Abstract
Genital herpes simplex virus (HSV) infections are very common worldwide. Approximately 22% of pregnant women are infected genitally with HSV, and a majority of them are unaware of this. The most devastating consequence of maternal genital herpes is HSV disease in the newborn. Though neonatal HSV infections remain uncommon, due to the significant morbidity and mortality associated with the infection, HSV infection in the newborn is often considered in the differential diagnosis of ill neonates. The use of newer diagnostic techniques and the development of safe and effective anti-viral therapy have revolutionized the management of affected infants. This review will summarize the epidemiology and management of neonatal HSV infections and discuss strategies to prevent HSV infection in the newborn.
Keywords: HSV, genital herpes, acyclovir, PCR, antiviral therapy
Viral Structure
Herpes Simplex Viruses (HSV-1 and HSV-2) are large, enveloped virions with a double stranded DNA core. There is considerable cross-reactivity between most HSV-1 and HSV-2 glycoproteins, which mediate attachment to and penetration into cells and evoke host immune responses. However, antibody responses to glycoprotein G allow for serologic distinction between HSV-1 and HSV-2.
Maternal genital infections during pregnancy
Terminology pertaining to herpes infections is outlined in Box 1. Genital herpes infections are caused by either HSV-1 or HSV-2, and the majority of infections are asymptomatic. HSV-2 seroprevalence among pregnant women is estimated to be 20-30%, with approximately 10% of HSV-2 seronegative women living with a seropositive partner and hence at risk for acquisition of genital herpes during pregnancy1, 2. Among discordant couples, women seronegative for both HSV-1 and HSV-2 have an estimated 3.7% chance for seroconversion, while the risk for women already seropositive for HSV-1 to seroconvert to HSV-2 is estimated to be 1.7%3. Similar to non-pregnant women, two-thirds of women with who acquire genital HSV infection during pregnancy are either asymptomatic or have non-specific symptoms. Among women with a history of genital herpes acquired prior to pregnancy, 75% will have at least one recurrence during pregnancy and 14% will have prodromal symptoms or lesions at the time of delivery4, 5. For peripartum neonatal transmission, women must be shedding the virus symptomatically or asymptomatically around the time of delivery. It has been shown that 0.2-0.39%6 of all pregnant women shed HSV in the genital tract around the time of delivery irrespective of prior history of HSV, and this incidence of shedding increases to 0.77-1.4% among women with prior history of recurrent genital herpes7, 8.
Box 1. Terminology pertaining to HSV infections.
Acquisition of HSV-1 or HSV-2 without prior exposure to either virus and hence no preformed antibodies is referred to as a first-episode primary infection.
Acquisition of HSV-2 in an individual with prior HSV-1 antibodies and vice-versa is referred to as a first-episode non-primary infection.
Reactivation refers to isolation of HSV-1 in a person who already has HSV-1 antibodies, or the isolation of HSV-2 in a person who already has HSV-2 antibodies.
Presence of lesions characteristic of genital herpes with detectable HSV-1 or HSV-2 from the lesions by culture or PCR is referred to as symptomatic shedding.
Detection of HSV-1 or HSV-2 from genital mucosa by culture or PCR in the absence of genital lesions is referred to as subclinical shedding.
The risk of transmission of HSV to the neonate remains significantly higher with primary maternal infections acquired closer to the time of delivery compared with recurrent infections (50-60% with primary infections vs <3% for recurrent infections), most likely due to lack of transplacentally acquired antibodies in the neonate of women with primary infection as well as exposure in the birth canal of those women to larger quantities of virus for longer durations of time9.
HSV in the newborn
Herpes simplex virus (HSV) infection of the neonate is uncommon, with an estimated rate of 1 in 3,200 deliveries10. Approximately 1500 cases of neonatal HSV disease occur annually in the United States11.
Risk factors for transmission of HSV to the newborn (Box 2)
Box 2. Risk Factors for acquisition of HSV in the newborn.
Primary maternal genital herpes during pregnancy > recurrent infection
Vaginal delivery > Cesarean section
Prolonged rupture of membranes
Use of fetal scalp electrodes
Genital herpes with HSV-1 > HSV-2
The risk of neonatal acquisition of HSV is significantly higher with first episode primary and first episode non-primary maternal infections when compared with recurrent genital infections10. The risk of neonatal transmission in a large study was identified as 57% with first-episode primary infection, compared with 25% with first-episode non-primary infection and 2% with recurrent genital HSV infections10. Other statistically significant risk factor in this large study for transmission of HSV to neonate was isolation of HSV-1 from genital lesions when compared with HSV-2.
Cesarean delivery has been proven to be effective in preventing the transmission of HSV to the neonate12 although neonatal HSV cases have occurred despite cesarean delivery prior to rupture of membranes2, 13. Evidence also exists for prolonged rupture of membranes14 and disruption of mucocutaenous barrier by the use of fetal scalp electrodes and other instrumentation to affect the acquisition of neonatal HSV disease10, 15. While it has been shown that the chances of acquisition of HSV-1 are decreased in women who are seropositive for HSV-2, transmission of HSV-1 to the neonate has been documented to be high irrespective of primary or recurrent infection when compared with HSV-2 transmission patterns10.
Times of HSV acquisition by the newborn
Neonatal HSV is acquired during one of three time periods as outlined below:
In-utero (5%)
Peripartum (85%)
Postnatal (10%)
Disease classification and clinical presentations
HSV infection acquired in the peripartum or postnatal period is classified by extent of disease, with disease classification being predictive of both mortality and morbidity16-20.
Disseminated disease
CNS disease (central nervous system)
SEM disease (skin, eye and/or mouth)
Disseminated Disease
Accounts for ∼25% of all neonatal herpes infections10
Usually presents around day 10 to 12 of life
Involves multiple organs, including the central nervous system, lungs, liver, adrenal, skin, eyes, and/or mouth
Presents with viral sepsis, respiratory failure, hepatic failure, and disseminated intravascular coagulation (DIC)
Two-thirds of infants with disseminated disease also have concurrent encephalitis, and 40% never develop a vesicular rash during the entire illness2, 20, 21
CNS Disease
Accounts for one-third of cases of neonatal herpes disease
Infants present around 16 to 19 days of life20
Clinical manifestations include focal/generalized seizures, lethargy, irritability, poor feeding, temperature instability, and bulging fontanelle.
60-70% of infants with CNS disease have skin lesions at some point during the course of the illness20
SEM Disease
Infection confined to the skin, eye, and/or mouth of newborns; by definition, does not involve visceral organs or the CNS
Since the introduction of antiviral therapy, ∼45% of infants with neonatal HSV disease present with SEM disease2
Infants present around day 10 to 12 of life.
80% of infants with SEM disease present with a vesicular rash
Diagnostic modalities for identifying infants with HSV
Viral culture
The definitive method for identifying newborns with HSV
Swabs from conjunctivae, nasopharynx, mouth, and anus (surface cultures) are inoculated into cell culture systems and monitored for cytopathic effect21
Other sites for HSV isolation include vesicular lesions, cerebrospinal fluid (CSF), and blood
Polymerase chain reaction (PCR)
The application of PCR to CSF samples has revolutionized the diagnosis of CNS neonatal herpes disease22-25
Sensitivity of CSF PCR in neonatal HSV disease ranges from 75% to 100%, and specificity ranges from 71% to 100%23, 24. A positive CSF PCR for HSV DNA defines that patient as having CNS involvement (categorized either as CNS disease, or disseminated disease with CNS involvement)
Blood PCR in neonatal HSV has been evaluated to a lesser extent in the diagnosis of neonatal HSV infections24-26. A positive blood PCR for HSV DNA confirms infection but does not define disease classification, since all clinical disease categories (SEM, CNS, and disseminated) can have viremia and DNAemia22, 24.
Blood PCR can be positive for weeks, with the clinical significance of this (if any) unknown. As such, no data exist to support use of serial blood PCR assay to monitor response to therapy.
Specimens to obtain from newborn before initiating antiviral therapy
Prior to initiation of empiric parenteral antiviral therapy, the following specimens should be collected to aid in the diagnosis of neonatal HSV disease or to determine if antiviral therapy may be discontinued if HSV has been excluded21:
CSF for indices, bacterial culture and HSV DNA PCR
Swab for viral culture from the base of vesicles, suspicious areas, and mucous membrane lesions for viral culture; PCR may be performed in addition to cultures
Swab from mouth, conjunctiva, nasopharynx and rectum (surface cultures) for viral culture; PCR may be performed in addition to cultures
Whole blood for HSV DNA PCR
Blood to determine Alanine aminotransferase (ALT)
Recommendations for treatment of HSV in the newborn
Treat with parenteral acyclovir given at 60mg/kg/day divided every 8 hours19, 21
Treat newborns with SEM disease for 14 days, and neonates with CNS and disseminated disease for 21 days21
In newborns with CNS involvement, repeat CSF PCR at the end of therapy to document a negative CSF PCR result
In neonates with positive CSF PCR near the end of 21 days of parenteral therapy, intravenous acyclovir should be continued until PCR negativity is achieved20, 21, 23
The significance of blood DNA PCR positivity remains unknown, and therefore serial measurement of blood DNA PCR for assessing response to therapy is not recommended at this time21
Prognosis of HSV in the newborn
With the utilization of the higher dose of acyclovir (60mg/kg/day divided in three doses for 21 days), 1 year mortality has been reduced to 29% for disseminated disease and 4% for CNS disease19. With high dose acyclovir, 83% of neonates with disseminated disease and 31% with CNS disease develop normally at 12 months of age16, 19. Morbidity following SEM disease has dramatically improved after initiation of antiviral therapy, likely due to preemption of SEM disease progressing to disseminated disease and due to improved diagnostic classification of CNS disease with the availability of CSF PCR testing. None of the infants with SEM disease in the high-dose acyclovir study developed developmental disabilities at 12 months age19.
Long term antiviral suppressive therapy
A phase III, placebo controlled trial performed by The National Institute of Allergy and Infectious Diseases (NIAID) Collaborative Antiviral Study Group (CASG) supports the use of acyclovir suppressive therapy for six months after completion of intravenous therapy for neonatal HSV disease. Infants with CNS disease randomized to receive oral acyclovir had better neurodevelopmental outcomes compared with placebo group, with 69% and 30% developing normally, respectively. Infants with SEM disease had less frequent recurrence of skin lesions while receiving suppressive therapy27. Although almost half of infants enrolled in an earlier small phase I/II trial had significant neutropenia28, the phase III trial of acyclovir suppressive therapy found similar rates of neutropenia in the treatment and placebo arms of the study27 (although the p-value approached statistical significance). Babies with neonatal HSV infection of any disease classification should receive oral acyclovir at 300mg/m2/dose, three times a day for six months. Absolute neutrophil counts should be monitored at 2 and 4 weeks and monthly thereafter after initiation of suppressive therapy21.
Approach to an infant exposed to active maternal primary or recurrent genital HSV
Recommendations for the management of infants exposed to HSV in the intrapartum period, until recently were based on expert opinion and did not take into consideration the change in epidemiology of genital HSV (primary vs. recurrent infections and HSV-1 vs. HSV-2 infections in women). The most recent Clinical Report endorsed by the American Academy of Pediatrics (AAP) provides evidence-based guidance on the management of neonates born to women with active genital herpetic lesions29.
The recommendations are applicable to institutions that have access to PCR and serologic test results in a rapid fashion. They require clinicians to work very closely across pediatric and obstetrical lines. Moreover, the guidelines are only applicable to the care of infants exposed to HSV from maternal genital lesions present at the time of delivery and not to situations of asymptomatic shedding.
Testing of women in labor
Viral culture and PCR from genital lesions suggestive of HSV
Characterize virus type as HSV-1 or HSV-2
Obtain maternal serologic status for HSV-1 and HSV-2
Use these data to determine status of maternal infection (primary vs recurrent)
The information obtained from viral culture/PCR of genital lesions and maternal serological status obtained at delivery allow the clinician to determine the type of maternal infection and the risk of transmission to the neonate, and guide the approach to management of the neonate. Management of newborns born to women with genital lesions suggestive of HSV at delivery is outlined in figures 1 and 2.
Fig 1. Algorithm for management of newborns born to women with HSV genital lesions at delivery and a prior history of genital herpes.
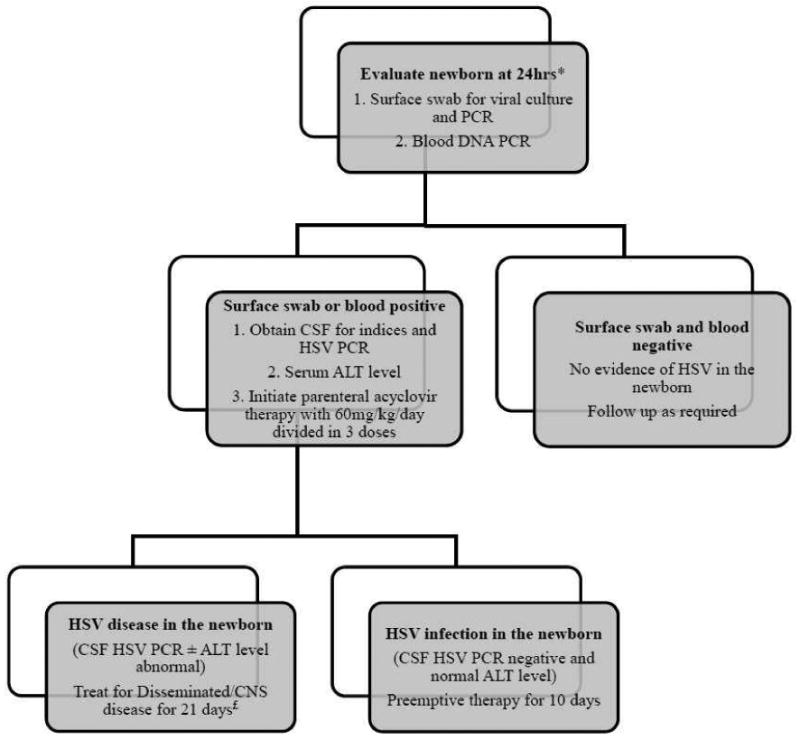
* Waiting for 24hrs after delivery is recommended to differentiate contamination of neonatal skin by maternal secretions versus true HSV infection of the baby.
£ After completion of parenteral therapy, treat with oral acyclovir suppressive therapy for 6 months (300mg/m2/dose three times per day)
Fig 2. Management of newborns born to women with lesions at delivery and no history of genital herpes prior to pregnancy.
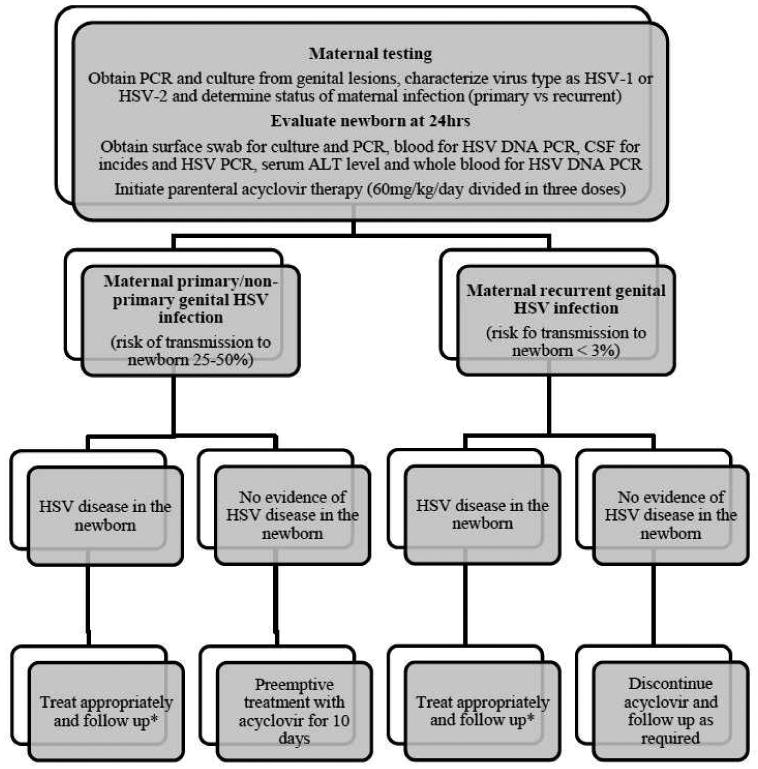
* For newborns with CNS/disseminated disease, treat for 21 days and 14 days for newborns with SEM disease. For babies with CNS involvement, repeat CSF analysis and CSF HSV PCR prior to stopping treatment. If CSF has detectable DNA by PCR at the end of therapy, continue treatment until negative CSF PCR result. After completion of parenteral therapy, treat with oral acyclovir suppressive therapy for 6 months (300mg/m2/dose three times per day).
Strategies for prevention of HSV in the newborn
1. Cesarean delivery
Delivery by Cesarean section decreases but does not entirely prevent transmission of HSV to the neonate. Transmission of HSV has been documented in circumstances where cesarean section was performed prior to rupture of membranes2, 13
This mode of delivery of the infant in women with active genital lesions can reduce the infant's risk of acquiring HSV10, 14 and is recommended when genital lesions or prodromal symptoms are present at the time of delivery12
Cesarean delivery is more likely to be effective if performed prior to rupture of membranes, but in situations where rupture of membranes has occurred and genital lesions are observed on physical examination cesarean delivery is recommended to minimize exposure of the neonate to HSV12
It is not recommended for women with a prior history of genital herpes and no active lesions/prodromal symptoms at the time of delivery12, 30
2. Antiviral suppressive therapy
In women with active recurrent genital herpes, antiviral suppressive therapy with acyclovir/valacyclovir initiated at 36 weeks of gestation is associated with decreased likelihood of genital lesions at the time of delivery and decreased viral detection by culture/PCR, with associated reduced need for C-section, and currently is recommended by ACOG12.
Subclinical viral shedding is not entirely suppressed and the utility of such a practice in preventing neonatal HSV disease is not defined. A recent multicenter case series reported eight cases of infants with neonatal HSV disease acquired from mothers despite receiving antiviral suppressive therapies beyond 36 weeks of gestation31
Although maternal antiviral suppressive therapy decreases the incidence of genital recurrences at labor, the extent to which these drugs prevent neonatal acquisition remains unknown and requires further research
3. HSV vaccine
Currently, no vaccine has proven to be effective for preventing acquisition of HSV-1 or HSV-2
An HSV-2 gD subunit vaccine, adjuvanted with alum, initially was found to be effective in preventing HSV-1 or HSV-2 genital herpes (∼75% vaccine efficacy) and HSV-2 infection, but the efficacy was limited only to women who were HSV-1 and HSV-2 seronegative prior to vaccination with no reported efficacy in men or women who were HSV-1 seropositive prior to vaccination32
In a subsequent randomized, double-blind trial evaluating the efficacy of the same HSV-2 gD subunit vaccine in women seronegative for HVS-1 and HSV-2, the vaccine was found to have an efficacy of 58% for preventing HSV-1 genital herpes but lacked efficacy for preventing HSV-2 genital herpes33
4. Prevention of maternal HSV acquisition during pregnancy
Various strategies have been recommended to prevent maternal acquisition during pregnancy but none have been tested in large scale trials34, 35.
The first approach is to screen all women with an IgG based assay at 24-28 weeks of gestation. Women identified to be seropositive but unaware of a prior infection would benefit from education regarding the significance of this finding and identification of recurrent lesions and prodromal symptoms, particularly at the time of labor. Women should be counseled regarding avoiding oral-genital. This strategy does not take into account the serostatus or exposure risk to the sexual partner.
The second approach recommends screening all couples for HSV serology at 14-18 weeks, with appropriate counseling based on serology results for both partners. This approach might not be applicable in situations where there are multiple partners or the partner changes during the course of pregnancy.
The third approach is to advise all pregnant women to abstain from all forms of sexual contact during the third trimester of pregnancy. The final approach might particularly be applicable in situations where serologic testing is either unavailable or is economically not feasible.
ACOG currently does not recommend routine screening of asymptomatic women for HSV during pregnancy12.
5. Prevention of postnatal acquisition
Approximately, 10% of cases are acquired in the postpartum period by exposure to the virus from open lesions of care takers, or following Jewish ritual circumcision involving oro-genital contact36.
Infected household contacts and family members are recommended to avoid contact with a newborn
Infected healthcare personnel with active herpetic whitlow lesions should not provide direct care for neonates21
Summary
While genital herpes infections remain common worldwide, HSV infection in the newborn fortunately is rare but when present contributes to significant long term morbidity. Significant advances have been made for diagnosing and effectively treating neonatal HSV disease. Efforts in the field of vaccine development to prevent maternal acquisition and subsequent transmission to the neonate have been unsuccessful so far.
Keypoints.
HSV infection in the newborn is an uncommon disease with devastating consequences
Early diagnosis, and parenteral anti-viral therapy followed by long term oral suppressive therapy have improved the prognosis of newborns with HSV infection
Vaccine development and interventions to decrease neonatal transmission remain a challenge
Outline.
Viral Structure
Maternal genital infections during pregnancy
HSV in the newborn
Risk Factors for transmission of HSV to the newborn
Times of HSV acquisition by the newborn
-
Disease classification and clinical presentations
Disseminated disease
CNS Disease
SEM Disease
-
Diagnostic modalities for identifying infants with HSV
Viral culture
Polymerase chain reaction
Specimens to obtain before starting antiviral therapy
Recommendations for treatment of HSV in the newborn
Prognosis of HSV in the newborn
Long term antiviral suppressive therapy
-
Management of infants exposed to active maternal primary and recurrent genital HSVStrategies for prevention of HSV in the newborn
Cesarean Delivery
Maternal antiviral suppressive therapy
Vaccine
Preventing maternal HSV acquisition during pregnancy
Prevention of postnatal acquisition
Acknowledgments
This work was supported under contract with the Division of Microbiology and Infectious Diseases of the National Institute of Allergy and Infectious Diseases (NIAID) (N01-AI-30025, N01-AI-65306, N01-AI-15113, N01-AI-62554).
Funding Sources:
Dr. Pinninti: None
Dr. Kimberlin: NIH, Cellex, GSK (All monies go directly to the University)
Footnotes
Conflict of Interest:
Dr. Pinninti: None
Dr. Kimberlin: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Swetha G. Pinninti, Email: spinninti@peds.uab.edu, Division of Pediatric Infectious Diseases, The University of Alabama at Birmingham, 1600 Seventh Avenue South, CHB 308, Birmingham, AL 35233, Phone: 205-996-7898, FAX: 205-975-6549.
David W. Kimberlin, Email: dkimberlin@peds.uab.edu, Division of Pediatric Infectious Diseases, The University of Alabama at Birmingham, 1600 Seventh Avenue South, CHB 303, Birmingham, AL 35233, Phone: 205-934-5316, FAX: 205-975-9972.
References
- 1.Kulhanjian JA, Soroush V, Au DS, et al. Identification of women at unsuspected risk of primary infection with herpes simplex virus type 2 during pregnancy. N Engl J Med. 1992;326(14):916–920. doi: 10.1056/NEJM199204023261403. [DOI] [PubMed] [Google Scholar]
- 2.Whitley RJ, Corey L, Arvin A, et al. Changing presentation of herpes simplex virus infection in neonates. J Infect Dis. 1988;158(1):109–116. doi: 10.1093/infdis/158.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med. 1997;337(8):509–515. doi: 10.1056/NEJM199708213370801. [DOI] [PubMed] [Google Scholar]
- 4.Sheffield JS, Hill JB, Hollier LM, et al. Valacyclovir prophylaxis to prevent recurrent herpes at delivery: a randomized clinical trial. Obstet Gynecol. 2006;108(1):141–147. doi: 10.1097/01.AOG.0000219749.96274.15. [DOI] [PubMed] [Google Scholar]
- 5.Watts DH, Brown ZA, Money D, et al. A double-blind, randomized, placebo-controlled trial of acyclovir in late pregnancy for the reduction of herpes simplex virus shedding and cesarean delivery. Am J Obstet Gynecol. 2003;188(3):836–843. doi: 10.1067/mob.2003.185. [DOI] [PubMed] [Google Scholar]
- 6.Brown ZA, Benedetti J, Ashley R, et al. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991;324(18):1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- 7.Arvin AM, Hensleigh PA, Prober CG, et al. Failure of antepartum maternal cultures to predict the infant's risk of exposure to herpes simplex virus at delivery. N Engl J Med. 1986;315(13):796–800. doi: 10.1056/NEJM198609253151303. [DOI] [PubMed] [Google Scholar]
- 8.Vontver LA, Hickok DE, Brown Z, et al. Recurrent genital herpes simplex virus infection in pregnancy: infant outcome and frequency of asymptomatic recurrences. Am J Obstet Gynecol. 1982;143(1):75–84. doi: 10.1016/0002-9378(82)90686-x. [DOI] [PubMed] [Google Scholar]
- 9.Sullender WM, Yasukawa LL, Schwartz M, et al. Type-specific antibodies to herpes simplex virus type 2 (HSV-2) glycoprotein G in pregnant women, infants exposed to maternal HSV-2 infection at delivery, and infants with neonatal herpes. J Infect Dis. 1988;157(1):164–171. doi: 10.1093/infdis/157.1.164. [DOI] [PubMed] [Google Scholar]
- 10.Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289(2):203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 11.Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev. 2004;17(1):1–13. doi: 10.1128/CMR.17.1.1-13.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. No. 82 June 2007. Management of herpes in pregnancy. Obstet Gynecol. 2007;109(6):1489–1498. doi: 10.1097/01.aog.0000263902.31953.3e. [DOI] [PubMed] [Google Scholar]
- 13.Peng J, Krause PJ, Kresch M. Neonatal herpes simplex virus infection after cesarean section with intact amniotic membranes. J Perinatol. 1996;16(5):397–399. [PubMed] [Google Scholar]
- 14.Nahmias AJ, Josey WE, Naib ZM, et al. Perinatal risk associated with maternal genital herpes simplex virus infection. Am J Obstet Gynecol. 1971;110(6):825–837. doi: 10.1016/0002-9378(71)90580-1. [DOI] [PubMed] [Google Scholar]
- 15.Kaye EM, Dooling EC. Neonatal herpes simplex meningoencephalitis associated with fetal monitor scalp electrodes. Neurology. 1981;31(8):1045–1047. doi: 10.1212/wnl.31.8.1045. [DOI] [PubMed] [Google Scholar]
- 16.Whitley RJ, Nahmias AJ, Soong SJ, et al. Vidarabine therapy of neonatal herpes simplex virus infection. Pediatrics. 1980;66(4):495–501. [PubMed] [Google Scholar]
- 17.Whitley R, Arvin A, Prober C, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324(7):444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- 18.Whitley R, Arvin A, Prober C, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991;324(7):450–454. doi: 10.1056/NEJM199102143240704. [DOI] [PubMed] [Google Scholar]
- 19.Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics. 2001;108(2):230–238. doi: 10.1542/peds.108.2.230. [DOI] [PubMed] [Google Scholar]
- 20.Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics. 2001;108(2):223–229. doi: 10.1542/peds.108.2.223. [DOI] [PubMed] [Google Scholar]
- 21.Pickering L, Baker C, Kimberlin D, et al. American Academy of Pediatrics. Red Book:2012 Report of the Committee on Infectious Diseases. 29th. Elk Grove Village, IL: American Academy of Pediatrics; 2012. Herpes Simplex; pp. 398–408. [Google Scholar]
- 22.Diamond C, Mohan K, Hobson A, et al. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J. 1999;18(6):487–489. doi: 10.1097/00006454-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Kimberlin DW, Lakeman FD, Arvin AM, et al. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis. 1996;174(6):1162–1167. doi: 10.1093/infdis/174.6.1162. [DOI] [PubMed] [Google Scholar]
- 24.Kimura H, Futamura M, Kito H, et al. Detection of viral DNA in neonatal herpes simplex virus infections: frequent and prolonged presence in serum and cerebrospinal fluid. J Infect Dis. 1991;164(2):289–293. doi: 10.1093/infdis/164.2.289. [DOI] [PubMed] [Google Scholar]
- 25.Malm G, Forsgren M. Neonatal herpes simplex virus infections: HSV DNA in cerebrospinal fluid and serum. Arch Dis Child Fetal Neonatal Ed. 1999;81(1):F24–29. doi: 10.1136/fn.81.1.f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewensohn-Fuchs I, Osterwall P, Forsgren M, et al. Detection of herpes simplex virus DNA in dried blood spots making a retrospective diagnosis possible. J Clin Virol. 2003;26(1):39–48. doi: 10.1016/s1386-6532(02)00019-7. [DOI] [PubMed] [Google Scholar]
- 27.Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med. 2011;365(14):1284–1292. doi: 10.1056/NEJMoa1003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kimberlin D, Powell D, Gruber W, et al. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to the skin, eyes and mouth: results of a phase I/II trial. Pediatr Infect Dis J. 1996;15(3):247–254. doi: 10.1097/00006454-199603000-00014. [DOI] [PubMed] [Google Scholar]
- 29.Kimberlin DW, Baley J. Committee on Infectious Diseases; Committee on Fetus and Newborn. Guidance on management of asymptomatic neonates born to women with active genital herpes lesions. Pediatrics. 2013 Feb;131(2):383–6. doi: 10.1542/peds.2012-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roberts SW, Cox SM, Dax J, et al. Genital herpes during pregnancy: no lesions, no cesarean. Obstet Gynecol. 1995;85(2):261–264. doi: 10.1016/0029-7844(94)00346-F. [DOI] [PubMed] [Google Scholar]
- 31.Pinninti SG, Angara R, Feja KN, et al. Neonatal Herpes Disease following Maternal Antenatal Antiviral Suppressive Therapy: A Multicenter Case Series. J Pediatr. 2012;161(1):134–138 e133. doi: 10.1016/j.jpeds.2011.12.053. [DOI] [PubMed] [Google Scholar]
- 32.Stanberry LR, Spruance SL, Cunningham AL, et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347(21):1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 33.Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown ZA, Gardella C, Wald A, et al. Genital herpes complicating pregnancy. Obstet Gynecol. 2005;106(4):845–856. doi: 10.1097/01.AOG.0000180779.35572.3a. [DOI] [PubMed] [Google Scholar]
- 35.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361(14):1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neonatal Herpes Simplex Virus Infection Following Jewish Ritual Circumcisions that Included Direct Orogenital Suction - New York City, 2000-2011. MMWR Morb Mortal Wkly Rep. 2012;61:405–409. [PubMed] [Google Scholar]


