Abstract
Small heat shock proteins (sHSPs) can regulate protein folding and protect cells from stress. To investigate the role of sHSPs in the silk-producing insect Antheraea pernyi response to microorganisms, a sHsp gene termed as Ap-sHSP21.4, was identified. This gene encoded a 21.4 kDa protein which shares the conserved structure of insect sHsps and belongs to sHSP21.4 family. Ap-sHSP21.4 was highly expressed in fat body and up-regulated in midgut and fat body of A. pernyi challenged with Escherichia coli, Beauveria bassiana and nuclear polyhedrosis virus (NPV), which was determined by quantitative real-time PCR. Meanwhile, knock down of Ap-sHSP21.4 with dsRNA result in the decrease at the expression levels of several immune response-related genes (defensin, Dopa decarboxylase, Toll1, lysozyme and Kazal-type serine protease inhibitor). Additionally, the impact of eicosanoid biosynthesis on the expression of Ap-sHSP21.4 response to NPV was determined using qPCR, inhibitors of eicosanoid biosynthesis significantly suppress Ap-HSP21.4 expression upon NPV challenge. All together, Ap-sHSP21.4 was involved in the immunity of A. pernyi against microorganism and possibly mediated by eicosanoids pathway. These results will shed light in the understanding of the pathogen-host interaction in A. pernyi.
Introduction
Heat shock proteins (HSPs) are evolutionarily highly-conserved proteins synthesized in cells when they are exposed to stress, which are found in almost all organisms [1–4]. Heat shock proteins can be divided into five families, including HSP100, HSP90, HSP70, HSP60, and small heat shock proteins (12–42 kDa) according to approximate molecular weight and protein characters. sHSPs were discovered in the salivary glands of Drosophila after heat shock [5,6] and are closely associated with the duration of stresses [7–9] such as cellular communication, immune response, protein transport, apoptosis and cell cycle regulation [10–13].
Insects are more or less constantly challenged with a daunting array of pathogenic organisms, including viruses, bacteria, fungi, protozoans as well as various metazoan parasites and parasitoids. Eicosanoids mediate melanotic nodulation reactions to pathogens infection in larvae of Pimpla turionellae and Bombyx mori [14–17] found that eicosanoid biosynthesis inhibitors significantly repressed the induction of the cecropin and lysozyme genes elicited by peptidoglycan. It is reported that both cyclooxygenase and lipoxygenase products were involved in nodulation responses to bacterial infections [18,19] and phagocytosis [20]. On the other hand, HSP induction represents an important adaptive response to stress and is also associated with local increase in tissue temperature. In addition, Bundey et al [21] also reported that eicosanoids mediate behavioral fever responses to infection in the locust Schistocerca gregaria. Taking together, we propose that eicosainods pathway is involved the HSP gene transcription in insect challenged by pathogens.
The Chinese oak silk moth Antheraea pernyi (Lepidoptera: Saturniidae; A. pernyi) is an economically valuable silk-producing insect that is commercially cultivated mainly in China, India, and Korea [22]. The sHSP21.4 genes have been found in a variety of insects [23,18], however, the HSP21.4 gene in the A. pernyi (Ap-sHSP21.4, GenBank accession number: KM881571) and its function remains unclear. In this study, we aimed to clone the genes of sHSP21.4 and investigate its role in the immune response against microorganisms.
Materials and Methods
Experimental insects and treatment
A. pernyi larvae were provided by the Sericultural Research Institute of Henan and were reared on oak leaves under indoor conditions. The larvae were reared on fresh oak leaves at 25 ± 1°C in 14 h light: 10 h dark (a long day length) with 70% humidity. Five 3rd day fifth instar larvae were randomly sampled at each time point after exposure to infection. The total RNA extracted from hemocytes, fat bodies, and midguts of A. pernyi larvae after challenges with heat-killed bacteria (Escherichia coli, E. coli and Beauveria bassiana, B. bassiana), A. pernyi nuclear polyhedrosis virus (NPV) and control sample (1.0 ×106 bacterial cells or 1.0 ×106 fungal spores or 1.0×109 virus particles were suspended in 10 μL of sterilized 0.85% NaCl, and then were separately injected into each larvae) [24]. Tissues were sampled for RNA extraction at 0 h, 3 h, 6 h, 12 h, 24 h, 48 h after infection and stored at -80°C and subjected to qPCR testing [25].
RNA extraction, cDNA synthesis, PCR primers, and conditions
Total RNA was isolated from fat bodies with TRIzol reagent (Invitrogen, USA) and first-strand cDNA was obtained using TransScript Synthesis SuperMix (TransGen, Beijing, China). The Ap-sHSP21.4 sequences from various animals were aligned by ClustalW (http://www.ebi.ac.uk/Tools/ClustalW). The degenerate oligonucleotide primers F2 and R2 (Table 1) were designed with Primer premier 5.0. PCR was performed using the amplification program with the following protocol: one cycle at 94°C for 5 min; followed by 35 cycles of 94°C for 30 s, 55°C for 35 s, and 72°C for 30 s; and a final elongation step of 72°C for 8 min. The PCR products were analyzed by 1% agarose gel electrophoresis, and sequenced at Invitrogen, Shanghai.
Table 1. Primers used in this study.
| Primer | sequences (5'–3') | Application |
|---|---|---|
| F1 | CAACACAGCATAGCGATAGCAG | qPCR |
| R1 | GATTTCTCCTCGTGTTTGGCAT | qPCR |
| F18S | CGATCCGCCGACGTTACTACA | qPCR |
| R18S | GTCCGGGCCTGGTGAGATTT | qPCR |
| F2 | ATGGCTGACAGTGGTCTCAAG | amplification |
| R2 | TCAGTGCTTCTGGATAGGAATG | amplification |
| F3 | CGCGGATCC ATGGCTGACAGTGGTCTCAAG | expression |
| R3 | ATAAGAATGCGGCCGCTCAGTGCTTCTGGATAGGAATG | expression |
| F4 | TGCCAAACACGAGGAGA | RACE PCR |
| F 5 | ACTCGCCATTACCGACAGG | RACE PCR |
| R4 | TCTGCTATCGCTATGCTGT | RACE PCR |
| R5 | TTTGCCGTCACCTTCATCC | RACE PCR |
Bioinformatics and phylogenetic analysis
NCBI bioinformatics tools (available at http://blast.ncbi.nlm.nih.gov/Blast.cgi) were used to detect the conserved domains in Ap-sHSP21.4. DNAman software was used to predict the secondary structure and search for open reading frame (ORF). Multiple sequence alignments were performed using ClustalX with its default parameters [26]. Phylogenetic analysis was performed using the neighbor-joining method with Molecular Evolutionary Genetics Analysis (MEGA version 6.0) software.
Expression analysis using quantitative RT-PCR
The total RNA from hemocytes, fat body, midgut, epidermis, silk gland, and Malpighian tubules of 3rd day fifth instar larvae were reverse transcribed into cDNAs. The housekeeping gene 18S rRNA (GenBank: DQ347469) was used as an internal control. RT- PCR was performed in a StepOne Plus Real-Time PCR System using the SYBR Premix Ex Taq kit (TaKaRa) with the specific primers in Table 1. A 20 μL reaction mixture contained 10 μL of 2× SYBR Premix Ex Taq buffer, 1 μL each of forward and reverse primers, 1 μL cDNA, and 7 μL Rnase-free H2O. The PCR procedure was as follows: 95°C for 10 s followed by 40 cycles each at 95°C for 15 s, 62°C for 15 s, and 72°C for 30 s. At the end of the reaction, a melting curve was produced by monitoring the fluorescence continuously while slowly heating the sample from 60°C to 95°C. Each independent experiment was conducted in triplicate and the data were analyzed using ANOVA method. It was considered statistically significant when P value less than 0.05 and the significance was indicated by an asterisk [27].
RNA interference of Ap-sHSP21.4 gene
The siRNAs (Table 1) were designed by the siRNA Selection Program (http://sirna.wi.mit.edu/home.php) and chemically synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). The BLAST homology search (http://www.ncbi.nlm.nih.gov/BLAST) was performed to avoid off-target effects on other genes or sequences. The siRNAs were purified by high-performance liquid chromatography and were dissolved in diethylpyrocarbonate-treated water (Milli-Q-grade). The final concentration of siRNA was 1 μg/μL H2O. The 10 μL of siRNA was injected into each larva using microliter syringes (Gaoge Co., Shanghai, China). To avoid leakage of siRNA from the insect body, needles were kept still at the injection point for 30 s. One set of siRNAs with random sequences was used as a negative control and injected alongside the experimental injection. Twenty-one or fourty-five hours after RNAi treatment, the larvae were injected with NPV (1.0×109 virus particles/larvae) followed by recovery at 25°C for 3 h. The fat bodies of the larvae were collected at 24 and 48 h after siRNA injection, frozen in liquid nitrogen and stored at -80°C. All experiments were conducted with two independent experiments in triplicate.
Effect of inhibitors of arachidonic acid on the expression of Ap-sHSP21.4 against NPV
All inhibitors and arachidonic acid were purchased from Sigma Chemicals. Test larvae were anesthetized with CO2 and first injected with inhibitor (phospholipase A2 inhibitor dexamethasone [DEX], lipoxygenase inhibitor nordihydroguaiaretic acid [NDGA], and cyclooxygenase inhibitor indomethacin [INDO]), arachidonic acid (AA) in 10 μL of DMSO, or DMSO alone (control). After incubating the mixtures for 30 min at room temperature, the larvae received a second injection of NPV (1.0×109 virus particles /larvae) or PBS alone, and were further incubated at 27°C. The fat body and midgut were collected at 3 h or 24 h respectively after the second injection, rinsed in ice-cold PBS solution, and frozen on dry ice [17].
Results
Cloning and sequence analysis of Ap-sHSP21.4 cDNA
The full-length cDNA of Ap-sHSP21.4 was 1,391 bp in length and encoded 187 amino acids with a calculated molecular mass of 21.4 kDa (S1A Fig).
This predicted protein shared 97.72, 97.26, and 97.26% identity with sHSP21.4 proteins from Bombyx mori, Helicoverpa armigera and Chilo suppressalis. It represents a conserved C-terminal domain of -crystallin-type sHSPs and belongs to the lepidopteran HSP21.4 family (S1B Fig). However, most insect sHSPs are species-specific. This strengthens the hypothesis that sHSP21.4 might have followed another evolutionary course [28].
Expression of Ap-sHSP21.4 in tissues and under microorganism challenges
The sHSP gene was commonly expressed in all examined tissues (Fig 1).
Fig 1. The expression of Ap-sHSP21.4 in different tissues and cells.
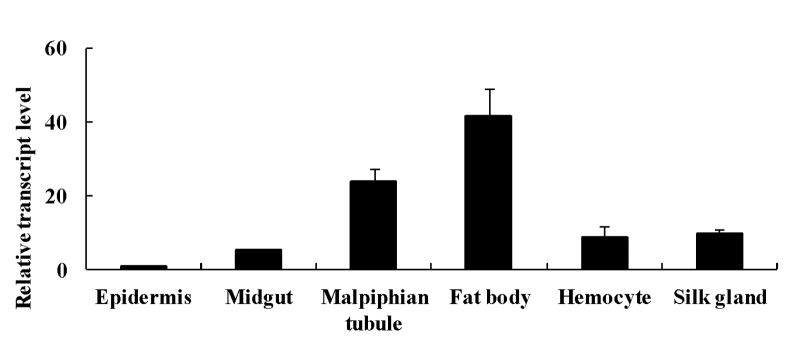
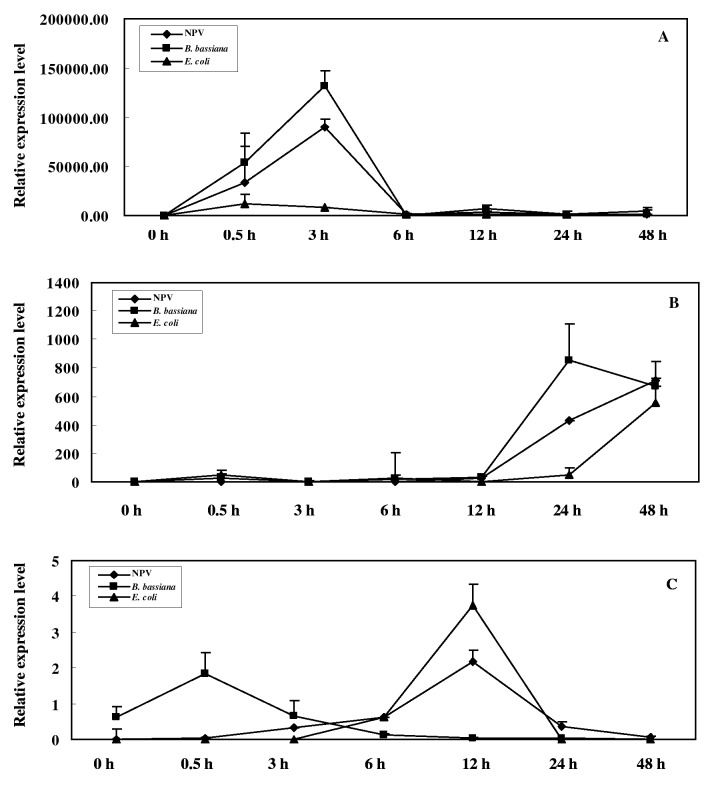
The expression level of Ap-sHSP21.4 transcript was highest in the fat body and lowest was epidermis. To investigate whether Ap-sHSP21.4 is involved in the immune response, three types of microorganisms were individually injected into the bodies of fifth-instar larvae. The Ap-sHSP21.4 transcripts quickly accumulated in the fat body and reached a maximum at 3 h, and then gradually decreased. The Ap-sHSP21.4 gene was found to be more susceptible to NPV and B. bassiana compared with E. coli in fat bodies (Fig 2A).
Fig 2. Expression patterns of the Ap-sHSP21.4 under microorganism challenges in different tissues (five larvae for each group).
(A): the expression of Ap-sHSP21.4 in the fat body, (B): the expression of Ap-sHSP21.4 in the midgut. (C): the expression of Ap-sHSP21.4 in the hemocytes.
In the midgut, the expression of Ap-sHSP21.4 was increased at 24 h after injected with NPV, B. bassiana or E. coli (Fig 2B). In the hemocytes, the expression of the Ap-sHSP21.4 showed a similar trend under NPV and E. coli challenge, and the highest expression level was detected at 12 h after the challenge, whereas the gene expression showed an almost immediate response to B. bassiana challenge (Fig 2C). These results suggest that Ap-sHSP21.4 has different expression patterns in various tissues upon different microorganisms.
RNAi of Ap-sHSP21.4 influences the expression of immune response-related genes
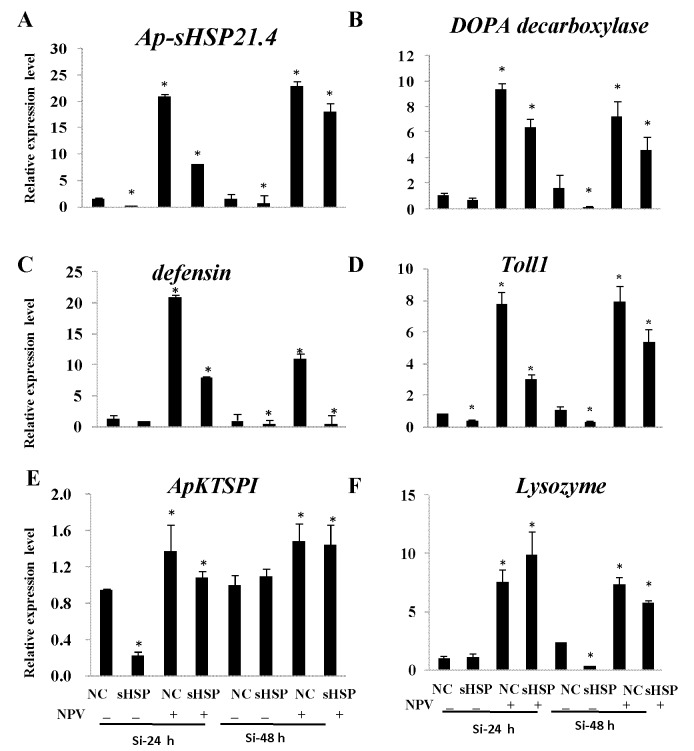
The expression level of Ap-sHSP21.4 in the fat bodies of A. pernyi larvae injected with siRNAs is significantly decreased compared with controls. When normalized to the housekeeping gene 18sRNA, the relative expression levels of Ap-sHSP21.4 dropped to 20% or 64% of the control values at 24 h and 48 h after siRNA injection (Fig 3A). This indicated that the expression of Ap-sHSP21.4 gene in A. pernyi was inhibited successfully when RNAi was administered by injection. The expression levels of Ap-sHSP21.4 were up-regulated significantly after injected with NPV at 24 and 48 h.
Fig 3. Relative mRNA expression levels of Ap-sHSP21.4 and immune response-related genes in response to the NPV stress by injection of siRNA at 24 and 48 h.
Expression levels were assessed using 18S rRNA gene for normalization. One set of negative control siRNAs (si-NC) was injected alongside the experimental injections. The data were analyzed by ANOVA and presented as mean ± SE of independent experiments done in triplicate and the asterisk represents the significant differences.
To further validate the transcripts level of immune response-related genes after si-Ap-sHSP21.4 injection, qRT-PCR was used to detect the transcription levels of the selected immune response-related genes in fat bodies. The transcription levels of most immune response-related genes (defensin, Dopa decarboxylase, Toll1, lysozyme and Kazal-type serine protease inhibitor) increased at least two fold compared to the control after NPV infection (Fig 3A). The transcription level of defensin significantly increased about 20-fold and Dopa decarboxylase 9 had the highest transcription level after NPV injection, while the expression levels down-regulated to 0.55 and 0.15-fold by treatment with si-Ap-sHSP21.4 (Fig 3B and 3C). The transcription levels of Toll1 were strongly induced almost 8-fold, then reduced to 0.28-fold by injection of Ap-sHSP21.4 dsRNA (Fig 3D). The transcription level of ApKTSPI reached the peak with 1.4-fold under NPV injection and decreased to 0.22-fold after RNA interference (Fig 3E), this result is similar to that for lysozyme (Fig 3F). All these data suggested that Ap-sHSP21.4 was probably involved in immune regulation.
Eicosanoid mediated the induction of Ap-sHSP21.4 by NPV
To study the effect of eicosanoid biosynthesis inhibitors on the induction of Ap-sHSP21.4 by NPV, silkworm larvae were firstly injected with drugs, then challenged with NPV. Three pharmaceutical inhibitors of eicosanoid biosynthesis dramatically suppress Ap-HSP21.4 expression upon NPV challenge (Fig 4).
Fig 4. Effect of eicosanoid biosynthesis inhibitors on the expression of Ap-sHSP21.4 in response to NPV.
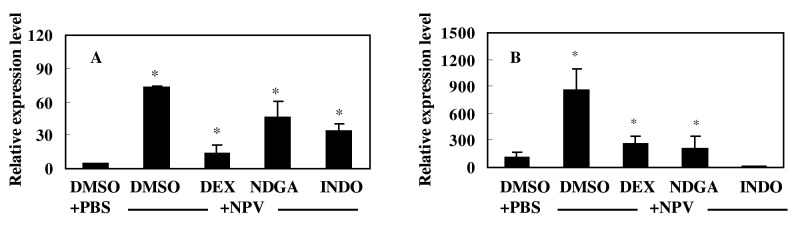
The larvae were at first injected with different concentrations (in 10 μL DMSO) of either dexamethasone (DEX), nordihydroguaiaretic acid (NDGA), or indomethacin (INDO). Control larvae were injected with 10 μL of DMSO alone. After keeping the larvae at room temperature for 30 min, the larvae then received a second injection of either insect PBS or 10 μL of NPV (109 /larva). Total RNA was isolated from the fat body (A) and midgut (B) 3 h or 24 h respectively after the second injection, and the Ap-sHSP21.4 gene expression was analyzed by quantitative real-time PCR. The mRNA levels are shown as a percentage of the levels in the DMSO control larvae. Bars represent the means ± SD (n = 5).
For example, the relative transcript level of Ap-HSP21.4 declined dramatically in a statistically manner from approximately 76-fold (treated with NPV) to 14-fold (treated with o.1 M DEX +NPV). The similar declines in relative transcript level of Ap-HSP21.4 were found in groups treated with INDO and NDGA. Furthermore, Ap-sHSP21.4 was significantly induced in the fat body and midgut by NPV injection. All the inhibitors injected prior to the treatment with NPV greatly suppressed the NPV-induced expression of the Ap-sHSP21.4.
Induction of Ap-sHSP21.4 by arachidonic acid
The previous results strongly suggested that arachidonic acid metabolites mediated the induction of immune gene in the fat body, hemocyte and midgut. To test the direct effect of arachidonic acid metabolites on the induction, the larvae were treated with arachidonic acid, and the Ap-sHSP21.4 mRNA levels in the fat body and midgut were examined. As shown in Fig 5, the arachidonic acid could induce the gene expression, although the levels were somewhat lower than that induced by NPV. The increase in Ap-sHSP21.4 gene expression by arachidonic acid was significantly up-regulation (P>0.05) compared to that in the control (treated with DMSO).
Fig 5. Induction of Ap-sHSP21.4 by arachidonic acid in the fat body (A) and midgut (B).
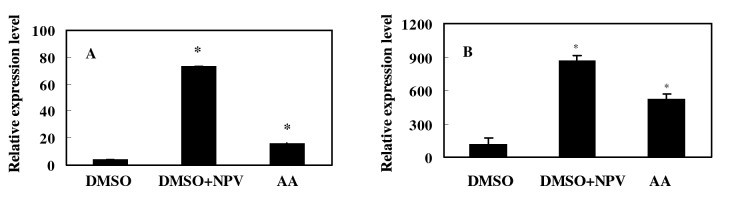
The larvae were injected with either 10 μL of DMSO (control), 10 μL of NPV (109 /larva) or arachidonic acid in 10 μL of DMSO (AA). The fat body (A) and midgut (B) 3 h or 24 h respectively after the second injection, The mRNA levels are shown as a percentage of the levels in the DMSO-treated control larvae. Bars represent the means ± SD (n = 5).
Discussion
sHSPs are a highly conserved, ubiquitously expressed family of proteins whose synthesis is induced in response to heat shock or adverse environments. Here, we identified a sHSP21.4 gene from A. pernyi. Multiple sequence alignment revealed that this sHSP from A. pernyi was highly homologous with those from other insects, suggesting that they may have a similar function to the sHSPs in the other species. The phylogenetic analysis of these superfamilies supports the traditional morphology-based classification [29].
Despite the main function of HSPs is to protect proteins from stress conditions, accumulating studies have shown that they are also involved the anti-biotic stress response. The expression level of Ap-sHSP21.4 was more abundant in the fat body than other tissues, which may indicate that the fat body was more sensitive to biotic stresses. In this experiment, the expression of Ap-sHSP21.4 was up-regulated by three types of microorganisms (bacteria, virus and fungi) challenges in the fat body and midgut. However, the expression patterns of Ap-sHSP21.4 varied in different tissues challenged with various microorganisms. For example, challenged with NPV and B. bassiana induced acute Ap-sHSP21.4 gene expression accumulation at 3 h pt with nearly 2 millions-fold in the fat body compared with the control (Fig 5A). But the response was much slower in the midgut, which appeared after 24 hours (Fig 5B). It is considered that these differential effects of sHSP upon different pathogens injection may be caused by their respective signaling pathway [30]. To further verify the role of Ap-sHSP21.4 in immune response, several immune response-related genes (defensin, decarboxylase, Toll, lysozyme and serine protease inhibitor) were selected for detection after si-Ap-sHSP21.4 injection. The transcription levels of most immunity response genes increased compared with the control after NPV infection (Fig 3A), while were significantly suppressed after RNAi Ap-sHSP21.4. The pattern of regulations is similar to Beetle, in which septic injury induces expression of genes involved in stress adaptation (e.g. heat-shock proteins) or potential antimicrobial effectors (e.g. ferritin, c-type lysozyme, serine proteinase inhibitors, and defensins), suggesting that there may be crosstalk between the immune and stress responses [11]. These results indicate the Ap-sHSP21.4 plays a role in immune response in A. pernyi against microorganism.
Aspirin and other non-steroidal anti-inflammatory drugs (NSAIDs) that act by inhibiting COX (the core enzyme in PG biosynthesis) have been studied in many areas of human and veterinary pathophysiology. Stanley-Samuelson and his colleagues have extensively studied the effects of pharmacological inhibitors of eicosanoid metabolism in insects [31–33]. The tobacco hornworm Manduca sexta injected with pharmaceutical inhibitors of eicosanoid biosynthesis (NSAIDs) could impair their ability to clear injected bacteria from hemolymph circulation [34]. Carton et al. reported that the PLA2-inhibiting glucocorticoid dexamethasone inhibited the encapsulation of parasitoid eggs in a resistant line of Drosophila melanogaster [35]. In the fat body of B. mori, specific inhibitors of phospholipase A2, cyclooxygenase, and lipoxygenase significantly inhibited the induction of the immune genes both in vivo and in cultured fat bodies. Arachidonic acid has been also found to induce the expression of the cecropin and lysozyme genes [17]. Our data clearly show that eicosanoid biosynthesis inhibitors suppress the expression of the Ap-sHSP21.4 elicited by NPV, while arachidonic acid induces the expression of Ap-sHSP21.4 in the fat body and midgut. All these suggest eicosanoid mediates the response of Ap-sHSP21.4 against immune challenges.
Supporting Information
A: The sHSPs proteins are from H. armigera (AGC39039.1), H. erato (ABS57447.1), C. suppressalis (AGC23338.1), B. mori (NM_001043520.1), E. pela (AGE92593.1), L. migratoria (ABC84493.1), A. darling (ETN64726.1), R. pedestris (BAN20225.1), M. domestica (NP_001273840.1). B: phylogenetic tree was constructed using the neighbor-joining algorithm method with a bootstrap test of 1000 repetitions.
(TIF)
Acknowledgments
We are grateful to Sericultural Research Institute, Henan Province for providing the experimental insects in this experiment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the earmarked fund for modern Argo-industry Technology Research System (CARS-22 SYZ10), Anhui High Schools Natural Science Foundation (KJ2013B320), National 863 plans projects of China (2011AA100306), Sericulture Biotechnology Innovation Team (2013xkdt-05), Ph.D. programs in Biochemistry and Molecular Biology (xk2013042). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat Struct Mol Biol. 2005; 12: 842–846. [DOI] [PubMed] [Google Scholar]
- 2. Kriehuber T, Rattei T, Weinmaier T, Bepperling A, Haslbeck M, Buchner J. Independent evolution of the core domain and its flanking sequences in small heat shock proteins. FASEB J. 2010; 24: 3633–3642. 10.1096/fj.10-156992 [DOI] [PubMed] [Google Scholar]
- 3. Poulain P, Gelly JC, Flatters D. Detection and architecture of small heat shock protein monomers. PLoS ONE 2010;5: e9990 10.1371/journal.pone.0009990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basha E, O'Neill H, Vierling E. Small heat shock proteins and α-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012; 37: 106–117. 10.1016/j.tibs.2011.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Velazquez JM, DiDomenico BJ, Lindquist S. Intracellular localization of heat shock proteins in Drosophila. Cell 1980; 20: 679–689. [DOI] [PubMed] [Google Scholar]
- 6. Arrigo AP, Ahmad-Zadeh C. Immunofluorescence localization of a small heat shock protein (hsp23) in salivary gland cells of Drosophila melanogaster . Mol Gen Genet. 1981;184: 73–79. [DOI] [PubMed] [Google Scholar]
- 7. Liu ZH, Xi DM, Kang MJ, Guo XQ, Xu BH. Molecular cloning and characterization of Hsp27.6: the first reported small heat shock protein from Apis cerana cerana . Cell Stress Chaperon. 2012;17: 539–551. 10.1007/s12192-012-0330-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li J, Moghaddam SHH, Du X, Zhong XX, Chen YY. Comparative analysis on the expression of inducible HSPs in the silkworm, Bombyx mori . Mol Biol Rep. 2012; 39: 3915–3923. 10.1007/s11033-011-1170-y [DOI] [PubMed] [Google Scholar]
- 9. Liu QN, Zhu BJ, Dai LS, Fu WW, Lin KZ, Liu CL. Overexpression of small heat shock protein 21 protects the Chinese oak silkworm Antheraea pernyi against thermal stress. J Insect Physiol. 2013;59: 848–854. 10.1016/j.jinsphys.2013.06.001 [DOI] [PubMed] [Google Scholar]
- 10. Liang D, Benko Z, Agbottah E, Bukrinsky M, Zhao RY. Anti-vpr activities of heat shock protein 27. Mol Med. 2007;13: 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Altincicek B, Knorr E, Vilcinskas A. Beetle immunity: Identification of immune-inducible genes from the model insect Tribolium castaneum . Dev Comp Immunol. 2008;32: 585–595. [DOI] [PubMed] [Google Scholar]
- 12. Sarkar S, Singha MD, Yadav R, Arunkumar KP, Pittman GW. Heat shock proteins: molecules with assorted functions. Fronti Microbiol. 2011; 6: 312–327. [Google Scholar]
- 13. Yang J, Mu Y, Dong S, Jiang Q, Yang J. Changes in the expression of four heat shock proteins during the aging process in Brachionus calyciflorus (rotifera). Cell Stress Chaperon. 2014;19: 33–52. 10.1007/s12192-013-0432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stanley-Samuelson DW, http://www.sciencedirect.com/science/article/pii/S0300962996004471-CORR1 Pedibhotla VK, Rana RL, Aliza Abdul Rahim N, Wyatt Hoback W, Miller JS. Eicosanoids mediate nodulation responses to bacterial infections in larvae of the silkmoth, Bombyx mori Comp Biochem Physiol. 1997;18: 93–100. [Google Scholar]
- 15. Buyukguzel E, Tunaz H, Stanley D, Buyukguzel K. Eicosanoids mediate Galleria mellonella cellular immune response to viral infection. J Insect Physiol. 2007;53: 99–105. [DOI] [PubMed] [Google Scholar]
- 16. Durmus Y, Buyukguzel E, Terzi B, Tunaz H, Stanley D, Büyükgüzel K. Eicosanoids mediate melanotic nodulation reactions to viral infection in larvae of the parasitic wasp, Pimpla turionellae . J Insect physiol. 2008;54: 17–24. [DOI] [PubMed] [Google Scholar]
- 17. Morishima I, Yamano Y, Inoue K, Matsuo N. Eicosanoids mediate induction of immune genes in the fat body of the silkworm, Bombyx mori . FEBS Lett. 1997;419: 83–86. [DOI] [PubMed] [Google Scholar]
- 18. Sakano D, Li B, Xia QY, Yamamoto K, Banno Y, Fujii H, et al. Genes encoding small heat shock proteins of the silkworm, Bombyx mori . Biosci Biotech Bioch. 2006;70: 2443–2450 [DOI] [PubMed] [Google Scholar]
- 19. Mikkelsen UR, Paulsen G, Schjerling P, Helmark IC, Langberg H, Kjaer M, et al. The heat shock protein response following eccentric exercise in human skeletal muscle is unaffected by local NSAID infusion. Eur J Appl Physiol. 2013;113: 1883–1893. 10.1007/s00421-013-2606-y [DOI] [PubMed] [Google Scholar]
- 20. Harlow E, Lane D. Using Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- 21. Bundey S, Raymond S, Dean P, Roberts SK, Dillon RJ, Charnley AK, et al. Eicosanoid involvement in the regulation of behavioral fever in the desert locust, Schistocerca gregaria . Arch Insect Biochem. 2003;52: 183–192. [DOI] [PubMed] [Google Scholar]
- 22. Zhou J, Han DX. Safety evaluation of protein of silkworm (Antheraea pernyi) pupae. Food Chemcal Toxicol. 2006;44: 1123–1130. [DOI] [PubMed] [Google Scholar]
- 23. Chen KK, Liu C, He Y, Jiang HB, Lu ZQ. A short-type peptidoglycan recognition protein from the silkworm: expression, characterization and involvement in the prophenoloxidase activation pathway. Dev Comp Immunol. 2014;45:1–9. 10.1016/j.dci.2014.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang T., Wu C, Li J, Ren GD, Huang DW, Liu FS. Stress-induced HSP70 from Musca domestica plays a functionally significant role in the immune system. J Insect Physiol. 2012;58:1226–1234. 10.1016/j.jinsphys.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 25. Wei ZJ, Yu M, Tang SM, Yi YZ, Hong GY, Jiang ST. Transcriptional regulation of the gene for prothoracicotropic hormone in the silkworm, Bombyx mori . Mol Biol Rep. 2011;38: 1121–1127. 10.1007/s11033-010-0209-9 [DOI] [PubMed] [Google Scholar]
- 26. Kokolakis G, Tatari M, Zacharopoulou A, Mintzas AC. The hsp27 gene of the Mediterranean fruit fly, Ceratitis capitata: structural characterization, regulation and developmental expression. Insect Mol Biol. 2008;17: 699–710. 10.1111/j.1365-2583.2008.00840.x [DOI] [PubMed] [Google Scholar]
- 27. Livak K, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25: 402–408. [DOI] [PubMed] [Google Scholar]
- 28. Li ZW, Li X, Yu QY, Xiang ZH, Kishino H, Zhang Z. The small heat shock protein (sHSP) genes in the silkworm, Bombyx mori, and comparative analysis with other insect sHSP genes. BMC Evol Biol. 2009;9: 215 10.1186/1471-2148-9-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kristensen NP, Skalski AW. Phylogeny and paleontology In: Kristensen N.P. (Ed.), Lepidoptera: Moths and Butterflies, 1 Evolution, Systematics, and Biogeography, Handbook of Zoology, vol IV, Part 35. De Gruyter, Berlin and New York, 1999. pp. 7–25. [Google Scholar]
- 30. Park JA, Kim Y. Toll recognition signal activates oenocytoid cell lysis via a crosstalk between plasmatocyte-spreading peptide and eicosanoids in response to a fungal infection. Cell Immunol. 2012;279: 117–123. 10.1016/j.cellimm.2012.11.005 [DOI] [PubMed] [Google Scholar]
- 31. Stanley D, Haas E, Miller J. Eicosanoids: Exploiting insect immunity toimprove biological control programs. Insects 2012;3: 492–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stanley DW, Miller JS. Eicosanoid actions in insect cellular immune functions. Entomol Exp Appl. 2006;119: 1–13. [Google Scholar]
- 33. Stanley D, Kim Y. Prostaglandins and their receptors in insect biology. Fronti Endocrinol. 2011;2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanley-Samuelson DW, Jensen E, Nickerson KW, Tiebel K, Ogg CL, Howard RW. Insect immune response to bacterial infection is mediated by eicosanoids. P Natl Acad Sci USA. 1991;88: 1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carton Y, Frey F, Stanley DW, Vass E, Nappi AJ. Dexameth as one inhibition of the cellular immune response of Drosophila melanogaster against a parasitoid. J Parasitol. 2002;288: 405–407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: The sHSPs proteins are from H. armigera (AGC39039.1), H. erato (ABS57447.1), C. suppressalis (AGC23338.1), B. mori (NM_001043520.1), E. pela (AGE92593.1), L. migratoria (ABC84493.1), A. darling (ETN64726.1), R. pedestris (BAN20225.1), M. domestica (NP_001273840.1). B: phylogenetic tree was constructed using the neighbor-joining algorithm method with a bootstrap test of 1000 repetitions.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.