Abstract
Background
Stress is associated with cardiovascular diseases.
Objective
This study aimed at assessing whether chronic stress induces vascular alterations, and whether these modulations are nitric oxide (NO) and Ca2+ dependent.
Methods
Wistar rats, 30 days of age, were separated into 2 groups: control (C) and Stress (St). Chronic stress consisted of immobilization for 1 hour/day, 5 days/week, 15 weeks. Systolic blood pressure was assessed. Vascular studies on aortic rings were performed. Concentration-effect curves were built for noradrenaline, in the presence of L-NAME or prazosin, acetylcholine, sodium nitroprusside and KCl. In addition, Ca2+ flux was also evaluated.
Results
Chronic stress induced hypertension, decreased the vascular response to KCl and to noradrenaline, and increased the vascular response to acetylcholine. L-NAME blunted the difference observed in noradrenaline curves. Furthermore, contractile response to Ca2+ was decreased in the aorta of stressed rats.
Conclusion
Our data suggest that the vascular response to chronic stress is an adaptation to its deleterious effects, such as hypertension. In addition, this adaptation is NO- and Ca2+-dependent. These data help to clarify the contribution of stress to cardiovascular abnormalities. However, further studies are necessary to better elucidate the mechanisms involved in the cardiovascular dysfunction associated with stressors. (Arq Bras Cardiol. 2014; [online].ahead print, PP.0-0)
Keywords: Stress, Physiological / physiopatology; Hypertension; Nitric Oxide / physiology; Rats; Vasoconstrictor Agents / pharmacology
Introduction
Stress is known as a complex and multidimensional condition1. The responses to stressor agents depend on the intensity, frequency, duration, and type of stressor agent. Hypothalamus-pituitary-adrenal (HPA) axis and sympathetic nervous system (SNS) are the major responsible systems that modulate the organism to stressor agents2. When stimulated, HPA and SNS release glucocorticoid hormone, such as cortisol, and biogenic amines, such as adrenaline and noradrenaline, respectively3.
Stress triggers different dysfunctions and pathologies including asthma, allergy, depression, anxiety, ulcer, metabolism dysfunction and cardiovascular diseases, such as stroke, hypertension and infarction2,4-8.
The literature and previous data from our laboratory have shown that stress induces cardiac alterations, such as fibrosis, systolic and diastolic left ventricle (LV) dysfunction and Ca2+ transit alteration6,9-13. Moreover, cardiovascular changes are not restricted only to the heart, some evidence implicates that different types of acute stress induce modulated vascular response to different agonists, such as increased acetylcholine response and decreased contractile effect of noradrenaline, which are nitric oxide (NO) and endothelium-dependent7,14-16. However, more studies are necessary to elucidate the mechanisms involved in modulated stress-induced responses.
Given that information, the aim of the present study was to assess whether chronic stress induces vascular alterations, and whether these modulations are NO- and Ca2+-dependent, in addition, the real involvement of a1-adrereceptor also was analyzed.. Our hypothesis was that chronic stress promotes adaptive vascular NO- and Ca2+-dependent responses and desensitization of α1-adrenoreceptor. To understand the involvement of mediators and of α1-adrenoreceptor, pharmacological tools were used.
Methods
Animals
Thirty-day-old male Wistar rats (70-100 g) obtained from the Animal Center of Botucatu Medical School (Botucatu, São Paulo, Brazil) were housed in individual cages. The environment was controlled in terms of light (12-hour light/dark cycle starting at 6 AM), clean-air, room temperature (23 ± 3°C), and relative humidity (60% ± 5%). After 7 days of acclimatization, the rats were distributed into two groups: control (C, n = 8) and chronic stress (St, n = 8). All experiments and procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals, published by the National Research Council (1996), and were approved by the Ethics Committee of the Instituto de Biociências UNESP-Botucatu (protocol nº 95/08-CEEA).
Chronic stress
The stress characteristics taken into consideration were quality, frequency, type, physical or emotional, as well as the animal species being studied17. Immobilization stress is a model of emotional stress and one of the most used in research18. After 37 days of age, the St group animals were immobilized individually in metal capsules at room temperature (25°C) for 1 hour per day, five days a week, for 15 weeks.
During the stress session, the C group animals remained in cage at room temperature (25°C), receiving neither food nor water. Then, the St group animals were returned to their cages. Forty-eight hours after the last stress session, the animals were subjected to experimental protocols.
Cardiac and adrenal hypertrophy test
The animals were sacrificed, and hypertrophy of adrenals glands and LV was assessed. The glands and LV were removed, dissected and weighed. Cardiac hypertrophy was assessed by using the LV/tibia (mm) ratio and, on echocardiography, the LV (g)/final body weight (FBW) ratio, according to our previous study7,8,13.
Systolic blood pressure (SBP)
Systolic blood pressure was assessed at the end of stress exposure by use of the non-invasive tail-cuff method with a Narco BioSystems® Electro-Sphygmomanometer (International Biomedical, Austin, TX, USA). The mean of two SBP readings was recorded for each animal.
Corticosterone level
Animals were submitted to a 12-15-hour fasting, anesthetized with sodium pentobarbital (50 mg/kg i.p.), and sacrificed by use of decapitation. Blood samples were collected in heparinized tubes, centrifuged at 3000 X g for 15 minutes at 4°C, and the serum was separated and stored at −80°C for further analysis. Corticosterone level was measured by using a specific radioimmunoassay kit (Coat-A-Count Rat Corticosterone - Diagnostic Products Corporation).
Vascular function
After 15 weeks of stress exposure, the rats were decapitated. The descending thoracic aorta was excised and trimmed free of adhering fat and connective tissue. Two transverse rings of the same artery, measuring 4 mm in length each, were cut and mounted at the optimal length for isometric tension recording in organ chambers. One ring served as control, while the endothelium was mechanically removed from the others by gently rubbing the luminal surface. The preparations were mounted in organ baths containing 7 mL of Krebs-Henseleit solution, whose composition in mM was as follows: NaCl 113.0; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.1; NaHCO3 25.0; glucose 11.0; ascorbic acid 0.11. The bathing fluid, kept at 37.0 ± 0.5 °C, was saturated with a gas mixture of 95% O2 and 5% CO2, and the pH was 7.4. The preparations were allowed to equilibrate for at least 1 h under a resting tension of 1.5 g, which is ideal for inducing maximum contraction. Tension was recorded by use of a myograph (Ugo Basile).
Cumulative concentration-effect (CCE) curves were constructed from the tissue response to potassium chloride (KCl) and to noradrenaline. Cumulative concentration-effect curves to noradrenaline were constructed in the absence and presence of L-NAME (3 x 10-4 M, inhibitor of NO synthase - NOS) or prazosin (10-8 M, α1-adrenoreceptor antagonist) (Sigma Chemical Co., St Louis, Missouri, USA).
In another set of experiments, CCE curves were constructed for acetylcholine, in intact aortic rings (+E), and for sodium nitroprusside (SNP), in endothelium denuded aortic rings (-E) (Sigma Chemical Co., St Louis, Missouri, USA).
Contribution of intracellular and extracellular Ca2+ in the decreased response of endothelium-free aortic rings to noradrenaline
Adapted from Tirapelli et al19, we investigated the contribution of intracellular Ca2+ release on the decreased vascular function to noradrenaline, contractile response to this agonist was obtained in Krebs' solution without Ca2+. The rings were exposed to this solution for 1 minute, then stimulated with 10-7 and 10-6 M noradrenaline, and then the tension was assessed. Furthermore, the role of extracellular Ca2+ mobilization was investigated by using CaCl2-induced contraction in the presence of noradrenaline. In Ca2+-free solution containing EDTA (1 mM), endothelium-free aortic rings were contracted with noradrenaline (10-4 M) to deplete the intracellular Ca2+ stores and then rinsed in Krebs solution without Ca2+ and EDTA and containing noradrenaline (0.1 mM). The process was repeated several times until the extinction of noradrenaline-induced contraction, when we considered that Ca2+ was completely depleted.
Statistical analysis
Data are reported as means ± standard error of the mean (SEM). The cardiac mass and adrenal hypertrophy, corticosterone levels and final SBP of the groups were compared by using t-test and post hoc Tukey-test with the GraphPad Prism 6.04 software. Individual concentration-effect curves were fitted into a curve by use of non-linear regression analysis. The negative logarithm of EC50 values (pD2) and the maximal response were compared by use of Student t test or ANOVA, when appropriate. The significance level of 5% was adopted.
Results
Chronic stress did not increase cardiac mass as follows: LV (g)/tibia (mm) values in the C and St groups were 0.15 ± 0.02 vs 0.16 ± 0.03, respectively; and the results of the echocardiography test [LV (g)/FBW (g)] in the C and St groups were 1.45 ± 0.16 vs 1.52 ± 0.11, respectively. However, chronic stress increased the wet adrenal weight (C = 0.57 ± 0.08 vs St = 0.76 ± 0.05). In addition, animals exposed to chronic stress developed high blood pressure [C = 118.3 ± 12.3 vs St = 148.8 ± 9.43* (mmHg)] and had increased corticosterone levels in plasma [C = 48.3 ± 10.2 vs St = 97.2 ± 16.3* (ng/mL)] *p < 0.05.
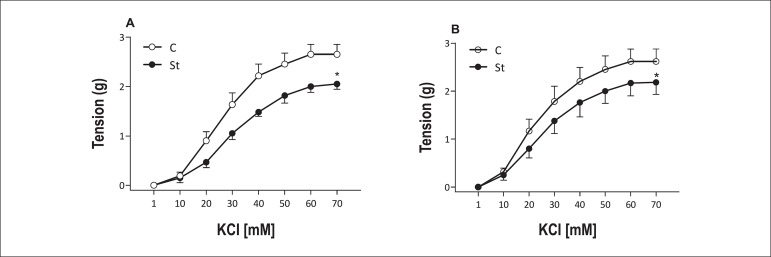
Chronic stress promoted decreased maximal response to KCl in aortic rings with or without endothelium [+E (C: 2.65 ± 0.48 vs St: 2.06 ± 0.26*); -E (C: 2.62 ± 0.64 vs St: 2.18 ± 0.62*)]. Moreover, no pD2 difference was observed in rings with and without endothelium [(C: 3.47 ± 0.10 vs St: 3.36 ± 0.09) and (C: 11.51 ± 2.80 vs St: 11.17 ± 2.81)] (Figure 1) *p < 0.05.
Figure 1.
Concentration-effect curves for KCl obtained with two rings, one with (A) and the other without (B) endothelium, of the same thoracic aorta from control (empty symbol) and stressed (solid symbol) rats. Data are reported as means ± SEM (n = 6) *p < 0.05.
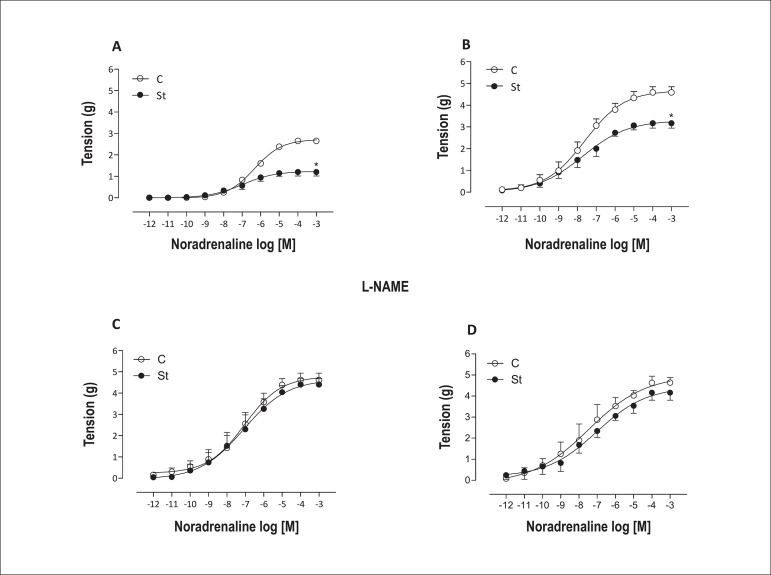
Similarly to the KCl response, aortic rings with or without endothelium from stressed rats also had decreased maximal response to noradrenaline. Pre-incubation with L-NAME blunted these changes. No pD2 difference was observed in the experimental groups without L-NAME pre-incubation. L-NAME pre-incubation increased the sensitivity to noradrenaline in both rings, endothelium-intact and denuded (Figure 2 and Table 1).
Figure 2.
Concentration-effect curves for noradrenaline obtained in intact endothelium (E+) (A and C) and in denuded endothelium (E-) aortic rings (B and D), in presence (C and D) or absence (A and B) of L-NAME (3x10-4 M), from control (empty symbol) and stressed (solid symbol) rats. Data are reported as means ± SEM (n = 5-7) *p < 0.05.
Table 1.
Vascular reactivity to noradrenaline In presence or absence of L-NAME
| Groups | Agonist | Parameters | |||
|---|---|---|---|---|---|
| Maximal response | pD2 | ||||
| +E | -E | +E | -E | ||
| C | Nor | 2.65 ± 0.21 | 4.58 ± 0.64# | 6.33 ± 0.07 | 7.60 ± 0.23# |
| Nor/L-NAME | 4.62 ± 0.70$ | 4.40 ± 0.63# | 7.05 ± 0.29$ | 7.47 ± 0.52 | |
| St | Nor | 1.20 ± 0.40* | 3.87 ± 0.58*# | 6.49 ± 0.32 | 7.27 ± 0.21# |
| Nor/L-NAME | 4.64 ± 0.55$ | 4.16 ± 0.78$# | 7.08 ± 0.38$ | 7.03 ± 0.44 | |
Effects of chronic stress on maximal response and pD2 (negative logarithm of the EC50) for noradrenaline (Nor) in aortic rings from Wistar rats, in L-NAME presence or absence (3x10-4 M). Concentration-effect curves (CCE) were constructed in intact endothelium (E+) and denuded endothelium (E-) aortic rings. Results are shown as means ± SEM of 5-7 rats in each experimental group.
p < 0.05 C vs St *p < 0.05 C vs St;
p < 0.05 L-NAME vs Nor;
p < 0.05 -E vs +E; C: control group; St: Stress group.
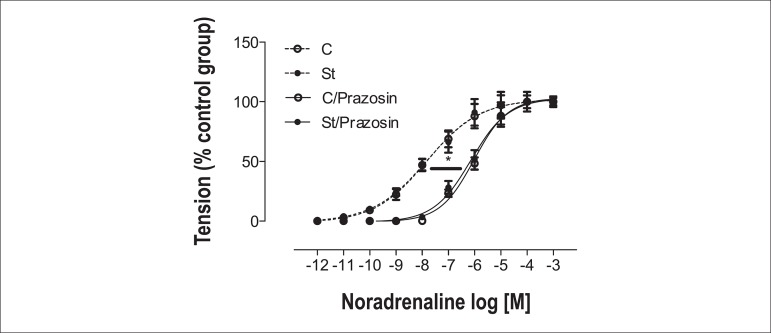
Prazosin, α1 competitive antagonist, was used to assess the real α1 adrenoreceptor involvement. It shifted the noradrenaline response to the right in endothelium-free aortic rings; however, there was no pD2 difference between the C and St groups (C = 7.82 ± 0.08; St = 7.81 ± 0.09; C/Prazosin = 6.02 ± 0.05*; St/Prazosin = 6.14 ± 0.06*) *p < 0.05 (Figure 3).
Figure 3.
Concentration-effect curves for noradrenaline in denuded endothelium (E-) aortic rings, in presence (solid line) or absence (dotted line) of prazosin (10-8 M) from control (empty symbol) and stressed (solid symbol) rats. Data are reported as means ± SEM (n = 6) *p < 0.05.
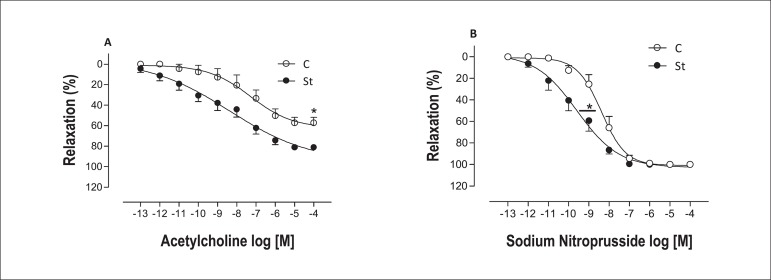
Chronic stress enhanced the maximal response to acetylcholine in aortic rings with endothelium, as well as its sensitivity (Figure 4.A and Table 2). Moreover, endothelium-denudedaortic rings from stressed rats showed shift to the left for NO donor, SNP, and no difference was observed in the maximal response parameter (Figure 4.B and Table 2).
Figure 4.
Concentration-effect curves for acetylcholine (A) obtained in intact endothelium (E+) aortic rings and sodium nitroprusside (B) obtained in denuded endothelium (E-) aortic rings from control (empty symbol) and stressed (solid symbol) rats. Data are reported as means ± SEM (n = 5-7) *p < 0.05.
Table 2.
Vascular reactivity to acetylcholine
| Groups | Agonist | Parameters | |
|---|---|---|---|
| Maximal response | pD2 | ||
| +E | |||
| C | ACh (%) | 62.4 ± 8.63 | 7.27 ± 0.47 |
| St | 95.3 ± 16.2* | 8.37 ± 0.63* | |
| -E | |||
| C | SNP (%) | 101 ± 5.4 | 8.38 ± 0.10 |
| St | 103 ± 5.7 | 9.58 ± 0.22* | |
Effects of chronic stress on maximal response and pD2 (negative logarithm of the EC50) for acetylcholine (ACh) in intact endothelium (E+) aortic rings and sodium nitroprusside (SNP) in denuded endothelium (E-) aortic rings from Wistar rats. Concentration-effect curves (CCE) were constructed. Results are shown as means ± SEM of 5-7rats in each experimental group.
p < 0.05 C vs St; C: control group; St: Stress group.
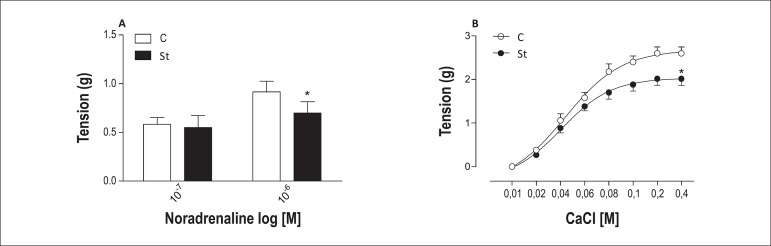
Stressed rats had decreased contractile response to noradrenaline in Krebs solution without Ca2+. Furthermore, CCE curves for CaCl2, in presence of noradrenaline, in endothelium-free aortic rings from chronically stressed rats also had decreased maximal response. No difference was observed in pD2 (Figure 5 and Table 3).
Figure 5.
Contractile response for noradrenaline in denuded endothelium (E-) aortic rings from control (empty bar) and stressed (solid bar) rats in Krebs solution without Ca2+ (A). Concentration- effect curves for CaCl2 in aortic rings without endothelium from control (empty symbol) and stressed (solid symbol) rats. Data are reported as means ± SEM (n = 5-7) *p < 0.05.
Table 3.
Vascular reactivity to CaCl2
| Groups | Agonist | Parameters | |
|---|---|---|---|
| Maximal response | pD2 | ||
| -E | |||
| C | CaCl2 | 2.76 ± 0.13 | 8.81 ± 0.34 |
| St | 2.03 ± 0.10* | 8.97 ± 0.37 | |
Effects of chronic stress on maximal response and pD2 (negative logarithm of the EC50) for CaCl2 in aortic rings from Wistar rats. Concentration-effect curves (CCE) were constructed in denuded endothelium (E-) aortic rings. Results are shown means ± SEM of 5-7 rats in each experimental group.
p < 0.05 C vs St; C: control group; St: Stress group.
Discussion
In the present study, chronic stress increased adrenal wet weight and plasma corticosterone levels, which suggest increased HPA axis activity, and corroborate the literature that shows these same effects in different stress models17,20,21. Stress can lead to hypertension through the production of several mediators or hyperactivation of some systems, including renin-angiotensin-aldosterone, and vasoactive amines, such as adrenaline, that are associated with blood pressure regulation22-24. Our stress model led to high blood pressure that might be associated with adrenaline release by adrenal glands, because we found increased adrenal mass, which indicates SNS hyperactivation, corroborating findings from literature13,22.
Stress, in acute or chronic models, improves vascular function to different agonists7,14,16,25. Our results corroborate these data, in which increased responses to noradrenaline and acetylcholine were observed. In addition, we can suggest that these responses are NO-dependent for two reasons: i) the previous incubation with NOS inhibitor abolished the decreased maximal response to noradrenaline in aortic rings from stressed rats compared with that of the control group; ii) we found that acetylcholine-induced relaxation was higher in aortic rings from the St group than from the C group. Acetylcholine, an endothelium-dependent agonist, is able to release NO when it binds to a muscarinic receptor located in endothelial cells, leading to vascular relaxation26.
Another interesting finding from our study shows that vascular smooth muscle from stressed rats is more sensitive to NO than that from the non-stressed group, because the NO donor, SNP, induced shift to the left in endothelium-free aortic rings. We did not assess pathways associated with NO production, such as AKT (protein kinase B), which is able to phosphorylate NO synthase27, but we can confirm that both NO releasing and sensitivity to NO are involved in the modulated vascular response to chronic stress.
We assessed whether α1-adrenoreceptor participates in the vascular function of stressed rats by using a competitive α1-adrenoreceptor antagonist. We concluded that α1-adrenoreceptor activity does not change in the aorta of stressed rats, and the decreased maximal response observed in the experimental group might be linked to downstream events to α1-adrenoreceptor, such as NO release and sensitivity28, or NO release might be associated with α2-adrenoreceptor activation by noradrenaline29.
Similarly to noradrenaline response, KCl, a contractile agonist not receptor-dependent, also had decreased maximal response in aorta rings of the St group. These data, together with prazosin and noradrenaline, strengthen that some intracellular mediator is involved in stress vascular response, NO being a strong candidate, since the KCl-induced vascular contraction does not depend on a specific receptor, but on action potential.
Ca2+ plays a crucial role in vascular homeostasis, modulating vascular function and structure, the intracellular Ca2+ and uptake being essential for perfect operation30. We examined whether intracellular Ca2+ and uptake are involved in the attenuated vascular contraction to noradrenaline in stressed rats. Attenuated vascular response to single concentrations of noradrenaline was observed in this study. So, we could suggest that intracellular Ca2+ release, which occurs in the endoplasmic reticulum (ER) after interaction of inositol trisphosphate (IP3) with its receptor located in the ER membrane31,32, or the low Ca2+ concentration in the ER could participate in this modulation. Moreover, Ca2+ uptake by some channel, such as L-type calcium channels32,33, might have low activity to uptake Ca2+ in the vasculature from the St group, because the CCE curve for Ca2+, in a Ca2+-free medium in presence of noradrenaline, was attenuated in endothelium-free aortic rings of stressed rats.
Conclusion
Our study advances the understanding and identifies new mediators involved in the attenuated vascular response to noradrenaline in stressed rats. Nitric oxide and Ca2+ fluxes are the possible mediators. We believe these mediators are positively activated to counterbalance the deleterious cardiovascular effects caused by stressful conditions. However, more studies are necessary to better elucidate the adaptive response to chronic stress.
Footnotes
Author contributions
Conception and design of the research, Statistical analysis and Obtaining financing: Bruder-Nascimento T, Cicogna AC, Cordellini S; Acquisition of data: Bruder-Nascimento T, Campos DHS; Analysis and interpretation of the data and Critical revision of the manuscript for intellectual content: Bruder-Nascimento T, Campos DHS, Cicogna AC, Cordellini S; Writing of the manuscript: Bruder-Nascimento T.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was funded by Fapesp add the process number 2009/03771-2.
Study Association
This article is part of the thesis of master submitted by Thiago Bruder do Nascimento, from Universidade do Estado de São Paulo.
References
- 1.McEwen BS, Seeman T. Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann NY Acad Sci. 1999;896:30–47. doi: 10.1111/j.1749-6632.1999.tb08103.x. [DOI] [PubMed] [Google Scholar]
- 2.Tsigos C, Chrousos GP. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J Psychosom Res. 2002;53(4):865–871. doi: 10.1016/s0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 3.Dunn AJ, Swiergiel AH. The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol. 2008;583(2-3):186–193. doi: 10.1016/j.ejphar.2007.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Viau V, Meaney MJ. Variations in the hypothalamic-pituitary- adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- 5.Hjemdahl P. Stress and the metabolic syndrome: an interesting but enigmatic association. Circulation. 2002;106(21):2634–2636. doi: 10.1161/01.cir.0000041502.43564.79. [DOI] [PubMed] [Google Scholar]
- 6.Rozanski A, Blumenthal JA, Davidson KW, Saab PG, Kubzansky L. The epidemiology, pathophysiology, and management of psychosocial risk factors in cardiac practice: the emerging field of behavioral cardiology. J Am Coll Cardiol. 2005;45(5):637–651. doi: 10.1016/j.jacc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Bruder-Nascimento T, Cordellini S. Vascular adaptive responses to physical exercise and to stress are affected differently by nandrolone administration. Braz J Med Biol Res. 2011;44(4):337–344. doi: 10.1590/s0100-879x2011007500043. [DOI] [PubMed] [Google Scholar]
- 8.Bruder-Nascimento T, Campos DH, Alves C, Thomaz S, Cicogna AC, Cordellini S. Effects of chronic stress and high-fat diet on metabolic and nutritional parameters in Wistar rats. Arq Bras Endocrinol Metabol. 2013;57(8):642–649. doi: 10.1590/s0004-27302013000800010. [DOI] [PubMed] [Google Scholar]
- 9.Costoli T, Bartolomucci A, Graiani G, Stilli D, Laviola G, Sgoifo A. Effects of chronic psychosocial stress on cardiac autonomic responsiveness and myocardial structure in mice. Am J Physiol Heart Circ Physiol. 2004;286(6):H2133–H2140. doi: 10.1152/ajpheart.00869.2003. [DOI] [PubMed] [Google Scholar]
- 10.Krepsova K, Micutkova L, Novotova M, Kubovcakova L, Kvetnansky R, Krizanova O. Repeated immobilization stress decreases mRNA and protein levels of the type 1 IP3 receptor in rat heart. Ann N Y Acad Sci. 2004;1018:339–344. doi: 10.1196/annals.1296.042. [DOI] [PubMed] [Google Scholar]
- 11.Burri MV, Nanda NC, Lloyd SG, Hsiung MC, Dod HS, Beto RJ, et al. Assessment of systolic and diastolic left ventricular and left atrial function using vector velocity imaging in Takotsubo cardiomyopathy. Echocardiography. 2008;25(10):1138–1144. doi: 10.1111/j.1540-8175.2008.00819.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Xu J, Gong JB, Qian L. L-type calcium channel current up-regulation by chronic stress is associated with increased α1c subunit expression in rat ventricular myocytes. Cell Stress Chaperones. 2009;14(1):33–41. doi: 10.1007/s12192-008-0052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruder-Nascimento T, Campos DH, Leopoldo AS, Lima-Leopoldo AP, Okoshi K, Cordellini S, et al. Chronic stress improves the myocardial function without altering L-type Ca+2 channel activity in rats. Arq Bras Cardiol. 2012;99(4):907–914. doi: 10.1590/s0066-782x2012005000082. [DOI] [PubMed] [Google Scholar]
- 14.Milakofsky L, Harris N, Vogel WH. Effects of repeated stress on plasma arginine levels in young and old rats. Physiol Behav. 1993;54(4):725–728. doi: 10.1016/0031-9384(93)90083-r. [DOI] [PubMed] [Google Scholar]
- 15.Manukhina EB, Lapshin AV, Meerson FZ. Effect of adaptation to periodic hypoxia on post infarction fall of blood pressure and hyperactivation of the endothelium. Fisiol Zh. 1998;37(3):98–105. [PubMed] [Google Scholar]
- 16.Cordellini S, Vassilieff VS. Decreased endothelium-dependent vasoconstriction to noradrenaline in acute-stressed rats is potentiated by previous chronic stress: nitric oxide involvement. Gen Pharmacol. 1998;30(1):79–83. doi: 10.1016/s0306-3623(97)00074-8. [DOI] [PubMed] [Google Scholar]
- 17.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav. 2007;91(4):449–458. doi: 10.1016/j.physbeh.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kvetnansky R, Weise VK, Thoa NB, Kopin IJ. Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and costicosterone in forcibly immobilized rats. J Pharmacol Exp Ther. 1979;209(2):287–291. [PubMed] [Google Scholar]
- 19.Tirapelli CR, Al-Khoury J, Bkaily G, D'Orléans-Juste P, Lanchote VL, Uyemura SA, et al. Chronic ethanol consumption enhances phenylephrine-induced contraction in the isolated rat aorta. J Pharmacol Exp Ther. 2006;316(1):233–241. doi: 10.1124/jpet.105.092999. [DOI] [PubMed] [Google Scholar]
- 20.Huizenga NA, Koper JW, de Lange P, Pols HA, Stolk RP, Grobbee DE, et al. Interperson variability but intraperson stability of baseline plasma cortisol concentrations, and its relation to feedback sensitivity of the hypothalamo-pituitaryadrenal axis to a low dose of dexamethasone in elderly individuals. J Clin Endocrinol Metab. 1998;83(1):47–54. doi: 10.1210/jcem.83.1.4498. [DOI] [PubMed] [Google Scholar]
- 21.Ricart-Jané D, Rodríguez-Sureda V, Benavides A, Peinado-Onsurbe J, López-Tejero MD, Llobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 2002;51(7):925–931. doi: 10.1053/meta.2002.33353. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni S, O'Farrell I, Erasi M, Kochar MS. Stress and hypertension. WMJ. 1998;97(11):34–38. [PubMed] [Google Scholar]
- 23.Pausova Z. From big fat cells to high blood pressure: a pathway to obesity-associated hypertension. Curr Opin Nephrol Hypertens. 2006;15(2):173–178. doi: 10.1097/01.mnh.0000214775.42103.a5. [DOI] [PubMed] [Google Scholar]
- 24.Santos PC, Krieger JE, Pereira AC. Renin-angiotensin system, hypertension, and chronic kidney disease: pharmacogenetic implications. J Pharmacol Sci. 2012;120(2):77–88. doi: 10.1254/jphs.12r03cr. [DOI] [PubMed] [Google Scholar]
- 25.Lanza Júnior U, Cordellini S. Differential vascular adaptive response to stress exposition in male and female rats: role of gonadal hormones and endothelial cells. Stress. 2007;10(1):27–36. doi: 10.1080/10253890601135426. [DOI] [PubMed] [Google Scholar]
- 26.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5798):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 27.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. 10. [DOI] [PubMed] [Google Scholar]
- 28.Jones CJ, DeFily DV, Patterson JL, Chilian WM. Endothelium-dependent relaxation competes with alpha 1- and alpha 2-adrenergic constriction in the canine epicardial coronary microcirculation. Circulation. 1993;87(4):1264–1274. doi: 10.1161/01.cir.87.4.1264. [DOI] [PubMed] [Google Scholar]
- 29.Rascado RR, Bendhack LM. Activation of alpha2-adrenoceptors is necessary to induce nitric oxide release in isoprenaline-induced relaxation. Vascul Pharmacol. 2005;42(2):63–68. doi: 10.1016/j.vph.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Orlov S, Resink TJ, Bernhardt J, Ferracin F, Buhler FR. Vascular smooth muscle cell calcium fluxes. Regulation by angiotensin II and lipoproteins. Hypertension. 1993;21(2):195–203. doi: 10.1161/01.hyp.21.2.195. [DOI] [PubMed] [Google Scholar]
- 31.Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. Pt 1J Physiol. 2006;570:5–11. doi: 10.1113/jphysiol.2005.095604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007;87(2):593–658. doi: 10.1152/physrev.00035.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callera GE, Bendhack LM. Contribution of sarcoplasmic reticulum calcium uptake and L type calcium channels to altered vascular responsiveness in the aorta of renal hypertensive rats. Gen Pharmacol. 1999;33(6):457–466. doi: 10.1016/s0306-3623(99)00042-7. [DOI] [PubMed] [Google Scholar]