Abstract
Background
The long-term course of schizophrenia is often characterized by relapses, induced by poor medication adherence. Early nonadherence after discharge is frequent.
Objective
To evaluate a skills-based inpatient training program for medication intake.
Methods
We developed a manual-based inpatient medication training program to be carried out by nurses and focusing on practical skills enabling autonomous intake of medication. Medication adherence was measured by three different methods: pill count, determination of serum levels, and self-assessment by the patient. The raters were blinded.
Results
Four weeks after discharge, 98% of the patients in the intervention group (N=52) were rated as adherent by pill count versus 76% in the control group (N=50; P<0.01). By measurement of serum level, 88.5% versus 70% were adherent (P<0.05).
Conclusion
The inpatient medication training program carried out by nurses seems to be an effective intervention for enhancing medication adherence after hospital discharge.
Keywords: adherence, psychopharmacotherapy, schizophrenia
Introduction
Schizophrenia is a common psychiatric disorder1 that is often complicated by recurring relapses.2 Thus, schizophrenia places a substantial burden on patients, their relatives, and health systems.3 Pharmacotherapy is crucial in the acute phase of the disorder and for the prevention of relapse.4 Every relapse can worsen the course of the disease; for example, by prolonging the time to remission5 or by further impairment of cognitive functioning,6 and even loss of brain tissue.7 Nonadherence is the most important reason for relapses in schizophrenia and schizoaffective disorders.8,9 Conversely, there is evidence that good adherence to medication and other therapies decreases relapses and rehospitalizations.10,11 Continuous antipsychotic maintenance therapy is therefore considered highly important. According to the World Health Organization, nonadherence is a common phenomenon, concerning approximately 50% of chronically ill people.12 Medication nonadherence is one of the strongest social factors affecting medical outcomes.13 “Compliance” describes the degree to which a patient correctly follows medical advice, and “adherence” means the extent to which a patient continues an agreed treatment. As suggested by Haynes et al14 we use these terms synonymously.
Several studies and reviews focus on nonadherence in patients with schizophrenic disorders, using oral antipsychotic maintenance therapy.15,16 Various factors for nonadherence have been discussed:14,15 insufficient knowledge of the disorder and its treatment, lack of insight into the illness, and deficient communication between inpatient units and community mental health workers. Interventions targeting the improvement of adherence in patients with schizophrenic disorders are therefore heterogeneous.17
Currently used techniques to improve patients’ adherence nearly exclusively use cognitive-behavioral or psychoeducative approaches.18,19 Their approaches generally follow the main reasons for nonadherence established by Lacro et al.15 Nearly all programs focus on a modification of attitudes and cognitive aspects to enhance adherence by the improvement of information and insight. Yet insight into the illness and adherence are only moderately associated.20 A considerable percentage of about 25% of patients discontinue their medication within the first week after discharge from inpatient treatment.21 Thus, the loss of adherence is by far the biggest at the interface of inpatient and early outpatient treatment. Hence, to address the problem of early nonadherence, we developed an inpatient training program for practical skills with regard to medication. The patients should be enabled to recognize their medication and to organize its intake autonomously in full self-responsibility.
This kind of intervention has rarely been described and investigated to our knowledge. Boczkowski et al22 tested a therapy program in a randomized controlled trial with 36 patients, using behavioral strategies to improve adherence to medication in patients with schizophrenia. The patients treated with cognitive-behavioral strategies showed better medication adherence than the patients receiving psychoeducation or the control group. Liberman et al23 evaluated a behavioral program for patients with schizophrenia in the United States. In this study, patients received 12 hours of training weekly over the course of 6 months, as well as 18 months of case management in their community setting. The patients showed a significant improvement in “social and independent living skills.”
So far, to our knowledge, no prospective controlled study evaluating a skills-based training program for patients with psychotic disorders with a sufficient number of patients has been conducted. The objective of our study was to test whether the rate of early nonadherence could be decreased by the introduction of a newly developed skills-based medication training program.
Methods
Study design
The study was a randomized controlled trial in three centers, using an intervention group and a control group. It was conducted in accordance with the Declaration of Helsinki. The study protocol, information brochure, and informed consent were approved by the Medical Ethics Committee of the University of Ulm. Recruitment was carried out from October 2008 to September 2010 in the Centres for Psychiatry in Zwiefalten and Weissenau and the Clinic for Psychiatry in Reutlingen in the south of Germany.
Inclusion/exclusion criteria
Inclusion criteria were voluntary, written, informed consent; diagnosis of schizophrenia (International Statistical Classification of Diseases and Related Health Problems, 10th Revision: F20.x) or schizoaffective disorder (F25.x); age 18–60 years; reachability for home visits; no earlier participation in such a training program; and outpatient visits for antipsychotic maintenance treatment after discharge.
Exclusion criteria were admission for crisis intervention (short stay), absence of written informed consent, high probability that support would be needed for medication intake over a longer period of time (eg, patient in a residential home in which medication is controlled by staff), and monotherapy with depot antipsychotics. Patients who attended the program for less than 12 days were excluded because this was the shortest period in which level 4, the highest level of the training program could be achieved.
Sample size/power
To calculate the required patient sample, we assumed that, in line with the literature, about 25% of the treated patients20 would stop taking medication against medical advice within 14 days. The aim of the intervention was a significant reduction of noncompliant patients. A decrease of about one-third, that is, to a level of 17%, was assumed as a clinically significant reduction. The confirmation of such an effect would require a sample of N=174, with a statistical power of 80% on a level of significance of P<0.05.
Randomization and blinding
Randomization was carried out with a computer-generated restrictive randomization list for all three participating hospitals. The software RandList, Version 1.2, was created by DataInf GmbH, Tuebingen. The study was financed by the Centre for Psychiatry, South-Württemberg. Recruitment took place between October 2008 and September 2010. The course of participation in this randomized controlled trial was documented according to the recommendations of the CONSORT (Consolidated Standard of Reporting Trials) group.24 To ensure similarity of all other treatment conditions, patients of the intervention group and control group were treated on the same wards by the same doctors, with routine clinical treatment.
The study workers who conducted the interviews were blinded with regard to the intervention. To ensure consistency, only three different nurses visited the patients at home. The interviews and the data collection were based on an interview manual developed for this purpose. The clinical experts who rated the adherence by means of the serum levels of antipsychotics were also blinded with regard to allocation to the intervention or control group. Severity of illness was measured using the Clinical Global Impression Scale–Severity.
Training program
Patients should know their prescribed medication; that is, the names of the substances, the packages, the doses, the required schedule for medication intake, and that they should learn to prepare their weekly medication in advance in a weekly dispenser containing the prescribed pills in separate compartments for each time of intake. A training manual was developed, describing four levels of autonomy in the use of medication and the required skills. For each level, the objectives of the training were defined. The training program is conducted in one-to-one lessons with skilled nurses. Patients should learn to prepare their medication by themselves during the hospital stay in the same way they are expected to do it autonomously after discharge. The patients are informed using an educational approach: color, shape, and name of the pills; operating mode of the dispenser they are given; and other technical aspects. The preparation of the medication follows a plan, describing all necessary steps and the criteria for an upgrade to the next level or, if problems occur, a downgrade. Level 1 focuses on the scheduled intake of medication; level 2 covers the arrangement of the next day’s medication coached by a nurse; in level 3, the dispenser is located in the patient’s room, and the next day’s medication is arranged without help by the patient; and in level 4, the patient arranges the medication for 1 week in a dispenser, which remains in the patient’s room in a locked cupboard. With every level, requirements get more complex. The training takes place in a low-stimulus room in one-to-one lessons. It is divided into small steps comprising demonstration, supporting lessons, and autonomous, structured lessons as the patients advance.
The participating nurses were instructed in the use of the training manual in a 1-day course.
The patients in the control group received nonspecific one-to-one nursing activities for a comparable amount of time. To approximate the attention by nurses in the control and the intervention group, the total time for the intervention group was estimated. We estimated 3–5 minutes of attention per patient and day. The average duration of inpatient treatment for patients with a schizophrenic or schizoaffective disorder in 2007 was 40 days (40 days ×4 minutes =160 minutes/patient). A further 20 minutes were added for the introductory information, so that the patients in the control group received 180 minutes of additional unspecified one-to-one nursing care with no relation to the medication.
Measurement of adherence
Several instruments to measure adherence to medication intake exist.17 The “expert consensus guideline 12 assessing compliance” recommends five strategies25: asking a relative or caregiver, asking the patient, pill count, blood levels, and a self-rating scale for compliance. For this study, three strategies were chosen.
Pill count
The number of available pills in the patient’s administration at discharge (t1) and the number of available pills at the time of the home visit (t2) were counted, and the difference was compared with the expected total intake, according to the prescribed dose. The adherence was evaluated according to the “levels of compliance.”26 Patients who had taken more than 75% of their medication were rated as adherent, patients who had taken more than 35%–75% of their medication were rated as partly adherent, and patients who had taken less than 35% of their medication were rated as nonadherent.
Measurement of the serum levels of the antipsychotic medication
One blood sample was taken close to the time of discharge (t1), at least 3 days after the last change of medication. The next sample was taken during the home visit (t2). Analysis of the antipsychotic serum concentrations were conducted in a certified laboratory, following international standards. Two highly experienced experts (a psychiatrist and a pharmacologist) assessed the serum levels independently in terms of compliance, following therapeutic ranges given in a German consensus guideline.27 Because no clear recommendations are available in terms of a cutoff in the literature, we decided in advance that a serum level at t2 of 75% or more compared with the serum level at t1 was classified as adherent. Patients with serum levels below 75% were classified as nonadherent. However, differences in the time span between taking the blood sample and last medication intake had to be considered individually, as well as changes of the prescribed dosage after discharge. In the case of differing opinions, the two experts sought a consensus by discussing all relevant aspects. All ratings referred to the prescribed antipsychotic, and in the case of combination therapy, to the one with the higher chlorpromazine-equivalent dosage.
Self-rating of medication intake
During home visits, the patients were asked in form of a structured interview about their present medication and their habits with respect to preparation and intake. Finally, they were asked to indicate how often they had taken their medication during the last 7 days, using a four-point Likert scale: took all the pills, did not take the pills once a week, did not take the pills two to three times a week, or did not take the pills four to six times a week. To compare the different outcome measures, we divided the patients into two groups: those who indicated they had taken all pills were classified as adherent, and all other patients were classified as nonadherent.
Follow-up
Two follow-ups with home visits by study nurses were conducted, which included taking blood samples to determine the serum level of the antipsychotic medication. The first visit (t1) took place shortly after discharge from hospital, and the second (t2) 4 weeks after. The patients were visited at home after being given notice by telephone the day before. Blood samples were taken, pills were counted, and the patients were asked to self-assess their medication intake. For participating in the study, the patients received €15.
Statistical analysis
The data sheets were numbered and anonymized. Data were entered into an Excel database and transferred to STATISTICA (StatSoft, Inc; STATISTICA for Windows, Version 8.0) to conduct statistical analysis.
To test the differences between the intervention and the control group, we performed t-tests for interval-scaled variables. These tests require samples taken from populations with a normal distribution and variance homogeneity of the groups.28 The assumption of a normal distribution was verified using the Kolmogorov–Smirnov test. In the case of the assumption being breached we used the Mann–Whitney U-test. The variance homogeneity was tested by means of Levene’s test. If there was no variance homogeneity, the degrees of freedom of the Student’s t-test were corrected.28
In the case of ordinal-scaled variables, we tested differences in the central tendency by means of the Mann–Whitney U-test. Assuming equal distribution patterns of the underlying populations, this test only responds to differences in the central tendency. Even when the underlying populations do not show an equal distribution pattern, the test mainly responds to differences in the central tendency.29 For dichotomous or polytomous variables, we used the chi-square test. This test requires expected cell numbers greater than 5. For a small sample size (N<20), the chi-square test has to be replaced by Fisher’s exact test.30
Interim analysis/termination of the study
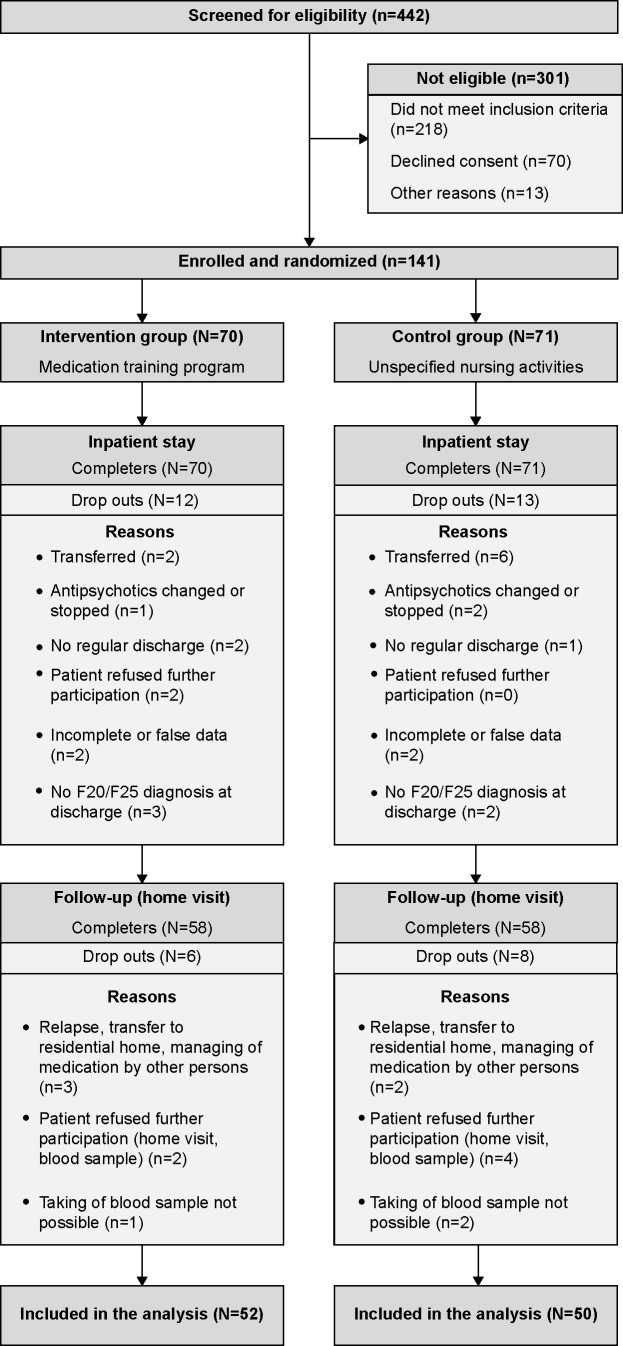
An early termination of the study was fixed in advance, in case of a clear effect in favor of one of the groups. In September 2010, the interim analysis was carried out as a result of the study plan. At that time, 141 patients were enrolled and randomized. One hundred and two patients could be included in the analysis (see Results section and Figure 1). The recruitment was stopped at that time because of a clear effect in favor of the study group.
Figure 1.
CONSORT diagram.
Results
A total of 442 patients were screened for participation in the study; 218 of the screened patients were excluded following the exclusion criteria, 167 of them because of intramuscular depot medication.
Another 13 patients were excluded because they discontinued the therapy, were transferred to a different hospital, or were discharged before they had signed the informed consent (n=9). Four patients had to be excluded because of homelessness.
In total, 211 patients were informed about the study and asked for a written informed consent, of whom 70 patients refused to participate. Reasons for the refusal were rejection of home visits (n=20), feeling of inability to meet demands of the training program (n=12), no declaration of reasons (n=12), refusal to take any medication (n=8), and other subjective reasons (n=18). The remaining 141 participants were randomized either into the intervention group (n=70) or the control group (n=71).
After having signed the informed consent before being discharged from inpatient treatment, 25 patients had to be excluded from the study because of incomplete data records or other exclusion criteria, including 12 patients from the intervention group and 13 from the control group. Another 14 patients were excluded from the study as a result of the home visit. Data from 102 patients could be analyzed (Figure 1). The drop-out rates did not differ significantly between the intervention group (25.7%) and the control group (29.6%).
Patient characteristics
There were no differences between the intervention group and the control group with respect to sex, age, housing, education, native language, diagnosis, and severity of symptoms (Table 1). The patients in the intervention group showed a tendency to stay longer, had more previous admissions, and were more often switched to a generic drug after discharge than the patients in the control group. With regard to the number of prescribed drugs, the type of antipsychotic medication, modification in dosage after discharge from hospital, prior attendance of psychoeducation groups, and use of community mental health services after discharge, we likewise found no differences between the two groups (results not reported in detail).
Table 1.
Clinical and sociodemographic details of both study groups
| Sociodemographic variables | Intervention group | Control group | Level of significance |
|---|---|---|---|
| Sex (m/f) | 27/25 | 23/27 | ns (χ2=0.36, df=1, P=0.55) |
| Average age, years | 39.8 | 40.4 | ns (t=0.27, df=100, P=0.79) |
| Living alone | 22 | 18 | ns (χ2=4.14, df=5, P=0.53) |
| Higher and intermediate educational level | 30 | 31 | ns (χ2=2.68, df=4, P=0.61) |
| Native language is German | 48 | 45 | ns (χ2=0.20, df=1, P=0.66) |
| F2-diagnosis of schizophrenia | 35 | 32 | ns |
| Clinical Global Impression Scale: moderate or definitely ill | 32 | 32 | ns (Mann–Whitney U=1,132.0, P=0.89) |
| Average length of stay, days | 76.7 (SD: 57.5) | 59.4 (SD: 36.4) | ns (t: −1.79, df=100, P=0.07) |
Abbreviations: m, male; f, female; SD, standard deviation; ns, not significant.
Achievement of training levels
All 52 patients in the intervention group achieved at least level 2. At the time of discharge, 34 patients (65.4%) had reached only level 2, 11 patients (21.1%) reached level 3, and 7 patients (13.5%) reached level 4 (Table 2). The mean duration of participation in the training program was 49.3 days, and the median was 42.5 days. The number of training days ranged between 15 and 241.
Table 2.
Training days in the different levels of the medication training program
| Level of training program at discharge | Patients, n | Training days, arithmetic mean (standard deviation) |
|---|---|---|
| 2 | 34 (65.4%) | 52.4 (43.6) |
| 3 | 11 (21.1%) | 43.6 (22.6) |
| 4 | 7 (13.5%) | 43.0 (17.4) |
| 2–4 | 52 (100%) | 49.3 (37.2) |
During their hospital stay, 48 patients were not downgraded. Overall, three patients were downgraded once, and one patient a second time. The control group consisted of 50 patients. All received unspecified nursing care for at least 3 hours.
Rating of adherence
After discharge from inpatient treatment, pill count showed that 95.2% (mean) of the prescribed medication had been taken from the dispenser by the 52 patients in the intervention group. In the control group, 86.9% (mean) of the prescribed medication had been taken. Thus, the pill count revealed a significant difference between both groups (Mann–Whitney U=844; P=0.002; Figure 2). In total, eight patients, five of them in the control group, had taken more medication than prescribed.
Figure 2.
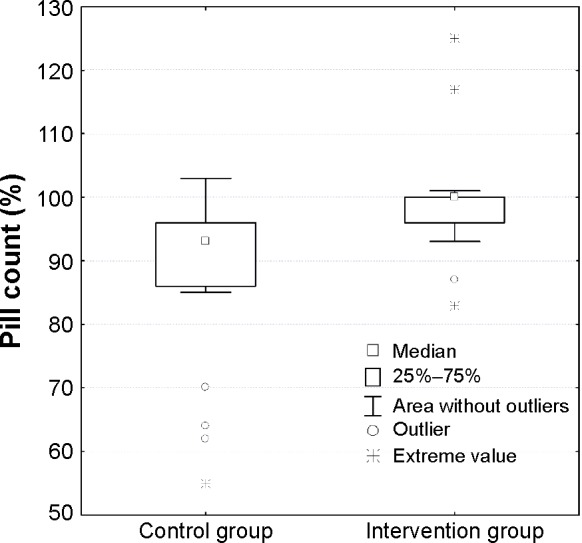
Box-whisker plot showing the number of antipsychotics taken out from the dispenser in percent.
In terms of the classification into three degrees of adherence (adherent, partly adherent, nonadherent), 51 patients (98.1%) from the intervention group and 38 patients (76.0%) from the control group were rated as adherent. None of the patients in the intervention group were classified as partly adherent, whereas nine patients in the control group met the criteria. One patient in the intervention group was nonadherent versus three patients in the control group. The difference was significant (P=0.003) in favor of the intervention group.
The assessment of adherence by means of the plasma serum levels of antipsychotics was conducted by two independent and blinded raters. In a dichotomous approach (adherent versus nonadherent patients), 46 patients (88.5%) from the intervention group and 35 patients (70.0%) from the control group were classified as adherent. In contrast, six patients (11.5%) from the intervention group and 15 patients (30.0%) from the control group were classified as nonadherent. The difference between both groups was statistically significant (P=0.02) in favor of the intervention group.
In the self-rating by the patients on their intake of antipsychotics in the previous 7 days, 48 patients (92.3%) from the intervention group and 42 patients (84.0%) from the control group stated that they had taken their medication “as prescribed.” The difference did not reach significance (χ2=4,86, df=3, P=0.18).
Correlation between the main outcome measures
There was a highly significant correlation between the results of the pill count and serum level methods with regard to the dichotomous outcome adherent/nonadherent (P<0.001): 75% of the patients rated as nonadherent by means of pill count were also rated as nonadherent by serum blood levels, and 85.4% of the patients rated as adherent by means of the pill count were also rated as adherent by blood serum levels (phi=0.40, kappa=0.25, P<0.001).
Comparing the ratings of adherence by pill count and self-assessment, there was a significant correlation by trend (P=0.06): 75% of the patients rated as nonadherent in the pill count were also rated as nonadherent in the self-assessment, and 74.2% of those rated as adherent by the pill count were also rated as adherent in the self-assessment (phi=0.23, kappa=0.14, P<0.05).
Rating of adherence by blood serum levels and self-assessment showed no significant correlation: 33.3% of the patients rated as nonadherent by blood serum levels were also rated as nonadherent by self-assessment, and 71.6% of the patients rated as adherent by blood serum levels were also rated as adherent by self-assessment (phi=0.04, kappa=0.04, not significant).
Discussion
To our knowledge, this is the first study to assess the influence of a standardized skills-based medication training program conducted by nursing staff on early medication nonadherence after hospital discharge in patients with schizophrenia. Pill count and blood serum levels showed that the training program effected a significant improvement in medication intake within the observation period of 32 days on average (median, 29 days): 98.1% of the patients in the intervention group were rated as adherent at this time versus 76.0% in the control group. In contrast to these objective measures, the self-rating by the patients as having taken the antipsychotics “as prescribed” did not differ significantly between the intervention group and the control group. This third method of evaluation yielded the highest level of adherence in the control group. This reflects the well-known tendency that patients retrospectively overestimate their adherence.31,32 However, it must be assumed that this tendency is represented in both groups to the same degree.
The number of adherent patients in the control group is in line with a large number of preceding reports. In the literature, the rate of nonadherence after 4–8 weeks is stated to be between 20% and 50%.33–35 A more recent study showed nonadherence in 25% 7 days after discharge from hospital.21 Comparable studies do not exist so far. Previous work in this field22,23 used insight-based approaches such as psychoeducation and aimed at a change of attitudes. In contrast, our approach basically aims at an increase of technical skills and tries to minimize the early loss of adherence to treatment at the interface between inpatient and outpatient treatment.
This study also reveals a highly significant correlation between the two outcome measures, pill count and serum levels of antipsychotics. In contrast, there was only a correlation by trend between self-rating and pill count and no correlation between self-rating and serum levels. Nearly 80% of the patients classified as nonadherent by at least one of the two other methods stated that they had taken their medication as prescribed. Our results confirm that self-ratings alone are not sufficient as an instrument for measuring adherence and should be complemented by other methods. However, each of the methods has its specific advantages and drawbacks, and thus the combination of several approaches as used in this case seems appropriate.
Taken together, this study shows that a standardized training program for medication intake carried out by nurses is an effective intervention to enhance early medication adherence in patients with schizophrenic or schizoaffective disorder after discharge. The practical training seems to facilitate the transfer of the patient into her/his home environment. Therefore, the training program can provide an important contribution to maintenance therapy and relapse prevention in patients with schizophrenic or schizoaffective disorder.
This study has several limitations. First, it is limited to a rather short observation period of 32 days on average. The study focused on early nonadherence in the weeks after discharge, which at least in part may be caused by a lack of knowledge and skills. A follow-up for a longer time might be desirable, but was out of the focus of this study, and the method of pill-count used here probably would not provide valid results in the long-term because of the many confounding factors. Similarly, the method of blood level controls during home visits could be increasingly biased during repeated measures; for example, by nonadherent patients refusing consent to taking the blood samples. The differing time span between taking the blood sample and last medication intake is another critical point. Second, although the sample was drawn from an unselected population in clinical routine care and attrition rates were rather low, it must be assumed that, as in all studies of this kind, patients with very poor insight and poor adherence refused to participate, and thus were underrepresented in this study. Third, we discontinued the study before reaching the desirable sample size calculated in advance. This decision was made on ethical grounds because of the clear results in favor of the intervention, which was from that point on implemented for all patients in routine care. This is a typical problem of interim analysis that may weaken the results. Fourth, the control group and the intervention group differed with respect to the average length of inpatient treatment in spite of random allocation.
Conclusion
We consider training programs for mentally ill patients an effective way to pass back responsibility to the patients, and therefore increase their self-efficacy and autonomy. In addition, medication training programs increase medication adherence. This is very important with regard to the prevention of relapses, which worsen the course of a mental disorder. Such feasible interventions are needed in modern mental health systems emphasizing outpatient treatment.
Acknowledgments
The research group is financed by a grant of the Ministry of Social Affairs of Baden-Wuerttemberg.
Footnotes
Author contributions
UBS was responsible for the implementation of the intervention and data collection procedures, TS participated in the design of the study, EF performed the statistical analysis, and RB conceived of the study, participated in the design and coordination. All authors contributed toward data analysis, drafting and critically revising the paper, agree to be accountable for all aspects of the work, and read and approved the final manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jablensky A. Schizophrenia: recent epidemiologic issues. Epidemiol Rev. 1995;17(1):10–20. doi: 10.1093/oxfordjournals.epirev.a036164. [DOI] [PubMed] [Google Scholar]
- 2.Ascher-Svanum H, Zhu B, Faries DE, et al. The cost of relapse and the predictors of relapse in the treatment of schizophrenia. BMC Psychiatry. 2010;10(1):2. doi: 10.1186/1471-244X-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mangalore R, Knapp M. Cost of schizophrenia in England. J Ment Health Policy Econ. 2007;10(1):23–41. [PubMed] [Google Scholar]
- 4.Buchanan RW, Kreyenbuhl J, Kelly DL, et al. Schizophrenia Patient Outcomes Research Team (PORT) The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr Bull. 2010;36(1):71–93. doi: 10.1093/schbul/sbp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayuso-Gutiérrez JL, del Río Vega JM. Factors influencing relapse in the long-term course of schizophrenia. Schizophr Res. 1997;28(2–3):199–206. doi: 10.1016/s0920-9964(97)00131-x. [DOI] [PubMed] [Google Scholar]
- 6.Levander S, Jensen J, Gråwe R, Tuninger E. Schizophrenia – progressive and massive decline in response readiness by episodes. Acta Psychiatr Scand Suppl. 2001;104(408):65–74. doi: 10.1034/j.1600-0447.2001.104s408065.x. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen NC, Liu D, Ziebell S, Vora A, Ho BC. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609–615. doi: 10.1176/appi.ajp.2013.12050674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2010;71(suppl 2):20–26. doi: 10.4088/JCP.9096su1cc.04. [DOI] [PubMed] [Google Scholar]
- 9.Bodén R, Brandt L, Kieler H, Andersen M, Reutfors J. Early non-adherence to medication and other risk factors for rehospitalization in schizophrenia and schizoaffective disorder. Schizophr Res. 2011;133(1–3):36–41. doi: 10.1016/j.schres.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 10.Verdoux H, Lengronne J, Liraud F, et al. Medication adherence in psychosis: predictors and impact on outcome. A 2-year follow-up of first-admitted subjects. Acta Psychiatr Scand. 2000;102(3):203–210. doi: 10.1034/j.1600-0447.2000.102003203.x. [DOI] [PubMed] [Google Scholar]
- 11.Morken G, Widen JH, Grawe RW. Non-adherence to antipsychotic medication, relapse and rehospitalisation in recent-onset schizophrenia. BMC Psychiatry. 2008;8(1):32. doi: 10.1186/1471-244X-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Adherence to Long-Term Therapies: Evidence for Action Section I – Setting the Scene. Geneva: World Health Organization; 2003. [Accessed November 23, 2014]. pp. 1–16. Available from: http://apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf. [Google Scholar]
- 13.Giles J. Social science lines up its biggest challenges. Nature. 2011;470(7332):18–19. doi: 10.1038/470018a. [DOI] [PubMed] [Google Scholar]
- 14.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005;(4):CD000011. doi: 10.1002/14651858.CD000011.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Lacro JP, Dunn LB, Dolder CR, Leckband SG, Jeste DV. Prevalence of and risk factors for medication nonadherence in patients with schizophrenia: a comprehensive review of recent literature. J Clin Psychiatry. 2002;63(10):892–909. doi: 10.4088/jcp.v63n1007. [DOI] [PubMed] [Google Scholar]
- 16.Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724–742. doi: 10.1093/schbul/sbj075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization . Adherence to Long-Term Therapies: Evidence for Action Section II – Improving Adherence Rates: Guidance for Countries. Geneva: World Health Organization; 2003. [Accessed November 23, 2014]. pp. 17–44. Available from: http://apps.who.int/medicinedocs/pdf/s4883e/s4883e.pdf. [Google Scholar]
- 18.Kemp R, Kirov G, Everitt B, Hayward P, David A. Randomised controlled trial of compliance therapy. 18-month follow-up. Br J Psychiatry. 1998;172(5):413–419. doi: 10.1192/bjp.172.5.413. [DOI] [PubMed] [Google Scholar]
- 19.Dolder CR, Lacro JP, Leckband S, Jeste DV. Interventions to improve antipsychotic medication adherence: review of recent literature. J Clin Psychopharmacol. 2003;23(4):389–399. doi: 10.1097/01.jcp.0000085413.08426.41. [DOI] [PubMed] [Google Scholar]
- 20.Beck EM, Cavelti M, Kvrgic S, Kleim B, Vauth R. Are we addressing the ‘right stuff’ to enhance adherence in schizophrenia? Understanding the role of insight and attitudes towards medication. Schizophr Res. 2011;132(1):42–49. doi: 10.1016/j.schres.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 21.Keith SJ, Kane JM. Partial compliance and patient consequences in schizophrenia: our patients can do better. J Clin Psychiatry. 2003;64(11):1308–1315. doi: 10.4088/jcp.v64n1105. [DOI] [PubMed] [Google Scholar]
- 22.Boczkowski JA, Zeichner A, DeSanto N. Neuroleptic compliance among chronic schizophrenic outpatients: an intervention outcome report. J Consult Clin Psychol. 1985;53(5):666–671. doi: 10.1037//0022-006x.53.5.666. [DOI] [PubMed] [Google Scholar]
- 23.Liberman RP, Wallace CJ, Blackwell G, Kopelowicz A, Vaccaro JV, Mintz J. Skills training versus psychosocial occupational therapy for persons with persistent schizophrenia. Am J Psychiatry. 1998;155(8):1087–1091. doi: 10.1176/ajp.155.8.1087. [DOI] [PubMed] [Google Scholar]
- 24.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. J Pharmacol Pharmacother. 2010;1(2):100–107. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velligan DI, Weiden PJ, Sajatovic M, et al. Adherence Problems in Patients with Serious and Persistent Mental Illness. J Clin Psychiatry. 2009;70(suppl 4):1–48. [PubMed] [Google Scholar]
- 26.Kane JM, Leucht S, Carpenter D, Docherty JP, Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders The expert consensus guideline series. Optimizing pharmacologic treatment of psychotic disorders. Introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5–19. [PubMed] [Google Scholar]
- 27.Hiemke C, Baumann P, Laux G, Kuss HJ. Therapeutisches Drug-Monitoring in der Psychiatrie. Konsensus-Leitlinie der AGNP. Psychopharmakotherapie. 2005;12:166–182. [Google Scholar]
- 28.Bortz J. Formulierung und Überprüfung von Hypothesen. In: Bortz J, editor. Statistik für Sozialwissenschaftler. 5th ed. Berlin: Springer Verlag; 1999. German. [Google Scholar]
- 29.Bortz J, Lienert GA, Boehnke K. Analyse von Rangdaten. In: Bortz J, Lienert GA, Boehnke K, editors. Verteilungsfreie Methoden in der Biostatistik. 2nd ed. Berlin: Springer Verlag; 2000. pp. 197–294. German. [Google Scholar]
- 30.Bortz J, Lienert GA, Boehnke K. Analyse von Häufigkeiten. In: Bortz J, Lienert GA, Boehnke K, editors. Verteilungsfreie Methoden in der Biostatistik. 2nd ed. Berlin: Springer Verlag; 2000. pp. 87–190. German. [Google Scholar]
- 31.Arnsten JH, Demas PA, Farzadegan H, et al. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33(8):1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buelow JM, Smith MC. Medication management by the person with epilepsy: perception versus reality. Epilepsy Behav. 2004;5(3):401–406. doi: 10.1016/j.yebeh.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Zito JM, Routt WW, Mitchell JE, Roerig JL. Clinical characteristics of hospitalized psychotic patients who refuse antipsychotic drug therapy. Am J Psychiatry. 1985;142(7):822–826. doi: 10.1176/ajp.142.7.822. [DOI] [PubMed] [Google Scholar]
- 34.McEvoy JP, Freter S, Everett G, et al. Insight and the clinical outcome of schizophrenic patients. J Nerv Ment Dis. 1989;177(1):48–51. doi: 10.1097/00005053-198901000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Olfson M, Mechanic D, Hansell S, Boyer CA, Walkup J, Weiden PJ. Predicting medication noncompliance after hospital discharge among patients with schizophrenia. Psychiatr Serv. 2000;51(2):216–222. doi: 10.1176/appi.ps.51.2.216. [DOI] [PubMed] [Google Scholar]