score. The p value for comparison of Z-scores between the two groups is provided on the figure.
Participants whose Fontan course was complicated by PLE were shorter compared with the rest of the Fontan cohort (height Z-score −1.73±0.72 vs −0.36±1.04, p=0.01). Leg lean mass Z-scores tended to be lower in Fontan participants with PLE compared with those without, although this difference was not statistically significant (−1.45±0.70 vs −0.8±0.92, p=0.2).
Lean mass and exercise capacity
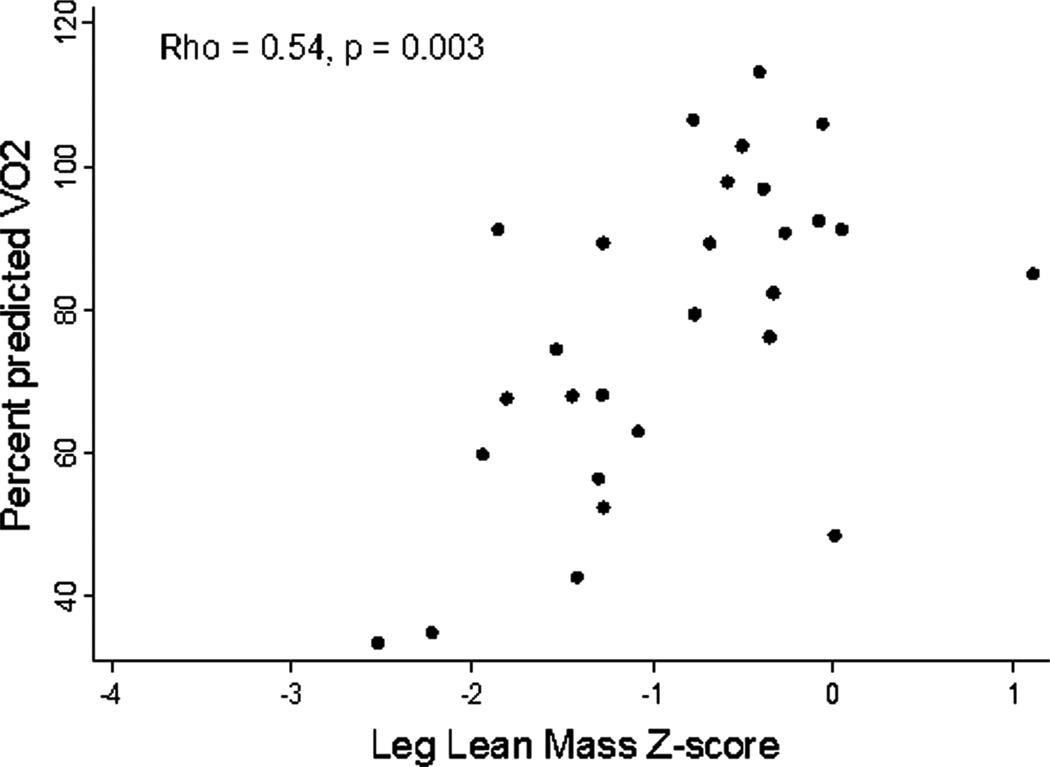
Cardiopulmonary exercise test measurements are summarised in table 3. Leg lean mass Z-score was associated with peak VO2 (Spearman’s r=0.53, p=0.004) and per cent predicted peak VO2 (Spearman’s r=0.54, p=0.003) on cardiopulmonary exercise test (figure 3). There was no association between leg lean mass Z-score and VO2 at the anaerobic threshold (p=0.72) or per cent predicted VO2 at the anaerobic threshold (p=0.68). Whole body lean mass Z-score was not associated with any measures of exercise capacity. The group differences between Fontan and reference participants and differences within Fontan participants remained significant when patients with PLE or genetic syndromes were excluded from analyses.
Table 3.
Oxygen consumption on CPET (n=28)
| Variable | Range |
|---|---|
| Measurements at peak exercise | |
| Oxygen consumption, mL/kg/min | 31 (16.7–44.5) |
| Per cent predicted VO2, % | 81 (33–113) |
| Measurements at anaerobic threshold | |
| Oxygen consumption, mL/kg/min | 17 (11.5–27) |
| Per cent predicted VO2, % | 77 (43–142) |
Results expressed as median (range).
CPET, cardiopulmonary exercise test.
Figure 3.
In the Fontan participants who completed exercise testing, leg lean mass Z-score was associated with per cent predicted peak VO2.
DISCUSSION
In this study, we demonstrate that children, adolescents and young adults with Fontan physiology have impaired growth and pubertal development as well as alterations in body composition with modest deficits in whole body lean mass and marked deficits in leg lean mass relative to a healthy reference population.9,13,14 These findings extend our recognition of the non-cardiac sequelae of the Fontan circulation during growth and development, and demonstrate the inadequacies of BMI as the sole index of nutritional status in this population. In addition, the association between leg lean mass deficits and decreased exercise capacity in this largely paediatric Fontan population adds to existing data in adult Fontan patients.12
Growth, body composition and vitamin D status
Fontan participants in this study demonstrated growth failure, delayed puberty and low insulin-like growth factor-1 Z-scores. These findings are consistent with previous reports of poor longitudinal growth in Fontan patients.4–6 Insufficient levels of insulin-like growth factor-1 have been demonstrated in infants and children with congenital heart disease;22 however, studies are limited by the small number of patients with Fontan physiology. Although insulin-like growth factor-1 is an important determinant of lean mass accrual with growth and development, we were unable to detect associations between insulin-like growth factor-1 and lean mass or height Z-scores, perhaps as a result of the cross-sectional design.
The discrepancy between whole body and leg lean mass deficits in Fontan participants is noteworthy. Since the measurement of whole body lean mass also includes organ mass, vasculature, interstitial space and other components, the measurement may be confounded by organomegaly or oedema. Given that passive congestion of the liver, spleen or intestinal tract as a result of elevated central venous pressure is an inherent characteristic of Fontan physiology, leg lean mass Z-scores may provide a more accurate representation of metabolically active lean mass in these patients. This is supported by the observation that vitamin D deficiency and lower VO2 were significantly associated with leg lean mass Z-scores, but not whole body lean mass Z-scores. Additionally, peripheral muscle contraction is more important for the augmentation of systemic venous return in the Fontan circulation, and therefore leg lean mass may actually be the more relevant measurement.
In our cohort, there was a trend toward an increase in whole body fat mass in the Fontan participants relative to reference participants. This increase in adiposity in conjunction with the impact of venous congestion on whole body lean mass may account for the absence of statistically significant differences in BMI Z-score between the Fontan and reference participants. These data suggest that BMI may be a misleading assessment of body composition in this population as it cannot distinguish between lean mass deficits and marginal excess adiposity.
This study did not identify cardiac-specific risk factors for lean mass deficits, again, potentially due to the cross-sectional design, although participants with protein-losing enteropathy tended to have markedly lower leg lean mass Z-scores compared with participants without protein-losing enteropathy. However, the number of subjects with protein losing enteropthy was small, and this numerically large difference did not reach statistical significance.
While vitamin D deficiency is relatively common in the general population, a full quarter of the Fontan cohort in this study was vitamin D deficient. Vitamin D is essential to calcium transport and protein synthesis in the muscle.23 Low vitamin D status has been associated with decreased skeletal muscle mass and impaired physical performance in both healthy young adults24 and frail elderly adults25 while vitamin D supplementation has been shown to increase muscle fibre size in mobility limited older women.26 It is not clear from this study whether vitamin D deficiency is a cause of lean mass deficits or a marker of disease burden associated with lean mass deficits, but there were clearly profound deficits in our Fontan cohort.
Lean mass and exercise capacity
The relationship between leg lean mass deficits and exercise capacity in our study is impressive and in agreement with other studies. A prior study of 16 adults (age 30±2 years) demonstrated a mean appendicular lean mass index (kg/m2) Z-score of −1.46±0.22 (p<0.0001). In this study, lean mass deficits were also associated with VO2 max (r 0.67).12 In addition, MR spectroscopy measures of phosphocreatine resynthesis in eight participants revealed impaired aerobic capacity, suggesting intrinsic muscle deficits above and beyond the muscle mass deficits. The more profound deficits in these adults compared with our younger cohort are likely related to the progression of muscle wasting over time. In a related study by the same investigators, high intensity resistance training over a 20-week interval in 11 adults with Fontan circulation resulted in significant improvements in muscle mass and strength, and was associated with improved stroke volume, cardiac output and exercise capacity.27
These findings suggest that muscle mass is important to peak exercise capacity in the paediatric Fontan circulation and that the peripheral musculature may be targeted to improve exercise capacity in young Fontan patients. While peak VO2 in Fontan patients is typically 60%–65% of predicted value for age and gender,28 there is considerable variability in patients’ exercise capacities that is not solely explained by cardiorespiratory factors.29 VO2 declines progressively at a rate of 2.6% per year during the second decade after Fontan.28 When VO2 falls below about 50% predicted for age and gender, the risk of symptomatic heart failure and sudden death significantly increases in congenital heart disease patients, specifically those with Fontan physiology.30 Some aspects of diminished exercise capacity are likely related to the inherent characteristics of Fontan physiology, but some may be modifiable. Longitudinal studies are needed to identify therapies that may improve exercise capacity and augment forward flow in the Fontan circulation in children and young adults with this physiology.
Limitations
The primary limitations of this study are the cross-sectional design, the considerable heterogeneity in age, and the absence of cardiac MRI and VO2 data in some patients. Longitudinal studies may identify a correlation between progressive lean mass deficits and progressive exercise impairment. Additionally, the absence of VO2 measures in the reference participants did not allow us to determine the degree to which adjustment for lean mass would explain the decrements in exercise capacity in patients with Fontan circulation.
CONCLUSIONS
In conclusion, this study demonstrated growth and pubertal delay and muscle deficits in children, adolescents and young adults with Fontan physiology. Importantly, worse skeletal muscle deficits were associated with worse functional outcome as characterised by decreased exercise capacity. Longitudinal studies are necessary to characterise the change in lean mass deficits over time, further understand the metabolic implications of these deficits, and identify therapies to promote muscle mass and function in paediatric and young adult Fontan patients.
Key messages.
What is known on this subject?
Body composition may be abnormal in children and young adults with Fontan physiology. Lean mass deficits correlate with diminished exercise capacity in the adult Fontan population and may contribute to exercise limitations in the younger cohort.
What might this study add?
This study demonstrates poor growth, pubertal delay and marked lean mass deficits in children, adolescents and young adults with Fontan physiology. In addition, the association between leg lean mass deficits and decreased exercise capacity in this paediatric Fontan population is similar to that reported in adult Fontan patients.
How might this impact on clinical practice?
Future studies may identify therapies that promote muscle mass and function in paediatric Fontan patients and may improve exercise capacity through modification of the peripheral musculature.
Acknowledgments
Funding This study was funded by a Children’s Hospital of Philadelphia Cardiac Center Grant. It was also supported by the Robert S and Dolores Harrington Endowment in Pediatric Cardiology at The Children’s Hospital of Philadelphia and by NIH grants T32 HL007915 (CMA), K23 HL089647 (KKW), K24 DK0768084 (MBL), R01 HL098252-01, and Clinical and Translational Science Award UL1 RR024134 and UL1 TR000003.
Footnotes
Contributors CMA (guarantor): conception and design, analysis and interpretation of data, drafting of the manuscript, revising the manuscript critically for important intellectual content, and final approval of the manuscript submitted. MBL, KKW and DJG: conception and design, analysis and interpretation of data, revising the manuscript critically for important intellectual content, and final approval of the manuscript submitted. BSZ and DL: analysis and interpretation of data, revising the manuscript critically for important intellectual content, and final approval of the manuscript submitted. JLB, EG and JR: conception and design, revising the manuscript critically for important intellectual content, and final approval of the manuscript submitted. KD, CH-R and SMP: revising the manuscript critically for important intellectual content and final approval of the manuscript submitted.
Competing interests None.
Ethics approval Institutional Review Board of the Children’s Hospital of Philadelphia.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 2.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 3.McCrindle BW, Williams RV, Mital S, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. doi: 10.1136/adc.2006.105239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogt KN, Manlhiot C, Van Arsdell G, et al. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MI, Bush DM, Ferry RJ, Jr, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MS, Zak V, Atz AM, et al. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098. 8e1. doi: 10.1016/j.ahj.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinto NM, Marino BS, Wernovsky G, et al. Obesity is a common comorbidity in children with congenital and acquired heart disease. Pediatrics. 2007;120:e1157–e1164. doi: 10.1542/peds.2007-0306. [DOI] [PubMed] [Google Scholar]
- 8.Ellis KJ. Human body composition: in vivo methods. Physiol Rev. 2000;80:649–680. doi: 10.1152/physrev.2000.80.2.649. [DOI] [PubMed] [Google Scholar]
- 9.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160:122–128. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethna CB, Salerno AE, McBride MG, et al. Cardiorespiratory fitness in pediatric renal transplant recipients. Transplantation. 2009;88:395–401. doi: 10.1097/TP.0b013e3181aed7d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster BJ, Kalkwarf HJ, Shults J, et al. Association of chronic kidney disease with muscle deficits in children. J Am Soc Nephrol. 2011;22:377–386. doi: 10.1681/ASN.2010060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordina R, O’Meagher S, Gould H, et al. Skeletal muscle abnormalities and exercise capacity in adults with a Fontan circulation. Heart. 2013;99:1530–1534. doi: 10.1136/heartjnl-2013-304249. [DOI] [PubMed] [Google Scholar]
- 13.Baker JF, Davis M, Alexander R, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53:34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, et al. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int. 2013;83:495–502. doi: 10.1038/ki.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris M, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adol. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 16.Saenger AK, Laha TJ, Bremner DE, et al. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 17.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 18.Cooper D, Weiler-Ravell D. Gas exchange response to exercise in children. Am Rev Respir Dis. 1984;129:S47–S48. doi: 10.1164/arrd.1984.129.2P2.S47. [DOI] [PubMed] [Google Scholar]
- 19.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 20.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 21.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy X-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soliman AT, Elawwa A, Khella A, et al. Linear growth in relation to the circulating concentration of insulin-like growth factor-I in young children with acyanotic congenital heart disease with left to right shunts before versus after surgical intervention. Indian J Endocrinol Metab. 2012;16:791–795. doi: 10.4103/2230-8210.100678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12:628–633. doi: 10.1097/MCO.0b013e328331c707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forney L, Earnest CP, Henagan TM, et al. Vitamin D status, body composition and fitness measures in younger, physically active individuals. J Strength Cond Res. 2014;28:814–824. doi: 10.1519/JSC.0b013e3182a35ed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tieland M, Brouwer-Brolsma EM, Nienaber-Rousseau C, et al. Low vitamin D status is associated with reduced muscle mass and impaired physical performance in frail elderly people. Eur J Clin Nutr. 2013;67:1050–1055. doi: 10.1038/ejcn.2013.144. [DOI] [PubMed] [Google Scholar]
- 26.Ceglia L, Niramitmahapanya S, Morais MD, et al. A randomized study on the effect of vitamin D3 supplementation on skeletal muscle morphology and vitamin D receptor concentration in older women. J Clin Endocrinol Metab. 2013;98:E1927–E1935. doi: 10.1210/jc.2013-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cordina RL, O’Meagher S, Karmali A, et al. Resistance training improves cardiac output, exercise capacity and tolerance to positive airway pressure in Fontan physiology. Int J Cardiol. 2012;168:780–788. doi: 10.1016/j.ijcard.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Giardini A, Hager A, Pace Napoleone C, et al. Natural history of exercise capacity after the Fontan operation: a longitudinal study. Ann Thorac Surg. 2008;85:818–821. doi: 10.1016/j.athoracsur.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Paridon SM, Mitchell PD, Colan SD, et al. A cross-sectional study of exercise performance during the first 2 decades of life after the Fontan operation. J Am Coll Cardiol. 2008;52:99–107. doi: 10.1016/j.jacc.2008.02.081. [DOI] [PubMed] [Google Scholar]
- 30.Diller GP, Giardini A, Dimopoulos K, et al. Predictors of morbidity and mortality in contemporary Fontan patients: results from a multicenter study including cardiopulmonary exercise testing in 321 patients. Eur Heart J. 2010;31:3073–3083. doi: 10.1093/eurheartj/ehq356. [DOI] [PubMed] [Google Scholar]