Abstract
Background
Innovative technologies for drug discovery and development, cancer models, stem cell research, tissue engineering, and drug testing in various cell-based platforms require an application similar to the in vivo system.
Materials and Methods
We developed for the first time nanomagnetically levitated three dimensional (3-D) cultures of breast cancer (BC) and colorectal cancer (CRC) cells using carbon encapsulated cobalt magnetic nanoparticles. BC and CRC xenografts grown in severe combined immunodeficient (SCID) mice were evaluated for N-cadherin and Epidermal growth factor receptor (EGFR) expressions. These phenotypes were compared with 2-D cultures and 3-D cultures grown in a gel matrix.
Results
The BC and CRC cells grown by magnetic levitation formed microtissues. The levitated cultures had high viability and were maintained in culture for long periods of time. It has been observed that N-cadherin and EGFR activities were highly expressed in the levitated 3-D tumor spheres and xenografts of CRC and BC cells.
Conclusions
Nanomagnetically levitated 3-D cultures tend to form stable microtissues of BC and CRC and may be more feasible for a range of applications in drug discovery or regenerative medicine.
Keywords: Nanomagnetic cell levitation, Scaffolds-free 3-D Cell cultures, Carbon encapsulated Cobalt, Cancer cells, Nanotechnology
1. Introduction
The advancement in cell culture methods from two dimensional (2-D) to three dimensional (3-D) is a big leap by demonstrating significant differences in cellular characteristics and behavior enabling the assessment of drug efficacy, pharmacokinetics and pharmacodynamics [1]. Using 3-D polymeric scaffolds, efforts have been made to achieve 3-D cell matrix structure with enhanced cell growth and improved functions [2], embryonic stem cell differentiation [3], active regulation of multi-cellular organization [4], cell migration [5] and angiogenic capability [6].
The three-dimensional polymeric systems have been shown to facilitate 3-D growth of cancer cells and tumors [7–9]. The use of 3-D scaffold materials such as hydrogel, matrigel and Alvetex polystyrene produces successful 3-D cultures but they have limitations and broad practical applications of such methods have not at been achieved. The use of these 3-D scaffold materials tend to act as mass transport barrier within the tissue construct resulting in impedance in drug delivery to target cells, limits nutrient supply to these cells and allows the accumulation of metabolic waste [10] resulting in delayed proliferation of cells and establishment of cell-cell interactions.
To overcome these problems, previous studies reported feasibility of magnetically levitated 3-D tissue culture for long term multicellular studies [11]. The biological application of magnetic forces in clinical diagnostic radiology has long been studied [12–16]. Magnets have also been used to levitate biological samples through the natural diamagnetism of organic material [17]. Internalization of nanoparticles has further supported cell sorting [13], mechano-conditioning of cells [13–15] and cellular micromanipulation [18]. However, development of magnetically levitated 3-D microtissues of breast cancer (BC) and colorectal cancer (CRC) cells using carbon encapsulated cobalt magnetic nanoparticles has not yet been studied. Therefore, in the present study, using encapsulated cobalt nanoparticles with carbon (C-Co) for biocompatibility and biosafety, we developed the modified, efficient, cost effective and scaffolds-free nanomagnetic levitation based 3-D cultures of BC and CRC microtissue for high throughput drug screening and xenograft studies. To compare the biological difference generated by magnetic levitation growth we evaluated the expression of N-cadherin and EGFR markers in our 2-D and 3-D cultures and in xenografts of BC and CRC cancer cells. These cultures are so phenotypically different that we considered the possible roles the presence of adhesion proteins such as N-cadherin and EGFR might play in their development. We hypothesized that an optimal in vitro tumorigenic model can be derived through improved 3-D cultures using nanotechnology. We have applied magnetic forces to levitate cells while they divide and grow.
2. Methods
2.1. 2-D cultures
BC (MDA-MB-231, MDA-MB-468 and MCF-7) and CRC (HT-29, WiDr, SW-480) cell lines (ATCC, Global Resource Center, Manassas, VA) were tested and found to be without pathogen, including mycoplasma. These cell lines were maintained in flasks with RPMI-1640 medium (ATCC) supplemented with 10% FBS (Thermo Scientific HyClone, Logan, UT), 25mM HEPES, penicillin/streptomycin (Pen/Step) (Mediatech, Manassas, VA), L-glutamine and sodium bicarbonate, and incubated at 37°C and 5% CO2. All cell lines were initially grown as 2-D culture monolayers. These cells were then harvested and used for the experimental culture parameters: 3-D cultures in hydrogel, and magnetic levitation based 3-D cell cultures.
2.2. 3-D cultures using sea prep hydrogel
BC (MDA-MB-231, MDA-MB-468 and MCF-7) and CRC (HT-29, WiDr, and SW-480) cells were mixed with 1 % Sea Prep hydrogel (Lonza, Rockland, ME) in Nonclon petri dishes (5 cm. diameters) diameter and allowed to solidify for 30 minutes at 4°C. Subsequently, the culture medium RPMI-1640 supplemented with 10% FBS, 25mM HEPES, Pen/Step, L-glutamine and sodium bicarbonate, was added to the gel with embedded cells and allowed to grow in the incubator at 37°C and 5% CO2. Tumor spheres developed in the gel matrix. These tumor spheres were used for comparison with microtissue developed in magnetic levitated 3-D cell cultures.
2.3. 3-D cultures based on magnetic levitation
A. Preparation of C-Co nanoparticles. Carbon encapsulated cobalt magnetic nanomaterial (<50 nm particle size; resistivity 6.24 μΩ-cm, 20°C) that has monodisperse magnetic nanoparticle property was sonicated in sterile aqueous solution (Sigma-Aldrich, St. Louis, MO). This nanomaterial (C-Co nanoparticles) was used for internalization by the cancer cells for the purposes of nanomagnetic levitation, and formation of 3-D cancer microtissues. The carbon coatings (approximately three graphitic layers used) will endow these magnetic particles with biocompatibility and stability in both biological and non-biological systems. B. Internalization of C-Co nanoparticles. BC (MDA-MB-231, MDA-MB-468 and MCF-7) and CRC (HT-29, WiDr, SW-480) cell lines were subjected to nanomagnetic levitation to assume 3-D growth in cultures by internalization of magnetic cobalt nanoparticles. The cancer cells were suspended in the warm solution of 0.25 % sea prep hydrogel (Lonza), mixed with 20 μL of sonicated carbon encapsulated cobalt magnetic nanomaterial (1μg/μL) and allowed to solidify in the petri dish for 30 minutes at 4°C. This hydrogel phase has been shown to effectively prevent the aggregation and clumping of the nanoparticles and to facilitate their passage into the cells. Subsequently, the growth medium RPMI-1640 supplemented with 10% FBS, 25mM HEPES, Pen/Step, L-glutamine and sodium bicarbonate, was added to the gel with embedded cells and allow to grow for 12 h at 37°C in 5% CO2. The cells with internalized magnetic nanoparticles were separated from the very dilute (0.25%) gel, and RPMI-1640 supplemented with 10% FBS, 25mM HEPES, Pen/Step, L-glutamine and sodium bicarbonate was added. A small block magnet was placed on the petri dish to promote magnetic forces levitation of cells while they divide and grow.
2.4. Animals and Xenografts
Severe combined immunodeficient female (SCID) mice were purchased from Taconic Farm (Taconic, NY) at four weeks of age and quarantined for one week prior to use. The mice were inoculated with 2-D cultures of BC (MCF-7 and MDA-MB231) and CRC (HT29 and SW480) cells to establish primary tumors (xenografts). All food, water, and bedding were sterilized by autoclaving. The mice resided in micro-filtered cages in a room designated for immune compromised mice. On a daily basis the animals were evaluated regarding health status and tumor growth. Body weight, nutritional intake, general activity level, and ruffling of the mice coats were used to determine the health status. All surgical procedures were done under the laminar flow hood and with strictly sterile protocols. A liquid sterilant, exspor (Alcide Co., Norwalk, CT) was used to sanitize the researcher’s gloves and the mouse coat at the site of planned surgery. Xenografts were stored in liquid nitrogen and used for N-cadherin and EGFR expression studies.
2.5. Characterization of the cells and Microtissues
A. Light and fluorescent microscope characterization of microtissue. The 3-D cellular matrix structure grown under magnetic forces was visualized by light and fluorescent microscopy. The cells and tumor spheres that make up the microtissue are clearly visible with both light and fluorescent microscopy. For cell viability testing, the cells were harvested, washed and diluted with phosphate-buffered Minimal Essential Medium or 1x phosphate buffered saline (1xPBS) to make an estimated 1–5 × 106 cells/mL. An equal amount of 25 μL volumes of cell suspension and ethidium bromide/acridine orange was added in a test tube, mixed and incubated for 10 minutes at RT, and visualized with fluorescent blue light using fluorescent microscopy (495 nm primary filter and a 515 nm secondary filter). Live cells will have fluoresced green (with acridine orange) and dead cells will have fluoresced orange (with ethidium bromide). B. Transmission electron microscope (TEM) characterization of internalized magnetic nanoparticles. To evaluate and confirm internalization of magnetic nanoparticles in the cells, we used JOEL 1200EX Transmission Electron Microscope (JEOL Ltd., Japan). In Transmission Electron Microscopy much of the electron beam makes it through the sample allowing a 3-D visualization of structures. MDA-MB-231 and MDA-MB-468 cells were grown on specific cover-slips, washed with PBS, fixed in primary (2.5% glutaldehyde) and secondary (1% Osmium tetroxide) fixatives, en block stained, dehydrated, and passed through transitional solvent (Propylene oxide), resin, filtered, sectioned, and visualized under TEM at 80KV. C. Immunofluorescence detection of N-cadherin and EGFR. Human BC (MCF-7, MDA-MB231) and CRC (HT29 and SW480) cells used for 2-D cell culture were prepared by plating cells (1×105) on glass slides with poly-D-lysine (Becton Dickinson, Franklin Lakes, NJ) and then allowing cells to attach overnight. Cells were subsequently washed with PBS and fixed with formalin free Zinc fixative (Becton Dickinson) for 30 min and washed again. Cells were then permeabilized with PBS containing 0.1% triton X-100 for 5 min and washed with PBS. Paraffin blocks for 3-D cell structures from magnetically levitated cultures or xenografts of BC and CRC cells were prepared with standard histpathology methods as noted above for 2-D cell cultures. These 3-D cultures were embedded in paraffin blocks and sectioned for 5 micron sections. Deparaffinization for paraffin sections was performed with xylene followed by rehydration procedure through a series of ethanol solutions (100%, 95%, 80% and 50%).
Blocking was performed for 2-D cells, 3-D cultures and xenografts sections with 200μL of serum blocking solution (Zyagen, San Diego, CA) followed by incubation at RT for 60 min in a humidified chamber. The slides of the samples were incubated with 200 μL (2 μg/mL) of primary antibody for N-cadherin (Life Technologies, Grand Island, NY) and EGFR (Santa Cruz Biotechnology Inc, Santa Cruz, CA) overnight at 4°C at RT. Samples were washed 3 times with PBS for 5 min each then incubated with 200 μL of biotinylated secondary antibody solution (Zyagen) for 30 min at RT. After washing with PBS, samples were covered with 200 μL of streptavidin-FITC conjugate solution (Zyagen) and incubated for 30 min at RT. Samples were washed 3 times again with PBS, then counterstained with DAPI solution (Zyagen) for 2 min. Samples were washed 3 times with PBS for 5 min each and covered with coverslip using anti-fade fluorescent mounting medium (Zyagen).
Confocal images were acquired using the Olympus FluoView FV1000 Confocal Laser Scanning Microscope (Olympus America, Inc., Center Valley, PA) configured on a fully automated inverted IX81microscope using a 40x UPLFLN oil (NA1.3) objective. DAPI fluorescence was excited using the 405nm diode laser line, and the emission was detected using a 430–470nm band pass filter. FITC fluorescence was excited using the 488nm Argon laser line, and the emission was detected using a 505nm long pass filter. Transmitted laser light was used to simultaneously record the Differential Interference Contrast (DIC) image.
3. Results
3.1. 3-D tumor spheres formation
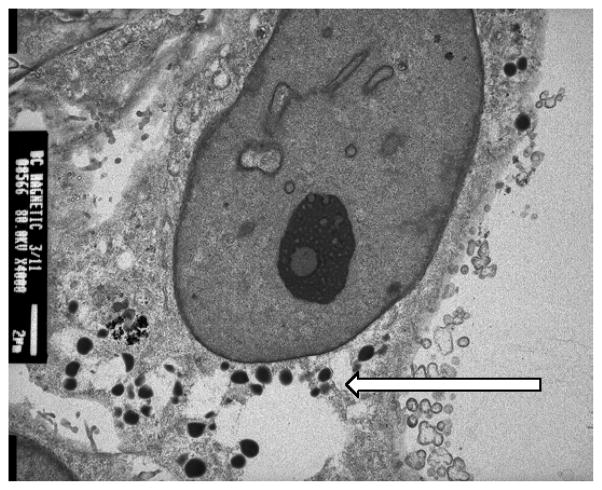
BC and CRC cell lines were initially grown as 2-D cultures and as monolayers. These cells were then harvested and cultured with 1% Sea Prep Hydrogel in supplemented RPMI1640 medium. After 3 weeks the 3-D cultures developed tumor spheres. Like in vivo conditions, these 3-D cultures had cell-cell interactions and the sphere was held together by an extracellular matrix as microtissue. The cells of 3-D culture were found to have multiple variations in morphology in the gel matrix. There is a distinct contrast in cell growth between 2-D and 3-D cultures (Figure 1). Transmission electron microscopy (TEM) image show the internalization of nanoparticle as a significant number of C-Co nanoparticles were internalized by MDA-MB-231 cells (Figure 2).
Figure 1.

The morphology of breast cancer cell cultures showing the monolayer of 2-D cultures and suspended tumor spheres in 3-D cultures. The inset is that of a single tumor sphere.
Figure 2.
TEM image of MDA-MB-231 cells with internalized magnetic nanoparticles. The nanoparticles are noted by the arrow and numerous particles have undergone phagocytosis.
3.2. 3-D microtissue growth pattern
The cancer cells with internalized magnetic nanoparticles could levitate into the culture media under the magnetic influence within a few minutes and concentrated below the magnet which facilitated cell clusters formation, triggering cell-to cell interactions, forming spheroid shapes in 3 days and ultimately assuming 3-D microtissue formation in 7 days. The 3-D microtissue has been found to be axially symmetric; reflecting the shape of the magnet used and with characteristic multicellular structure (figure 3).
Figure 3.

Tumor microtissue growing in culture in the petrie dish. The block magnet is shown in place on the lid of the dish as well as an image of the microtissue after removal of the block magnet.
Unlike in 2-D and 3-D with scaffolds, using magnetic levitation method, a large amount of the 3-D microtissue can be grown without detachment and replating cycles by simply changing the growth medium twice weekly. These 3-D cultures were maintained up to 5 months without any deterioration of the tissue. It has been observed that those 3-D cultures with the hydrogel matrix could only be maintained in culture for about 8 weeks before cellular degeneration started11. Cells growing embedded in the gel matrix were impossible to separate out without disrupting the spheres. The magnetic levitated cultures were shown to remain fully viable in culture for 12 weeks and it was at this time that cultures were used for other studies. There was near 100% cell viability and no discernible cell death within the microspheres.
3.3. Comparison of N-cadherin or EGFR expression in 2D, 3D and xenografts of breast and colon cancer cells
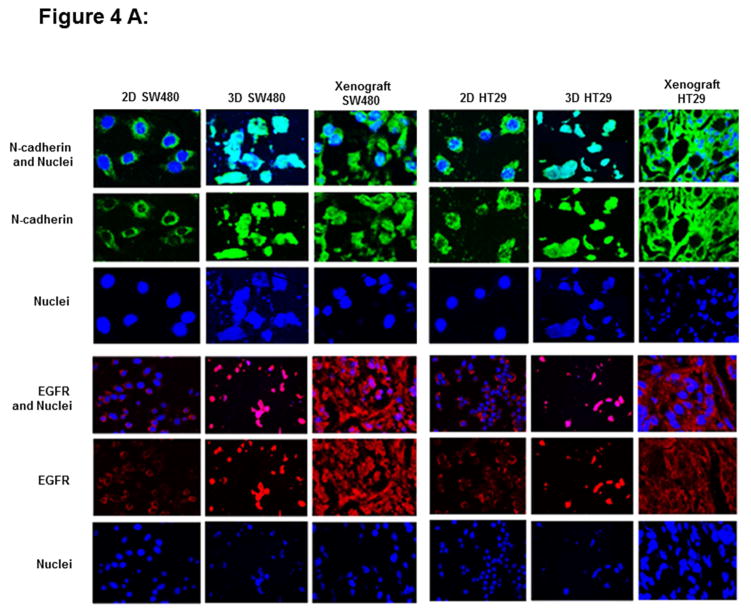
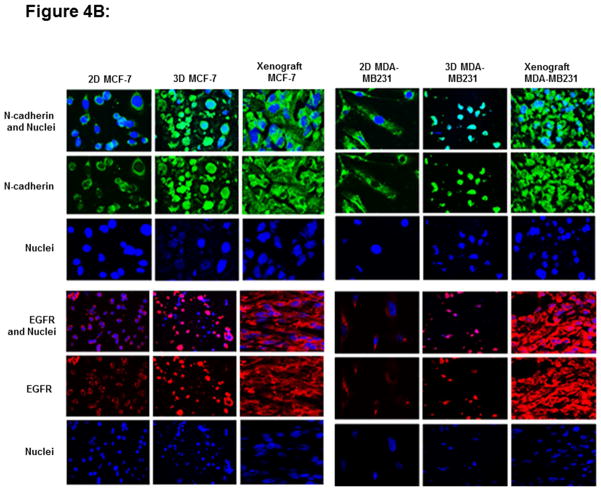
To study the biological characteristics afforded by magnetic levitation, we have evaluated the expression status of N-cadherin and EGFR markers in our 2-D, 3-D and xenografts of breast (MCF-7, MDA-MB231) and colon (HT29 and SW480) cancer cells. The 2D, 3D cultures were stained for N-cadherin or EGFR and nuclei were counterstained with DAPI. In the present study, it has been observed that similar expression patterns of N-cadherin and EGFR in levitated 3-D cells and xenografts of CRC (Fig 4A) and BC (Fig 4B) cells. The 2-D cultures show expression of N-cadherin in cytoplasm and nucleus. In contrast, levitated 3-D cells or matching xenografts shows large N-cadherin activity upon cellular aggregation and demonstrates expression of N-cadherin in the cytoplasm, nucleus, membrane or cell-cell junctions. There are differential expression patterns of N-cadherin and EGFR in 3-D cells compared to 2-D cells. However, molecular similarity exists between the levitated 3-D cultures and xenografts that originated from immunodeficient mice. EGFR was predominantly punctate and intracellular in 2D and it was found in membrane and at points of cell-cell contact in 3D cells or xenografts. Data for N-cadherin or EGFR expression in colon cancer cell line (WiDr) and in breast cancer cell line (MDA-MB468) was not shown as those xenografts were not available.
Figure 4.
The expression of N-cadherin and EGFR markers in 2-D and 3-D cultures and xenografts of colon (HT29 and SW480) (Fig 4A) and breast cancer cells (MCF-7, MDA-MB231) (Fig 4B). Two dimensional cultures show expression of N-cadherin (green fluorescence) in the cytoplasm and nucleus. In contrast, levitated 3-D cells and matching xenografts shows large N-cadherin activity upon cellular aggregation and demonstrates expression of N-cadherin in the cytoplasm, nucleus, cell membrane or cell-cell junctions. EGFR (red fluorescence) is predominantly punctate and intracellular in 2D cultures and it is found in high expression in membrane and at points of cell-cell contact in 3D cells or xenografts. These figures were subjectively quantified based on color intensity.
4. Discussion
Many schemes for 3-D culturing with profuse cell proliferation have been developed [19–23] to address the challenges of 2-D cell culturing where the cells grow as a monolayer in culture medium and attached to the flask bottom. However, current 3-D culturing provides a poor representation of in vivo conditions and are widely acknowledged as being insufficient for demanding technological needs. The magnetic levitation based 3-D cell matrix structure developed in this study mitigates the short comings of the conventional 3-D cell cultures with some kind of bioscaffolds. A comparative analysis was made between the cells grown in 3-D culture using hydrogel and nanomagnetic cell levitation system. Unlike in 2-D and 3-D with scaffolds, using magnetic levitation method, a large amount of the 3-D microtissue can be grown and these 3-D cultures were maintained up to 5 months without any deterioration of the tissue. This improved nanomagnetically levitated scaffolds-free 3-D cell culture system is efficient for evaluating cell characteristics and growth, cost effective and offers alternative to the conventional 3-D cell culture system. We have not specifically assessed the doubling time for 3-D cultured cells compared to 2-D culture. The model was phenotypically compared to in 2-D derived cultures and xenografts. Due to their rate of proliferation there may be some limitations for its applicability. However our data suggest that the proposed magnetic levitation for 3-D in vitro breast and colorectal tumors will have applicable value due to their abilities to: (1) rapidly expand tumor spheres in 24 hours, (2) control tumor cell composition and density, (3) mimic the in vivo tumor microenvironment, and (4) demonstrate phenotypic changes in an in vitro model that is comparable to in vivo tumors.
Previous studies reported feasibility of magnetically levitated in 3-D tissue culture for long term multicellular studies [11]. The biological application of magnetic forces in clinical diagnostic radiology has long been studied [12–16]. Magnets have also been used to levitate biological samples through the natural diamagnetism of organic material [17]. Internalization of nanoparticles has further supported cell sorting [13], mechano-conditionong of cells [13–15] and cellular micromanipulation [18]. However, development of magnetically levitated 3-D microtissues of breast and CRC cells using carbon encapsulated cobalt magnetic nanoparticles has not yet been studied.
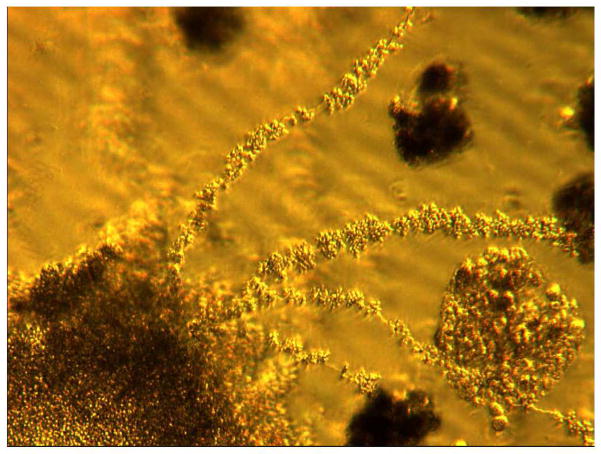
The very novel components of the experiment is in using, for the first time, the carbon encapsulated magnetic nanoparticles for stability and biocompatibility, and developing partially grown in vitro cancer cell colonies as tumor tissues. Cell culturing by magnetic levitation using carbon encapsulated magnetic nanoparticles is based on magnetization and levitation of the cells by spatially varying magnetic fields and we believe this technical strategy can be applied to develop 3-D microtissues from any cell type. In addition, magnetic levitation raises microtissue formation with better cell viability and no discernible cell death within the microspheres. The presence of the magnetic field levitates and spatially guides cells together, thus promoting cell-cell interaction in a manner that allows cells to self-assemble, expand, and migrate in 3-D. Our results have shown that cells start to generate their tiny stalks and assemble cells into biologically relevant 3-D cellular structures that resemble the vivo system within hours of levitation. Figure 5 shows how tumor spheres have aggregated to form tumor tissue in the levitated cultures. Here we also study the biological characteristics of levitated cultured through the evaluation of their expression of N-cadherin and EGFR. N-cadherin, a calcium dependent cell-cell adhesion glycoprotein comprising five extracellular cadherin repeats, a transmembrane region and a highly conserved cytoplasmic tail. It can be inferred that N-cadherin activity plays a significant role in cellular aggregation through homotypic cell adhesion interactions during the self-assembling process [24]. Indeed, this may have broad implications for future tissue engineering studies attempting to recapitulate the biological traits in 3-D architecture. In the present study, it has been observed that similar expression status of N-cadherin in levitated 3-D cultures and xenografts of BC and CRC cells. Two dimensional cultures show expression of N-cadherin in the cytoplasm and in nucleus. In contrast, levitated 3-D cells or xenografts of cells shows large N-cadherin activity upon cellular aggregation and demonstrates expression of N-cadherin in the cytoplasm, nucleus, membrane and cell-cell junctions. The large N-cadherin and EGFR activity upon cellular aggregation in 3-D cells suggests that magnetic levitation may recapitulate some in vivo-like traits. The results of present study, specifically for N-cadherin expression are qualitatively consistent with previous study [24] in which cartilage grown in vitro also resulted differential N-cadherin expression patterns in 3-D culture relative to 2-D culture.
Figure 5.
Forty-eight days of growth of 3-D levitated cultures of MD-MB468 cells. Tumor spheres are in aggregate and adhered together as microtissue forms. This extended growth is noted to establish stalks composed of tumor spheres with extension of the tissue formation throughout the culture medium (40 × magnifications).
3-D microtissue will more closely recapitulate in vivo protein expression and may be more feasible for long-term multi-cellular studies. The 3-D cell culture model systems will provide information on the complex interacting roles of matrix composition, integrins, growth factor receptors, and signaling in developing cancers. This is essential to understand the plasticity, regulation, and suppression of these processes and evolve strategies to identify therapeutic targets for future cancer therapy [25]. The microtissue developed by nanomagnetic levitation will be utilized to study pharmacokinetics of the cancer therapeutics with our novel apoptotic peptide (Nef-M1 peptide) and to produce xenografts in the SCID mouse. We will further evaluate the advantage of nanomagnetically levitated 3-D cell culture system by analyzing cell surface markers from 2-D cultures and making comparison with 3-D cell cultures using various bioscaffolds. At this time the 3-D magnetic levitated cultures have not been placed in vivo. It is of interest to determine if this could increase the rate of xenograft production. Furthermore, angiogenesis is important to tumor growth therefore understanding the role of proangiogenic factors, such as VEGF, on these tumor spheres would prove to be significant to this model. We believe that it is important to understand the rate of VEGF (circulatory and in situ) and neo-angiogenetic activity in tumor xenografts of 2-D and 3-D cancer cells that may facilitate development of disease models specifically for cancer or drug testing. We have found that in the tumor microenvironment, tumor cells secrete a high level of VEGF. This binds to receptors on surrounding endothelial cells, promoting endothelial cell migration, proliferation, differentiation and tube formation. Western blot analyses on lysates of tumors from mice revealed that these tumors express VEGF-A protein suggesting VEGF activation (in situ) in the SCID mice. However, measurement of the levels of VEGF-A has not been performed in the blood (circulatory). Immunostaining analyses for endothelial marker (CD31) indicated that these tumors have well established vascularity and microvesel density associated with neo-angiogenic activity (data not shown). However, VEGF and neo-angiogenesis within the 3-D cultures of BC and CRC cells will be completed in future studies.
Future studies will also compare microtissue biomarkers to those associated with in vivo xenografts grown in SCID mice. Nanomagnetic levitated 3-D cultures tend to form microtissue BCs and CRCs and may facilitate drug discovery and development, disease models specifically for cancer, stem cell research, tissue engineering, drug testing and the production of xenografts in an animal model.
Acknowledgments
This work was supported by Department of Defense Award W81XWH-08-1-0476. This work was also supported in part by National Institute of Health R21CA171251-02.
Footnotes
Author Contributions
H.L.B and D.G.J. are responsible for conception and design of the research; H.L.B, D.G.J and V.K. Performed the experiments, collected, and analyzed the data; H.L.B, D.G.J, M.D.B. and V.K. interpreted the results of the experiments; D.G.J and V.K. prepared the figures and drafted the manuscript; H.L.B and U.M. edited the manuscript and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Haycock JW. 3-D Cultures-Methods and Protocols XI. Vol. 343. Springer; 2011. p. 104. illus., 6 in color. [Google Scholar]
- 2.Bokharia M, Carnachana RJ, Cameronb NR, Przyborski SA. Novel cell culture device enabling three-dimensional cell growth and improved cell function. Biochem Biophys Res Commun. 2007;354 (4):1095. doi: 10.1016/j.bbrc.2007.01.105. [DOI] [PubMed] [Google Scholar]
- 3.Baharvand H, Hashemi SM, Ashtiani SK, Farrokhi A. Differentiation of human embryonic stem cells into hepatocytes in 2D and 3D culture systems in vitro. Int J Dev Bio. 2006;50 (7):645. doi: 10.1387/ijdb.052072hb. [DOI] [PubMed] [Google Scholar]
- 4.Ranucci CS, Moghe PV. Polymer substrate topography actively regulates the multi-cellular organization and liver-specific functions of cultured hepatocytes. Tissue Eng. 1999;5 (5):407. doi: 10.1089/ten.1999.5.407. [DOI] [PubMed] [Google Scholar]
- 5.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005;17 (5):524. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Fischbach C, Joon Kong H, Hsiong SX, Evangelista MB, Yuen W, Mooney DJ. Cancer cell angiogenic capability is regulated by 3D culture and integrin engagement. Proc Natl Acad Sci USA. 2009;106 (2):399. doi: 10.1073/pnas.0808932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu F, Burg KJL. Three-dimensional polymeric systems for cancer cell studies. Cytotechnology. 2007;54 (3):135. doi: 10.1007/s10616-007-9065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu M, Yang Z, Liu Y, Liu B, Zhao X. The 3-D culture and in vivo growth of the human hepatocellular carcinoma cell line HepG2 in a self-assembling peptide nanofiber scaffold. J Nanomater. 2010;2010:1–7. [Google Scholar]
- 9.Wang R, Xu J, Juliette L, et al. Three-dimensional culture models to study prostate cancer growth, progression, and metastasis to bone. Semin Cancer Biol. 2005;15:353. doi: 10.1016/j.semcancer.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Elliott NT, Yuan F. A review of three-dimensional in vitro tissue models for drug discovery and transport studies. J Pharm Sci. 2011;100 (1):59. doi: 10.1002/jps.22257. [DOI] [PubMed] [Google Scholar]
- 11.Souza GR, Molina JR, Raphael RM, et al. Three-dimensional tissue culture based on magnetic cell levitation. Nature Nanotechnology. 2010;5:291. doi: 10.1038/nnano.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A, Ino K, Kobayashi T, Honda H. The effect of RGD peptide conjugated magnetite cationic liposomes on cell growth and cell sheet harvesting. Biomaterials. 2005;26:6185. doi: 10.1016/j.biomaterials.2005.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Pankhurst Q, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J Phys. 2003;D 36:R167. [Google Scholar]
- 14.Alsberg E, Feinstein E, Joy MP, Prentiss M, Ingber DE. Magnetically guided self-assembly of fibrin matrices with ordered nano-scale structure for tissue engineering. Tissue Eng. 2006;12:3247. doi: 10.1089/ten.2006.12.3247. [DOI] [PubMed] [Google Scholar]
- 15.Dobson J, Cartmell SH, Keramane A, El Haj AJ. Principles and design of a novel magnetic force mechanical conditioning bioreactor for tissue engineering, stem cell conditioning and dynamic in vitro screening. IEEE Trans Nanobiosci. 2006;5:173. doi: 10.1109/tnb.2006.880823. [DOI] [PubMed] [Google Scholar]
- 16.Meyer CJ, Alenghat FJ, Rim P, Fong JH, Fabry B, Ingber DE. Mechanical control of cyclic AMP signaling and gene transcription through integrins. Nature Cell Biol. 2000;2:666. doi: 10.1038/35023621. [DOI] [PubMed] [Google Scholar]
- 17.Coleman CB, Gonzalez-Villalobos RA, Allen PL, et al. Diamagnetic levitation changes growth, cell cycle and gene expression of Saccharomyces cerevisiae. Biotechnol Bioen. 2007;98:854. doi: 10.1002/bit.21526. [DOI] [PubMed] [Google Scholar]
- 18.Matthews BD, La Van DA, Overby DR, Karavitis J, Ingbera DE. Electromagnetic needles with submicron pole tip radii for nanomanipulation for biomolecules and living cells. Appl Phys Lett. 2004;85:2968. [Google Scholar]
- 19.Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- 20.Abbott A. Biology’s new dimension. Nature. 2003;424:870. doi: 10.1038/424870a. [DOI] [PubMed] [Google Scholar]
- 21.Pampaloni F, Reynaud EG, Stelzer EH. The third dimension bridges the gap between cell culture and live tissue. Nature Rev Mol Cell Biol. 2007;8:839. doi: 10.1038/nrm2236. [DOI] [PubMed] [Google Scholar]
- 22.Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nature Rev Mol Cell Biol. 2006;7:211. doi: 10.1038/nrm1858. [DOI] [PubMed] [Google Scholar]
- 23.Atala A. Engineering tissues, organs and cells. J Tissue Eng Regen Med. 2007;1:83. doi: 10.1002/term.18. [DOI] [PubMed] [Google Scholar]
- 24.Ofek G, Revell CM, Hu JC, Allison DD, Grande-Allen KJ, Athanasiou KA. Matrix development in self-assembly of articular cartilage. PLoS ONE. 2008;3 (7):e2795. doi: 10.1371/journal.pone.0002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bissell MJ, LaBarge MA. Context, tissue plasticity, and cancer: are tumor stem cells also regulated by the microenvironment? Cancer Cell. 2005;7:17. doi: 10.1016/j.ccr.2004.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]