Abstract
Stress-induced activation of the sympathoadrenal medullary system activates both the coagulation and fibrinolysis system resulting in net hypercoagulability. The evolutionary interpretation of this physiology is that stress-hypercoagulability protects a healthy organism from excess bleeding should injury occur in fight-or-flight situations. In turn, acute mental stress, negative emotions and psychological trauma also are triggering factors of atherothrombotic events and possibly of venous thromboembolism. Individuals with pre-existent atherosclerosis and impaired endothelial anticoagulant function are the most vulnerable to experience onset of acute coronary events within two hours of intense emotions. A range of sociodemographic and psychosocial factors (e.g., chronic stress and negative affect) might critically intensify and prolong stress-induced hypercoagulability. In contrast, several pharmacological compounds, dietary flavanoids, and positive affect mitigate the acute prothrombotic stress response. Studies are needed to investigate whether attenuation of stress-hypercoagulability through medications and biobehavioral interventions reduce the risk of thrombotic incidents in at-risk populations.
Keywords: Blood coagulation, cardiovascular disease, fibrinolysis, psychological stress, risk factor, thrombosis
Introduction
In the first half of the 20th century, the famous Harvard physiologist Walter B. Cannon demonstrated with a series of experiments that stimulation of the splanchnic nerve, pain, fear, and enragement all shortened blood clotting in the cat. Rapid coagulation did not occur if adrenals had been removed or were exhausted because of previous excitement, when cats had been caged near dogs. Cannon's evolutionary interpretation of these observations was that “rapid coagulation may reasonably be considered as an instance of adaptive reaction serviceable to the organism in the injury which may follow the struggle that fear or rage may occasion” [1]. Since Cannon's groundbreaking work on the human stress response he coined "fight-or-flight", abundant evidence has been accumulated from naturalistic, experimental, and mechanistic studies showing that hemostatic responses to acute mental stress result in net hypercoagulability [2–5]. This research has also revealed sociodemographic factors, certain diseases, affective states, coping strategies, and life circumstances as modulating variables of the acute prothrombotic stress response.
The role of enhanced coagulation, impaired fibrinolysis, and hyperactive platelets in the development of atherogenesis, atherothrombosis, and acute coronary syndromes (ACS) has been established [6]. Specifically, coronary plaque disruption and subsequent thrombotic occlusion are the underlying pathophysiological processes marking the transition from stable coronary heart disease to ACS [4]. Moreover, against a background risk of acquired and inherited prothrombotic conditions (e.g., immobilization, dehydration, and thrombophilia), mental stress might bring forward a prothrombotic milieu triggering the onset of venous thromboembolism (VTE) as well [2, 7].
The aim of this paper is to provide a succinct overview of the current understanding of hemostatic changes in response to acute mental stress and their potential role in the pathophysiology of the clinical manifestation of acute thrombotic events.
Acute mental stress and thrombotic events
Acute mental stress has been identified as an important triggering factor of ACS. For instance, of 849 myocardial infarction patients, 18.4% reported emotional upset as a possible trigger [8]. More recent studies have shown that intense emotions such as outbursts of anger and acute depressed mood increase the risk of ACS onset within two hours at least two-fold [9,10]. Research supports a key role of stress-induced hemostatic changes for this link. If tested one year after having survived an ACS, patients who reported emotional triggering showed significantly greater increase of and delayed recovery from stress-induced platelet activation [11]. Moreover, regular aspirin user had a relatively reduced risk of ACS onset following acute anger [12]. Those with atherosclerotic cardiovascular disease (CVD) are the most vulnerable to suddenly die from a cardiac cause in the week after acute traumatic stress such as inflicted by a natural disaster [13]. In the four weeks after an earthquake, the prevalence of pulmonary embolism as well as D-dimer levels, a predictor of incident VTE, were increased [5,14]; contextual factors likely contributed to the risk of VTE, including immobilization and dehydration of victims having endured the disaster for hours in their cars.
Taken together, this research concurs with the current understanding that against a background of several risk factors, including genetic, sociodemographic, medical, and psychosocial ones, the thrombosis risk increases at times of acute mental stress, whereas in a healthy individual, stress-hypercoagulability is not ultimately harmful to the vasculature [2]. Yet, there remains a possibility that even in initially healthy individuals, repetitive, exaggerated and prolonged hypercoagulability following acute stress episodes might critically contribute to the development of atherothrombotic CVD over time through, for instance, facilitating fibrin deposition in the vessel wall [2,6].
Psychobiological mechanisms in the onset of atherothrombotic events
Factors that underlie the manifestation of overt acute atherothrombotic events through triggering factors have extensively been scrutinized and include increased hemodynamic activity, shear stress, vasoconstriction, proinflammatory changes, cellular adhesion, and activation of the hemostatic system [15]. Stress-induced alterations in the cardiovascular system are mainly launched through activation of the sympathetic nervous system. Changes in the hemostatic system in response to stress are a key mechanism in that following rupture of an atherosclerotic plaque, coronary thrombus growth depends upon coagulation activation initiated by tissue factor and platelets at the site of endothelial lesions. Sympathetic activation of blood-borne tissue factor was recently shown to partially contribute to injury-induced arterial thrombotic occlusion in rodents exposed to restraint stress [16]. Moreover, atherosclerotic vessels are characterized by endothelial dysfunction with decreased nitric oxide production, resulting in loss of anticoagulant and profibrinolytic properties of endothelial cells resulting in exaggerated hypercoagulability during acute stress [2].
Acute mental stress and hemostasis activation
Half a century of research has been showing that acute mental stress elicits activation of coagulation molecules, platelets, and fibrinolysis resulting in net hypercoagulability [5]. More recent studies typically applied standardized laboratory stress paradigms, including the Stroop color-word inference test, mental arithmetic and speech stress. Stressors eliciting uncontrollability and social evaluative threat, i.e., when to be given in front of an audience, are most potent in mounting biological stress responses. Table 1 provides an overview of hemostatic parameters previously been shown to be responsive to acute mental stress. Particularly, several studies have demonstrated increased activity of clotting factor VIII (FVIII:C), platelets, and tissue-type plasminogen activator (t-PA) with a concomitant increase in D-dimer, indicating enhanced fibrin turnover (i.e., fibrin formation and degradation).
Table 1.
Acute stress-induced changes in hemostatic factors
| Fibrinogen | ↑ |
| Factor XII:C | ↑ |
| Factor VII:C | ↑ |
| Factor VIII:C | ↑ |
| Von Willebrand factor antigen | ↑ |
| Platelet activity | ↑ |
| Thrombin-antithrombin complex | ↑ |
| Fibrin D-dimer | ↑ |
| Percent prothrombin time | ↑ |
| Activated partial thromboplastin time | ↓ |
| Tissue-type plasminogen activator activity | ↑ |
| Tissue-type activator antigen | ? |
| Plasminogen activator inhibitor-1 | - |
Qualitative changes in levels of hemostatic factors are indicated: ↑ = increased level; ↓ = decreased levels; ? = unclear; - = no change
Unlike stress-induced changes in blood pressure and cortisol, healthy subjects did not show adaptation in the magnitude of the coagulation response across stress repetitions, likely because stress-hypercoagulability should protect from too much blood loss in any fight-or-flight situation [17]. In healthy subjects, activity of several clotting factors, including FVIII:C, as well as fibrinogen and von Willebrand factor (VWF) antigen levels increase between 5% and 10% from baseline in response to acute stress with coagulation and platelet activity returning to pre-stress levels within 20–45 minutes after stress cessation [2,15]. Therefore, factors contributing to prolonged recovery of prothrombotic changes from stress might critically augment hypercoagulability in the two hours after stress during which the risk of onset of an emotionally triggered ACS is highest.
Mechanisms of hemostatic activation with acute mental stress
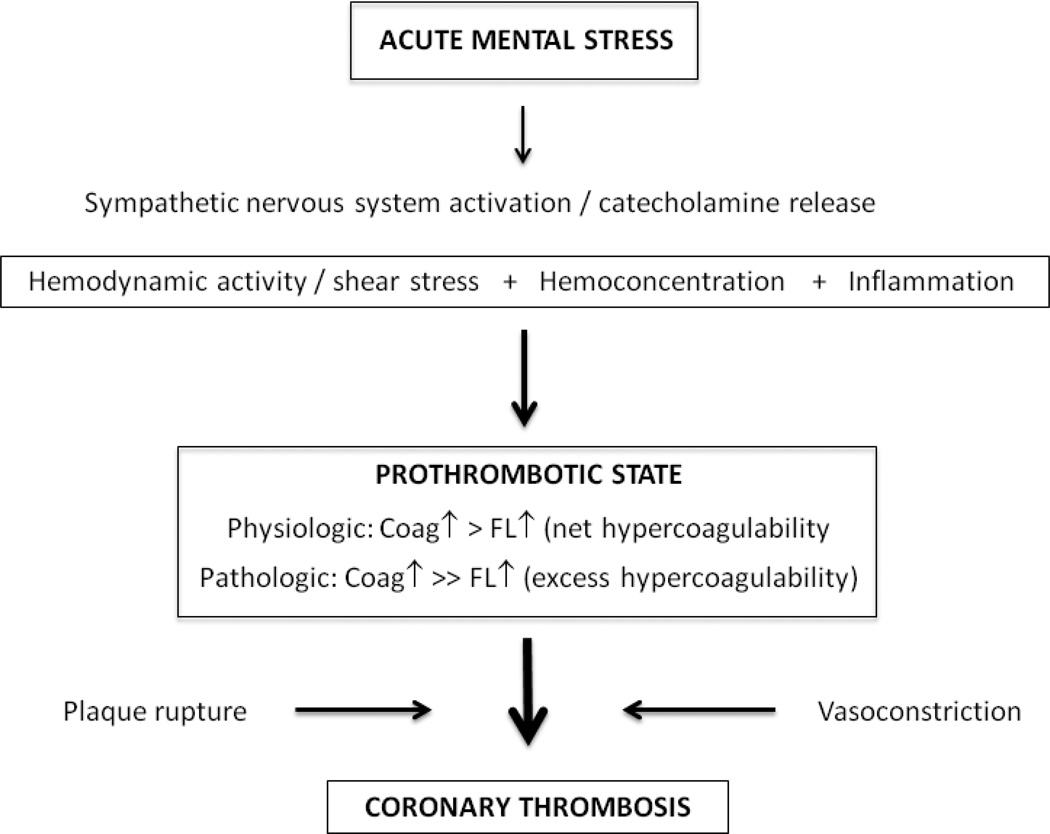
The mechanisms of acute stress-induced hemostatic activation underlying physiologic hypercoagulability as well as the subsequent formation of a coronary thrombus leading to an ACS are summarized in Figure 1. Catecholamines, released from the adrenalmedullary system and sympathetic nerve endings, dose-dependently stimulate vascular endothelial beta2-adrenergic receptors. Within a few minutes, preformed FVIII, hemostatically active VWF and profibrinolytic t-PA are released from endothelial storage pools into the circulation [18]. Catecholamines also stimulate hepatic release of FVIII and affect hepatic clearance of t-PA and likely D-dimer [2]. Sympathetic nerves in artery walls are a further important source of acute-stress induced increase in circulating t-PA [19]. Catecholamines also activate platelets through stimulation of alpha2-adrenergic receptors [18]. Thrombin that is formed during acute mental stress [20] is another important platelet agonist. There is much cross-talking between hemostasis and inflammation [6] with one study showing a significant and direct relationship between D-dimer and interleukin-6 stress reactivity over a two-hour interval [21]. Stress-induced hemodynamic shear forces together with the inflammatory response may further destabilize a vulnerable plaque [2,15]. Stress-hemoconcentration is facilitated by an acute increase in blood pressure and net efflux of plasma volume into the interstitial space with a resulting intravascular concentration of non-diffusable large (i.e. >69 kDa) hemostatic molecules [2]. As a consequence, the endothelium "sees" more of these molecules which, moreover, come in closer contact with each other [3]. Arithmetic adjustment for stress-hemoconcentration accounts for a sizeable portion of stress-induced elevations in hemostasis molecules (e.g., fibrinogen, VWF antigen) with the notable exception of FVIII:C, suggesting the intrinsic coagulation pathway is genuinely activated during acute mental stress [22].
Figure 1. Mechanisms underlying acute stress-induced hemostatic activation.
Acute mental stress activates the sympathetic nervous system, whereby catecholamine release from the adrenal medulla and sympathetic nerve endings trigger coagulation (coag) and fibrinolysis (FL) activation to result in net hypercoagulability. Excess or pathological hypercoagulability occurs if there is exaggerated coagulation activation and/or reduced fibrinolysis activation. Atherosclerotic plaque rupture and vasoconstriction initiate coronary thrombus occlusion and acute coronary syndromes, respectively. Hemodynamic activation, hemoconcentration, a proinflammatory state and shear stress to the vulnerable plaque are additional mechanisms which modulate the prothrombotic response to acute mental stress under physiological and pathological conditions.
Modulating variables of the acute prothrombotic stress response
Exaggerated hypercoagulability may occur against a background of a range of sociodemographic, biological and psychosocial factors which have previously been reviewed in detail elsewhere [2] and are summarized in Table 2. In terms of sociodemographic factors, only one study has previously addressed gender differences with men showing greater net stress-hypercoagulability than women: men had a greater FVII:C increase, while t-PA activity was greater in women [23]. In healthy men, age showed a direct association with an increase of D-dimer levels during acute stress and 20 minutes post-stress, suggesting elderly individuals might be particularly vulnerable to experience stress-triggered thrombosis [24].
Table 2.
Modulators of the prothrombotic response to acute mental stress
| Factors increasing stress-induced hypercoagulability | Factors decreasing stress-induced hypercoagulability |
| Older age | Dietary flavanoids |
| Male sex | Non-selective beta-blockade |
| Low socioeconomic status | Aspirin |
| Cardiovascular disease | Melatonin |
| Chronic psychosocial stress | Calcium antagonists |
| Negative affect | Positive affect |
| Perceived threat and challenge | Adaptive coping strategies |
Compared to individuals with high and intermediate socioeconomic status, as indexed by grade of employment, those with a low socioeconomic status showed elevated FVIII:C levels 45 min post-stress [25]. Compared to subjects without CVD, those with coronary heart disease and/or systemic hypertension had greater and prolonged platelet activation [26], greater D-dimer increase [27], and less fibrinolysis activation [28] in response to acute stress.
Chronic psychosocial stress and negative affect were also shown to exaggerate the acute prothrombotic stress response [2,5]. For instance, men with higher job stress showed a greater VWF antigen response to acute stress [29]. In chronically stressed caregivers of a spouse with dementia, but not in non-caregiving controls, depressive and anxiety symptoms were related to increased expression and delayed recovery of platelet P-selectin in response to acute mental stress, controlling for age, sex, history of coronary heart disease, and use of aspirin and antidepressants [30].
Studies on factors with a buffering effect on the acute prothrombotic stress response are only emerging; these suggest that positive affect (e.g., happiness) [31], adaptive coping strategies with stress [32], and medications, including aspirin, beta-blockers, calcium antagonists, and melatonin, variously mitigate stress reactivity of fibrinogen, FVIII:C, VWF antigen, platelets, and D-dimer (cf. [2] for data from randomized placebo-controlled drug trials). Likewise, dietary flavanoids from black tea (catechin) consumed over six weeks and from a single intake of 50g of dark chocolate with 72% cocoa content (epicatechin) significantly attenuated acute stress-induced platelet activation and D-dimer formation, respectively, compared with placebo [33,34].
Summary and conclusions
A truly physiologic prothrombotic stress response to acute mental stress is part of the fight-or-flight response, but can be exaggerated and prolonged in vulnerable individuals, thereby leading to excess/pathologic hypercoagulability. Plausible psychobiological processes have been identified to partially explain how acute stress affects hemostasis. As a prothrombotic state plays a key role in atherothrombotic CVD and VTE, excess/pathologic stress-hypercoagulability provides one mechanism that might underlie thrombotic manifestations triggered by emotional upset and psychological trauma such as ACS.
Prospective studies are needed to estimate the predictive value of the acute prothrombotic stress response for the risk of incident and recurrent thrombotic events in healthy individuals and in patients with established vascular disease. Such studies ought to consider sociodemographic and psychosocial factors which may modulate the acute prothrombotic stress response. Medications, polyphenolic flavanoids, and behavioral factors like positive mood and coping processes might have the potential to mitigate the acute prothrombotic stress response. Prospectively designed biobehavioral intervention studies would be needed to test whether translation of this knowledge into clinical practice would ultimately reduce the risk of emotional triggering of thrombotic events.
Supplementary Material
Acknowledgements
Own research and research from co-workers cited in this article has financially been supported by grants from various sources, including the Swiss National Science Foundation (81BE-56155, 32-68277, PP00P1_128565/1); the National Institutes of Health (MO1 RR-00827, HL-57265, AG-13332, HL-36005, HL-44915); the National Institute on Aging (AG-15301, AG-23989); the Swiss Federal Institute of Technology Zurich; the University of Bern, Switzerland; the University of Zurich, Switzerland; Novartis Foundation Switzerland; and the Swiss Cocoa and Chocolate Foundation. The funding sources had no involvement in study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit this article for publication.
REFERENCES
- 1.Cannon WB. An account of recent researches into the function of emotional excitement. 2nd ed. Boston: Charles T. Branford Company; 1953. Bodily changes in pain, hunger, fear, and rage. [Google Scholar]
- 2.Austin AW, Wissmann T, von Känel R. Stress and hemostasis: an update. Semin Thromb Hemost. 2013;39:902–912. doi: 10.1055/s-0033-1357487. [DOI] [PubMed] [Google Scholar]
- 3.Austin AW, Patterson SM, von Känel R. Hemoconcentration and hemostasis during acute stress: interacting and independent effects. Ann Behav Med. 2011;42:153–173. doi: 10.1007/s12160-011-9274-0. [DOI] [PubMed] [Google Scholar]
- 4.Thrall G, Lane D, Carroll D, Lip GY. A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: Implications for the pathogenesis of acute coronary syndromes. Thromb Res. 2007;120:819–847. doi: 10.1016/j.thromres.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 5.von Känel R, Mills PJ, Fainman C, Dimsdale JE. Effects of psychological stress and psychiatric disorders on blood coagulation and fibrinolysis: a biobehavioral pathway to coronary artery disease? Psychosom Med. 2001;63:531–544. doi: 10.1097/00006842-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Borissoff JI, Spronk HM, ten Cate H. The hemostatic system as a modulator of atherosclerosis. N Engl J Med. 2011;364:1746–1760. doi: 10.1056/NEJMra1011670. [DOI] [PubMed] [Google Scholar]
- 7.Lippi G, Franchini M, Favaloro EJ. Unsuspected triggers of venous thromboembolism trivial or not so trivial? . SeminThrombHemost. 2009;35:597–604. doi: 10.1055/s-0029-1242713. [DOI] [PubMed] [Google Scholar]
- 8.Tofler GH, Stone PH, Maclure M, Edelman E, Davis VG, Robertson T, et al. Analysis of possible triggers of acute myocardial infarction (the MILIS study) . Am J Cardiol. 1990;66:22–27. doi: 10.1016/0002-9149(90)90729-k. [DOI] [PubMed] [Google Scholar]
- 9.Mostofsky E, Penner EA, Mittleman MA. Outbursts of anger as a trigger of acute cardiovascular events: a systematic review and meta-analysis. Eur Heart J. 2014;35:1404–1410. doi: 10.1093/eurheartj/ehu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steptoe A, Strike PC, Perkins-Porras L, McEwan JR, Whitehead DL. Acute depressed mood as a trigger of acute coronary syndromes. Biol Psychiatry. 2006;60:837–842. doi: 10.1016/j.biopsych.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 11.Strike PC, Magid K, Whitehead DL, Brydon L, Bhattacharyya MR, Steptoe A. Pathophysiological processes underlying emotional triggering of acute cardiac events. Proc Natl Acad Sci U S A. 2006;103:4322–4327. doi: 10.1073/pnas.0507097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, et al. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 13.Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334:413–419. doi: 10.1056/NEJM199602153340701. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Kodama M, Tanabe N, Nakamura Y, Nagai T, Sato M, Okabe M, et al. Impact of earthquakes on risk for pulmonary embolism. Int J Cardiol. 2008;129:152–154. doi: 10.1016/j.ijcard.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 15.von Känel R. Psychosocial stress and cardiovascular risk : current opinion. Swiss Med Wkly. 2012;142:w13502. doi: 10.4414/smw.2012.13502. [DOI] [PubMed] [Google Scholar]
- 16.Stämpfli SF, Camici GG, Keller S, Rozenberg I, Arras M, Schuler B, et al. Restraint stress enhances arterial thrombosis in vivo--role of the sympathetic nervous system. Stress. 2014;17:126–132. doi: 10.3109/10253890.2013.862616. [DOI] [PubMed] [Google Scholar]
- 17.von Känel R, Preckel D, Zgraggen L, Mischler K, Kudielka BM, Haeberli A, et al. The effect of natural habituation on coagulation responses to acute mental stress and recovery in men. Thromb Haemost. 2004;92:1327–1335. doi: 10.1160/TH04-04-0223. [DOI] [PubMed] [Google Scholar]
- 18.von Känel R, Dimsdale JE. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur J Haematol. 2000;65:357–369. doi: 10.1034/j.1600-0609.2000.065006357.x. [DOI] [PubMed] [Google Scholar]
- 19.Hao Z, Jiang X, Sharafeih R, Shen S, Hand AR, Cone RE, et al. Stimulated release of tissue plasminogen activator from artery wall sympathetic nerves: implications for stress-associated wall damage. Stress. 2005;8:141–149. doi: 10.1080/10253890500168098. [DOI] [PubMed] [Google Scholar]
- 20.von Känel R, Mills PJ, Ziegler MG, Dimsdale JE. Effect of beta2-adrenergic receptor functioning and increased norepinephrine on the hypercoagulable state with mental stress. Am Heart J. 2002;144:68–72. doi: 10.1067/mhj.2002.123146. [DOI] [PubMed] [Google Scholar]
- 21.von Känel R, Kudielka BM, Hanebuth D, Preckel D, Fischer JE. Different contribution of interleukin-6 and cortisol activity to total plasma fibrin concentration and to acute mental stress-induced fibrin formation. ClinSci (Lond) 2005;109:61–67. doi: 10.1042/CS20040359. [DOI] [PubMed] [Google Scholar]
- 22.Austin AW, Wirtz PH, Patterson SM, Stutz M, von Känel R. Stress-induced alterations in coagulation: assessment of a new hemoconcentration correction technique. Psychosom Med. 2012;74:288–295. doi: 10.1097/PSY.0b013e318245d950. [DOI] [PubMed] [Google Scholar]
- 23.Jern C, Manhem K, Eriksson E, Tengborn L, Risberg B, Jern S. Hemostatic response to mental stress during the menstrual cycle. Thromb Haemost. 1991;66:614–618. [PubMed] [Google Scholar]
- 24.Wirtz PH, Redwine LS, Baertschi C, Spillmann M, Ehlert U, von Känel R. Coagulation activity before and after acute psychosocial stress increases with age. Psychosom Med. 2008;70:476–481. doi: 10.1097/PSY.0b013e31816e03a5. [DOI] [PubMed] [Google Scholar]
- 25.Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, et al. Socioeconomic status and stress-related biological responses over the working day. Psychosom Med. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- 26.Strike PC, Magid K, Brydon L, Edwards S, McEwan JR, Steptoe A. Exaggerated platelet and hemodynamic reactivity to mental stress in men with coronary artery disease. Psychosom Med. 2004;66:492–500. doi: 10.1097/01.psy.0000130492.03488.e7. [DOI] [PubMed] [Google Scholar]
- 27.von Känel R, Dimsdale JE, Ziegler MG, Mills PJ, Patterson TL, Lee SK, Grant I, et al. Effect of acute psychological stress on the hypercoagulable state in subjects (spousal caregivers of patients with Alzheimer's disease) with coronary or cerebrovascular disease and/or systemic hypertension. Am J Cardiol. 2001;87:1405–1408. doi: 10.1016/s0002-9149(01)01564-8. [DOI] [PubMed] [Google Scholar]
- 28.Palermo A, Bertalero P, Pizza N, Amelotti R, Libretti A. Decreased fibrinolytic response to adrenergic stimulation in hypertensive patients. J Hypertens Suppl. 1989;7:S162–S163. doi: 10.1097/00004872-198900076-00077. [DOI] [PubMed] [Google Scholar]
- 29.Hamer M, Williams E, Vuonovirta R, Giacobazzi P, Gibson EL, Steptoe A. The effects of effort-reward imbalance on inflammatory and cardiovascular responses to mental stress. Psychosom Med. 2006;68:408–413. doi: 10.1097/01.psy.0000221227.02975.a0. [DOI] [PubMed] [Google Scholar]
- 30.Aschbacher K, Mills PJ, von Känel R, Hong S, Mausbach BT, Roepke SK, et al. Effects of depressive and anxious symptoms on norepinephrine and platelet P-selectin responses to acute psychological stress among elderly caregivers. Brain Behav Immun. 2008;22:493–502. doi: 10.1016/j.bbi.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steptoe A, Wardle J, Marmot M. Positive affect and health-related neuroendocrine, cardiovascular, and inflammatory processes. Proc Natl Acad Sci USA. 2005;102:6508–6512. doi: 10.1073/pnas.0409174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aschbacher K, Patterson TL, von Känel R, Dimsdale JE, Mills PJ, Adler KA, et al. Coping processes and hemostatic reactivity to acute stress in dementia caregivers. Psychosom Med. 2005;67:964–971. doi: 10.1097/01.psy.0000188458.85597.bc. [DOI] [PubMed] [Google Scholar]
- 33.Steptoe A, Gibson EL, Vuononvirta R, et al. The effects of tea on psychophysiological stress responsivity and post-stress recovery: a randomised double-blind trial. Psychopharmacology (Berl) . 2007;190:81–89. doi: 10.1007/s00213-006-0573-2. [DOI] [PubMed] [Google Scholar]
- 34.von Känel R, Meister R, Stutz M, Kummer P, Arpagaus A, Huber S, et al. Effects of dark chocolate consumption on the prothrombotic response to acute psychosocial stress in healthy men. Thromb Haemost. doi: 10.1160/TH14-05-0450. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.