Abstract
Rationale
Chronic cocaine exposure produces unconditioned enhancement in impulsive decision making; however, little is known about the effects of cocaine-paired conditioned stimuli on this behavior. Thus, this study explored the effects of cocaine-paired contextual stimuli on impulsive decision making and the contribution of nicotinic acetylcholine receptors (nAChRs) to this phenomenon.
Methods
Rats were trained to achieve stable performance on a delay discounting task, which involved lever press-based choice between a single food pellet (small reward) available immediately and three food pellets (large reward) available after a 10-, 20-, 40-, or 60-s time delay. Rats then received Pavlovian context-cocaine (15 mg/kg, i.p.) and context-saline (1 ml/kg, i.p.) pairings in two other, distinct contexts. Subsequently, delay discounting task performance was assessed in the previously cocaine-paired or saline-paired context following pretreatment with saline or cocaine (15 mg/kg, Experiment 1) or with saline or the nAChR antagonist, mecamylamine (0.2, 2 mg/kg, Experiment 2), using counterbalanced within-subjects testing designs.
Results
Independent of cocaine pretreatment, rats exhibited greater decrease in preference for the large reward as a function of delay duration in the cocaine-paired context, relative to the saline-paired context. Furthermore, systemic mecamylamine pretreatment dose-dependently attenuated the decrease in preference for the large reward in the cocaine-paired context, but not in the saline-paired context, as compared to saline.
Conclusion
Cocaine-paired contextual stimuli evoke a state of impulsive decision making, which requires nAChR stimulation. Drug context-induced impulsivity likely increases the propensity for drug relapse in cocaine users, making the nAChR an interesting target for drug relapse prevention.
Keywords: Cocaine, Context, Impulsive choice, Delay discounting, Relapse, Nicotinic acetylcholine receptor
Cocaine abusers display impulsive decision making which likely facilitates their propensity for drug relapse (Coffey et al. 2003; Kirby and Petry 2004; Bornovalova et al. 2005; Heil et al. 2006). Similarly, laboratory animals with a history of cocaine exposure exhibit enduring impairment in delay discounting, an index of impulsive decision making (Simon et al. 2007; Dandy and Gatch 2009; Roesch et al. 2007; Mendez et al. 2010; Broos et al. 2012). While these findings establish the existence of unconditioned cocaine effects on impulsive decision making, little is known about the possible impact of drug-paired contextual stimuli on this phenomenon. Given that exposure to previously drug-paired environmental stimuli elicits drug-like motivational effects in humans and laboratory animals (Fuchs et al. 2008; Crombag et al. 2008), it can be hypothesized that exposure to drug-paired contextual stimuli also triggers a drug-like, conditioned state of impulsive decision making.
The stimulation of nicotinic acetylcholine receptors (nAChRs) plays a critical role in cocaine-induced behaviors (for reviews, see Williams and Adinoff 2008; Crunelle et al. 2010) and decision making (Mitchell et al. 2011; Locey and Dallery 2009; 2011; Kolokotroni et al. 2011). In laboratory animals, stimulation of nAChRs is necessary for the maintenance and escalation of cocaine self-administration (Levin et al. 2000; Hansen and Mark 2007) as well as for the development of cocaine-induced behavioral sensitization (Schoffelmeer et al. 2002). In cocaine users, nicotine exposure enhances, while the non-selective nAChR antagonist, mecamylamine, reduces indices of conditioned cue-induced craving (Reid et al. 1998; 1999). Furthermore, in rats, inhibition of nAChRs using mecamylamine moderately attenuates the expression of cocaine-conditioned place preference (Sershen et al. 2010). In addition to regulating the unconditioned and conditioned effects of cocaine, acute nicotine pretreatment enhances impulsive decision making (i.e. delay discounting), whereas systemic administration of mecamylamine inhibits this effect (Kolokotroni et al. 2011). Thus, it can be postulated that nAChR stimulation is also critical for the ability of drug-paired contextual stimuli to produce a state of increased impulsive decision making.
To test the above hypotheses, experiment 1 evaluated the effects of cocaine-paired Pavlovian contextual stimuli on impulsive choice behavior in rats, using the delay discounting paradigm. In this model, impulsive choice is indicated by preference for an immediately available small food reward over a delayed large food reward (for a review, see Mar and Robbins 2007). As predicted, exposure to the cocaine-paired context elicited a robust increase in delay discounting. Hence, experiment 2 expanded upon this finding by exploring the contribution of nAChRs to this phenomenon in a second group of rats. To this end, the effects of systemic mecamylamine pretreatment on impulsive choice behavior were assessed in the presence and absence of drug-paired contextual stimuli.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles-River, N=16; 250–275 g) were individually housed in a temperature- and humidity-controlled vivarium on a reversed light/dark cycle. Rats were maintained on 15–20 g of rat chow per day and water ad libitum. The rats were acclimated to handling over five consecutive days prior to instrumental training. The housing and care of rats were in accordance with the guidelines defined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, 2011), and were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Instrumental Training
On day 6, rats were food deprived for 24 h in order to facilitate the acquisition of food-reinforced lever pressing behavior starting on day 7. The sessions were conducted in standard operant conditioning chambers, each equipped with two levers and a food magazine located at equal distance from each lever (Coulbourn Instruments, Whitehall, PA). Initially, each lever press on either lever resulted in the delivery of a food pellet (45 mg, Purina Test Diets, Richmond, IN, USA) under a continuous reinforcement schedule. Training continued until rats reached a criterion of 100 presses on each lever during a single 16-hour session (mean number of sessions needed = 1.25±0.16). Water was available ad libitum throughout the session. After reaching the acquisition criterion, rats were trained to respond for food reinforcement using a discrete trial procedure during daily 60-min sessions. Each trial was initiated by the insertion of one of the two levers into the chamber and the illumination of the tray light. The rats were required to press the lever within 30 s in order to extinguish the tray light and initiate the immediate delivery of a single food pellet. Failure to respond within 30 s led to the termination of the trial. The left and right levers were presented an equal number of times during each session with no more than two consecutive presentations of the same lever. Training on the discrete trial schedule continued until rats reached a criterion of at least 60 successful trials during a 60-min session (mean number of sessions needed = 1.45±0.21).
Delay discounting task training
The experimental timeline is shown in Figure 1. Delay discounting task training consisted of 5 blocks of 8 trials per day, during the rats’ dark cycle. Each block consisted of 2 forced-choice trials and 6 free-choice trials. The forced-choice trials introduced the reinforcement contingency. During the 2 forced-choice trials of each block, one lever was inserted and the tray light was illuminated. A lever press resulted in lever retraction and the extinction of the tray light, accompanied by the immediate delivery of a single food pellet or the delivery of three food pellets after a programmed time delay (0, 10, 20, 40, or 60 s). The order of lever presentations was random. The pairing of a particular lever with the reward size (1 or 3 food pellets) remained constant throughout the experiment, but it was counterbalanced across subjects. The delay period that preceded the delivery of the large reward increased systematically (i.e. 0, 10, 20, 40, then 60 s) across the 5 blocks. During the 6 free-choice trials of each block, both levers were presented and the tray light was illuminated. A press on one of the two levers resulted in a single food pellet delivered immediately, whereas a press on the other lever resulted in the delivery of 3 food pellets after the same delay as during the preceding forced-choice trial. A new trial started every 100s, resulting in a uniform number of choice opportunities but in variable inter-trial intervals across blocks, depending on the delay value programmed. If no lever press occurred within 30 s of trial onset (i.e. response omission), both levers were retracted and the tray light was extinguished. During the course of the 5 blocks of each session, the time delay to the presentation of the 3-pellet reward increased systematically (i.e. 0, 10, 20, 40, then 60 s). Delay discounting training sessions were conducted Monday through Friday. On Saturdays, delay discounting probe sessions were conducted, during which the delay to the presentation of the 3-pellet reward was maintained at 0 s in all blocks. These probe sessions examined whether the behavior remained sensitive to reward magnitude. If a rat selected the larger reward fewer than 5 out of 6 times in any one block during a probe session, the rat received additional probe sessions until this criterion was met before delay discounting task training resumed. Delay discounting task training continued until a stability criterion was reached. Stability was defined as the absence of a statistically significant main or interaction effect involving session on seven consecutive days in a repeated-measures analysis of variance (ANOVA), accompanied by a significant main effect of trial block (i.e., delay).
Figure 1.

Schematic illustration of the experimental timeline for Experiment 1 (EXP1) and Experiment 2 (EXP2), performed in different groups of rats. Asterisk represents that, after initial training on the delay discounting (DD) task, rats received seven cocaine – cocaine-paired context (COC context) and seven saline – saline-paired context (SAL context) pairings in alternation during Pavlovian conditioning. After conditioning, rats were required to re-obtain the DD stability criterion. In Experiment 1, four food-reinforced DD tests were then conducted. Bidirectional arrows indicate that, during these DD test session, the order of exposure to the two testing contexts (SAL context, COC context) and the order of intraperitoneal pretreatments (15 mg/kg of cocaine, 1 ml/kg of saline) were counterbalanced. In Experiment 2, eight food-reinforced DD tests were conducted. Bidirectional arrows indicate that, during these DD test sessions, the order of exposure to the two testing contexts, the order of intraperitoneal pretreatment types (mecamylamine, saline) and the order of mecamylamine doses (0.2 mg/kg, 2 mg/kg) were counterbalanced. Rats received additional DD task training in the training context on a minimum of two days before each test session.
Pavlovian conditioning
After delay discounting task training, rats underwent 14 Pavlovian conditioning sessions. During conditioning, cocaine injection (15 mg/kg, i.p.) was followed immediately by placement into a distinct context, and saline injection (1 ml/kg, i.p.) was followed immediately by placement into a different context, on alternating days (7 exposures to each context). Contexts 1 and 2 consisted of modified operant conditioning chambers in which the levers were retracted. In addition, context 1 contained a continuous red house light (0.4 fc brightness), intermittent pure tone (80 dB, 1 kHz; 2 s on, 2 s off), pine-scented air freshener strip, and wire mesh flooring (26 cm × 27 cm). Context 2 contained an intermittent white stimulus light above the left lever (1.2 fc brightness; 2 s on, 2 s off), continuous pure tone (75 dB, 2.5 kHz), vanilla-scented air freshener strip, and a slanted ceramic tile wall that bisected the bar floor (19 cm × 27 cm). Each conditioning session lasted 30 min, after which the rats were returned to their home cages. Assignment for cocaine versus saline to be paired with context 1 or 2, and the order of cocaine and saline conditioning sessions were counterbalanced based on the pre-cocaine delay discounting performance (i.e., indifference point) during the last seven days of training. The indifference point was defined as the time delay at which the large reward was chosen 50% of the time, and it was calculated for each subject using a linear regression model constructed from the data points averaged across the five trial blocks (Mendez et al. 2010; Diller et al. 2008; Winstanley et al. 2004).
Testing
After the last Pavlovian conditioning session, rats received additional delay discounting task training in the training context until they re-obtained the stability criterion (see above). Subsequently, 4 test sessions were conducted in Experiment 1 and 8 test sessions were conducted in Experiment 2. Between testing sessions that fell within experiments, rats received a minimum of two delay discounting training sessions in the training context until their responding reached the stability criterion. The stability criterion was defined as the absence of a statistically significant session main or interaction effect on two consecutive days in a repeated-measures ANOVA, accompanied by a significant main effect of trial block.
Experiment 1
On the test days, rats (N = 8) received an i.p. cocaine (15 mg/kg) or saline (1 ml/kg) injection. Immediately after the pretreatment, the rats were placed into the testing context (previously saline- or cocaine-paired), and their delay discounting performance was assessed. During four test sessions, the order of testing in the two contexts (saline-paired context first, cocaine-paired context first) and the order of pretreatments (saline first, cocaine first) were counterbalanced across subjects based on their pre-cocaine delay discounting task performance (i.e. indifference point).
Experiment 2
On the test days, a separate group of rats (N = 8) received an i.p. injection of 0.2 mg/kg of mecamylamine, 2 mg/kg of mecamylamine or 1 ml/kg of saline. Immediately after the pretreatment, the rats were placed into the testing context (previously saline- or cocaine-paired), and their delay discounting performance was assessed. During testing, mecamylamine and saline test sessions alternated in the two contexts, such that a total of eight test sessions were conducted. The order of testing in the two contexts (saline-paired context first, cocaine-paired context first), the order of pretreatment type (mecamylamine first, saline first), and the order of mecamylamine dose (0.2 mg/kg first, 2 mg/kg first) were counterbalanced across subjects based on their pre-cocaine delay discounting task performance (i.e. indifference point).
Data analysis
The percentage of trials with choice of large reward was calculated based on the number of trials with a choice of large reward relative to the total number of choice trials in each block (i.e. 6). The percentage of trials with choice of large reward during delay discounting task training before and after the Pavlovian context-cocaine conditioning was analyzed using analysis of variance (ANOVA) with time (day) and delay interval (0, 10, 20, 40, 60 s) as within-subjects factors. In Experiment 1, the percentage of trials with choice of large reward during the four test sessions was analyzed using ANOVA with testing context (previously saline-, cocaine-paired), pretreatment (saline, cocaine), and delay interval (0, 10, 20, 40, 60 s) as within-subjects factors. In Experiment 2, the percentage of trials with choice of large reward during the four vehicle test sessions was analyzed using ANOVA with test day (first saline test, second saline test), testing context (previously saline-, cocaine-paired), and delay interval (0, 10, 20, 40, 60 s) as within-subjects factors. This ANOVA did not indicate any test day effects. Subsequently, the percentage of trials with choice of large reward during the two collapsed saline tests and four mecamylamine test sessions was analyzed using ANOVA with testing context (saline-paired, cocaine-paired), pretreatment (0, 0.2, 2 mg/kg mecamylamine), and delay interval (0, 10, 20, 40, 60 s) as within-subjects factors. In all analyses, significant ANOVA main and interaction effects were further investigated using Tukey’s HSD post hoc tests. Alpha was set at 0.05. Only statistically significant effects are reported below.
RESULTS
Delay discounting task performance
After 37.4±0.6 (Mean ± SEM) delay discounting training sessions, rats (N=16) exhibited stable delay discounting performance in the training context during the last 7 days preceding the Pavlovian context-cocaine conditioning phase (ANOVA time main and delay×time interaction effects, F<1, data not shown). There was no statistically significant difference in delay discounting task performance during the first re-training session after Pavlovian conditioning relative to the delay discounting task performance during the last training session before Pavlovian conditioning (ANOVA time main and delay×time interaction effects, F<1, Figure 2A). Thus, in 7.1±0.2 days, rats reacquired the stability criterion for delay discounting task performance in the training context (ANOVA time main and delay×time interaction effects, F<1, data not shown). Furthermore, once the stability criterion was reached, the baseline delay discounting performance was not different before versus after Pavlovian conditioning (ANOVA all main and interaction effects, F<1, Figure 2B).
Figure 2.

Delay discounting task performance (mean percentage of trials with choice of the large reward ± SEM) in the training context (A) during the last session before and during the first session after Pavlovian conditioning and (B) after achieving the stability criterion before and after Pavlovian conditioning. Asterisks represent significant difference relative to the large reward available with no delay (ANOVA delay main effect, Tukey test, p<0.05).
Experiment 1: Effects of cocaine-paired context exposure on impulsive decision making
On the test days (Figure 3A), rats exhibited preference for the large reward when it was available with no delay (0 s), regardless of testing context and pretreatment (Tukey test, p>0.05). However, preference for the large reward decreased as a function of delay and testing context (context×delay interaction effect, F(4, 28)=4.10, p=0.01; delay main effect, F(4, 28)=22.30, p=0.0001; context main effect, F(1, 7)=63.41, p=0.0001). This effect was independent of pretreatment (cocaine versus saline; pretreatment two- and three-way interaction effects, F<1), with no significant difference in choice omissions between the test conditions (mean number of choice omissions=1.2±0.2; F<1, data not shown). Thus, collapsed across the pretreatment variable (Figure 3B), in the saline-paired context, rats exhibited decreased preference for the large reward after 20-, 40-, or 60-s, but not 10-s delay, relative to 0-s delay (Tukey test, p<0.05; Figure 3B). In contrast, in the previously cocaine-paired context, rats exhibited decreased preference for the large reward after all delay periods (Tukey test, p<0.05; Figure 3B). Importantly, rats exhibited significantly less preference for the large reward after 10-, 20-, 40-, or 60-s delay in the cocaine-paired context, relative to the saline-paired context (Tukey tests, p<0.05; Figure 3B).
Figure 3.

Delay discounting task performance (mean percentage of trials with choice of the large reward ± SEM) during testing, (A) following pretreatment with cocaine (15 mg.kg, i.p.) or saline (1 ml/kg, i.p.) in the saline-paired (SAL CTX) and cocaine-paired (COC CTX) contexts, and (B) the same data set displayed collapsed across the non-significant pretreatment variable. Asterisks represent significant difference relative to the large reward available with no delay (ANOVA delay simple main effect, Tukey test, p<0.05). Daggers represent significant difference relative to the large reward available with comparable delay in the saline-paired context (ANOVA context simple main effect, Tukey test, p<0.05).
Experiment 2: Effects of systemic administration of mecamylamine on drug context-induced impulsive decision making
There was no difference in preference for the large reward following saline pretreatment as a function of test day (all test day main or interaction effects, F<1). Therefore, data were collapsed to form a single saline condition for each testing context. Preference for the large reward decreased as a function of delay and testing context (context×delay interaction effect, F(4, 28)=3.32, p=0.02; delay main effect, F(4, 28)=24.65, p=0.0001; context main effect, F(1, 7)=44.03, p=0.0001). Furthermore, rats’ preference for the large reward was altered in the cocaine-paired context, relative to the saline-paired context as a function of mecamylamine pretreatment (context×pretreatment interaction effect, F(2, 14)=5.97, p=0.01; pretreatment main effect, F(2, 14)=3.81, p=0.04). Specifically, on the test days (Figure 4A), rats exhibited preference for the large reward when it was available with no delay (0 s), regardless of testing context and pretreatment (0, 0.2, 2 mg/kg mecamylamine; Tukey test, p>0.05). In the saline-paired context, rats exhibited decreased preference for the large reward after 20-, 40-, or 60-s, but not 10-s delay, relative to 0-s delay, regardless of pretreatment (Tukey test, p<0.05; Figure 4B–D). In contrast, in the previously cocaine-paired context, rats exhibited decreased preference for the large reward after all delay periods, regardless of pretreatment (Tukey test, p<0.05; Figure 4B–D). Collapsed across delay, following saline or 0.2 mg/kg of mecamylamine pretreatment, rats exhibited significantly less preference for the large reward in the cocaine-paired context, relative to the saline-paired context (Tukey test, p<0.05; Figure 4B–C). Conversely, following 2 mg/kg of mecamylamine pretreatment, rats failed to exhibit a preference for the large reward in the cocaine-paired context, relative to the saline-paired context (Tukey test, p>0.05; Figure 4D). Furthermore, there was no significant difference in choice omissions between the test conditions (mean number of choice omissions=1.1±0.2; F<1, data not shown).
Figure 4.
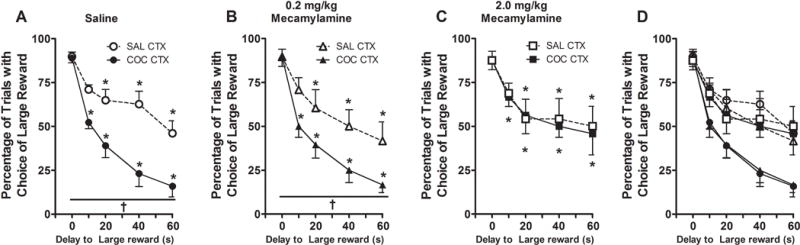
Delay discounting task performance (mean percentage of trials with choice of large reward ± SEM) during testing in the saline-paired (SAL CTX) and cocaine-paired (COC CTX) contexts, following i.p. pretreatment with (A) 1 ml/kg of saline, (B) 0.2 mg/kg of mecamylamine, and (C) 2 mg/kg of mecamylamine. Panel D represents a composite of the data in Panels A–C. Asterisks represent significant difference relative to the same reward with no delay (ANOVA delay simple main effect, Tukey test, p<0.05). Daggers represent significant difference relative to the large reward with comparable delay in the saline-paired context (ANOVA context simple main effect, Tukey test, p<0.05).
DISCUSSION
In the present study, rats displayed greater delay discounting behavior (i.e. greater preference for the immediately available small reward) in the previously cocaine-paired context than in the saline-paired context (Figure 3B). This behavioral effect reflected increased sensitivity to delay, rather than a decreased sensitivity to reward magnitude or an instrumental performance deficit, given that the context manipulation failed to alter the preference for the large reward over the small reward when both were available with no delay (Figure 3A) and failed to increase choice omissions during the test sessions, respectively. In the follow-up experiment, systemic mecamylamine pretreatment dose-dependently decreased the preference for the immediately available small reward in the previously cocaine-paired context, but not in the saline-paired context, as compared to saline (Figure 4A–C). However, mecamylamine pretreatment failed to alter the preference for the large reward over the small reward when both were available with no delay during the test session (Figure 4D) and failed to increase choice omissions during the test sessions. Taken together, these findings indicated that exposure to a cocaine-paired environmental context produces a state of increased impulsive decision making and the stimulation of nAChRs is critical for this phenomenon.
In the present study, as in earlier work by Winstanley et al. (2007), the repeated cocaine administration regimen (15 mg/kg, i.p.) failed to alter subsequent delay discounting performance in a drug-free state in the training context (Figure 2). Conversely, more extensive passive cocaine exposure regimens (3×15 mg/kg or 30 mg/kg i.p. per day for 14 days) in the home cage or cocaine self-administration regimens in distinct self-administration chambers can increase subsequent delay discounting performance in the training context (Paine et al., 2003; Simon et al. 2007; Roesch et al. 2007; Mendez et al. 2010; Broos et al. 2012). Furthermore, it appears that a less extensive cocaine regimen (e.g., 15 mg/kg i.p. per day for 9 days) can also produce a prolonged increase in delay discounting performance as long as cocaine is administered in the delay discounting training context (Dandy and Gatch 2009). Thus, contextual conditioning and cocaine history may interact to determine the effects of cocaine exposure on subsequent delay discounting performance and these factors will need to be explored systematically in future studies.
Interestingly, cocaine challenge (15 mg/kg i.p.) on the test day failed to alter delay discounting performance relative to saline challenge in either the previously cocaine-paired or the previously saline-paired context (Figure 3). There are several possible explanations for this negative effect. First, tolerance may develop to the unconditioned effects of cocaine on impulsive choice. Consistent with this possibility, a previous study reports that a cocaine challenge injection alters impulsive choice in cocaine-naive rats, but not in cocaine-experienced rats (Winstanley et al. 2007). Adding to this line of research, the present findings suggest that the possible development of tolerance to the unconditioned effects of cocaine does not inhibit cocaine-paired contextual stimuli from maintaining critical control over impulsive decision making. Second, individual differences in pre-cocaine delay discounting, or trait impulsivity, may influence the unconditioned effects of cocaine on this behavior (for review, see Perry and Carroll 2008). In support of this idea, D-amphetamine challenge increases impulsive choice in low impulsive rats, but decreases impulsive choice in high impulsive rats (Perry et al. 2008). Furthermore, based on the asymptote of the delay discounting curve, rats in the present study were relatively low in impulsivity, as compared with those in other studies (Winstanley et al. 2007; Broos et al., 2012). Third, independent of the development of tolerance or trait impulsivity, cocaine challenge may enhance impulsive choice under experimental conditions (e.g. dose, task parameters) other than those employed in these studies, warranting further exploration of this question.
While we may postulate that cocaine-paired contextual stimuli enhance impulsive decision making and therefore maladaptive behaviors in cocaine users, the results from clinical and preclinical studies on the contextual control of impulsive decision making have been inconsistent. Exposure to a gambling-associated context enhances impulsive choice in habitual gamblers (Dixon et al. 2006), while smoking-related environmental cues fail to facilitate delay discounting in smokers (Field et al. 2007). The latter negative finding may be related to drug-specific differences in sensitivity to the motivational effects of drug-paired contextual stimuli, given that a recently published study demonstrates that exposure to heroin-related video images enhances risk-based decision making, as measured using the Iowa gambling task, in heroin users (Wang et al. 2011). However, other factors may also regulate the development of context-dependent impulsive decision making. Consistent with this, exposure to cocaine-paired contextual stimuli robustly enhanced impulsive decision making in rats in the present study, whereas exposure to a context, in which rats had self-administered cocaine, resulted in a transient reduction in delay discounting in a recent study by Broos et al. (2012). In addition to methodological differences between these studies, rats exhibited greater impulsivity prior to cocaine exposure in the study by Broos et al. (2012) than in the present study. Thus, the effects of cocaine-paired contextual stimuli on impulsive decision making may depend on trait impulsivity, similar to the unconditioned effects of psychostimulants (Perry et al. 2008). Overall, these findings provide strong rationale for comparative studies exploring the effects of drug-related contextual stimuli on impulsive decision making in various populations of substance users.
The finding that mecamylamine administration decreased delay discounting in the present study indicates that populations of nAChRs play a critical role in drug context-induced impulsive decision making. These receptor populations are likely in limbic and paralimbic brain regions that receive dense cholinergic innervations (Mesulam 1996; Papez 1995; Calabresi et al. 2000; Pisani et al, 2001; Lautin 2001; Zhou et al. 2002). Such brain regions include the nucleus accumbens, basolateral amygdala, hippocampus, and orbitofrontal cortex (OFC), which are also recognized for controlling impulsive decision making (Cardinal et al. 2001; Winstanley et al. 2004; Cheung and Cardinal 2005; Churchwell et al. 2009; Galtress and Kirkpatrick 2010; Zeeb et al. 2010; Mar et al. 2011; Zuo et al. 2012). Remarkably, the functional integrity of the same brain regions is critical for the ability of cocaine-paired contextual stimuli to elicit goal-directed behaviors (e.g. cocaine-seeking behavior; Fuchs et al. 2005; 2008; Lasseter et al. 2009). Among these brain regions, the OFC critically regulates unconditioned impulsive decision making by encoding reward delay (Roesch et al. 2006) given that baclofen/muscimol-induced temporary neuronal inactivation of the OFC decreases delay discounting in high-impulsive rats (Zeeb et al. 2010). The OFC also exhibits Fos protein expression, an index of neuronal activation, concomitant with cocaine-seeking behavior in a cocaine-paired context (Hearing et al. 2008). Furthermore, baclofen/muscimol-induced temporary neuronal inactivation of the OFC inhibits drug context-induced reinstatement of extinguished cocaine-seeking behavior in rats (Lasseter et al. 2009). High impulsive choice predicts robust cue-induced nicotine-seeking behavior (Diergaarde et al. 2008), Thus, conditioned activation of neuronal ensembles within the OFC, and in similar brain regions, may increase impulsive decision making and the reinstatement of extinguished cocaine-seeking behavior. Given the involvement of nAChRs in various forms of drug-seeking behavior (Biala et al. 2010; Liu et al. 2007; Schmidt et al. 2009; Zhou et al. 2007), future studies will need to examine the role of distinct neuronal ensembles and nAChR populations in drug context-induced impulsive decision making in relation to cocaine-seeking behavior per se.
In summary, nAChR stimulation is necessary for the ability of cocaine-paired contextual stimuli to facilitate impulsive decision making, as measured using delayed discounting performance in rats. Such drug-paired context-induced increase in impulsivity may augment the propensity to drug relapse in cocaine users, making nAChRs an interesting therapeutic target for reducing cocaine relapse. However, different forms of impulsivity exist and these may be differentially modulated by exposure to drug-paired contextual stimuli (Evenden 1999; Moeller et al. 2001). In support of this, preclinical and clinical studies find little correlation between different measures of impulsivity (e.g. impulsive choice and impulsive action; McDonald et al, 2003; Winstanley et al., 2004). Furthermore, a study in attention deficit hyperactivity disorder patients suggests that individual differences or clinical conditions may influence the efficacy of nAChR manipulations on impulsive decision making (Potter et al. 2009). Thus, it will be imperative to evaluate the impact of drug-paired contextual stimuli and nAChR manipulations on multiple indices of impulsivity in various populations in the future. Such studies may inform the development of effective anti-impulsivity pharmacotherapies for cocaine addiction as well as for impulse control disorders.
Acknowledgments
The authors thank Antonia Bista, Christopher Hanlin and Dr. Heather Lasseter for excellent technical assistance and insightful comments on an earlier version of this manuscript. This work was supported by National Institute on Drug Abuse grants DA017673 and DA025646.
Footnotes
The authors declare no conflict interest.
References
- Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:361–70. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Daughters SB, Hernandez GD, Richards JB, Lejuez CW. Differences in impulsivity and risk-taking propensity between primary users of crack cocaine and primary users of heroin in a residential substance-use program. Exp Clin Psychopharmacol. 2005;13:311–8. doi: 10.1037/1064-1297.13.4.311. [DOI] [PubMed] [Google Scholar]
- Broos N, Diergaarde L, Schoffelmeer AN, Pattij T, De Vries TJ. Trait Impulsive Choice Predicts Resistance to Extinction and Propensity to Relapse to Cocaine Seeking: A Bidirectional Investigation. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2011.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–6. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Cardinal RN. Hippocampal lesions facilitate instrumental learning with delayed reinforcement but induce impulsive choice in rats. BMC Neurosci. 2005;6:36. doi: 10.1186/1471-2202-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell JC, Morris AM, Heurtelou NM, Kesner RP. Interactions between the prefrontal cortex and amygdala during delay discounting and reversal. Behav Neurosci. 2009;123:1185–96. doi: 10.1037/a0017734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Bossert JM, Koya E, Shaham Y. Context-induced relapse to drug seeking: a review. Philos Trans R Soc Lond B Biol Sci. 2008;363:3233–43. doi: 10.1098/rstb.2008.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelle CL, Miller ML, Booij J, van den Brink W. The nicotinic acetylcholine receptor partial agonist varenicline and the treatment of drug dependence: a review. Eur Neuropsychopharmacol. 2010;20:69–79. doi: 10.1016/j.euroneuro.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Dandy KL, Gatch MB. The effects of chronic cocaine exposure on impulsivity in rats. Behav Pharmacol. 2009;20:400–5. doi: 10.1097/FBP.0b013e328330ad89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Diller JW, Saunders BT, Anderson KG. Effects of acute and repeated administration of caffeine on temporal discounting in rats. Pharmacol Biochem Behav. 2008;89:546–55. doi: 10.1016/j.pbb.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Dixon MR, Jacobs EA, Sanders S. Contextual control of delay discounting by pathological gamblers. J Appl Behav Anal. 2006;39(4):413–22. doi: 10.1901/jaba.2006.173-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Field M, Rush M, Cole J, Goudie A. The smoking Stroop and delay discounting in smokers: effects of environmental smoking cues. J Psychopharmacol. 2007;21:603–10. doi: 10.1177/0269881106070995. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Ledford CC, Parker MP, Case JM, Mehta RH, See RE. The role of the dorsomedial prefrontal cortex, basolateral amygdala, and dorsal hippocampus in contextual reinstatement of cocaine seeking in rats. Neuropsychopharmacology. 2005;30:296–309. doi: 10.1038/sj.npp.1300579. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–258. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–56. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtress T, Kirkpatrick K. The role of the nucleus accumbens core in impulsive choice, timing, and reward processing. Behav Neurosci. 2010;124:26–43. doi: 10.1037/a0018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen ST, Mark GP. The nicotinic acetylcholine receptor antagonist mecamylamine prevents escalation of cocaine self-administration in rats with extended daily access. Psychopharmacology (Berl) 2007;194:53–61. doi: 10.1007/s00213-007-0822-z. [DOI] [PubMed] [Google Scholar]
- Hearing MC, Miller SW, See RE, McGinty JF. Relapse to cocaine seeking increases activity-regulated gene expression differentially in the prefrontal cortex of abstinent rats. Psychopharmacology (Berl) 2008;198:77–91. doi: 10.1007/s00213-008-1090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil SH, Johnson MW, Higgins ST, Bickel WK. Delay discounting in currently using and currently abstinent cocaine-dependent outpatients and non-drug-using matched controls. Addict Behav. 2006;31:1290–4. doi: 10.1016/j.addbeh.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99:461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- Kolokotroni KZ, Rodgers RJ, Harrison AA. Acute nicotine increases both impulsive choice and behavioural disinhibition in rats. Psychopharmacology (Berl) 2011;217:455–73. doi: 10.1007/s00213-011-2296-2. [DOI] [PubMed] [Google Scholar]
- Lasseter HC, Ramirez DR, Xie X, Fuchs RA. Involvement of the lateral orbitofrontal cortex in drug context-induced reinstatement of cocaine-seeking behavior in rats. Eur J Neurosci. 2009;30:1370–81. doi: 10.1111/j.1460-9568.2009.06906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautin A. The Limbic Brain. Kluwer Academics/Plenum Publishers; New York: 2001. [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–70. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–8. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locey ML, Dallery J. Isolating behavioral mechanisms of intertemporal choice: nicotine effects on delay discounting and amount sensitivity. J Exp Anal Behav. 2009;91:213–23. doi: 10.1901/jeab.2009.91-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locey ML, Dallery J. Nicotine and the behavioral mechanisms of intertemporal choice. Behav Processes. 2011;87:18–24. doi: 10.1016/j.beproc.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar AC, Robbins TW. Delay discounting and impulsive choice in the rat. Curr Protoc Neurosci. 2007 doi: 10.1002/0471142301.ns0822s39. Chapter 8: Unit 8.22. [DOI] [PubMed] [Google Scholar]
- Mar AC, Walker AL, Theobald DE, Eagle DM, Robbins TW. Dissociable effects of lesions to orbitofrontal cortex subregions on impulsive choice in the rat. J Neurosci. 2011;31:6398–404. doi: 10.1523/JNEUROSCI.6620-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, de Wit H. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–65. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Mendez IA, Simon NW, Hart N, Mitchell MR, Nation JR, Wellman PJ, Setlow B. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124:470–7. doi: 10.1037/a0020458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. The systems-level organization of cholinergic innervation in the human cerebral cortex and its alterations in Alzheimer’s disease. Prog Brain Res. 1996;109:285–297. doi: 10.1016/s0079-6123(08)62112-3. [DOI] [PubMed] [Google Scholar]
- Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218:703–12. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001;158:1783–93. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Paine TA, Dringenberg HC, Olmstead MC. Effects of chronic cocaine on impulsivity: relation to cortical serotonin mechanisms. Behav Brain Res. 2003;147:135–47. doi: 10.1016/s0166-4328(03)00156-6. [DOI] [PubMed] [Google Scholar]
- Papez J. A proposed mechanism of emotion. J Neuropsychiatry Clin Neurosci. 1995;7:103–112. doi: 10.1176/jnp.7.1.103. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Stairs DJ, Bardo MT. Impulsive choice and environmental enrichment: effects of d-amphetamine and methylphenidate. Behav Brain Res. 2008;193:48–54. doi: 10.1016/j.bbr.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Picconi B, Tolu M, Giacomini P, Scarnati E. Role of tonically-active neurons in the control of striatal function: cellular mechanisms and behavioral correlates. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:211–30. doi: 10.1016/s0278-5846(00)00153-6. [DOI] [PubMed] [Google Scholar]
- Potter AS, Ryan KK, Newhouse PA. Effects of acute ultra-low dose mecamylamine on cognition in adult attention-deficit/hyperactivity disorder (ADHD) Hum Psychopharmacol. 2009;24:309–17. doi: 10.1002/hup.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Takahashi Y, Gugsa N, Bissonette GB, Schoenbaum G. Previous cocaine exposure makes rats hypersensitive to both delay and reward magnitude. J Neurosci. 2007;27:245–50. doi: 10.1523/JNEUROSCI.4080-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G. Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron. 2006;51:509–20. doi: 10.1016/j.neuron.2006.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358–69. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelmeer AN, De Vries TJ, Wardeh G, van de Ven HW, Vanderschuren LJ. Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci. 2002;22:3269–76. doi: 10.1523/JNEUROSCI.22-08-03269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Differences between nicotine and cocaine-induced conditioned place preferences. Brain Res Bull. 2010;81:120–4. doi: 10.1016/j.brainresbull.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Simon NW, Mendez IA, Setlow B. Cocaine exposure causes long-term increases in impulsive choice. Behav Neurosci. 2007;121:543–9. doi: 10.1037/0735-7044.121.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GB, Zhang XL, Zhao LY, Sun LL, Wu P, Lu L, Shi J. Drug-related cues exacerbate decision making and increase craving in heroin addicts at different abstinence times. Psychopharmacology (Berl) 2011 doi: 10.1007/s0021301126175. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology. 2008;33:1779–97. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Cardinal RN, Robbins TW. Contrasting roles of basolateral amygdala and orbitofrontal cortex in impulsive choice. J Neurosci. 2004;24:4718–22. doi: 10.1523/JNEUROSCI.5606-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeeb FD, Floresco SB, Winstanley CA. Contributions of the orbitofrontal cortex to impulsive choice: interactions with basal levels of impulsivity, dopamine signalling, and reward-related cues. Psychopharmacology (Berl) 2010;211:87–98. doi: 10.1007/s00213-010-1871-2. [DOI] [PubMed] [Google Scholar]
- Zhou FM, Wilson CJ, Dani JA. Cholinergic interneuron characteristics and nicotinic properties in the striatum. J Neurobiol. 2002;53:590–605. doi: 10.1002/neu.10150. [DOI] [PubMed] [Google Scholar]
- Zhou W, Liu H, Zhang F, Tang S, Zhu H, Lai M, Kalivas PW. Role of acetylcholine transmission in nucleus accumbens and ventral tegmental area in heroin-seeking induced by conditioned cues. Neuroscience. 2007;144:1209–18. doi: 10.1016/j.neuroscience.2006.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y, Wang X, Cui C, Luo F, Yu P, Wang X. Cocaine-induced impulsive choices are accompanied by impaired delay-dependent anticipatory activity in basolateral amygdala. J Cogn Neurosci. 2012;24:196–211. doi: 10.1162/jocn_a_00131. [DOI] [PubMed] [Google Scholar]


