Abstract
The aim of this study was to describe the fate of patients with newly diagnosed acute myeloid leukemia (AML) who did not achieve an initial remission while being treated on a contemporary cooperative group trial. We analyzed the outcome of patients entered into S0106, a recently reported cooperative group trial for patients with newly diagnosed AML. A total of 589 eligible patients was treated, of whom 150 (25%) did not achieve a remission while on study and were available for further analysis. The 4-year survival rate for the entire cohort of 150 patients was 23%. Among the 64 patients who received an allogeneic hematopoietic cell transplant, the 4-year survival rate was 48% compared with 4% for the 86 patients who did not undergo transplantation. Among those transplanted, we could not detect a difference in outcome according to remission status, donor source, type of preparative regimen, or cytogenetic risk category. More than 20% of patients with newly diagnosed AML who fail induction therapy can still be cured, particularly if they are able to receive an allogeneic hematopoietic cell transplant. These results suggest that early HLA typing and donor identification are important components of the initial therapy of AML.
Introduction
Based on results of contemporary cooperative group studies, approximately 70–75% of younger patients (age <65) with newly diagnosed acute myeloid leukemia (AML) will achieve complete remission (CR) if treated with standard induction therapy (1, 2). The treatment-related mortality associated with induction therapy has fallen to 5% or less (3). Thus, a sizeable proportion of patients, perhaps 20–25%, will survive induction therapy but not achieve an initial CR with protocol-directed therapy. The fate of such patients is not well described, particularly in the current era where the spectrum of subsequent therapies available to patients who fail induction has expanded, especially with the broader availability of hematopoietic cell transplantation. We hypothesized that knowledge of the outcome of subsequent therapies in patients who fail induction might be useful in developing further clinical studies and treatment recommendations. Accordingly, we followed up on all patients who failed to achieve a CR on a recently reported cooperative group trial.
Methods
S0106 Patient Population
S0106 is a recently reported study testing the efficacy of the addition of gemtuzumab ozogamicin to conventional AML therapy. The patient population, study design and treatment groups have been reported (1). Briefly, patients with de novo AML, aged 18 to 60 years, with a Zubrod performance score (PS) of 0–3 and adequate organ function were eligible. All patients provided written informed consent in accordance with local policies, federal regulations, and the Declaration of Helsinki.
S0106 Study Design
At registration, patients were randomly assigned 1:1 to receive either daunorubicin 45mg/m2 by IV push on days 1 through 3, cytarabine 100 mg/m2 by continuous IV infusions on days 1 through 7, and gemtuzumab ozogamicin 6 mg/m2 by 2 hour IV infusion on day 4 (DA+GO), or daunorubicin 60mg/m2 by IV push on days 1 through 3 and cytarabine 100mg/m2 by continuous IV infusions on days 1 through 7 (DA). Marrow response was assessed on day 14. For both groups, a second course of induction using DA was recommended for patients with marrows having more than 20% cellularity and more than 5% blasts at day 14. If the day 14 marrow was hypocellular, repeat marrows were suggested until cellularity returned to greater than 20% and either a CR was documented or greater than 5% blasts were seen, at which point repeat treatment with DA was recommended. Patients who achieved a CR were eligible to receive 3 courses of consolidation therapy with cytarabine 3g/m2 by 3-hour continuous IV infusions every 12 hours on days 1, 3 and 5. Consolidation courses were administered monthly. After completing consolidation therapy, patients were eligible for post-consolidation randomization (1:1) between GO (5mg/m2, 3 doses at least 28 days apart) or observation.
Induction Failure Patient Cohort
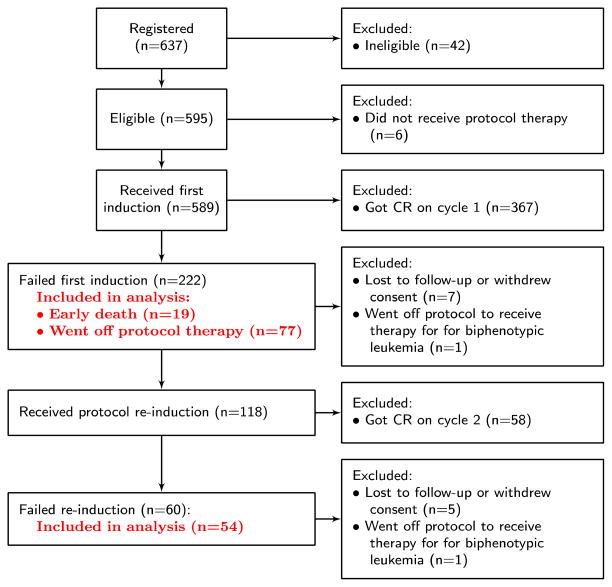
The S0106 patients included in and excluded from this analysis are summarized in Figure 1. A total of 637 patients were registered to S0106. Five hundred ninety-five were eligible, 589 received protocol therapy, and 425 achieved a CR (71%). Of the 164 eligible patients who received protocol therapy but did not achieve a CR, 14 were excluded from further analysis because they were lost to follow-up, withdrew consent for the study, or went off protocol therapy to receive therapy for biphenotypic leukemia. A total of 150 are included in the following analysis.
Figure 1.
Flowchart of S0106 patients included in analysis
Statistical Methods
Date of induction failure was defined as date taken off of protocol therapy due to death or documented induction failure. Survival after induction failure was measured from date of induction failure to death from any cause, with patients last known to be alive censored at the date of last contact. Survival after transplant was defined from the date of transplant to death from any cause, with patients last known to be alive censored at the date of last contact. Patient characteristics were tabulated and summarized. Survival was estimated using the Kaplan-Meier method. Log-rank tests were used to compare survival curves.
Results
Patient Characteristics
The characteristics of the 150 patients who failed induction are summarized in Table 1. The median age was 48 (range 18–60) years, 49% were female, and 84% had a PS of 0 or 1. Cytogenetic analysis was available on 116 patients (68%); 34 patients did not have specimens submitted for central review. Of those with centrally reviewed cytogenetics, 7% were favorable, 47% were intermediate, 43% were unfavorable and 3% were clonal but unclassified according to the SWOG classification schema. FLT3-ITD and NMP1 mutational status was available on 73 patients. Among patients with molecular data, 3% were NPM1 mutated but FLT3-ITD wild-type, and 30% were FLT3 mutated. Median time between registration and date of induction failure was 27 days, with a range of 2–138 days.
Table 1. Patient Characteristics.
Median (range) and N (%) reported. (N = 150)
| Age | |
| Median (range) | 48 (18, 60) |
| Sex | |
| Female | 73 (49%) |
| Male | 77 (51%) |
| PS | |
| 0 | 52 (35%) |
| 1 | 73 (49%) |
| 2 | 14 (9%) |
| 3 | 10 (7%) |
| Missing | 1 |
| FAB | |
| M0 | 16 (11%) |
| M1 | 34 (23%) |
| M2 | 39 (27%) |
| M4 | 35 (24%) |
| M4EOS | 2 (1%) |
| M5 | 13 (9%) |
| M6 | 5 (3%) |
| M7 | 2 (1%) |
| Missing | 4 |
| Cytogenetic risk | |
| Favorable | 7 (6%) |
| Intermediate | 55 (47%) |
| Unknown risk | 4 (3%) |
| Unfavorable | 50 (43%) |
| Not done | 34 |
| NPM1/FLT3-ITD | |
| NPM1+/FLT3-ITD+ | 7 (10%) |
| NPM1+/FLT3-ITD− | 2 (3%) |
| NPM1−/FLT3-ITD+ | 15 (21%) |
| NPM1−/FLT3-ITD− | 49 (67%) |
| Not done | 77 |
The reasons for induction failure are summarized in Table 2. Seventeen patients died following induction therapy without ever having a marrow exam and three patients were removed from protocol therapy without a bone marrow exam. Of the 130 patients who had at least one marrow exam following induction, 25 patients achieved a CRi, 6 were classified as a PR, and the remainder (99) were classified as having resistant disease. Excluding 19 patients who died within 28 days of registration to the study, 85 patients were taken off S0106 and declared induction failures after receiving one cycle of therapy, including 14 with a CRi. Sixty patients were taken off study after failing a second cycle of induction.
Table 2.
Primary induction failure summary (n=150)
| Reason | N (%) |
|---|---|
| Died without bone marrow exam | 17 (11%) |
| Off protocol without bone marrow exam | 3 (2%) |
| CRi | 25 (17%) |
| PR | 6 (4%) |
| Resistant disease | 99 (66%) |
Clinical Course Following Induction Failure
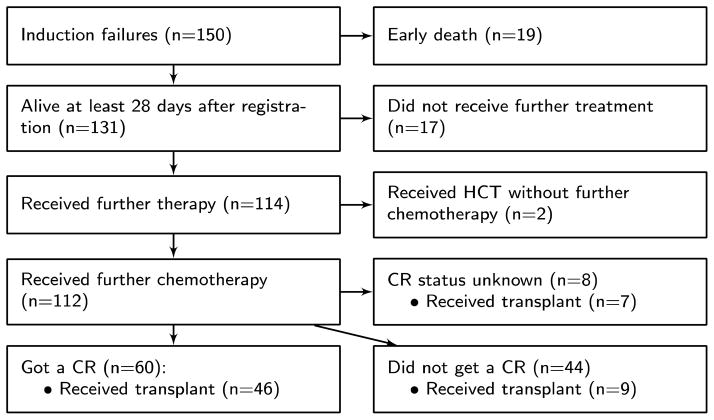
Figure 2 illustrates the course of patients following failure of induction on S0106. Thirty-six patients, including the 19 who died within 28 days of study registration, received no further therapy. Among the 114 patients who are known to have received further therapy, 112 received some form of chemotherapy while the other two were transplanted without further treatment. The majority of patients treated with chemotherapy received at least one cycle that included some form of high-dose cytarabine (1.5–3.0 gm/m2/day 2–6 days) (HDAC) (84/112, 75%). Overall, 53% of patients who received re-induction or salvage therapy achieved a CR. Of the 60 patients who obtained a CR, 50 (83%) received some form of HDAC. Among patients who received reinduction or salvage therapy, 62 (56%) went on to receive a transplant, and among these 62 patients 50 (81%) received HDAC. Among the 46 patients who got a CR and went on to get a transplant, 32 were in CR at the time of transplant and CR status at time of transplant was not known for 4 patients. Among the 46 patients who got a CR and went on to get a transplant, 40 (87%) received some form of HDAC.
Figure 2.
Flowchart of S0106 induction failure patients post-protocol therapy
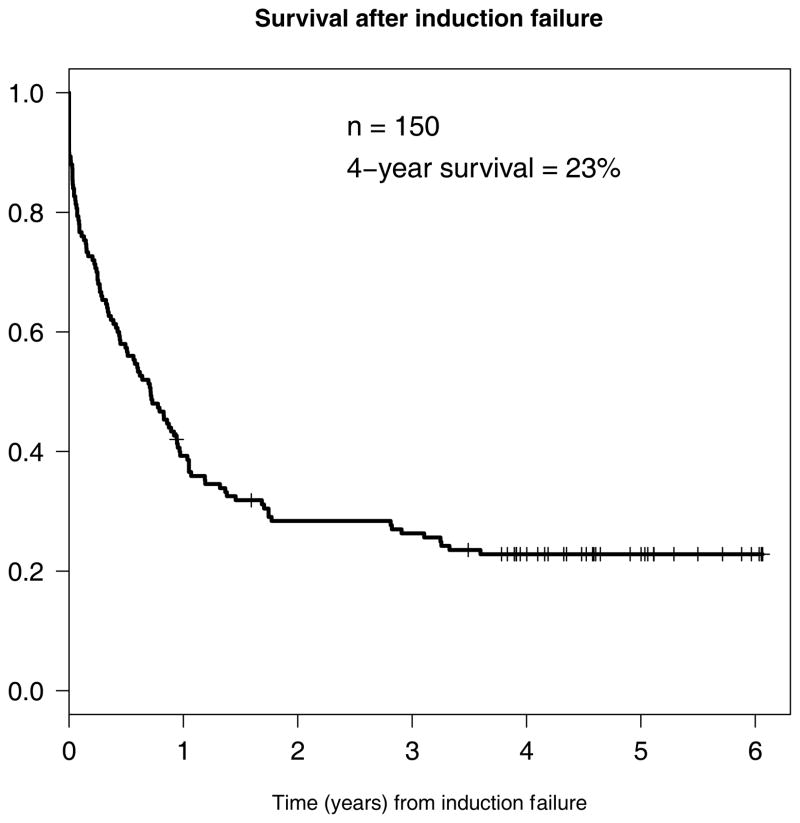
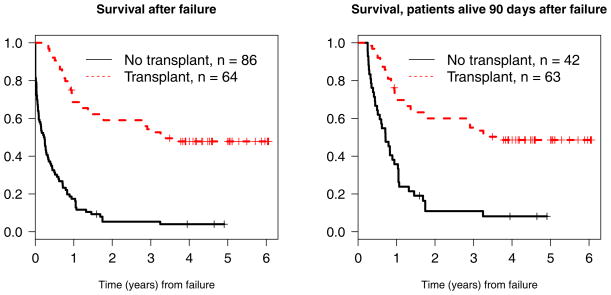
The four-year survival for the entire cohort of 150 patients with primary induction failure was 23% (95% confidence interval 17%–31%) (Figure 3). The four-year survival rates after primary induction failure for patients who received a transplant was 48%, while for those who were not transplanted, the four-year survival rate was 4% (Figure 4). In an attempt to account for the lead time bias associated with patients needing to live long enough to receive a transplant, we also performed a landmark survival analysis restricted to patients alive 90 days after induction failure. As shown, four-year survivals from day 90 were 49% for those transplanted versus 8% for those not transplanted. Among the entire cohort of 150 patients, those with unfavorable cytogenetics had significantly worse survival than the favorable (p=0.020), intermediate (p=0.002), and not done (p=0.001) groups. There were no significant differences among the NPM1/FLT3 cohorts.
Figure 3.
Survival of full induction failure cohort
Figure 4.
Survival after failure stratified by transplant
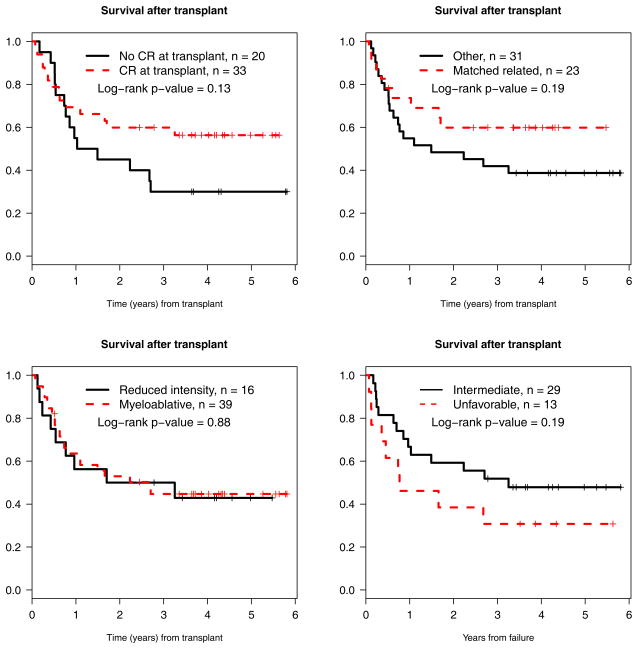
When comparing survival after transplant by CR at time of transplant (yes versus no), type of donor (matched related versus other), transplant conditioning regimen (ablative versus reduced intensity), and cytogenetic risk category (intermediate versus unfavorable), there were no significant differences, although there was a trend towards improved long-term survival in patients who were in CR at the time of transplant (p=0.13, Figure 5).
Figure 5.
Survival after transplant stratified by: CR at time of transplant (yes versus no), donor (matched related versus not), conditioning regimen intensity (myeloablative versus reduced intensity), and cytogenetic risk (intermediate versus unfavorable).
Discussion
The results of this analysis demonstrate that a substantial proportion (over 20%) of patients who were determined to have failed primary induction therapy for AML on a contemporary cooperative group trial became long-term survivors. Even after attempting to account for the lead time bias associated with having to live long enough to be transplanted, the likelihood of long-term survival appeared to be significantly higher (49%) if patients were transplanted than if they weren’t (8%). These results suggest that early HLA-typing and donor identification are important components of the initial therapy of AML.
In reviewing the results of this study, a number of topics deserve discussion. First is the issue of the definition of primary induction failure. The definition of resistant disease adopted by the International Working Group and used by the European LeukemiaNet requires the patient to be alive for 7 or more days after the completion of the first course of chemotherapy and to have persistent leukemia cells in the peripheral blood or bone marrow at that time (4, 5). Primary induction failure then combines those patients who die early and those with resistant disease. Other studies use alternative definitions, most commonly defining patients as having primary induction failure if they die early or fail to achieve a CR following two cycles of standard dose cytarabine and daunorubicin therapy or one cycle of a high dose cytarabine-containing regimen. As reviewed by Ravandi et al, there is no single widely accepted definition of primary induction failure (6). In this report, we used the pragmatic definition of not achieving a CR while on protocol therapy.
In the S0106 protocol, patients were treated with an initial cycle of induction therapy and a bone marrow was mandated 14 days after the start of therapy. If on the day 14 marrow >5% blasts were seen and cellularity was >20%, a second induction course using standard dose daunorubicin and cytarabine was recommended. If cellularity was <20%, repeat marrows were suggested until patients either entered remission or >5% blasts with >20% cellularity was seen, at which time a second induction cycle could be initiated. Despite this recommendation, a substantial number of patients were removed from S0106 after receiving only one induction cycle. Most of these patients subsequently were treated with high dose cytarabine-containing regimens.
Data were not collected to explain why physicians removed patients from study after a single cycle of induction, but it is reasonable to presume that the treating physician felt that the patients would be better served if treated with a high-dose cytarabine containing regimen. This experience is not unique to S0106; similar behavior was seen in a recently reported ECOG trial (2) where over 50% of those failing the first cycle of induction were removed from study instead of receiving a second cycle of therapy (Tallman, personal communication). There are no data to prove or disprove that switching from a second cycle of standard induction to an alternative therapy is of benefit, but a study formally testing this would be of great interest.
In the S0106 study, 118 patients who failed to achieve a CR with their first cycle of therapy were treated with a second cycle of standard induction therapy on study and 49% achieved a CR. Among the 69 patients who were removed from study after one cycle and treated with alternative regimens, 57% eventually got a CR. It might be mentioned that the 49% CR rate on study was with a single second cycle, whereas the 57% off study includes patients who may have gotten a CR after second, third or subsequent salvage cycles. The only difference we were able uncover between those receiving a second cycle on protocol versus those taken off was a greater likelihood for those being treated at SWOG member (academic) sites to be removed from study. Whether this reflects the availability of more alternative therapeutic options or studies at academic sites or some other bias is unknown. Greater understanding of who is likely to benefit or not from a second cycle of conventional induction, versus those for whom an alternative therapy is a better choice would be of great practical importance. Until such facts are known, clinical trials would be more interpretable if there were increased uniformity in treatment approaches. The removal of a substantial proportion of patients after a single cycle of induction artificially lowers the CR rate with that approach, making comparisons across studies difficult, and if the practice is not applied equivalently in both arms of a randomized trial, such behavior could potentially bias the results of such studies.
The fact that hematopoietic cell transplantation can cure some patients who never achieve an initial remission with conventional chemotherapy has been documented several times. Reports from both single institutions and registry data suggest that approximately 20–25% of patients transplanted from matched siblings for primary refractory AML can become long-term disease-free survivors (7–9). More recently, similar results have been reported in recipients of unrelated donor transplants for primary induction failure (10). Transplantation requires donor identification, insurance clearance, and referral to a transplant center, which can be formidable barriers to an urgent transplant for patients with primary induction failure. Thus, it is difficult to gauge how highly selected the transplanted patients were in these previous reports.
S0106 contained no specific instructions about how to handle patients with primary induction failure. Nonetheless, fully 43% of those who failed primary induction were able to undergo transplantation. If the analysis were restricted to those who lived at least 90 days after failure and thus presumably would have had enough time for a donor to be identified and to initiate the transplant process, 60% were actually transplanted. Several factors probably account for this high rate of transplantation. First, there has been impressive expansion of alternative donor availability in the form of unrelated donors and cord blood banks. Second, many of the institutions participating in S0106 have large, active transplant programs. This high rate of transplantation for patients with primary induction failure is not typical of practice across the United States. According to SEER statistics, there were approximately 6890 new cases of AML in patients age <66 last year, and assuming a CR rate of 70%, there should have been 2067 patients failing induction (11). According to the Center of International Bone Marrow Transplantation Research, there were only 316 transplants conducted in the US last year for patients with primary refractory AML (12). This suggests that a procedure with a curative potential was not made available to a large number of patients. Further research into the reasons for the restricted access to transplantation in this patient population would be of interest.
Among those who were transplanted, 48% were alive more than four years later. This proportion is higher than that previously reported for patients transplanted after primary induction failure. This difference may be due, at least in part, to improvements in allogeneic hematopoietic cell transplantation technologies and supportive care measures over the last two decades, as documented in numerous reports (13–17). Part of the difference may also be due to differences in the patient population. Some previous reports defined their patient population as those who “had never achieved a first CR with chemotherapy” without regard to the number of cycles of induction. In the current study, there were 2 patients who went directly to transplant after failing one cycle of induction, and 1 of these patients is alive in remission more than four years after the transplant. Other previous reports were restricted to those patients who had failed to achieve a CR after two or more courses of induction. Most of these studies do not define what proportion, if any, of the patients achieved a remission with alternative therapy prior to transplant. In the current study, 21 patients had failed two cycles of protocol induction and went on to get a transplant, and of these, 4-year survival after transplant was 37%. Our best results were seen in the subset of patients who failed to achieve a CR on study, but managed to achieve a CR with alternative therapy prior to transplant. Their outcomes were essentially the same as reported for patients transplanted in first remission with a 4-year survival after transplant of 56%. Among those transplanted with active disease, the four-year survival was 30%, a proportion very similar to that reported in other studies of transplantation for primary refractory AML. We could not detect an impact of donor source (related versus unrelated), intensity of preparative regimen (myeloablative versus reduced intensity), or cytogenetic risk category (intermediate versus unfavorable) on outcome. However, the small number of patients and non-randomized nature of the study limit the interpretability of these results.
In summary, over 20% of patients who failed to achieve a CR on a contemporary cooperative group trial became long-term survivors, largely because of the availability of allogeneic hematopoietic cell transplantation. This result underscores the potential importance of early HLA-typing, donor search, and referral to a transplant center. Our experience also highlights a number of unsettled issues. Is there a population of patients, such as those who achieve a CRi, who should go straight to transplant after failing their initial cycle of induction therapy? Can we identify those patients who, even though they fail the first round of standard induction, are likely to achieve a CR with a second cycle? Does switching to an alternative chemotherapy after failing a first cycle benefit selected patients? Answers to these questions should improve cure rates in AML.
References
- 1.Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Othus M, Kantarjian H, Petersdorf S, et al. Declining rates of treatment-related mortality in patients with newly diagnosed AML given ‘intense’ induction regimens: a report from SWOG and MD Anderson. Leukemia. 2013 doi: 10.1038/leu.2013.176. Epub ahead of print 2013 Jul 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia [erratum appears in J Clin Oncol. 2004 Feb 1; 22(3):576 Note: LoCocco, Francesco [corrected to Lo-Coco, Francesco]] J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 5.Döhner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 6.Ravandi F. Primary refractory acute myeloid leukaemia - in search of better definitions and therapies (Review) Br J Haematol. 2011;155:413–419. doi: 10.1111/j.1365-2141.2011.08869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biggs JC, Horowitz MM, Gale RP, et al. Bone marrow transplants may cure patients with acute leukemia never achieving remission with chemotherapy. Blood. 1992;80(4):1090–1093. [PubMed] [Google Scholar]
- 8.Fung HC, Stein A, Slovak M, et al. A long-term follow-up report on allogeneic stem cell transplantation for patients with primary refractory acute myelogenous leukemia: impact of cytogenetic characteristics on transplantation outcome. Biol Blood Marrow Transplant. 2003;9:766–771. doi: 10.1016/j.bbmt.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Duval M, Klein JP, He W, et al. Hematopoietic stem-cell transplantation for acute leukemia in relapse or primary induction failure. J Clin Oncol. 2010;28:3730–3738. doi: 10.1200/JCO.2010.28.8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craddock C, Labopin M, Pillai S, et al. Factors predicting outcome after unrelated donor stem cell transplantation in primary refractory acute myeloid leukaemia. Leukemia. 2011;25:808–813. doi: 10.1038/leu.2011.13. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2010), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2013, based on the November 2012 submission.
- 12.Center for International Blood and Marrow Transplant Research. [Accessed August 15, 2013]; Available at: www.cibmtr.org.
- 13.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–2101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Appelbaum FR. Improved outcomes with allogeneic hematopoietic cell transplantation. Best Practice & Research Clinical Haematology. 2012;25:465–471. doi: 10.1016/j.beha.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remberger M, Ackefors M, Berglund S, et al. Improved survival after allogeneic hematopoietic stem cell transplantation in recent years. A single-center study. Biol Blood Marrow Transplant. 2011;17:1688–1697. doi: 10.1016/j.bbmt.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Giebel S, Labopin M, Holowiecki J, et al. Outcome of HLA-matched related allogeneic hematopoietic stem cell transplantation for patients with acute leukemia in first complete remission treated in Eastern European centers. Better results in recent years. Ann Hematol. 2009;88:1005–1013. doi: 10.1007/s00277-009-0719-5. [DOI] [PubMed] [Google Scholar]
- 17.Horan JT, Logan BR, Agovi-Johnson MA, et al. Reducing the risk for transplantation-related mortality after allogeneic hematopoietic cell transplantation: how much progress has been made? J Clin Oncol. 2011;29:805–813. doi: 10.1200/JCO.2010.32.5001. [DOI] [PMC free article] [PubMed] [Google Scholar]