Improved CT colonography interpretation can be achieved with computer-aided detection subject to avoidable false-positive interpretations, which are described and illustrated.
Abstract
Computed tomography (CT) colonography is a screening modality used to detect colonic polyps before they progress to colorectal cancer. Computer-aided detection (CAD) is designed to decrease errors of detection by finding and displaying polyp candidates for evaluation by the reader. CT colonography CAD false-positive results are common and have numerous causes. The relative frequency of CAD false-positive results and their effect on reader performance on the basis of a 19-reader, 100-case trial shows that the vast majority of CAD false-positive results were dismissed by readers. Many CAD false-positive results are easily disregarded, including those that result from coarse mucosa, reconstruction, peristalsis, motion, streak artifacts, diverticulum, rectal tubes, and lipomas. CAD false-positive results caused by haustral folds, extracolonic candidates, diminutive lesions (<6 mm), anal papillae, internal hemorrhoids, varices, extrinsic compression, and flexural pseudotumors are almost always recognized and disregarded. The ileocecal valve and tagged stool are common sources of CAD false-positive results associated with reader false-positive results. Nondismissable CAD soft-tissue polyp candidates larger than 6 mm are another common cause of reader false-positive results that may lead to further evaluation with follow-up CT colonography or optical colonoscopy. Strategies for correctly evaluating CAD polyp candidates are important to avoid pitfalls from common sources of CAD false-positive results.
©RSNA, 2014
See discussion on this article by Yee.
SA-CME LEARNING OBJECTIVES
After completing this journal-based SA-CME activity, participants will be able to:
■ List the common causes of CAD and reader false-positive results at CT colonography.
■ Describe the problem-solving techniques for evaluating a polyp candidate identified at CT colonography.
■ Discuss the benefits and drawbacks of use of CAD for reader performance in interpreting CT colonography studies.
Introduction
CT Colonography and Computer-aided Detection
Computed tomography (CT) colonography is a screening modality used to detect colonic polyps before they progress to colorectal cancer (1–3). Colorectal cancer screening can reduce mortality from colorectal cancer by 13%–18% (4). Current screening guidelines include optical colonoscopy, the most frequently recommended study (5). However, patient compliance with optical colonoscopy is poor for several reasons, including discomfort with the procedure and bowel cleansing, as well as cost (6). CT colonography has rates of polyp detection comparable to those of optical colonoscopy, and its use may improve overall adherence to screening guidelines because it is minimally invasive, requires no sedation, has the potential for reduced cathartic examinations, and has faster patient throughput (4,7–10). Data support the cost-effectiveness of CT colonography (11). Current challenges of CT colonography include variable sensitivity and specificity among readers and long learning curves for new readers (similar to mammography). Most reader errors are avoidable and result from errors of detection (12–14). Computer-aided detection (CAD) is designed to decrease errors of detection by finding and displaying possible polyps to be evaluated by the reader (15–18).
CAD has three modes: primary, secondary, and concurrent. The primary mode involves an exclusive review of CAD marks. The secondary CAD mode involves reading the examination first, without the CAD marks, to avoid satisfaction-of-search bias, and then viewing the CAD marks. The concurrent CAD mode involves reading the examination normally but with the CAD marks visible. Substantial training is required to learn how to accurately interpret CT colonography results (19,20). Experienced readers take 5–10 minutes to read most examinations, whereas novices, and even some experienced readers interpreting complex cases, can take substantially longer. Nevertheless, on average, reading times are substantially less than those for optical colonoscopy (21–23). Multiple CT colonography trials with CAD have shown improved reader times, particularly for inexperienced readers, although the use of CAD in secondary mode has been shown to add about 1–3 minutes to the reading time for most cases (24–27). The increased sensitivity for polyp detection is accompanied by a small decrease in specificity because, often, possible polyps according to CAD are not true polyps (a CAD false-positive result), which can contribute to reader false-positive results.
In this article, we review the most common causes of CAD false-positive results and present strategies for evaluating possible polyps, with potential pitfalls that may lead to incorrect reader interpretations (Table 1). Data for the relative frequency of CAD false-positive results and its effect on reader performance are presented from a large multiple-reader, multiple-case trial, and the readers’ performance is summarized according to the causes of CAD false-positive results (Table 2) (24). Specifically, the percentage of CAD false-positive results in each category with at least one reader false-positive result and the average percentage of readers who selected a polyp within each category (which we refer to as “incidence”) are presented. These data show that most CAD false-positive results are dismissed by readers. This systematic review of the causes of CAD false-positive results can help improve the performance of CT colonography readers in general and novice readers in particular.
Table 1:
Causes of False-Positive Results at CT colonography CAD
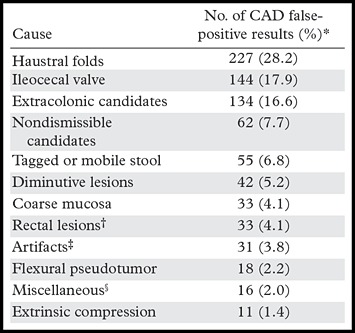
Based on an analysis of 806 CAD false-positive results from a 100-case, 19-reader trial (24).
Includes anal papillae, internal hemorrhoids, and varices.
Caused by reconstruction, peristalsis or motion, high-attenuation contrast material, or streak artifact.
Includes the following categories that represent <2% of CAD false-positive results each: diverticula (n = 9), rectal tube (n = 5), and lipoma (n = 2).
Table 2:
Rates of Reader False-Positive Results Related to CAD False-Positive Results by Cause
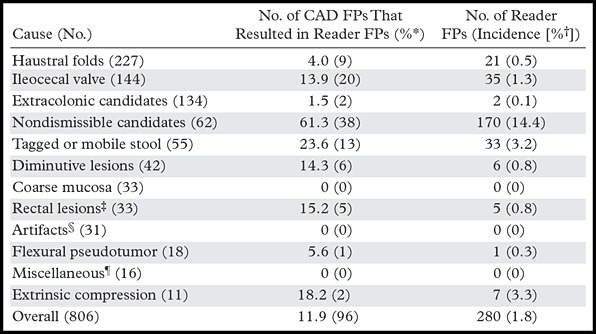
Note.—FPs = false-positive results.
Percentage of CAD false-positive results in a given category that resulted in at least one reader FP during the CAD-assisted session.
The average percentage of the 19 readers who selected a polyp candidate in that category during the CAD-assisted session.
Includes anal papillae, internal hemorrhoids, and varices.
Caused by reconstruction, peristalsis or motion, high-attenuation contrast material, or streak artifact.
Includes the following categories that represent <2% of CAD false-positive results each: diverticula (n = 9), rectal tube (n = 5), and lipoma (n = 2).
Frequency of CAD False-Positive Results
Our experience with CAD false-positive results is based on a study in which 19 readers interpreted 100 cases in two separate sessions: once without CAD (the CAD-unassisted session) and once with CAD implemented in the second-reader mode (the CAD-assisted session), which were at least 27 days apart (24). The 19 readers were adequately trained nonexpert radiologists from both academic and community environments with an average of 5 years of experience interpreting CT colonography cases (range, 1–10 years) and who interpreted an average of 92 cases per year (range, 20–300 cases). The CAD system had 8.1 CAD false-positive results per patient (including supine and prone series) with a stand-alone performance sensitivity of 93.2% for large polyps (≥10 mm) and 91.8% for small adenomas (6–10 mm), a rate that is comparable to previous reports on CAD system performance (28,29).
Sensitivity of the average reader improved with use of CAD by approximately five percentage points in each of the segment-, patient-, and polyp-level analyses, with a smaller decrease in specificity. The 806 CAD false-positive polyp candidates were reviewed and categorized according to the common causes of CAD false-positive results versus nondismissible polyp candidates by two trained residents. All 806 false-positive polyp candidates were then reviewed by a board-certified radiologist with extensive CT colonography experience. All reviewers were blinded to the readers’ performance with respect to the CAD false-positive results.
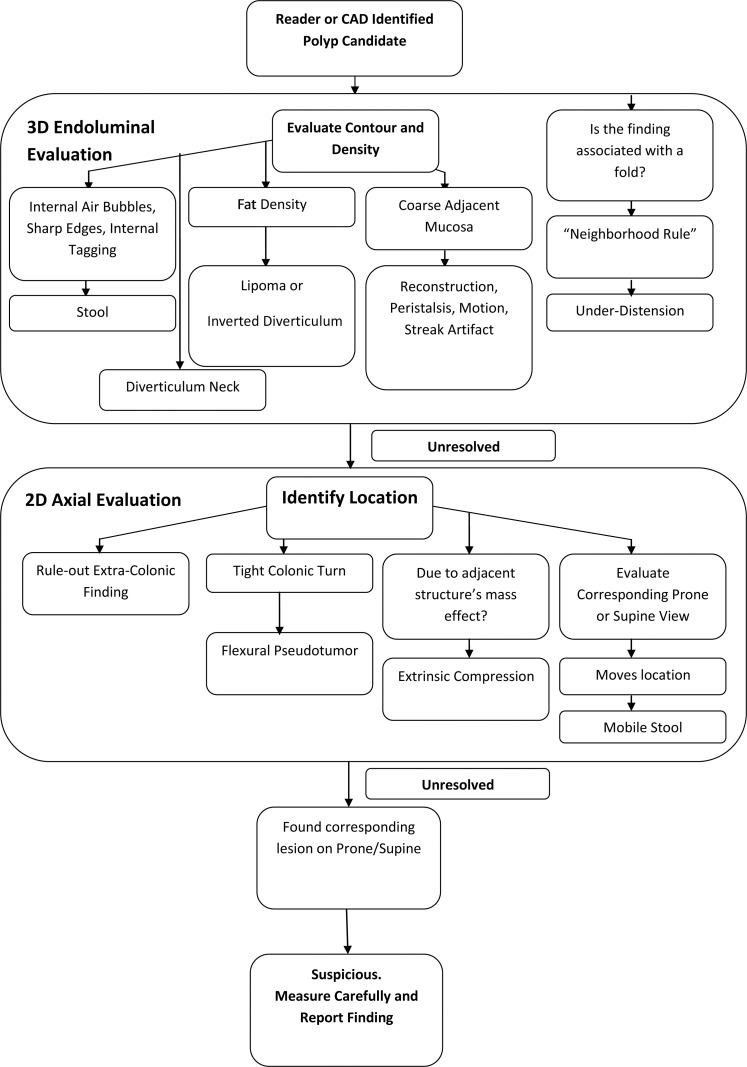
Many normal anatomic structures and lesions that mimic polyps can be selected as a polyp candidate by CAD software and presented to the reader for further characterization. Some causes of CAD false-positive results are similar to those found without CAD, whereas others result from limitations of CAD algorithms and are often easy for readers to recognize. Previous studies reported that approximately 20% of CAD false-positive results are difficult for readers to dismiss (30,31). The number of CAD false-positive results per study has steadily decreased as algorithms for recognizing normal anatomic structures have improved, leaving polyp candidates that require reader analysis (32,33). Although the incidence of CAD false-positive results for any CAD software purchase and CT colonography data set varies, the causes are common to all software programs. Every CAD polyp candidate should be evaluated with a logical, systematic approach (Fig 1) (34,35). In this article, we review the causes of CAD false-positive results and give strategies for properly characterizing and dismissing them.
Figure 1.
Flowchart shows the general approach for evaluating a polyp candidate.
General Approach to Evaluating Polyp Candidates
The specific process for interpreting a CT colonography examination is software and user dependent. The reader must be familiar with the workstation tools to accurately and efficiently interpret a study. User preference determines whether CAD is implemented in the first-, concurrent-, or second-reader mode. Usually, CT colonography software automatically segments the colon and asks the reader to verify. At this time, it is important to note whether any extracolonic gas-filled lumen is included in the segmentation (and, if possible, exclude it), the degree of colon distention, and whether any major artifacts are present. Many CT colonography experts initially read the study in a three-dimensional (3D) endoluminal view and navigate through the colon antegrade and retrograde, with multiplanar two-dimensional (2D) views available for problem solving. Software tools should be used to display any regions that were not included in the field of view during navigation.
In general, the target lesion for colorectal cancer screening is a polypoid lesion with soft-tissue attenuation or, less commonly, a flat lesion that is at least 6 mm in diameter, does not significantly move (unless it is pedunculated), and is not a normal anatomic structure. Once a polyp candidate is identified by the reader or the CAD program, the following general steps are taken (Fig 1): The intrinsic properties and context of the polyp candidate are essential for proper characterization. Its contour and attenuation should be evaluated to exclude artifact, stool (eg, air bubbles, sharp edges, or internal tagging), lipoma (eg, fat attenuation), diverticula, and coarse mucosa. When a polyp candidate is associated with a fold, the adjacent folds should be evaluated for similar thickness and nodular findings (the “neighborhood” rule), the presence of which would make the candidate most likely an artifact related to underdistention, reconstruction, peristalsis or motion, contrast material, or streak artifacts.
If suspicion remains, 2D views should be used to further characterize the finding. An intracolonic location should be reconfirmed on 2D multiplanar reconstructions. The contour and attenuation should be reassessed. Extrinsic compression (a result of mass effect from adjacent structures), a flexural pseudotumor (a sharp turn in the colon), and colonic underdistention should be considered as possible causes for the finding. A search for a lesion in the same position on corresponding supine or prone views should be performed to rule out untagged mobile stool. The presence of a corresponding nonmobile lesion should raise suspicion, although adherent untagged stool may still be a consideration. If the lesion is in the rectum, common benign entities, such as anal papillae, internal hemorrhoids, and varices, should be considered. The finding should be considered in the context of the quality of the study. For example, are numerous similar findings present that may result from untagged stool or poor preparation? Finally, if the lesion remains suspicious, an accurate measurement of its maximum diameter should be obtained and the finding reported if it is 6 mm or larger.
Haustral Folds
Haustral folds are a frequent source of CAD false-positive results (Figs 2, 3). They can markedly vary in their degree of difficulty to dismiss. Simple straight folds are an infrequent source of CAD polyp candidates, often at the site of converging folds or the taenia coli, and are easily dismissible once an infiltrative lesion is excluded with 2D multiplanar correlation (Figs 2a, 2b, 4). Minimally nodular folds may result from reconstruction artifacts or motion and are another cause of CAD polyp candidates that may be easily dismissed. Folds that are very nodular require careful evaluation to rule out an infiltrative flat lesion. Thickened folds are frequently seen in patients with a poorly distended colon or sigmoid muscular hypertrophy and can result in a CAD polyp candidate (Fig 3). By applying the so-called neighborhood rule, if most of the folds in a segment look similar, the thickened folds can be confidently dismissed. In more difficult cases in which the fold is very different from nearby folds, careful examination of 2D views should be performed for discrete focal or infiltrative lesions. In some cases, a lesion cannot be entirely excluded.
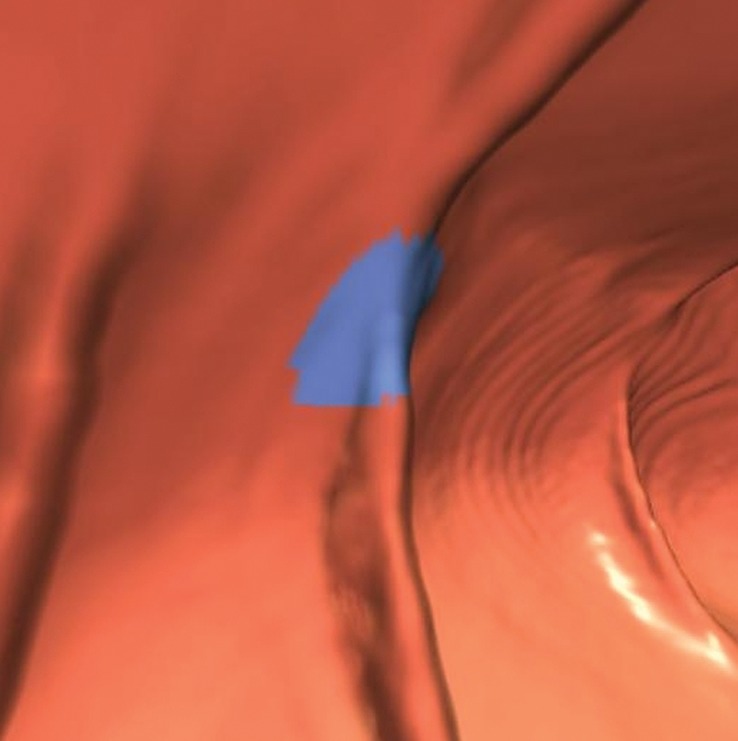
Figure 2a.
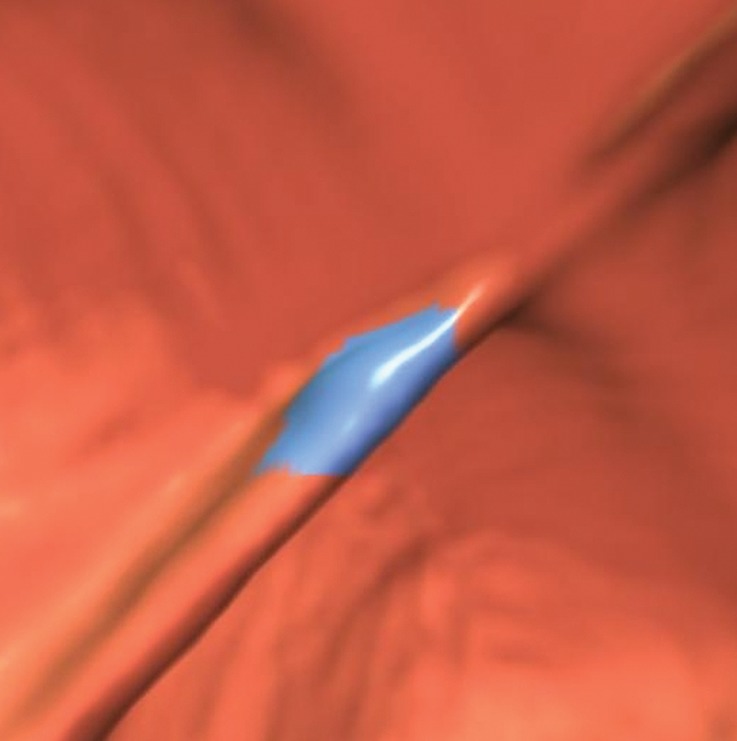
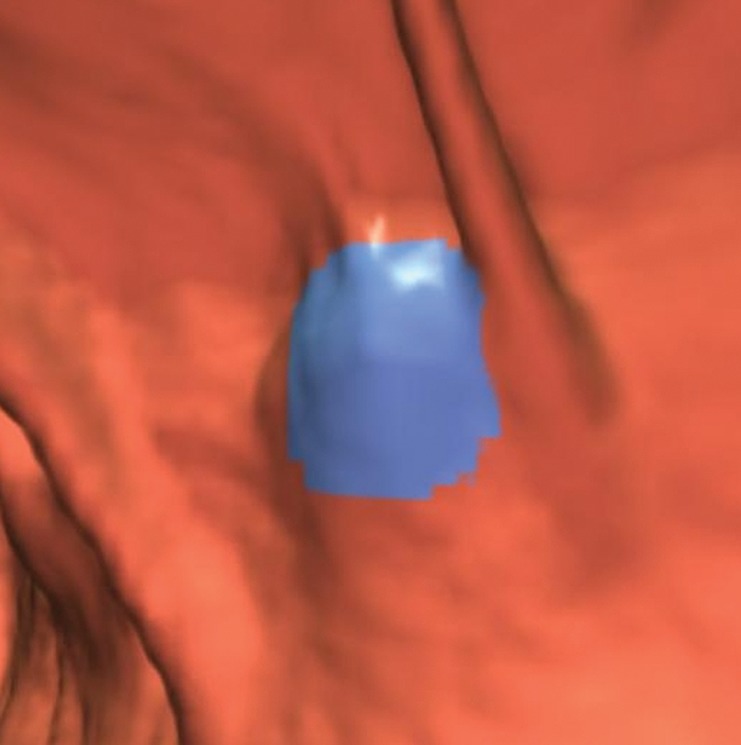
Haustral folds. (a) Endoluminal 3D CT colonography image shows a CAD polyp candidate (blue area) on a normal haustral fold. Minimal irregularity on a convex structure can generate CAD polyp candidates that are easily disregarded by the reader. (b) Endoluminal 3D CT colonography image shows a CAD polyp candidate (blue area) at the site of the convergence of two normal haustral folds that is easily disregarded by the reader.
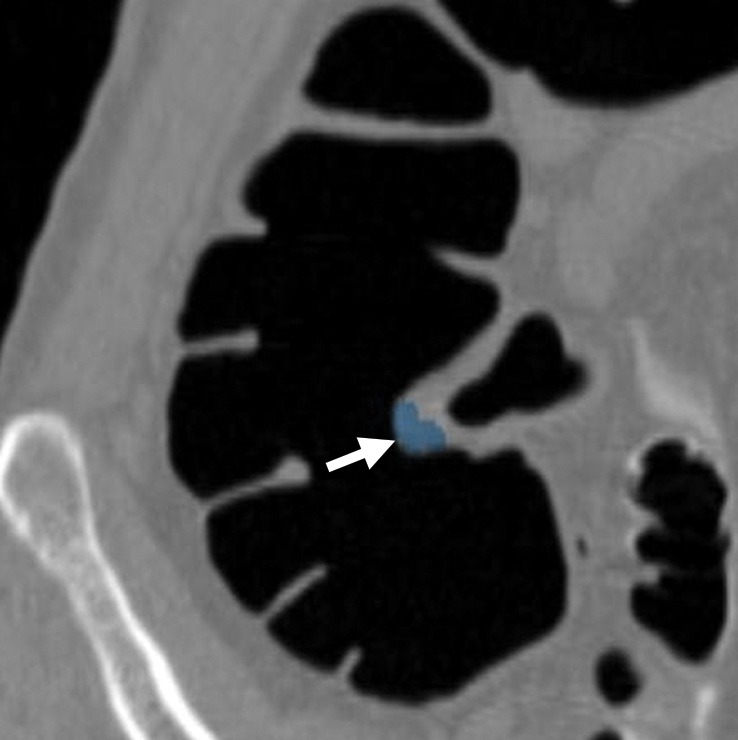
Figure 3a.
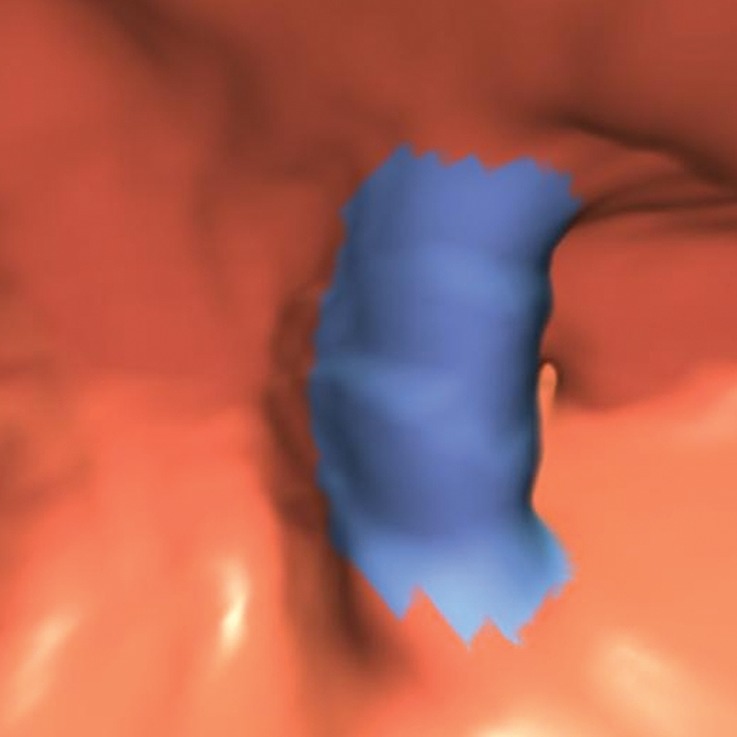
Haustral folds. (a) Endoluminal 3D CT colonography image shows a nodular thickened haustral fold that resulted in a CAD polyp candidate (blue area). This finding requires careful comparison with surrounding folds on 3D and 2D views and assessment for an overlying polyp or infiltrating carcinoma. The appearance of multiple convexities and similar adjacent folds suggests underdistention or normal anatomy of the sigmoid colon. (b) Coronal 2D CT colonography image shows the thickened haustral fold CAD polyp candidate (arrow). No distinct polypoid lesion is seen. Its location in the sigmoid colon and endoluminal appearance suggest underdistention or muscular hypertrophy, which is common in the sigmoid colon, particularly in association with diverticulosis.
Figure 2b.
Haustral folds. (a) Endoluminal 3D CT colonography image shows a CAD polyp candidate (blue area) on a normal haustral fold. Minimal irregularity on a convex structure can generate CAD polyp candidates that are easily disregarded by the reader. (b) Endoluminal 3D CT colonography image shows a CAD polyp candidate (blue area) at the site of the convergence of two normal haustral folds that is easily disregarded by the reader.
Figure 4a.
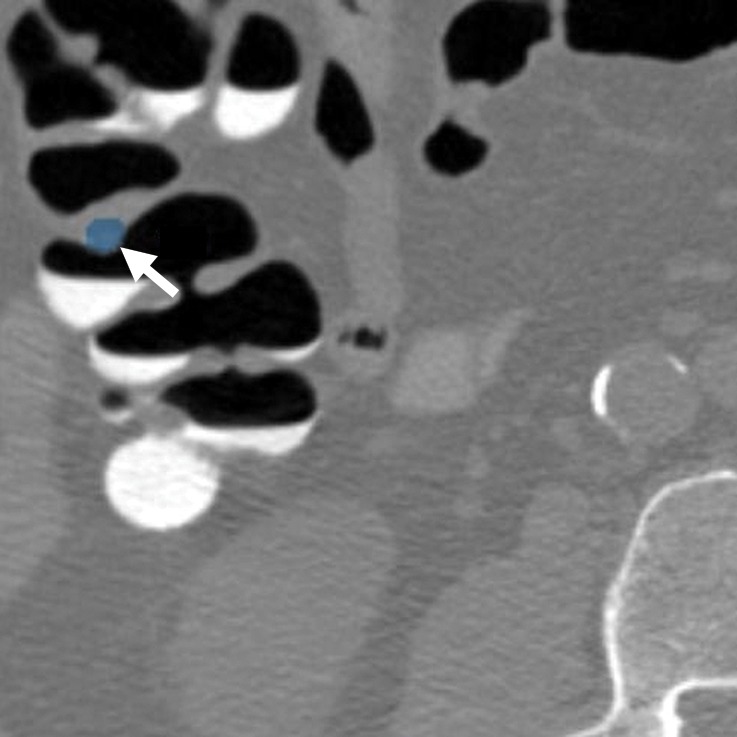
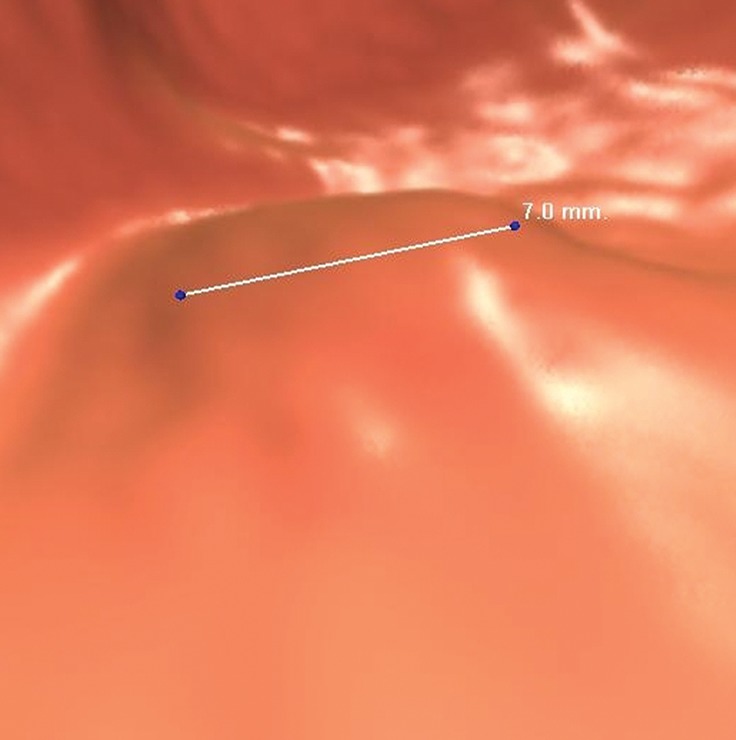
Normal anatomy. Axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) at the site of a protuberance at the junction of colonic folds and the taenia coli that measures approximately 7.2 mm in its maximal dimension. This is a difficult polyp candidate to disregard, with six readers reporting it as a polyp during the CAD-assisted session and two reporting it as a polyp during the CAD-unassisted session. The location of the polyp candidate at this junction, poor distention of the colon at this site, and the bulbous appearance of adjacent folds are most consistent with normal anatomy and a CAD false-positive result.
Figure 3b.
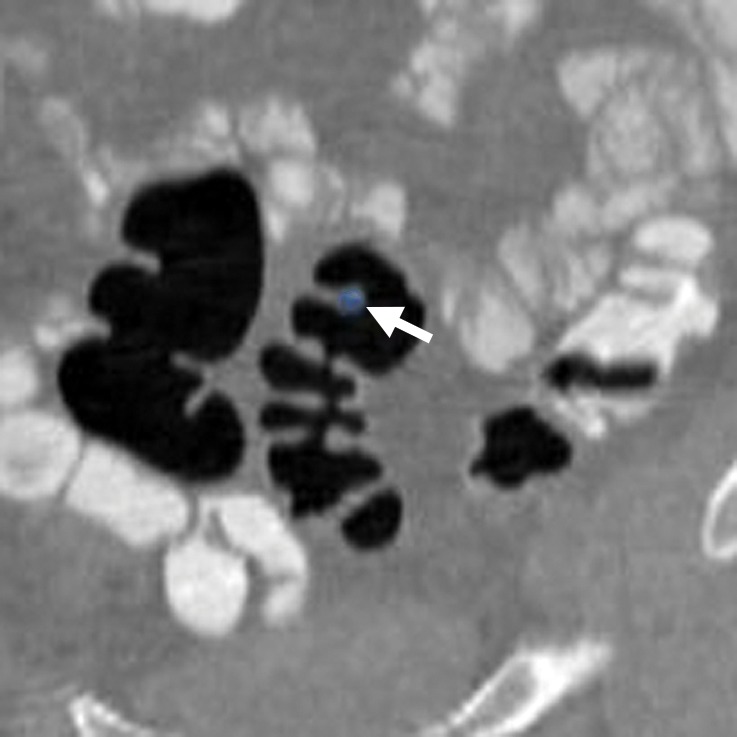
Haustral folds. (a) Endoluminal 3D CT colonography image shows a nodular thickened haustral fold that resulted in a CAD polyp candidate (blue area). This finding requires careful comparison with surrounding folds on 3D and 2D views and assessment for an overlying polyp or infiltrating carcinoma. The appearance of multiple convexities and similar adjacent folds suggests underdistention or normal anatomy of the sigmoid colon. (b) Coronal 2D CT colonography image shows the thickened haustral fold CAD polyp candidate (arrow). No distinct polypoid lesion is seen. Its location in the sigmoid colon and endoluminal appearance suggest underdistention or muscular hypertrophy, which is common in the sigmoid colon, particularly in association with diverticulosis.
Figure 4b.
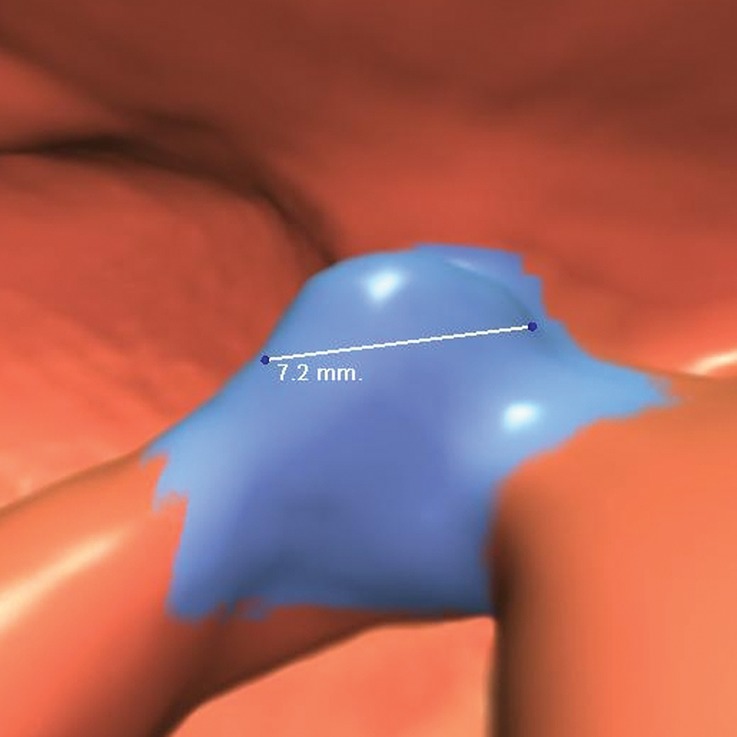
Normal anatomy. Axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) at the site of a protuberance at the junction of colonic folds and the taenia coli that measures approximately 7.2 mm in its maximal dimension. This is a difficult polyp candidate to disregard, with six readers reporting it as a polyp during the CAD-assisted session and two reporting it as a polyp during the CAD-unassisted session. The location of the polyp candidate at this junction, poor distention of the colon at this site, and the bulbous appearance of adjacent folds are most consistent with normal anatomy and a CAD false-positive result.
In our review, haustral folds were the most frequent source of CAD false-positive results (227 out of 806 [28.2%]). Nine of these were associated with 21 reader false-positive results. Seven of these nine were selected by only one or two readers, and two were selected by six and seven readers, respectively (Fig 4).
Ileocecal Valve
The normal curved and bulbous shape of the ileocecal valve (ICV) is a frequent cause of CAD polyp candidates because it can mimic the appearance of a polyp or mass (Fig 5). The 3D endoluminal view of the ICV correlates well with the endoscopically determined morphologic classification; namely, the ICV can have a papillary appearance, a rounded protrusion with the orifice at the apex, or a labial appearance. The orifice, which is occasionally open, is frequently seen at CT colonography.
Figure 5a.
Bulbous ICV in two patients. (a) Magnified coronal 2D CT colonography image obtained in the plane of the ICV, cecum, and ascending colon shows a CAD polyp candidate (arrow) that corresponds to the lips of the ICV, which, in this patient, is sufficiently bulbous to simulate a polyp candidate. No evidence of an overlying polyp or infiltrating mass is seen. The appearance of the ICV is within the normal range and should be easily dismissed by the reader. (b) Endoluminal 3D CT colonography image shows the CAD polyp candidate (blue area) in part a. Most, but not all, of the ICV surface is marked as a CAD polyp candidate. In a primary 3D read, the colon is first viewed on an endoluminal 3D image, and the 2D image is used for problem solving. Interactive 2D and 3D viewing is needed to confirm the expected location and appearance of the ICV. On 3D images, the bulbous lips of the ICV often have polypoid morphologic characteristics and require evaluation on 2D images. (c) Endoluminal 3D CT colonography image shows a bulbous ICV that generates two CAD polyp candidates (blue area), which can be reliably dismissed with 2D problem-solving images obtained to first verify that this is the location of the ICV and, second, to ensure that there is no evidence of an overlying soft-tissue polyp or infiltrating carcinoma. (d) Axial 2D magnified CT colonography image shows the CAD polyp candidate (arrow) from part c, the terminal ileum with tagged fluid, the ICV, the cecum, and the adjacent ascending colon. The lips of the ICV are bulbous and could be concerning for a polyp in any other colonic location. However, this appearance is within the normal range for the ICV.
Figure 5b.
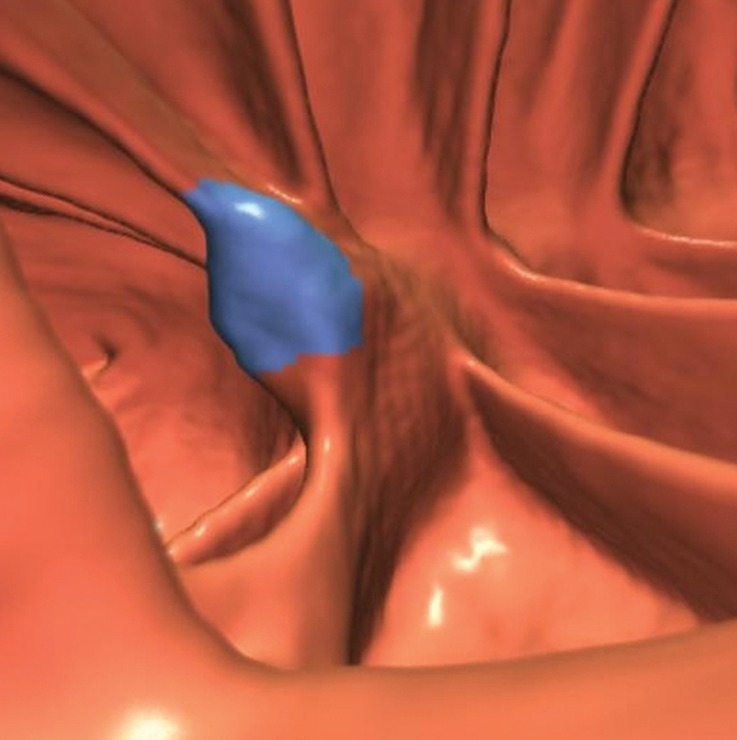
Bulbous ICV in two patients. (a) Magnified coronal 2D CT colonography image obtained in the plane of the ICV, cecum, and ascending colon shows a CAD polyp candidate (arrow) that corresponds to the lips of the ICV, which, in this patient, is sufficiently bulbous to simulate a polyp candidate. No evidence of an overlying polyp or infiltrating mass is seen. The appearance of the ICV is within the normal range and should be easily dismissed by the reader. (b) Endoluminal 3D CT colonography image shows the CAD polyp candidate (blue area) in part a. Most, but not all, of the ICV surface is marked as a CAD polyp candidate. In a primary 3D read, the colon is first viewed on an endoluminal 3D image, and the 2D image is used for problem solving. Interactive 2D and 3D viewing is needed to confirm the expected location and appearance of the ICV. On 3D images, the bulbous lips of the ICV often have polypoid morphologic characteristics and require evaluation on 2D images. (c) Endoluminal 3D CT colonography image shows a bulbous ICV that generates two CAD polyp candidates (blue area), which can be reliably dismissed with 2D problem-solving images obtained to first verify that this is the location of the ICV and, second, to ensure that there is no evidence of an overlying soft-tissue polyp or infiltrating carcinoma. (d) Axial 2D magnified CT colonography image shows the CAD polyp candidate (arrow) from part c, the terminal ileum with tagged fluid, the ICV, the cecum, and the adjacent ascending colon. The lips of the ICV are bulbous and could be concerning for a polyp in any other colonic location. However, this appearance is within the normal range for the ICV.
Flat masses can mimic a normal ICV; therefore, it is important to identify the normal ICV in every examination. Its location is usually easy to ascertain in the multiple views provided on a standard CT colonography workstation. Typically, it is located along the medial wall of the cecum, with a reported lateral and posterior wall position of 15% and 7%, respectively (36,37). However, confirmation that a CAD polyp candidate is related to the ICV does not preclude a pathologic condition on or adjacent to the ICV. Therefore, an overlying polyp or infiltrating carcinoma must be excluded. In most cases, excluding an overlying polyp or infiltrating carcinoma is straightforward, but in some cases, nodularity of the ICV resulting from variable fatty infiltration or lipoma can present a diagnostic challenge (Fig 5c). The attenuation of a normal ICV varies (eg, fatty, soft-tissue, and mixed) and cannot be used to distinguish a normal ICV from one that is pathologically involved (Fig 5d) (38). Furthermore, there is no consensus regarding the normal size of the valve; studies have reported a normal thickness for each ICV lip of as much as 1.5 cm and an overall maximal height of approximately 4 cm (37,39,40). On the basis of experience from air-contrast barium enemas, histologically normal valves may be large, asymmetric, or smoothly lobulated, making these characteristics generally nonspecific and unhelpful at CT colonography (37).
Figure 5c.
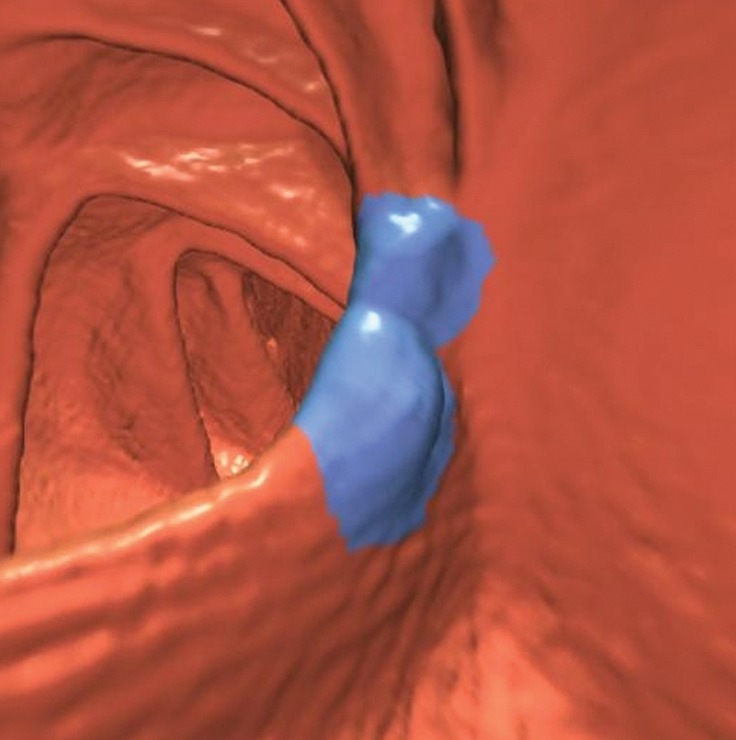
Bulbous ICV in two patients. (a) Magnified coronal 2D CT colonography image obtained in the plane of the ICV, cecum, and ascending colon shows a CAD polyp candidate (arrow) that corresponds to the lips of the ICV, which, in this patient, is sufficiently bulbous to simulate a polyp candidate. No evidence of an overlying polyp or infiltrating mass is seen. The appearance of the ICV is within the normal range and should be easily dismissed by the reader. (b) Endoluminal 3D CT colonography image shows the CAD polyp candidate (blue area) in part a. Most, but not all, of the ICV surface is marked as a CAD polyp candidate. In a primary 3D read, the colon is first viewed on an endoluminal 3D image, and the 2D image is used for problem solving. Interactive 2D and 3D viewing is needed to confirm the expected location and appearance of the ICV. On 3D images, the bulbous lips of the ICV often have polypoid morphologic characteristics and require evaluation on 2D images. (c) Endoluminal 3D CT colonography image shows a bulbous ICV that generates two CAD polyp candidates (blue area), which can be reliably dismissed with 2D problem-solving images obtained to first verify that this is the location of the ICV and, second, to ensure that there is no evidence of an overlying soft-tissue polyp or infiltrating carcinoma. (d) Axial 2D magnified CT colonography image shows the CAD polyp candidate (arrow) from part c, the terminal ileum with tagged fluid, the ICV, the cecum, and the adjacent ascending colon. The lips of the ICV are bulbous and could be concerning for a polyp in any other colonic location. However, this appearance is within the normal range for the ICV.
Figure 5d.
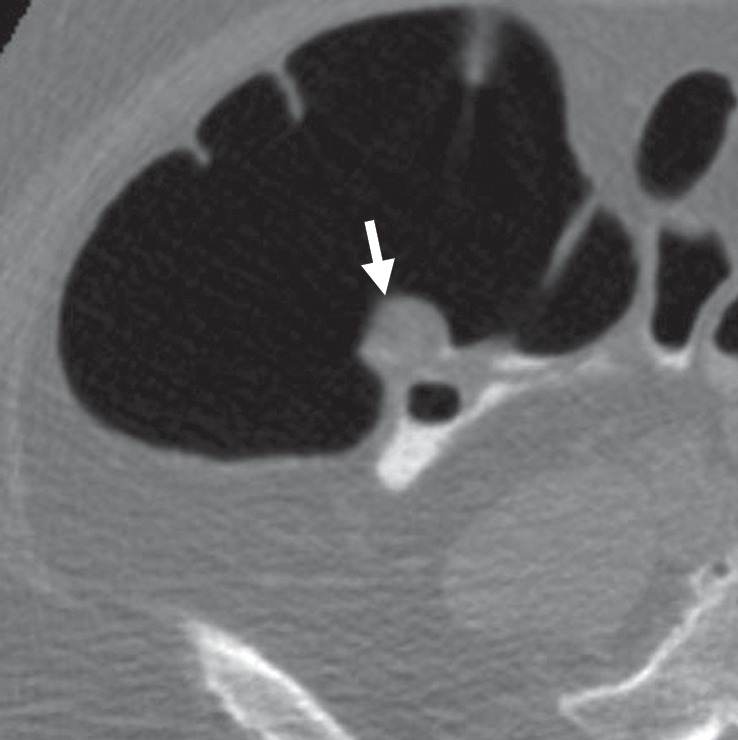
Bulbous ICV in two patients. (a) Magnified coronal 2D CT colonography image obtained in the plane of the ICV, cecum, and ascending colon shows a CAD polyp candidate (arrow) that corresponds to the lips of the ICV, which, in this patient, is sufficiently bulbous to simulate a polyp candidate. No evidence of an overlying polyp or infiltrating mass is seen. The appearance of the ICV is within the normal range and should be easily dismissed by the reader. (b) Endoluminal 3D CT colonography image shows the CAD polyp candidate (blue area) in part a. Most, but not all, of the ICV surface is marked as a CAD polyp candidate. In a primary 3D read, the colon is first viewed on an endoluminal 3D image, and the 2D image is used for problem solving. Interactive 2D and 3D viewing is needed to confirm the expected location and appearance of the ICV. On 3D images, the bulbous lips of the ICV often have polypoid morphologic characteristics and require evaluation on 2D images. (c) Endoluminal 3D CT colonography image shows a bulbous ICV that generates two CAD polyp candidates (blue area), which can be reliably dismissed with 2D problem-solving images obtained to first verify that this is the location of the ICV and, second, to ensure that there is no evidence of an overlying soft-tissue polyp or infiltrating carcinoma. (d) Axial 2D magnified CT colonography image shows the CAD polyp candidate (arrow) from part c, the terminal ileum with tagged fluid, the ICV, the cecum, and the adjacent ascending colon. The lips of the ICV are bulbous and could be concerning for a polyp in any other colonic location. However, this appearance is within the normal range for the ICV.
In our review, the ICV was the second most frequent source of CAD false-positive results (144 out of 806 [18%]). Twenty of these polyp candidates were related to 35 reader false-positive results, for an incidence of 1.3%, 18 of which were selected by only one or two readers and one of which was selected by three readers. A particularly large and bulbous ICV was selected by 10 readers during the CAD-assisted session and 12 readers during the CAD-unassisted session (Fig 6).
Figure 6.
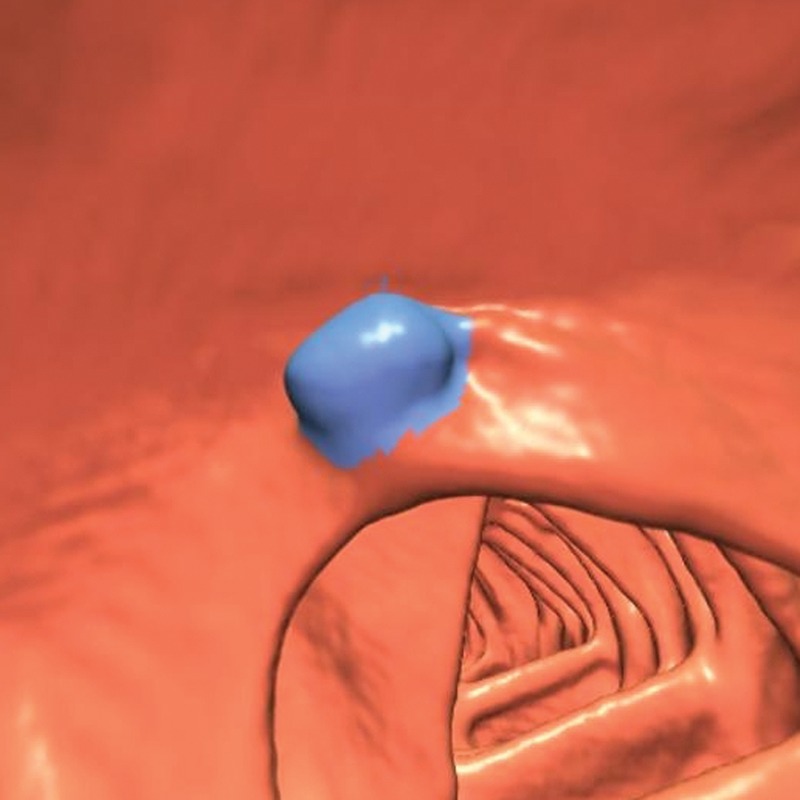
Endoluminal 3D CT colonography image shows a polyp candidate (blue area) that corresponds to a markedly polypoid ICV. This polyp candidate is difficult to disregard because polyps can occur on the ICV, the appearance of which can markedly vary. Ten readers reported this finding during the CAD-assisted session, and 12 readers reported it during the CAD-unassisted session.
Extracolonic CAD Polyp Candidates
CAD software is designed to evaluate intracolonic lesions; however, the segmentation of the colon may be imperfect, and extracolonic polyp candidates can be found in other gastrointestinal organs, such as an air-distended stomach or small bowel. Readers must be familiar with their workstation setup and know whether these entities are automatically excluded from display once the colon is automatically segmented by the CAD software or manually segmented by the reader. Often, even a properly segmented colon can include adjacent small intestinal lumina, a result of gaseous communication, which can lead to additional CAD false-positive results that should be easily dismissed during problem-solving with 2D views (Fig 7). However, in cases in which adjacent redundant colon or poor colonic distention may be present, care is required on the part of the reader to make sure these findings are truly extracolonic.
Figure 7a.
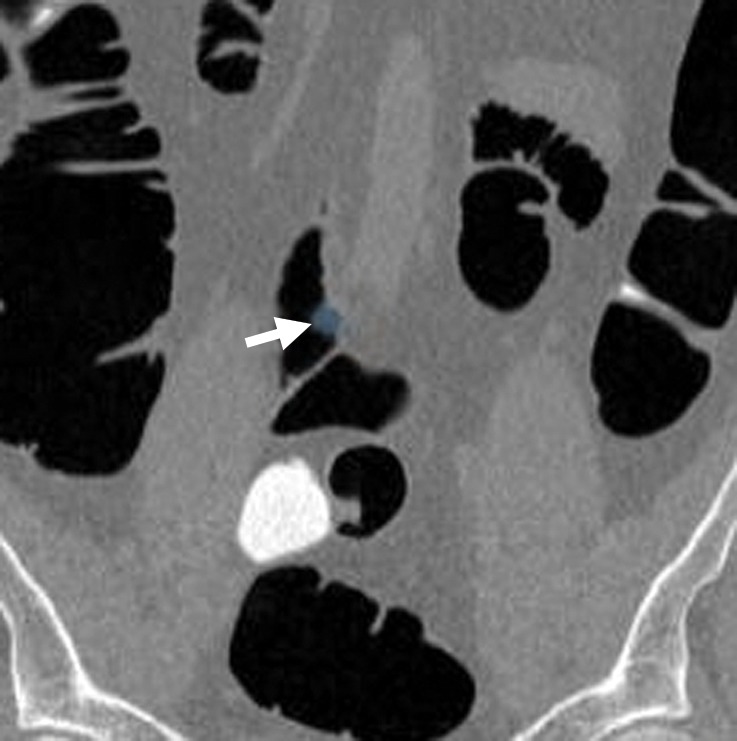
Extracolonic location. Coronal 2D (a) and corresponding magnified endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) in a segment of small bowel that could not be excluded during segmentation of the colon. Thus, the polyp candidate was displayed to the readers. Acquisition of multiplanar problem-solving images is necessary to confirm the extracolonic location. No readers reported this finding as a polyp.
Figure 7b.
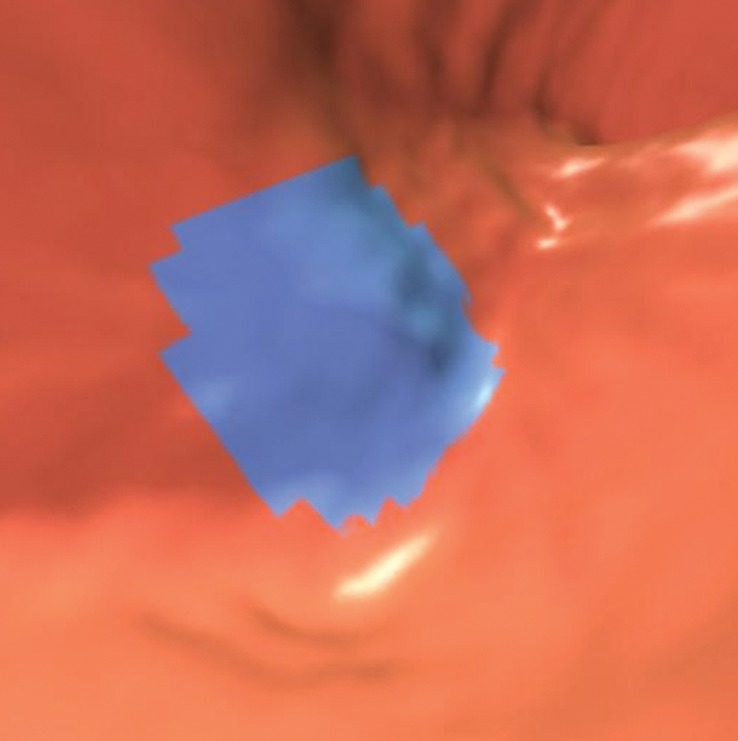
Extracolonic location. Coronal 2D (a) and corresponding magnified endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) in a segment of small bowel that could not be excluded during segmentation of the colon. Thus, the polyp candidate was displayed to the readers. Acquisition of multiplanar problem-solving images is necessary to confirm the extracolonic location. No readers reported this finding as a polyp.
Among the 806 CAD false-positive polyp candidates in our review, 134 (17%) were extracolonic, almost all of which were properly segmented either automatically by the CAD software or during manual review by the radiologist. Two extracolonic CAD false-positive results were associated with two reader false-positive results.
Tagged or Mobile Stool
Stool can appear polypoid and lack typical internal gas bubbles, resulting in a CAD polyp candidate, the most frequent cause of CAD false-positive results in poorly cleansed colons or intentionally limited or noncathartic CT colonography studies (Fig 8). It is less frequent in the setting of good bowel cleansing. Most experts recommend the routine use of fecal tagging with oral contrast material as part of the pre-imaging preparation to make many such candidates identifiable as stool. Tagging agent within the volume of the polyp candidate should be distinguished from tagging material, which may adhere to the surface of true polyps, a characteristic that is particularly common with polyps with villous histologic characteristics. This process is best accomplished with the use of interactive windowing of 2D images, although color maps may provide similar information (Fig 8b). In one study, 46% of polyps that were not touching a pool of contrast material had adherent contrast, a characteristic most frequently associated with polyps with villous histologic characteristics (41).
Figure 8a.
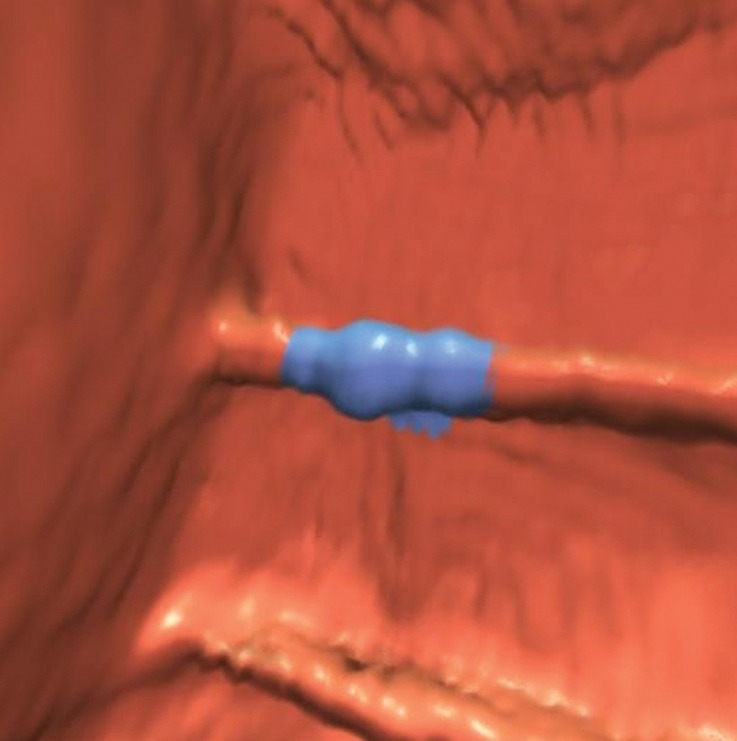
Tagged stool. (a) Endoluminal 3D CT colonography image shows a haustral fold that was marked as a CAD polyp candidate (blue area). The haustral fold has a nodular appearance due to adherent stool. Without further evaluation of the attenuation of this polyp candidate, it could be mistaken for a polyp. (b) Endoluminal 3D CT colonography image with an overlying color map shows high-attenuation (white areas) internal tagging, a finding consistent with tagged stool adhering to the normal haustral fold. (A soft-tissue polyp would be shaded red.) Care should be taken when evaluating attenuation on 2D images to confirm internal tagging rather than external surface tagging, which is sometimes seen with true polyps. (c) Magnified axial 2D CT colonography image shows the CAD polyp candidate (arrow). Interactive window width and level adjustment may be necessary to evaluate and confirm that the hyperattenuating material is internal tagging rather than the external surface tagging that is sometimes seen in true polyps.
Figure 8b.
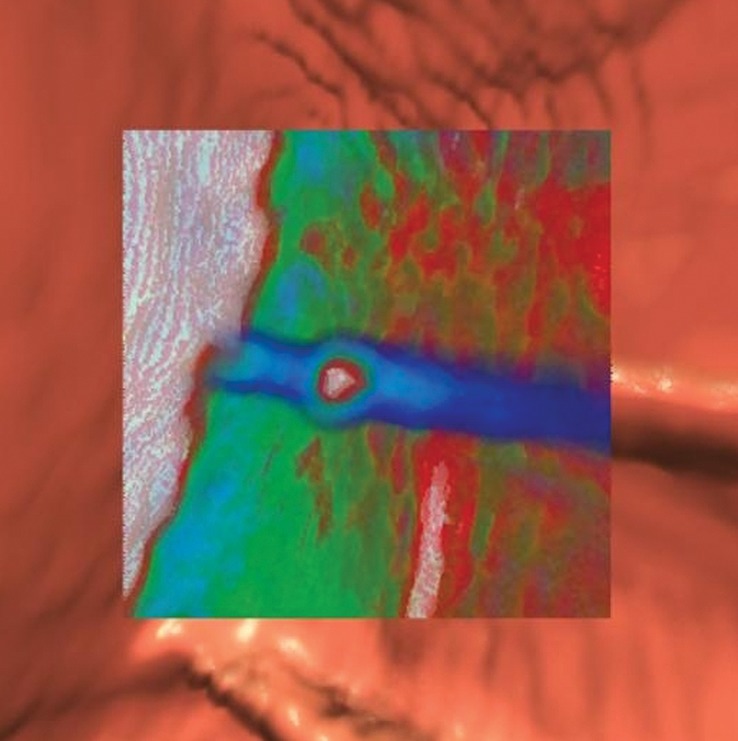
Tagged stool. (a) Endoluminal 3D CT colonography image shows a haustral fold that was marked as a CAD polyp candidate (blue area). The haustral fold has a nodular appearance due to adherent stool. Without further evaluation of the attenuation of this polyp candidate, it could be mistaken for a polyp. (b) Endoluminal 3D CT colonography image with an overlying color map shows high-attenuation (white areas) internal tagging, a finding consistent with tagged stool adhering to the normal haustral fold. (A soft-tissue polyp would be shaded red.) Care should be taken when evaluating attenuation on 2D images to confirm internal tagging rather than external surface tagging, which is sometimes seen with true polyps. (c) Magnified axial 2D CT colonography image shows the CAD polyp candidate (arrow). Interactive window width and level adjustment may be necessary to evaluate and confirm that the hyperattenuating material is internal tagging rather than the external surface tagging that is sometimes seen in true polyps.
Figure 8c.
Tagged stool. (a) Endoluminal 3D CT colonography image shows a haustral fold that was marked as a CAD polyp candidate (blue area). The haustral fold has a nodular appearance due to adherent stool. Without further evaluation of the attenuation of this polyp candidate, it could be mistaken for a polyp. (b) Endoluminal 3D CT colonography image with an overlying color map shows high-attenuation (white areas) internal tagging, a finding consistent with tagged stool adhering to the normal haustral fold. (A soft-tissue polyp would be shaded red.) Care should be taken when evaluating attenuation on 2D images to confirm internal tagging rather than external surface tagging, which is sometimes seen with true polyps. (c) Magnified axial 2D CT colonography image shows the CAD polyp candidate (arrow). Interactive window width and level adjustment may be necessary to evaluate and confirm that the hyperattenuating material is internal tagging rather than the external surface tagging that is sometimes seen in true polyps.
Often, nontagged stool can be identified by the presence of internal gas, low attenuation, or a mottled internal texture. Additional characteristics of stool include angulated margins. Finally, movement to the dependent surfaces between supine and prone series or distally through the colon is characteristic of mobile stool, although a mobile head of a pedunculated polyp or a mobile or peristaltic colon should be excluded (Fig 9).
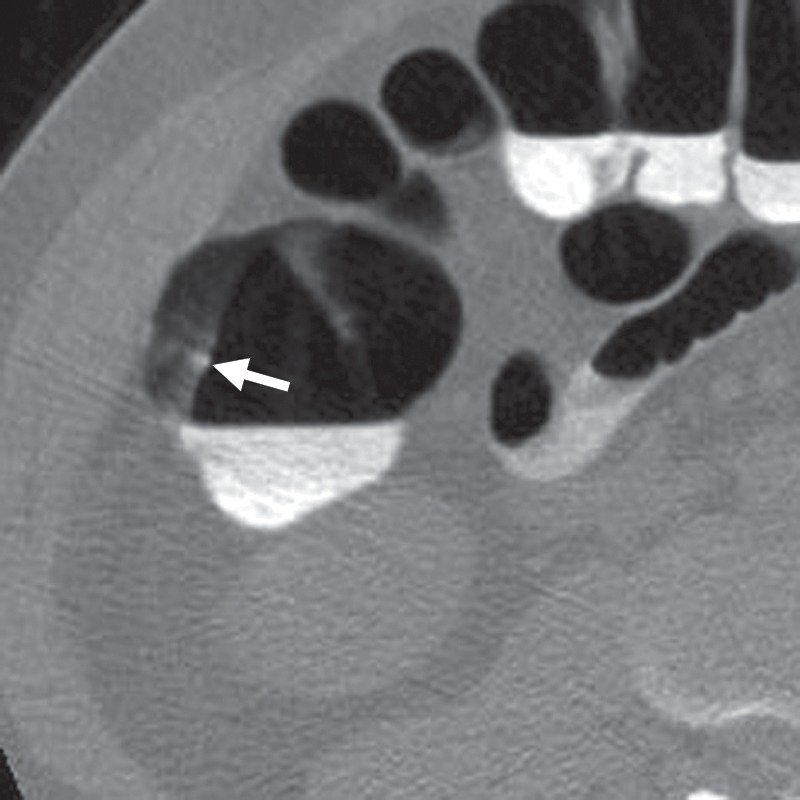
Figure 9a.
Mobile stool. (a) Magnified axial “flipped” 2D CT colonography image obtained in a prone position shows a submerged CAD polyp candidate (arrow) that is impossible to disregard without comparing it to the findings on supine images. Although it appears detached from the colon wall, all adjacent sections must be viewed to ascertain the absence of a stalk. (b) Magnified axial 2D CT colonography image obtained in the supine position shows the CAD polyp candidate (arrow), which is mobile to the dependent dorsal surface of the rectum. After correlating with the supine images, it is clear that this structure is mobile because it settles to the dependent region of the rectum in both the supine and prone series, a finding consistent with mobile stool. A mobile head of a pedunculated polyp must be excluded. In other potentially mobile colon segments, the possibility of bowel rotation should be excluded.
Figure 9b.
Mobile stool. (a) Magnified axial “flipped” 2D CT colonography image obtained in a prone position shows a submerged CAD polyp candidate (arrow) that is impossible to disregard without comparing it to the findings on supine images. Although it appears detached from the colon wall, all adjacent sections must be viewed to ascertain the absence of a stalk. (b) Magnified axial 2D CT colonography image obtained in the supine position shows the CAD polyp candidate (arrow), which is mobile to the dependent dorsal surface of the rectum. After correlating with the supine images, it is clear that this structure is mobile because it settles to the dependent region of the rectum in both the supine and prone series, a finding consistent with mobile stool. A mobile head of a pedunculated polyp must be excluded. In other potentially mobile colon segments, the possibility of bowel rotation should be excluded.
In our review, tagged or mobile stool accounted for 6.8% (55 out of 806) of the CAD false-positive results. Thirteen of these polyp candidates were related to 33 reader false-positive results, for an incidence of 3.2%. One tagged stool CAD false-positive result was selected by seven readers during the CAD-assisted session, and eight were related to reader false-positive results during the CAD-unassisted session, albeit at a lower frequency (Fig 10). Among the six moving stool CAD false-positive results, three were related to six reader false-positive results during the CAD-assisted session.
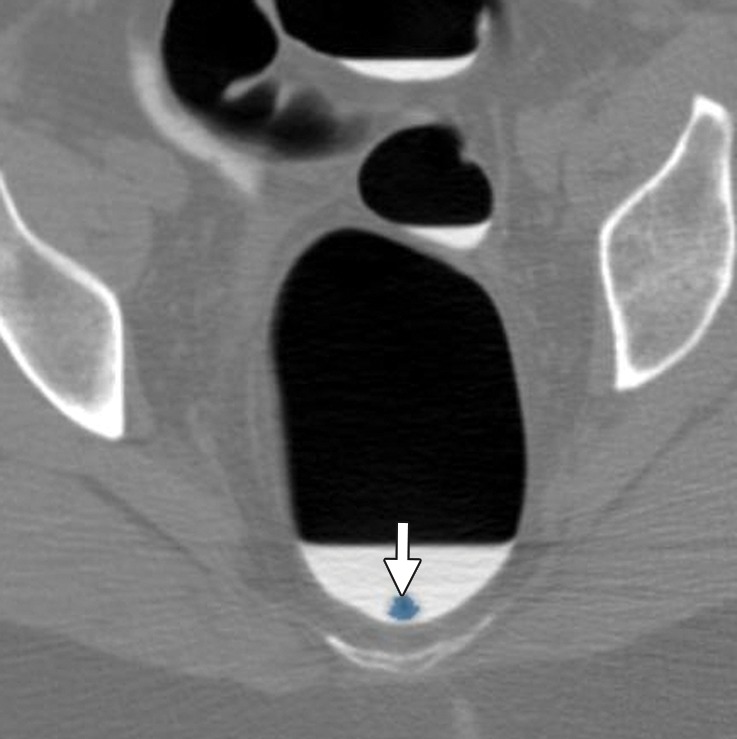
Figure 10a.
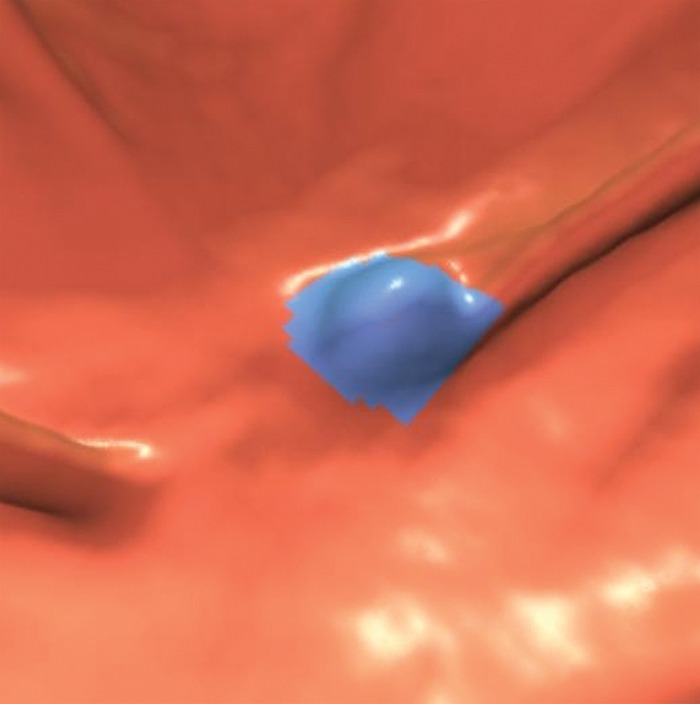
Stool. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b). (c) Endoluminal 3D CT colonography image with an overlying attenuation-dependent color map generated with a translucency rendering tool shows the internal architecture of the polyp candidate. The color map confirms internal contrast tagging of the polyp candidate, a finding characteristic of stool. Five and seven readers reported this finding as a polyp during the CAD-unassisted and CAD-assisted sessions, respectively.
Figure 10b.
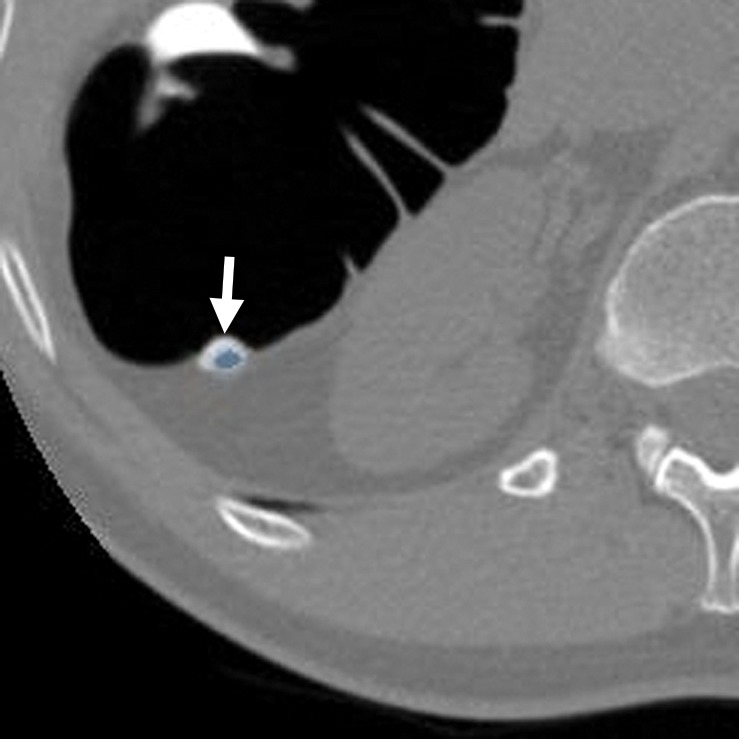
Stool. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b). (c) Endoluminal 3D CT colonography image with an overlying attenuation-dependent color map generated with a translucency rendering tool shows the internal architecture of the polyp candidate. The color map confirms internal contrast tagging of the polyp candidate, a finding characteristic of stool. Five and seven readers reported this finding as a polyp during the CAD-unassisted and CAD-assisted sessions, respectively.
Figure 10c.
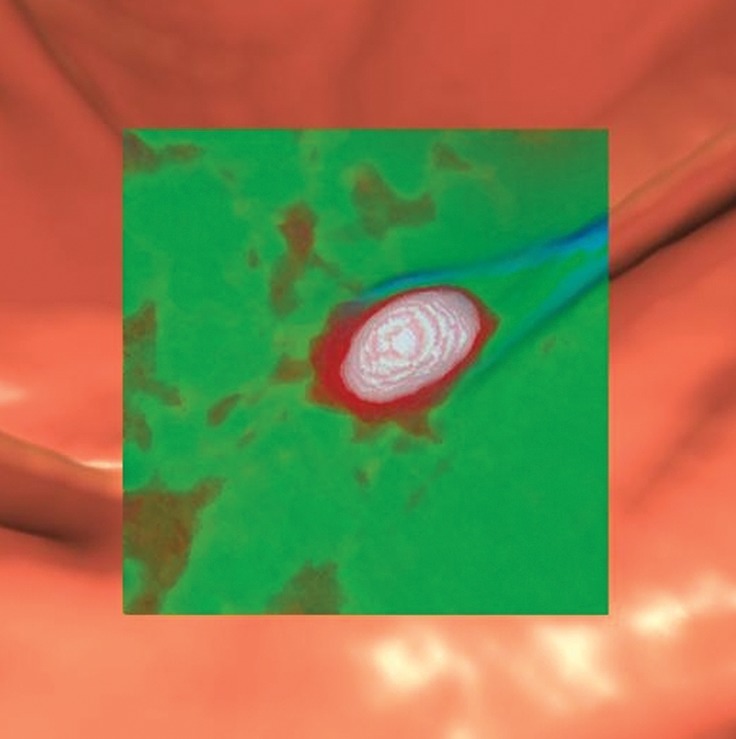
Stool. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b). (c) Endoluminal 3D CT colonography image with an overlying attenuation-dependent color map generated with a translucency rendering tool shows the internal architecture of the polyp candidate. The color map confirms internal contrast tagging of the polyp candidate, a finding characteristic of stool. Five and seven readers reported this finding as a polyp during the CAD-unassisted and CAD-assisted sessions, respectively.
Diminutive and Nondismissible Polyp Candidates
The target lesion for screening is an advanced adenoma, which is pathologically defined as a lesion that is 10 mm or larger, has villous features, or has high-grade cellular dysplasia. Polyp candidates that are smaller than 6 mm are often stool or hyperplastic with no malignant potential. Adenomatous polyps that are 5 mm or smaller have a slow growth rate and a low incidence of dysplasia, and CT colonography and conventional colonoscopy have low sensitivity for depicting them; thus, the CT colonography reporting and data system (C-RADS) recommends that these lesions not be reported. The risk for malignancy in adenomatous polyps that are 5 mm or smaller is estimated to be much less than 1% (42,43).
C-RADS recommends that short-term surveillance be considered in patients with one or two 6–9-mm polyps and no additional risk factors, although there is continued debate and research relating to the appropriate polyp size and number to determine whether optical colonoscopy or a surveillance strategy should be recommended (42–50). In this size range, the likelihood that a lesion contains invasive carcinoma is less than 1% (42,43).
The size of a lesion is determined by its maximal dimension and excludes the stalk in pedunculated lesions. The best view for accurately measuring lesions should be carefully determined; a combined 2D and 3D approach has been shown to be optimal (51). Appropriate window settings (eg, window width, 2000 HU; window level, 0 HU) should be used to discriminate colon wall from mesenteric fat. Readers must be familiar with the measurement tools of the software being used to correctly and reproducibly measure lesions. In general, irregularly shaped lesions may be underestimated on multiplanar views; a potential pitfall includes the calipers “falling off” the edge of a polyp (ie, depositing the cursor on a distant colon wall) on the 3D endoluminal view. Although CAD software attempts to measure the size of the polyp candidates it identifies, these measurements should not be reported because they often are incorrect. Instead, readers should carefully measure lesions manually. In our review, diminutive polyp candidates (those with a diameter <6 mm) accounted for 5% of the CAD false-positive results (42 out of 806), six of which resulted in six reader false-positive results during the CAD-assisted portion of the trial, for an incidence of 0.8% (Fig 11).
Figure 11a.
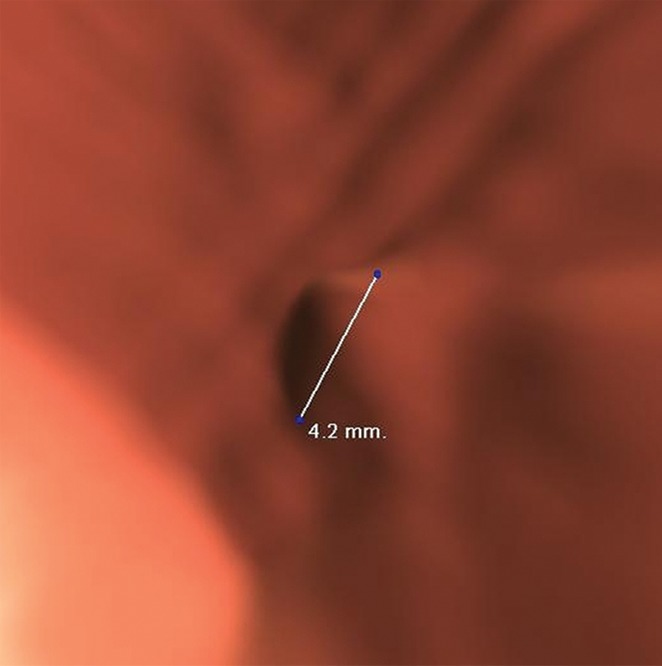
Diminutive lesion. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (arrow) that measures approximately 4.2 mm in its maximal dimension, which is below the 6-mm size criterion. During the CAD-assisted session, this polyp candidate was reported by one reader, who over-measured it as 7 mm.
Figure 11b.
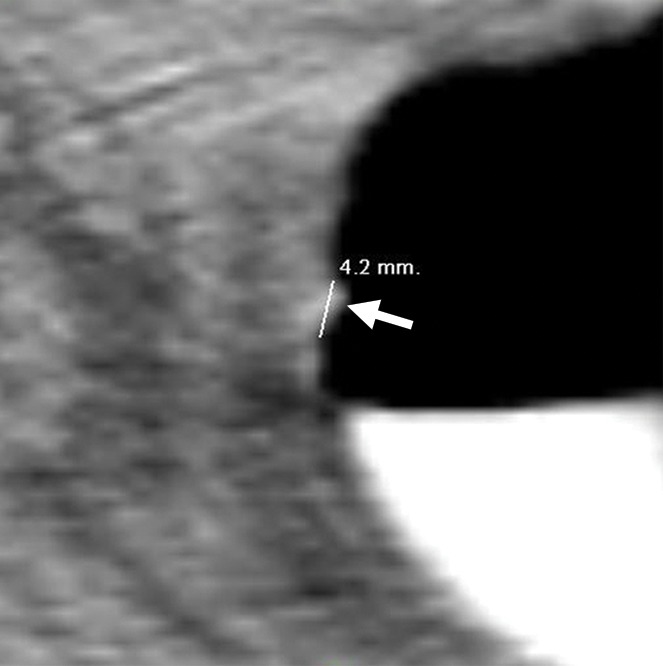
Diminutive lesion. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (arrow) that measures approximately 4.2 mm in its maximal dimension, which is below the 6-mm size criterion. During the CAD-assisted session, this polyp candidate was reported by one reader, who over-measured it as 7 mm.
CAD soft-tissue polyp candidates that did not strictly fit into the other causes of CAD false-positive results and were 6 mm or larger were classified as nondismissible polyp candidates, a category that accounted for 7.7% (62 out of 806) of the CAD false-positive results. Thirty-eight such nondismissable polyp candidates were selected by at least one reader, for a total of 170 reader false-positive results and an incidence of 14.4%. Of these, 33 were 6–9 mm, and five were larger than 9 mm. Eighteen were also selected during the CAD-unassisted session, albeit less frequently (usually). Four polyp candidates were selected by more than 10 readers during the CAD-assisted session (Fig 12).
Figure 12a.
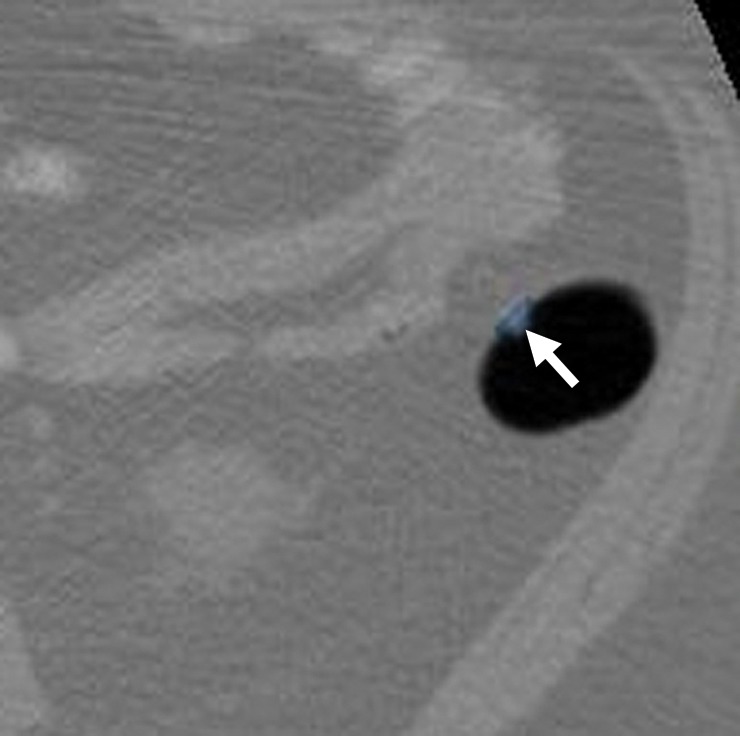
Suspected polyp. (a, b) Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b). The soft-tissue polypoid structure is not secondary to a normal anatomic structure or artifact and has no stool characteristics. Its maximal diameter is 6.1 mm, meeting the target lesion size threshold. This lesion may be reported as a suspected polyp and referred for optical colonoscopy for confirmation and biopsy. Alternatively, it may be conservatively managed with short-term surveillance according to the C-RADS recommendations for a patient with fewer than three 6–9-mm lesions. This lesion was reported by 11 and eight readers in the CAD-unassisted and CAD-assisted sessions, respectively.
Figure 12b.
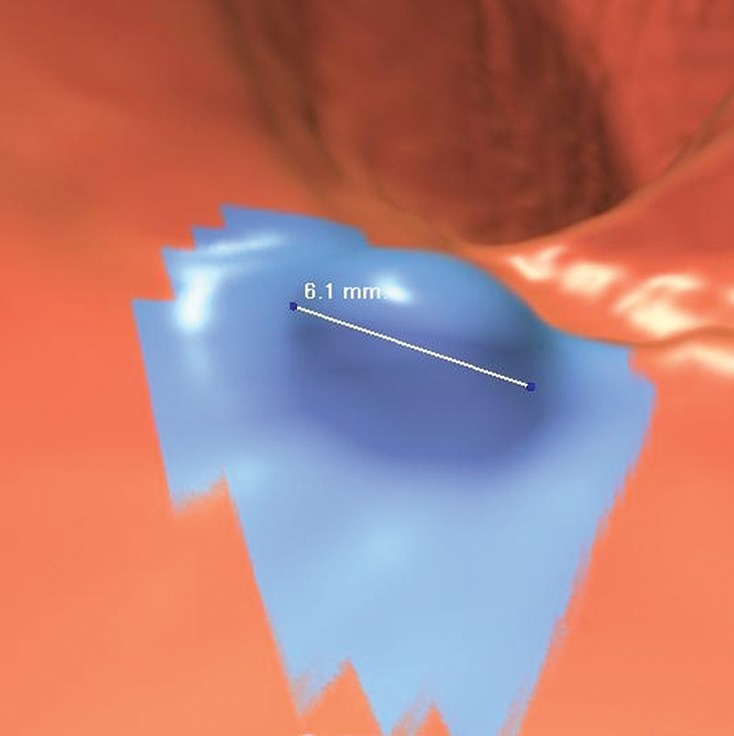
Suspected polyp. (a, b) Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b). The soft-tissue polypoid structure is not secondary to a normal anatomic structure or artifact and has no stool characteristics. Its maximal diameter is 6.1 mm, meeting the target lesion size threshold. This lesion may be reported as a suspected polyp and referred for optical colonoscopy for confirmation and biopsy. Alternatively, it may be conservatively managed with short-term surveillance according to the C-RADS recommendations for a patient with fewer than three 6–9-mm lesions. This lesion was reported by 11 and eight readers in the CAD-unassisted and CAD-assisted sessions, respectively.
Coarse Mucosa
Coarse mucosa that results from reconstruction artifacts, volume rendering, poor colonic distention, extracolonic artifacts, or amorphous particulate stool is rarely a diagnostic dilemma and easily dismissed in most cases (Fig 13). Coarse mucosa is more frequently seen in low-dose examinations and in thick or bony areas, such as the rectum and pelvis. The size of a nodular-appearing coarse mucosa may be overmeasured by the CAD software and presented as a polyp candidate. In our review, 4% (33 out of 806) of CAD false-positive results were attributed to coarse mucosa. None were related to reader false-positive results.
Figure 13a.
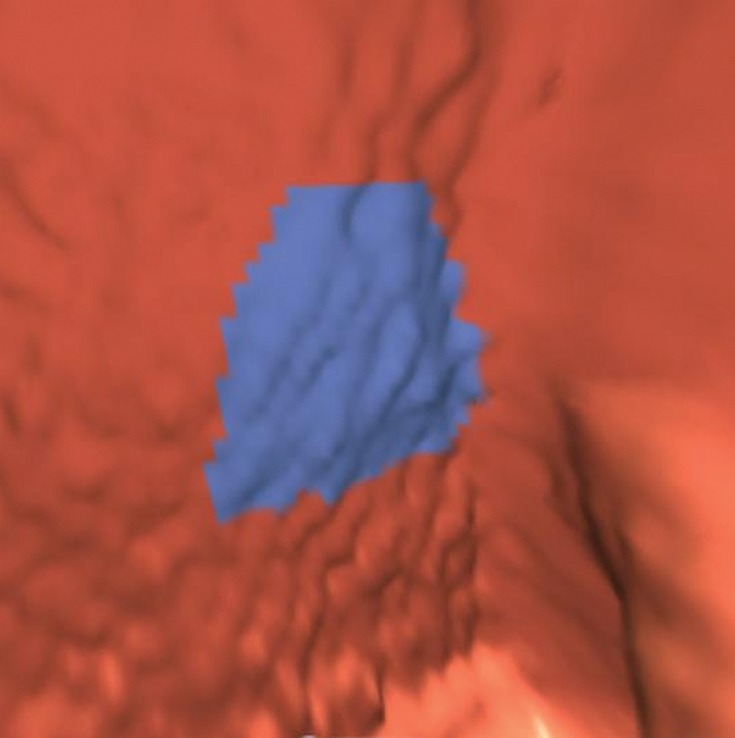
Coarse rectal mucosa.(a, b) Endoluminal 3D CT colonography image obtained along the posterior rectal wall (a) and corresponding magnified sagittal 2D CT colonography image (b) show a polyp candidate with nodular-appearing coarse rectal mucosa (blue area in a and arrow in b), a finding that may result from reconstruction artifacts, volume rendering, or the presence of fuzzy particulate stool. This is a common appearance, particularly in low-dose examinations and in the portions of colon in the pelvis where the x-ray beam is attenuated by the pelvic bones, such as the rectum.
Figure 13b.
Coarse rectal mucosa.(a, b) Endoluminal 3D CT colonography image obtained along the posterior rectal wall (a) and corresponding magnified sagittal 2D CT colonography image (b) show a polyp candidate with nodular-appearing coarse rectal mucosa (blue area in a and arrow in b), a finding that may result from reconstruction artifacts, volume rendering, or the presence of fuzzy particulate stool. This is a common appearance, particularly in low-dose examinations and in the portions of colon in the pelvis where the x-ray beam is attenuated by the pelvic bones, such as the rectum.
Rectal Lesions
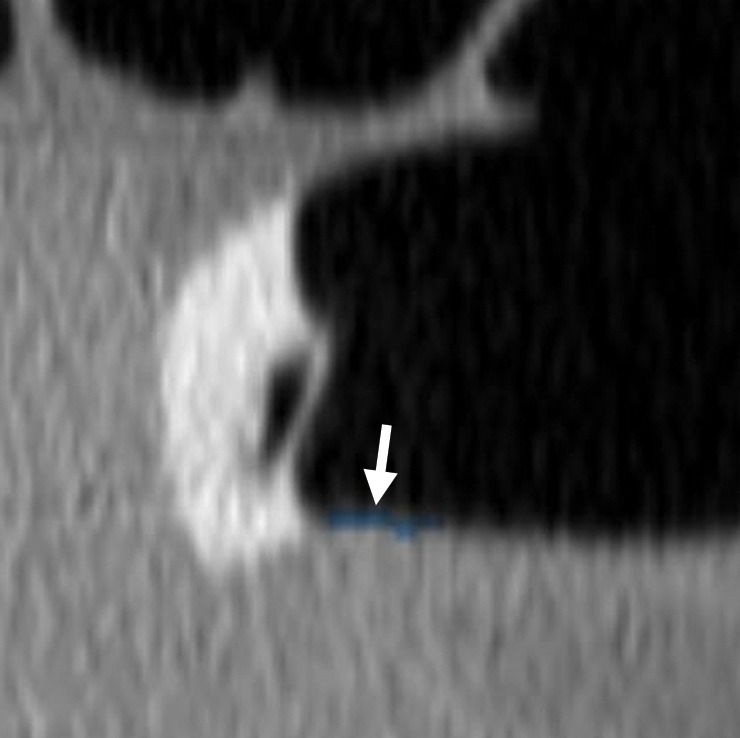
The rectum ends at the anal margin, a region that may be difficult to evaluate and is a source of CAD false-positive results due to technical and anatomic factors. The rectum is not distended, and the rectal tube or balloon distorts the mucosa. Anatomically, it is the site of anal papillae; anterior rectal bars; and internal hemorrhoids and varices, structures that can mimic polyps.
Anal papillae are typically located at the anal verge and have a pyramidal shape in a spoke-wheel–like arrangement (Fig 14a). (Hemorrhoids may have a similar configuration.) Anal papillae frequently resemble a polypoid lesion in terms of shape and attenuation. Hypertrophied anal papillae occur at the anorectal junction, are usually multiple (but can be solitary), and may be impossible to differentiate from polyps at CT (Fig 14b). Typically, internal hemorrhoids are broad-based, grape-like defects that surround the catheter and may change shape on supine and prone views. Classically, rectal varices cause a serpiginous impression on the rectal mucosa and flatten when in the prone position, a result of better rectal distention. On 2D views, the presence of perirectal vascular structures confirms the cause.
Figure 14a.
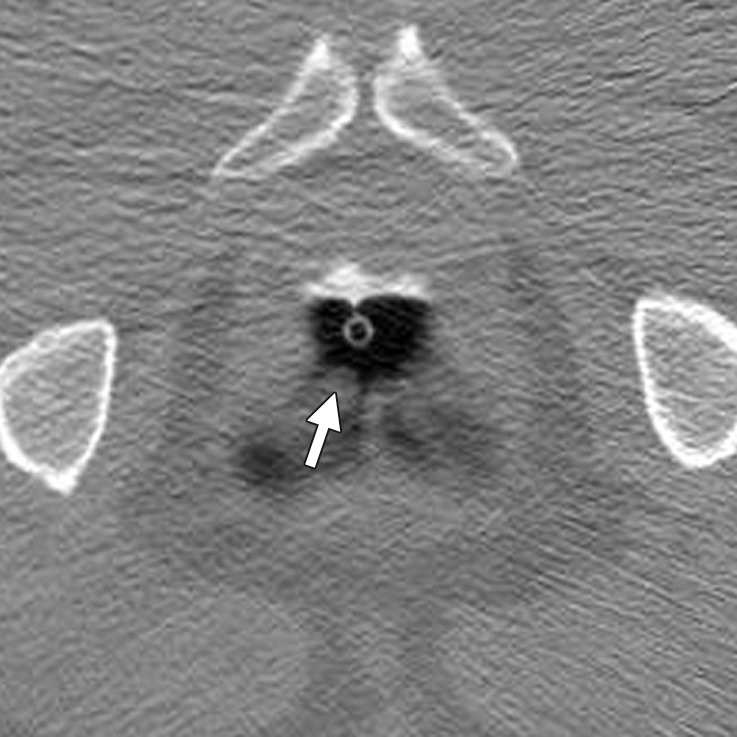
Anal papilla. (a) Magnified axial 2D CT colonography image shows a polyp candidate (arrow) at the anal verge that has a pyramidal shape in a spoke-wheel arrangement, a finding consistent with multiple anal papillae. (b) Magnified sagittal 2D CT colonography image shows the polyp candidate (arrow) adjacent to the rectal tube. On the basis of its characteristic location and morphologic findings, the differential diagnosis includes redundant mucosa; a solitary hypertrophied anal papilla, the most likely diagnosis; and a hemorrhoid.
Figure 14b.
Anal papilla. (a) Magnified axial 2D CT colonography image shows a polyp candidate (arrow) at the anal verge that has a pyramidal shape in a spoke-wheel arrangement, a finding consistent with multiple anal papillae. (b) Magnified sagittal 2D CT colonography image shows the polyp candidate (arrow) adjacent to the rectal tube. On the basis of its characteristic location and morphologic findings, the differential diagnosis includes redundant mucosa; a solitary hypertrophied anal papilla, the most likely diagnosis; and a hemorrhoid.
If the anal region has a concerning appearance, limited anoscopy or a digital rectal examination may be used to further evaluate the region (Fig 14b). CAD polyp candidates that are located near, but not at, the anal verge should be evaluated without taking into account their proximity to a region with frequent normal anatomic structures (35).
In our review, 4% (33 out of 806) of CAD false-positive results were attributed to one of these rectal lesions (ie, anal papillae, anterior rectal bars, internal hemorrhoids, and varices). Five were related to five reader false-positive results during the CAD-assisted portion of the trial, with an incidence of 0.8%; three of the reader false-positive results were from the same reader.
Flexural Pseudotumor
A flexural pseudotumor is a phenomenon that can occur inside a sharp turn of the colon, where the folds of the inner aspect of the curve form a pseudo-thickening or a polypoid structure (Fig 15). Flexural pseudotumor can be easily identified on axial, sagittal, and coronal reconstructions. Often, both directions of the colon can be simultaneously seen on 3D endoluminal views, a finding that indicates a sharp turn. Care should be taken to check for true polyps at or near the site of a flexural pseudotumor. In our review, 2% (18 out of 806) of CAD false-positive results were attributed to flexural pseudotumor. One was selected by a single reader during the CAD-assisted portion of the trial, for an incidence of 0.3% (Fig 16).
Figure 15a.
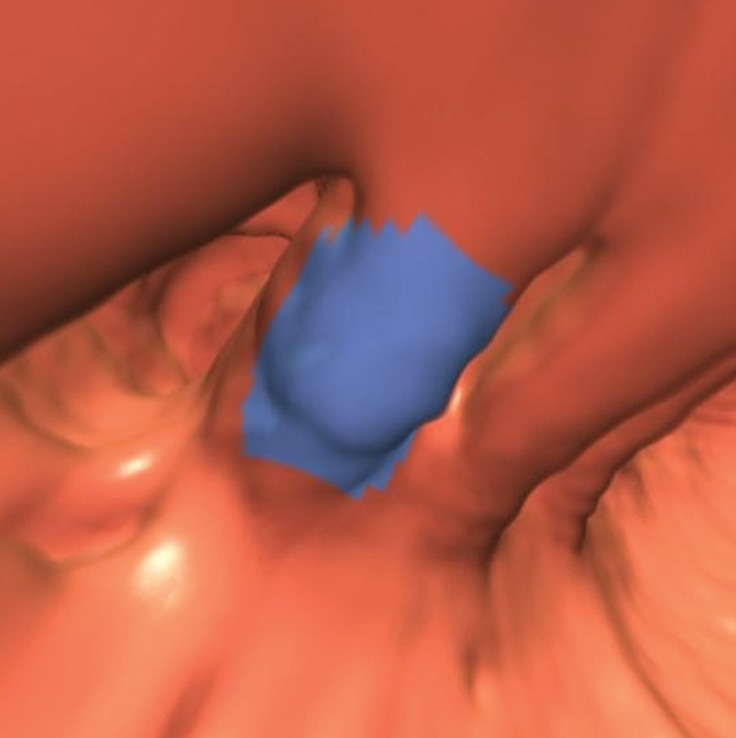
Flexural pseudotumor. Endoluminal 3D (a), magnified coronal (b), axial (c), and sagittal 2D (d) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b–d) at the location of a sharp turn in the sigmoid colon. The haustral folds along the inner bend appear thickened and bulbous, a finding often referred to as a flexural pseudotumor. This example illustrates the utility of multiple projections to clearly delineate the suspicious structure. The endoluminal and axial views demonstrate that the candidate is along a sharp turn of the colon, a finding that is more difficult to appreciate on the coronal and sagittal views.
Figure 16a.
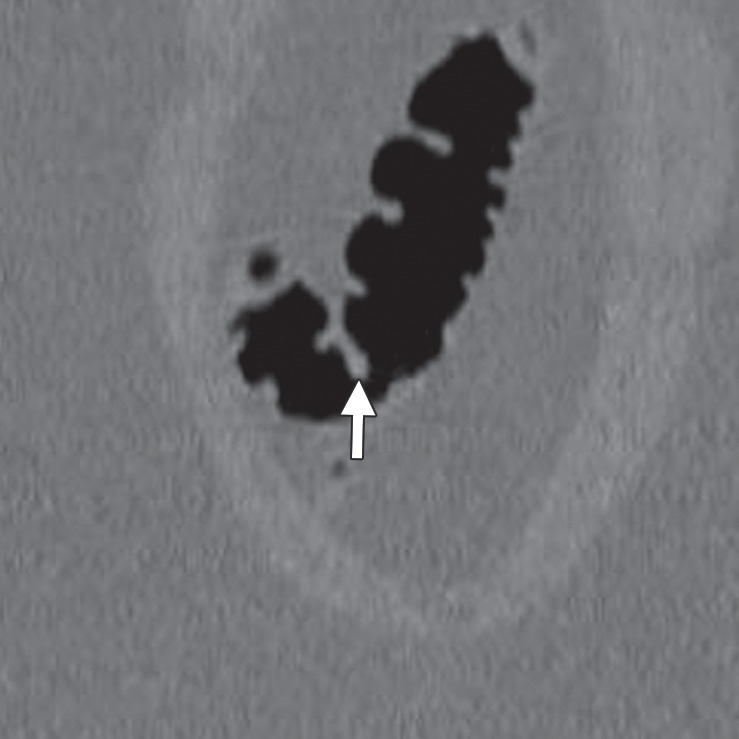
Flexural pseudotumor. (a, b) Magnified coronal 2D (a) and corresponding endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) located along the inner aspect of a sharp turn of the colon. On the 3D view, both directions of the colon are seen, a finding that confirms a flexural pseudotumor. The bulbous appearance of this turn should not be mistaken for a polyp, as one reader did during the CAD-assisted session.
Figure 15b.
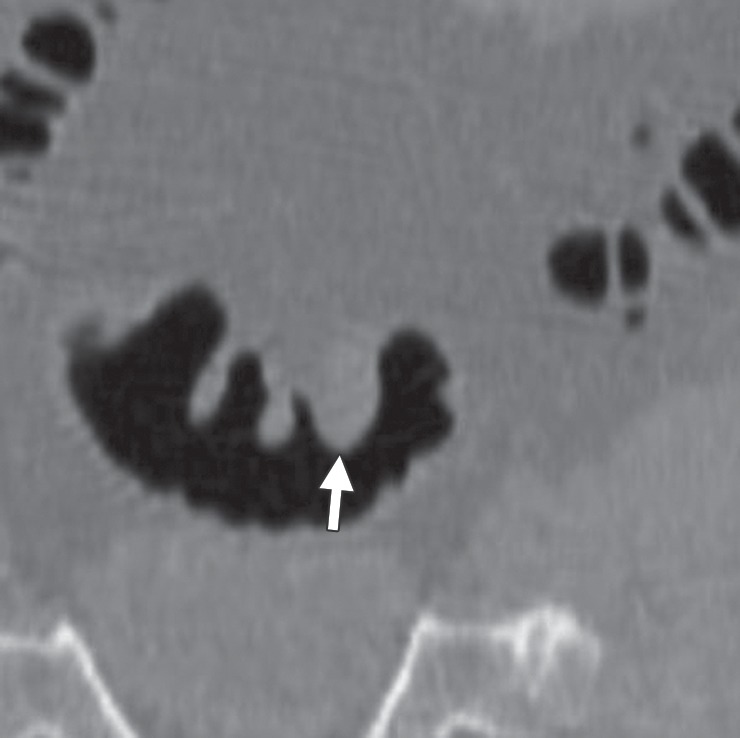
Flexural pseudotumor. Endoluminal 3D (a), magnified coronal (b), axial (c), and sagittal 2D (d) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b–d) at the location of a sharp turn in the sigmoid colon. The haustral folds along the inner bend appear thickened and bulbous, a finding often referred to as a flexural pseudotumor. This example illustrates the utility of multiple projections to clearly delineate the suspicious structure. The endoluminal and axial views demonstrate that the candidate is along a sharp turn of the colon, a finding that is more difficult to appreciate on the coronal and sagittal views.
Figure 15c.
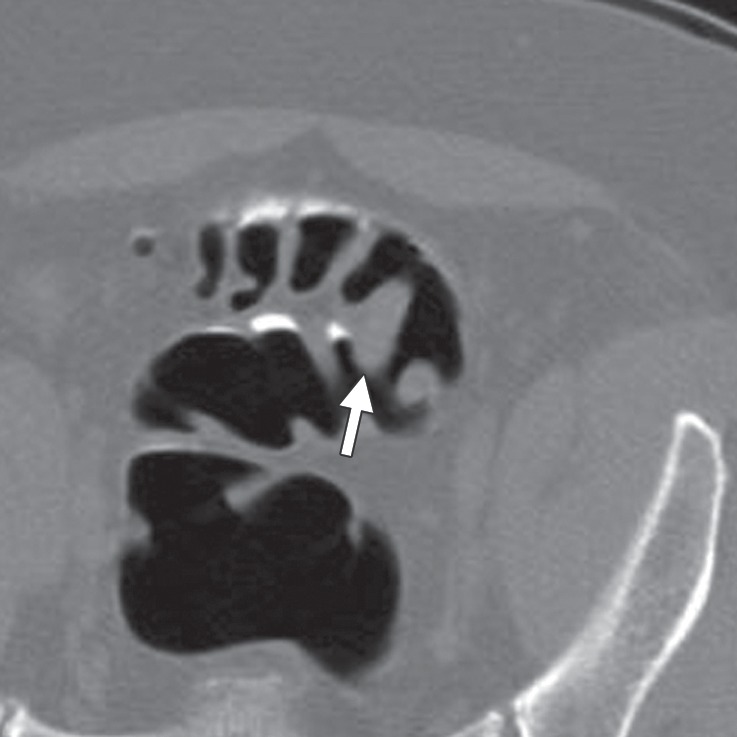
Flexural pseudotumor. Endoluminal 3D (a), magnified coronal (b), axial (c), and sagittal 2D (d) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b–d) at the location of a sharp turn in the sigmoid colon. The haustral folds along the inner bend appear thickened and bulbous, a finding often referred to as a flexural pseudotumor. This example illustrates the utility of multiple projections to clearly delineate the suspicious structure. The endoluminal and axial views demonstrate that the candidate is along a sharp turn of the colon, a finding that is more difficult to appreciate on the coronal and sagittal views.
Figure 15d.
Flexural pseudotumor. Endoluminal 3D (a), magnified coronal (b), axial (c), and sagittal 2D (d) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b–d) at the location of a sharp turn in the sigmoid colon. The haustral folds along the inner bend appear thickened and bulbous, a finding often referred to as a flexural pseudotumor. This example illustrates the utility of multiple projections to clearly delineate the suspicious structure. The endoluminal and axial views demonstrate that the candidate is along a sharp turn of the colon, a finding that is more difficult to appreciate on the coronal and sagittal views.
Figure 16b.
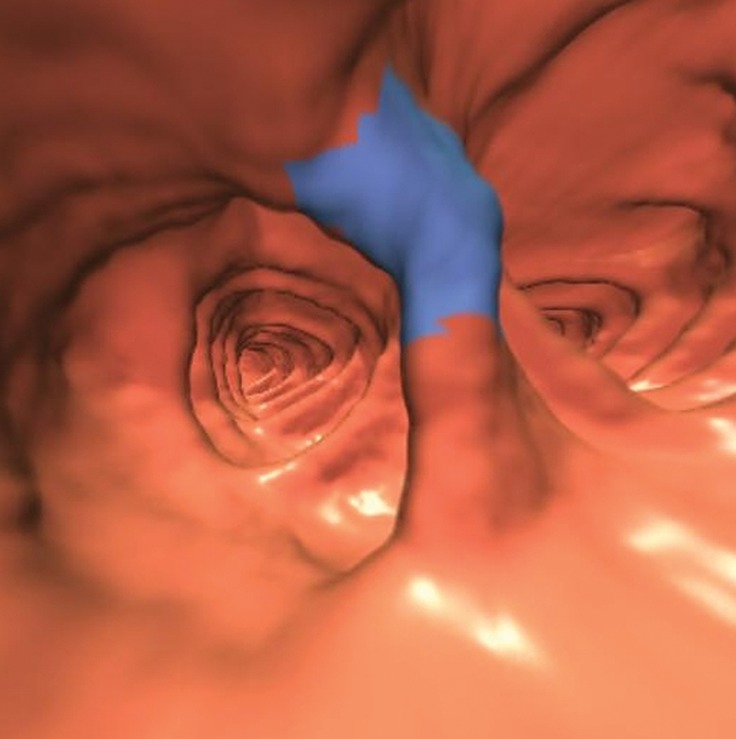
Flexural pseudotumor. (a, b) Magnified coronal 2D (a) and corresponding endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) located along the inner aspect of a sharp turn of the colon. On the 3D view, both directions of the colon are seen, a finding that confirms a flexural pseudotumor. The bulbous appearance of this turn should not be mistaken for a polyp, as one reader did during the CAD-assisted session.
Extrinsic Compression
Structures adjacent to the colon—such as the bowel, blood vessels, liver, gallbladder, spleen, psoas muscle, osseous structures, uterus, and diaphragmatic crus—can cause an impression on the colonic wall that can mimic a polypoid structure or flat lesion, particularly on 3D endoluminal views (Figs 17, 18) (52). On 2D views obtained with conventional soft-tissue window and level settings, extrinsic compression from an adjacent structure is apparent and easily dismissible. In our review, 1% (11 out of 806) of CAD false-positive results were attributed to extrinsic compression. Two were related to seven reader false-positive results during the CAD-assisted portion of the trial, for an incidence of 3.3%, and three were related to reader false-positive results during the CAD-unassisted portion of the trial.
Figure 17a.
Extrinsic compression. (a, b) Endoluminal 3D (a) and corresponding magnified axial 2D (b) CT colonography images show a CAD polyp candidate (arrow in b). On the endoluminal view, this polyp candidate is difficult to disregard and measures approximately 7 mm in its maximal diameter. However, the axial view shows that an external structure, a right iliac vessel, is adjacent to the bowel wall and is extrinsically compressing the sigmoid colon lumen.
Figure 18a.
Extrinsic compression. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b) that is secondary to extrinsic compression by the anterior tip of the spleen.
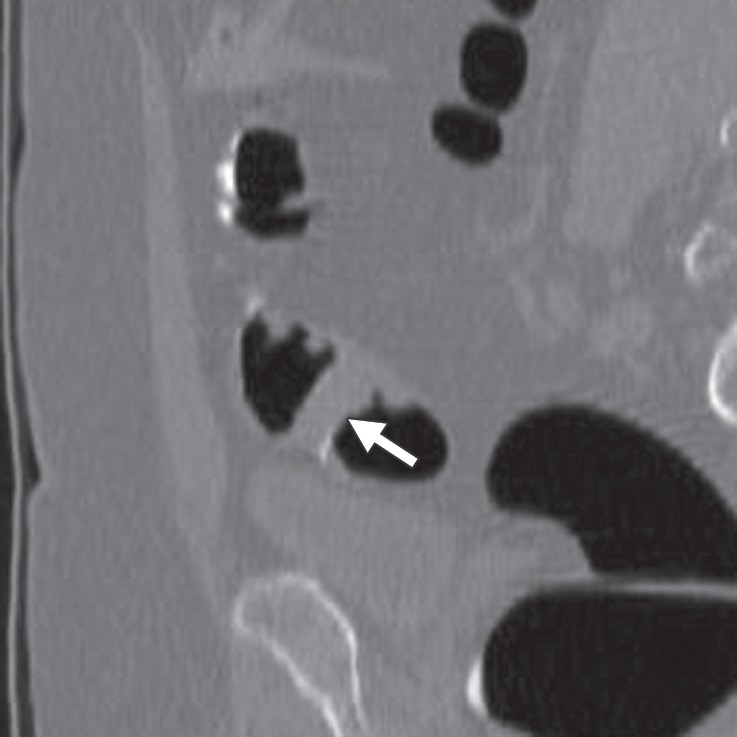
Figure 17b.
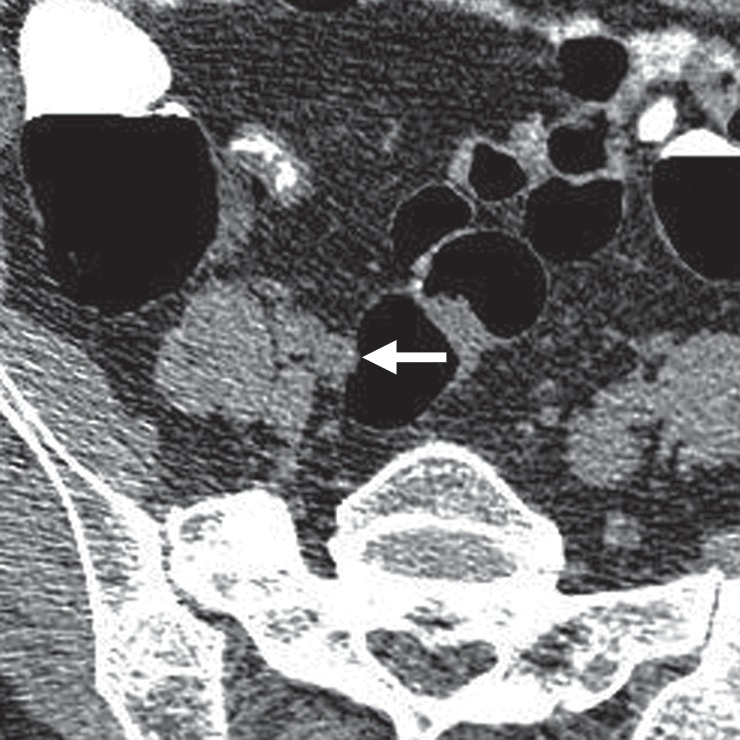
Extrinsic compression. (a, b) Endoluminal 3D (a) and corresponding magnified axial 2D (b) CT colonography images show a CAD polyp candidate (arrow in b). On the endoluminal view, this polyp candidate is difficult to disregard and measures approximately 7 mm in its maximal diameter. However, the axial view shows that an external structure, a right iliac vessel, is adjacent to the bowel wall and is extrinsically compressing the sigmoid colon lumen.
Figure 18b.
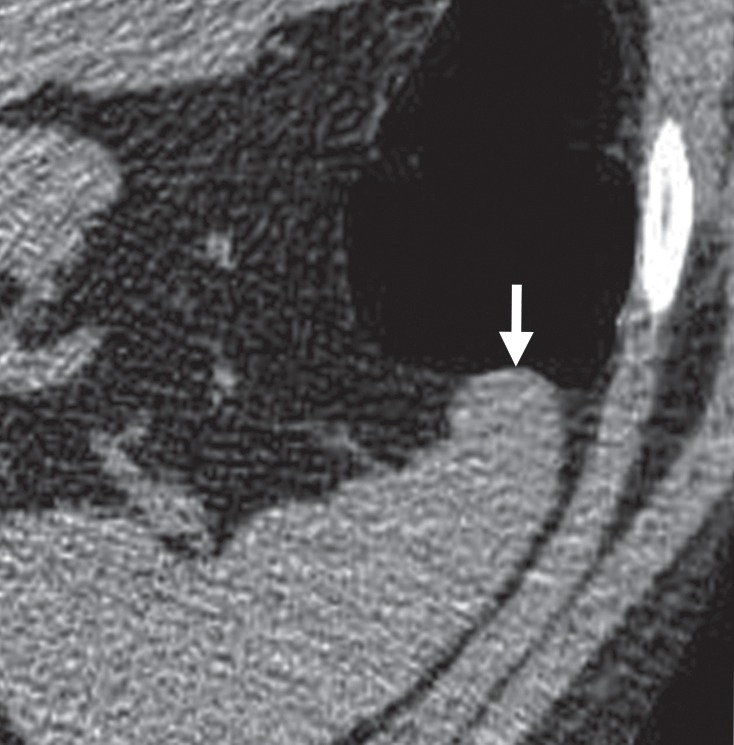
Extrinsic compression. (a, b) Endoluminal 3D (a) and magnified axial 2D (b) CT colonography images show a CAD polyp candidate (blue area in a and arrow in b) that is secondary to extrinsic compression by the anterior tip of the spleen.
Reconstruction, Peristalsis, Streak, and Metal Artifacts
Multiple regular ripples across the mucosal surface or folds can “fool” CAD but are usually easily dismissed on 3D endoluminal views by applying the neighborhood rule (Fig 19). Such ripples often result from stair-step artifacts. If symmetric findings are seen in adjacent folds, the CAD polyp candidate can be confidently dismissed. However, a discrete overlying abnormality should be ruled out by evaluating the region on 2D and additional views (eg, prone).
Figure 19.
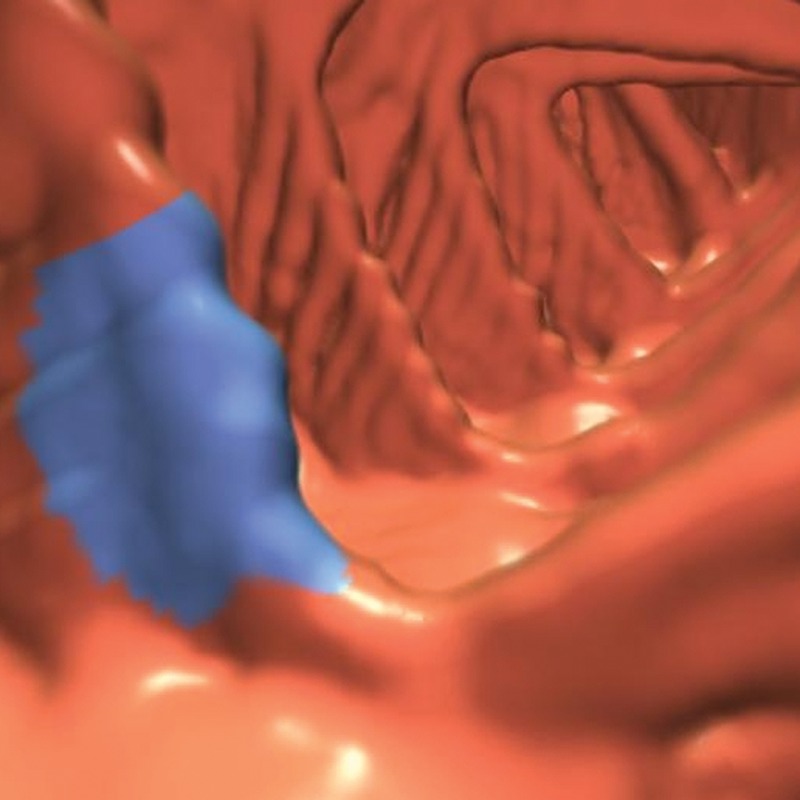
Endoluminal 3D CT colonography image shows a CAD polyp candidate (blue area) in the context of a symmetric, repeating rippling effect on the folds and surface of the colon. This appearance may be seen in cases of reconstruction artifact or, less commonly, respiratory or patient motion and requires careful evaluation for any asymmetric findings.
Electronic cleansing techniques have been developed to increase patient comfort and compliance by replacing cathartic pre-examination bowel preparation regimens with ingestion of positive contrast material to label the fecal contents of the bowel, which can be subsequently subtracted from source CT images with postprocessing techniques. The effect of postprocessing techniques on subsequent CAD software processing can result in an increased number of CAD polyp candidates (53).
Motion from bowel peristalsis and patient movement can potentially create soft-tissue polyp candidates that are impossible to dismiss on 3D endoluminal views. Similarly, streak artifacts from the interface of low-attenuation gas and high-attenuation tagged fluid or from other high-attenuation objects in the abdomen can lead to CAD polyp candidates (Fig 20). A related phenomenon was recently coined the “dense waterfall” sign and is associated with opacified fluid that flows from a high level to a low level relative to the examination table (54). Similarly, beam hardening and streak artifacts from metal objects in the vicinity of the colon can lead to suspicious contour abnormalities of the colonic mucosa and CAD false-positive results. In general, peristalsis, streak, and metal artifacts are easily recognizable on 2D multiplanar reconstructions. Although these artifacts are generally not confused with underlying pathologic conditions, they may potentially obscure relevant findings. CAD false-positive results related to these artifacts resulted in 31 (4%) CAD false-positive results and no reader false-positive results.
Figure 20a.
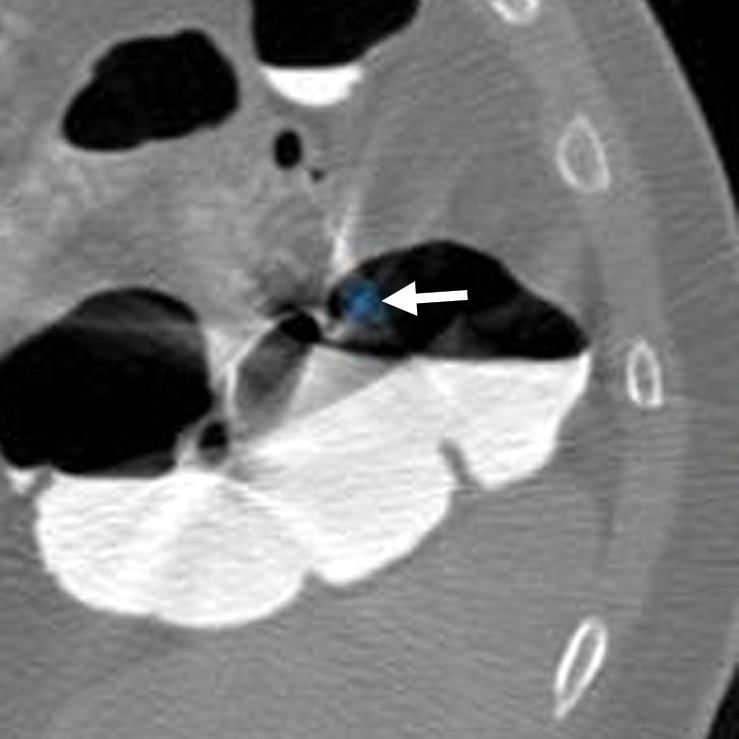
Artifacts. (a, b) Magnified axial 2D supine (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) that is difficult to disregard on the endoluminal view. However, the 2D view shows streak artifacts from peristalsis and the interface between air and densely-tagged liquid that may obscure large polyps. This region should be carefully evaluated on the prone view to confidently exclude polyps.
Figure 20b.
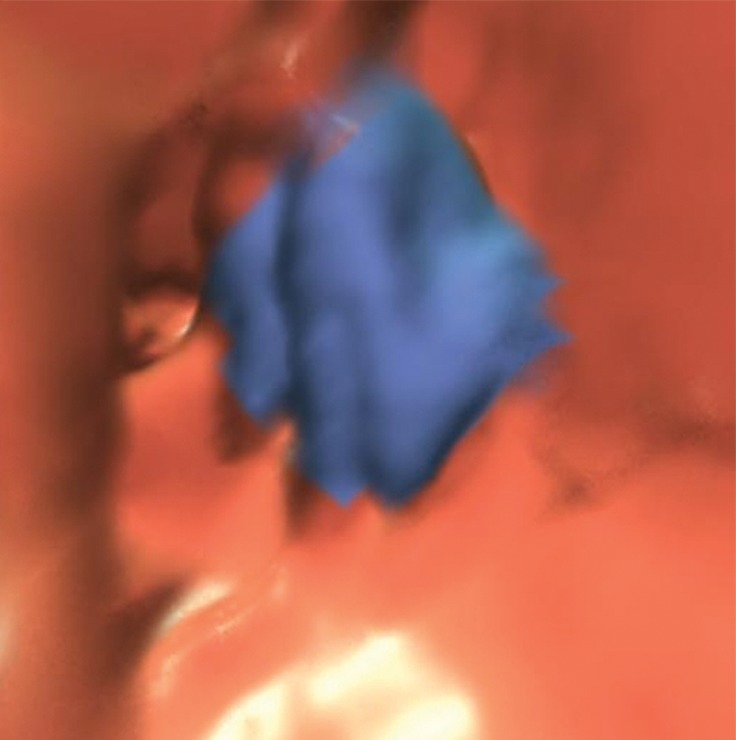
Artifacts. (a, b) Magnified axial 2D supine (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) that is difficult to disregard on the endoluminal view. However, the 2D view shows streak artifacts from peristalsis and the interface between air and densely-tagged liquid that may obscure large polyps. This region should be carefully evaluated on the prone view to confidently exclude polyps.
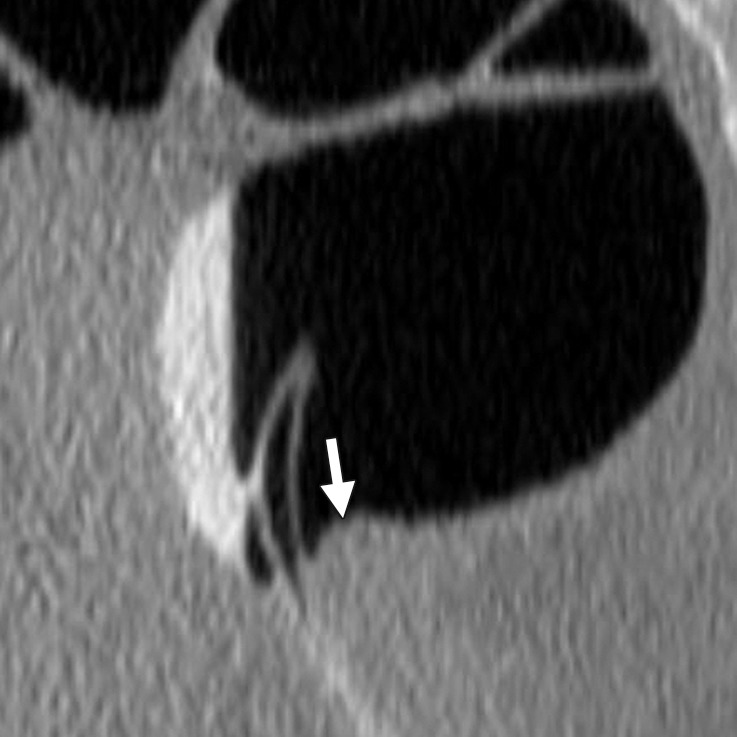
Diverticulum
Impacted stool inside a diverticulum (ie, diverticular fecalith) or the neck of the diverticulum can simulate a polypoid structure and lead to a CAD false-positive result (Fig 21). In our review, 1% (9 out of 806) of CAD false-positive results were related to diverticulum, and none were related to reader false-positive results. On 2D images, diverticulum classically appears as a luminal defect with a hypoattenuating center and a hyperattenuating peripheral ring. Diverticulum is easily seen on 2D and 3D views, making it an easily avoided pitfall. An inverted diverticulum can mimic a polyp and is impossible to dismiss on 3D endoluminal views. However, careful evaluation of 2D images may reveal mesenteric fat on the serosal side or air, findings that exclude a soft-tissue polyp.
Figure 21a.
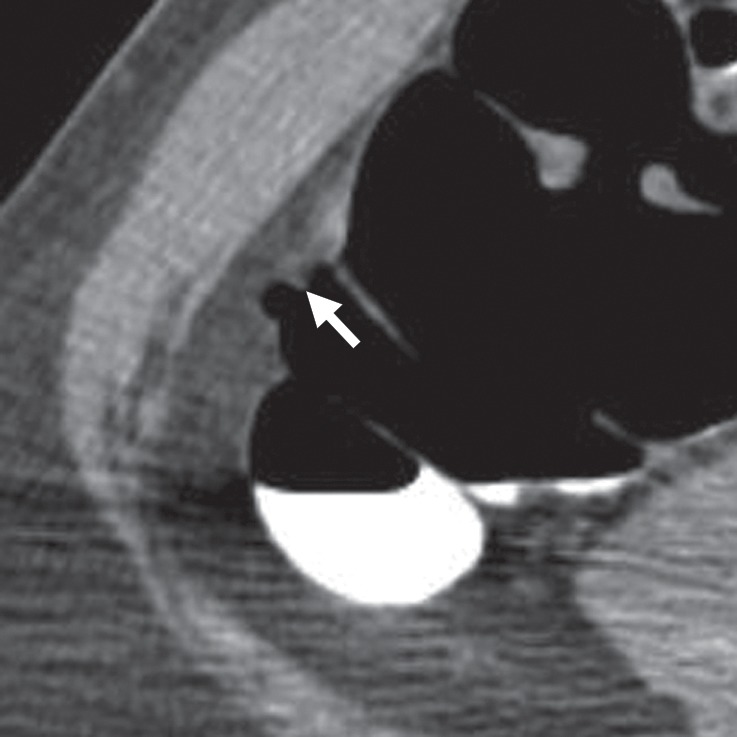
Diverticulum. (a, b) Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) at the margin of the neck of a diverticulum, which causes the CAD to “see” a round surface that is concerning for a polyp.
Figure 21b.
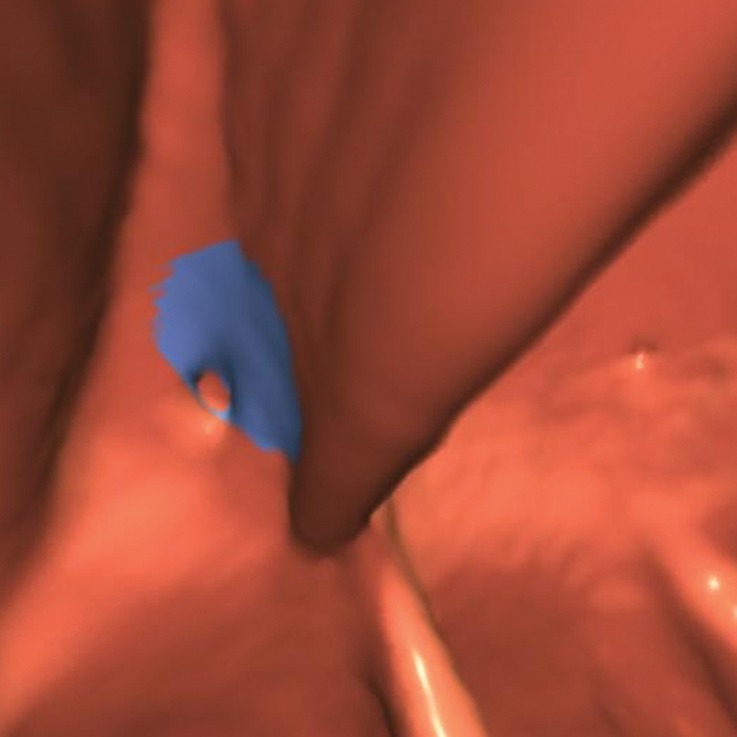
Diverticulum. (a, b) Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) at the margin of the neck of a diverticulum, which causes the CAD to “see” a round surface that is concerning for a polyp.
Lipomas
Lipomas are rare benign tumors in the gastrointestinal tract. However, they are the second most common benign tumors of the colon (usually sigmoid) and rectum after adenomas (55). On 3D endoluminal views, lipomas are indistinguishable from any other polypoid lesion. On 2D views, they are characterized by internal fat attenuation similar to that of surrounding pericolic fat (Fig 22). CAD infrequently detects lipomas and presents them as polyp candidates for review. In our review, two of the 806 CAD false-positive results were related to lipomas, and none were related to reader false-positive results.
Figure 22.
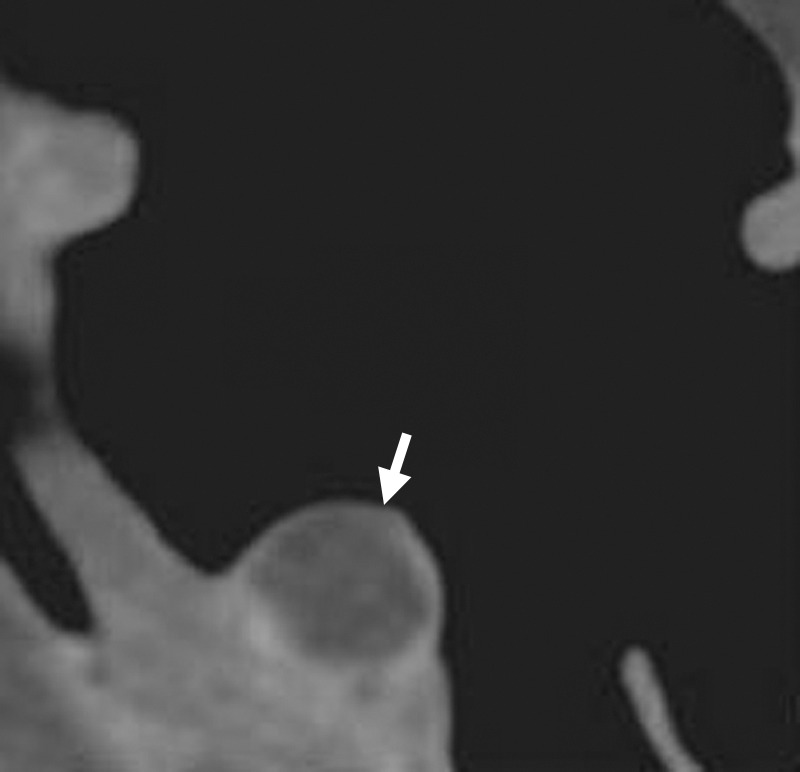
Benign lipoma. Magnified axial 2D CT colonography image shows a polyp candidate (arrow) with intrinsic macroscopic fat attenuation, consistent with a benign lipoma.
Rectal Tube
The contour of the rectal tube often results in a CAD false-positive result that is easily dismissed on 3D endoluminal and 2D views (Fig 23). The adjacent mucosa should be carefully evaluated to rule out an underlying or adjacent lesion.
Figure 23a.
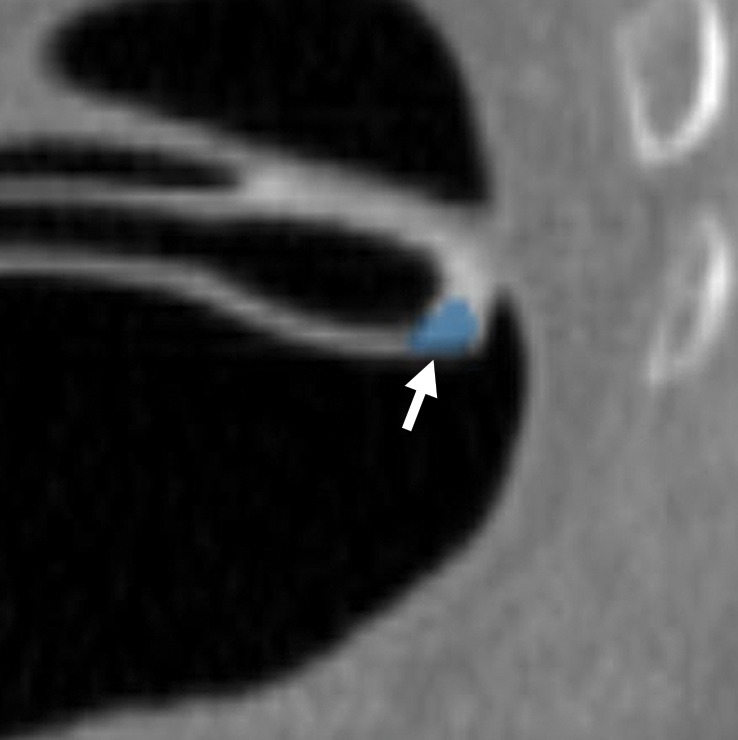
Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) on the convex contour of the rectal tube. Note the symmetric ripples of the surrounding rectal mucosal surface.
Figure 23b.
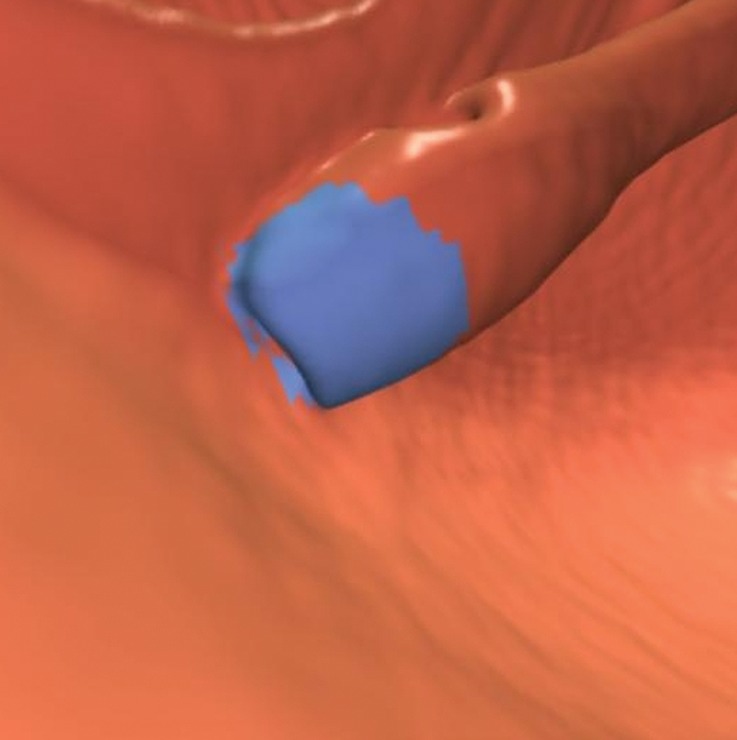
Magnified axial 2D (a) and endoluminal 3D (b) CT colonography images show a CAD polyp candidate (arrow in a and blue area in b) on the convex contour of the rectal tube. Note the symmetric ripples of the surrounding rectal mucosal surface.
Discussion
CAD has been shown to improve reader performance, particularly for the detection of 6–9-mm polyps. To maximize the positive predictive value of CT colonography, readers must be aware of and recognize the causes of reader and CAD false-positive results. A direct, conscientious attempt should be made to use the teaching points and examples herein to minimize the rate of reader false-positive results.
Many CAD false-positive results are easily disregarded, including those that result from coarse mucosa, reconstruction, peristalsis, motion, streak artifacts, diverticulum, rectal tubes, and lipomas. Of the 806 CAD false-positive results we reviewed, only 96 (12%) were mistaken for a polyp by at least one of the 19 readers during the CAD-assisted session, most of which (49 [51%]) were selected by only one reader. Among the 96 CAD false-positive results that were selected by at least one reader during the CAD-assisted session, 43 (45%) were selected by at least one reader during the CAD-unassisted session, eighteen (42%) of which were selected by an equal or greater number of readers in the CAD-unassisted session, indicating that they were difficult findings to disregard, even without the direct prompting of CAD.
These findings help explain the relatively small drop in specificity with the use of CAD compared with the moderate gains in sensitivity. The current goal of CAD is to decrease errors of detection, not errors of characterization. By directing the readers’ focus to areas of increased suspicion, obvious polyp candidates are given a second, directed evaluation. This evaluation comes at the expense of showing several easily dismissible polyp candidates that, fortunately, have a small effect on specificity.
Frequent CAD false-positive results from haustral folds, extracolonic polyp candidates, diminutive lesions (<6 mm), anal papillae, internal hemorrhoids, varices, and flexural pseudotumors are almost always recognized. (The incidence of related reader false-positive results is <1%.) With the neighborhood rule, haustral fold CAD false-positive results that result from reconstruction artifacts and underdistention can be recognized and dismissed. Proper and meticulous 2D localization is needed to exclude extracolonic polyp candidates. Careful measurement of the polyp candidate should be performed before the finding is reported. Knowledge of the normal anatomy and appearance of the rectal region is necessary to prevent mischaracterizing and over-reporting lesions in this region.
The categories of CAD false-positive results with the highest numbers of related single- and multireader false-positive results were stool, the ICV, and nondismissible polyp candidates (Table 2). CAD false-positive results related to stool are common and often difficult to evaluate and dismiss. Every polyp candidate should be carefully evaluated for features of stool, including internal gas, internal tagging, sharp edges, and movement.
The morphologic characteristics of an ICV are so variable and bulbous that it is often difficult to exclude a polyp. Given the inherent difficulty in evaluating the ICV and the lack of a standardized approach, it would be valuable to know the true incidence of polyps on the ICV in a screening population. Although a single report evaluating 91 consecutive cecal and ascending colon tumors reported that 15% involved the level of the ICV, the study included symptomatic patients with 3–12-cm lesions, including mass lesions that would be evident on 2D multiplanar CT colonography images and are clearly not the target lesion for screening CT colonography (56). Therefore, this data set is not generalizable to screening CT colonography. A review of more recent literature reveals a paucity of data regarding the incidence of polyps on the ICV and the rate of positive optical colonoscopy biopsy results involving the ICV. Rather, there are infrequent case reports of tumors affecting the ICV, which suggests its rarity (57). Further data are necessary to determine how best to manage suspicious ICV findings at CT colonography, given the likely low prevalence of adenomatous polyps. When patients will be referred to endoscopy because of other suspicious findings, the endoscopist should be made aware of any suspicious ICV findings.
The management of diminutive polyps and those that are 6–9 mm plays an important role in determining the net effect of CT colonography, both with and without CAD, on patient care and costs. Nondismissible polyp candidates (6–9 mm) are frequent causes of CAD-assisted and CAD-unassisted reader false-positive results. Having a 6-mm threshold increases false-positive results, with a questionable benefit in cancer detection and long-term mortality. Current C-RADS recommendations stipulate that fewer than three 6–9-mm lesions can be managed with short-term surveillance after discussion with the patient rather than immediate endoscopy and biopsy (44–50). Additionally, there are limitations to the use of optical colonoscopy as the reference standard, with a reported miss rate for adenomas that are 6–9 mm and 1 cm or larger as high as 13% and 6%, respectively (58–61). Although rare, extrinsic compression should be considered a possible source for polyp candidates, a question that may be resolved by careful evaluation for adjacent structures that cause mass effect on 2D views.
Conclusions
CT colonography has the potential to improve patient compliance with colon cancer screening recommendations and has been demonstrated to improve reader sensitivity in interpreting CT colonography studies. However, to maintain and improve specificity, readers must be familiar with the many sources of CAD false-positive results—including normal anatomic structures, underdistention of the colon, and stool—to recognize and properly categorize these polyp candidates. Strategies for correctly evaluating CAD polyp candidates are important to avoid pitfalls related to the most common sources of CAD false-positive results, especially the ICV and tagged stool. In a large multiple-reader, multiple-case trial, the vast majority of CAD false-positive results were dismissed by readers. As CT colonography becomes more widely implemented, the management of nondismissible polyp candidates and diminutive lesions will require further investigation.
Presented as an education exhibit at the 2011 RSNA Annual Meeting.
For this journal-based SA-CME activity, the authors A.H.D. and K.W. have provided disclosures (see “Disclosures of Potential Conflicts of Interest”); all other authors, the editor, and the reviewers have disclosed no relevant relationships.
Funding: The research was supported by the National Cancer Institute.
Disclosures of Conflicts of Interest.—A.H.D.:Activities related to the present article: cases from the clinical trial were supported by ICAD. Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships. K.W.: Activities related to the present article: grant from the National Cancer Institute. Activities not related to the present article: disclosed no relevant relationships. Other activities: disclosed no relevant relationships.
Abbreviations:
- ICV
- ileocecal valve
- 3D
- three-dimensional
- 2D
- two-dimensional
References
- 1.Kim DH, Pickhardt PJ, Hoff G, Kay CL. Computed tomographic colonography for colorectal screening. Endoscopy 2007;39(6):545–549. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Harmsen WS, Wilson LA, et al. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology 2003;125(2): 311–319. [DOI] [PubMed] [Google Scholar]
- 3.Pickhardt PJ, Choi JR, Hwang I, et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003;349(23):2191–2200. [DOI] [PubMed] [Google Scholar]
- 4.Whitlock EP, Lin J, Liles E, et al. Screening for Colorectal Cancer: An Updated Systemic Review [Internet]. Rockville, Md: Agency for Healthcare Research and Quality (U.S.). http://www.ncbi.nlm.nih.gov/books/NBK35179/. Published October 2008. Accessed March 15, 2013. [PubMed] [Google Scholar]
- 5.Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices—2006-2007. Am J Prev Med 2009;37(1): 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention . Behavioral Risk Factor Surveillance System Survey Data. http://apps.nccd.cdc.gov/brfss/. Published 2008. Accessed March 15, 2013.
- 7.Cotton PB, Durkalski VL, Pineau BC, et al. Computed tomographic colonography (virtual colonoscopy): a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA 2004;291(14):1713–1719. [DOI] [PubMed] [Google Scholar]
- 8.Rockey DC, Paulson E, Niedzwiecki D, et al. Analysis of air contrast barium enema, computed tomographic colonography, and colonoscopy: prospective comparison. Lancet 2005;365(9456):305–311. [DOI] [PubMed] [Google Scholar]
- 9.Kim DH, Pickhardt PJ, Taylor AJ, et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007;357(14): 1403–1412. [DOI] [PubMed] [Google Scholar]
- 10.Pooler BD, Baumel MJ, Cash BD, et al. Screening CT colonography: multicenter survey of patient experience, preference, and potential impact on adherence. AJR Am J Roentgenol 2012;198(6): 1361–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knudsen AB, Lansdorp-Vogelaar I, Rutter CM, et al. Cost-effectiveness of computed tomographic colonography screening for colorectal cancer in the Medicare population. J Natl Cancer Inst 2010;102(16):1238–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doshi T, Rusinak D, Halvorsen RA, Rockey DC, Suzuki K, Dachman AH. CT colonography: false-negative interpretations. Radiology 2007;244(1): 165–173. [DOI] [PubMed] [Google Scholar]
- 13.Näppi JJ, Nagata K. Sources of false positives in computer-assisted CT colonography. Abdom Imaging 2011;36(2):153–164. [DOI] [PubMed] [Google Scholar]
- 14.Fidler JL, Fletcher JG, Johnson CD, et al. Understanding interpretive errors in radiologists learning computed tomography colonography. Acad Radiol 2004;11(7):750–756. [DOI] [PubMed] [Google Scholar]
- 15.Krupinski EA. What can the radiologist teach CAD: lessons from CT colonoscopy. Acad Radiol 2009;16(1):1–3. [DOI] [PubMed] [Google Scholar]
- 16.Bogoni L, Cathier P, Dundar M, et al. Computer-aided detection (CAD) for CT colonography: a tool to address a growing need. Br J Radiol 2005; 78(Spec No 1):S57–S62. [DOI] [PubMed] [Google Scholar]
- 17.Summers RM, Yao J, Pickhardt PJ, et al. Computed tomographic virtual colonoscopy computer-aided polyp detection in a screening population. Gastroenterology 2005;129(6):1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida H, Näppi J, MacEneaney P, Rubin DT, Dachman AH. Computer-aided diagnosis scheme for detection of polyps at CT colonography. RadioGraphics 2002;22(4):963–979. [DOI] [PubMed] [Google Scholar]
- 19.Dachman AH, Kelly KB, Zintsmaster MP, et al. Formative evaluation of standardized training for CT colonographic image interpretation by novice readers. Radiology 2008;249(1):167–177. [DOI] [PubMed] [Google Scholar]
- 20.Fletcher JG, Chen MH, Herman BA, et al. Can radiologist training and testing ensure high performance in CT colonography? Lessons from the National CT Colonography Trial. AJR Am J Roentgenol 2010;195(1):117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2002;97(6):1296–1308. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2006; 63(4 suppl):S16–S28. [DOI] [PubMed] [Google Scholar]
- 23.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol 2008;6(10):1091–1098. [DOI] [PubMed] [Google Scholar]
- 24.Dachman AH, Obuchowski NA, Hoffmeister JW, et al. Effect of computer-aided detection for CT colonography in a multireader, multicase trial. Radiology 2010;256(3):827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawrence EM, Pickhardt PJ, Kim DH, Robbins JB. Colorectal polyps: stand-alone performance of computer-aided detection in a large asymptomatic screening population. Radiology 2010;256(3): 791–798. [DOI] [PubMed] [Google Scholar]
- 26.Petrick N, Haider M, Summers RM, et al. CT colonography with computer-aided detection as a second reader: observer performance study. Radiology 2008;246(1):148–156. [DOI] [PubMed] [Google Scholar]
- 27.Regge D, Della Monica P, Galatola G, et al. Efficacy of computer-aided detection as a second reader for 6–9-mm lesions at CT colonography: multicenter prospective trial. Radiology 2013;266(1):168–176. [DOI] [PubMed] [Google Scholar]
- 28.Park HS, Kim SH, Kim JH, et al. Computer-aided polyp detection on CT colonography: comparison of three systems in a high-risk human population. Eur J Radiol 2010;75(2):e147–e157. [DOI] [PubMed] [Google Scholar]
- 29.Hein PA, Krug LD, Romano VC, Kandel S, Hamm B, Rogalla P. Computer-aided detection in computed tomography colonography with full fecal tagging: comparison of standalone performance of 3 automated polyp detection systems. Can Assoc Radiol J 2010;61(2):102–108. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida H, Näppi J. CAD in CT colonography without and with oral contrast agents: progress and challenges. Comput Med Imaging Graph 2007;31 (4-5):267–284. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida H, Masutani Y, MacEneaney P, Rubin DT, Dachman AH. Computerized detection of colonic polyps at CT colonography on the basis of volumetric features: pilot study. Radiology 2002;222(2): 327–336. [DOI] [PubMed] [Google Scholar]
- 32.Suzuki K, Yoshida H, Näppi J, Armato SG, 3rd, Dachman AH. Mixture of expert 3D massive-training ANNs for reduction of multiple types of false positives in CAD for detection of polyps in CT colonography. Med Phys 2008;35(2):694–703. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki K, Rockey DC, Dachman AH. CT colonography: advanced computer-aided detection scheme utilizing MTANNs for detection of “missed” polyps in a multicenter clinical trial. Med Phys 2010;37(1): 12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dachman AH, Lefere P, Gryspeerdt S, Morin M. CT colonography: visualization methods, interpretation, and pitfalls. Radiol Clin North Am 2007;45(2): 347–359. [DOI] [PubMed] [Google Scholar]
- 35.Pickhardt PJ. Differential diagnosis of polypoid lesions seen at CT colonography (virtual colonoscopy). RadioGraphics 2004;24(6):1535–1556; discussion 1557–1559. [DOI] [PubMed] [Google Scholar]
- 36.Fleischner FG, Bernstein C. Roentgen-anatomical studies of the normal ileocecal valve. Radiology 1950;54(1):43–58, illust. [DOI] [PubMed] [Google Scholar]
- 37.El-Amin LC, Levine MS, Rubesin SE, Shah JN, Kochman ML, Laufer I. Ileocecal valve: spectrum of normal findings at double-contrast barium enema examination. Radiology 2003;227(1):52–58. [DOI] [PubMed] [Google Scholar]
- 38.Yitta S, Tatineny KC, Cipriani NA, Dachman AH. Characterization of normal ileocecal valve density on CT colonography. J Comput Assist Tomogr 2006;30(1):58–61. [DOI] [PubMed] [Google Scholar]
- 39.Lasser EC, Rigler LG. Ileocecal valve syndrome. Gastroenterology 1955;28(1):1–16. [PubMed] [Google Scholar]
- 40.Hinkel CL. Roentgenological examination and evaluation of the ileocecal valve. Am J Roentgenol Radium Ther Nucl Med 1952;68(2):171–182. [PubMed] [Google Scholar]
- 41.O’Connor SD, Summers RM, Choi JR, Pickhardt PJ. Oral contrast adherence to polyps on CT colonography. J Comput Assist Tomogr 2006;30(1): 51–57. [DOI] [PubMed] [Google Scholar]
- 42.Shinya H, Wolff WI. Morphology, anatomic distribution and cancer potential of colonic polyps. Ann Surg 1979;190(6):679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bond JH. Clinical relevance of the small colorectal polyp. Endoscopy 2001;33(5):454–457. [DOI] [PubMed] [Google Scholar]
- 44.Zalis ME, Barish MA, Choi JR, et al. CT colonography reporting and data system: a consensus proposal. Radiology 2005;236(1):3–9. [DOI] [PubMed] [Google Scholar]
- 45.Pickhardt PJ, Hassan C, Laghi A, et al. Small and diminutive polyps detected at screening CT colonography: a decision analysis for referral to colonoscopy. AJR Am J Roentgenol 2008;190(1):136–144. [DOI] [PubMed] [Google Scholar]
- 46.Pickhardt PJ, Hassan C, Laghi A, et al. Clinical management of small (6- to 9-mm) polyps detected at screening CT colonography: a cost-effectiveness analysis. AJR Am J Roentgenol 2008;191(5):1509–1516. [DOI] [PubMed] [Google Scholar]
- 47.Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin 2006;56(3):143–159; quiz 184–185. [DOI] [PubMed] [Google Scholar]
- 48.Lostumbo A, Suzuki K, Dachman AH. Flat lesions in CT colonography. Abdom Imaging 2010;35(5): 578–583. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman D, Moravec M, Holub J, Michaels L, Eisen G. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology 2008;135(4):1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rex DK, Overhiser AJ, Chen SC, Cummings OW, Ulbright TM. Estimation of impact of American College of Radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol 2009;104(1): 149–153. [DOI] [PubMed] [Google Scholar]
- 51.Pickhardt PJ, Lee AD, McFarland EG, Taylor AJ. Linear polyp measurement at CT colonography: in vitro and in vivo comparison of two-dimensional and three-dimensional displays. Radiology 2005;236(3):872–878. [DOI] [PubMed] [Google Scholar]
- 52.Pickhardt PJ, Kim DH, Menias CO, Gopal DV, Arluk GM, Heise CP. Evaluation of submucosal lesions of the large intestine: part 2—nonneoplastic causes. RadioGraphics 2007;27(6):1693–1703. [DOI] [PubMed] [Google Scholar]
- 53.Wi JY, Kim SH, Lee JY, Kim SG, Han JK, Choi BI. Electronic cleansing for CT colonography: does it help CAD software performance in a high-risk population for colorectal cancer? Eur Radiol 2010; 20(8):1905–1916. [DOI] [PubMed] [Google Scholar]
- 54.Boyce CJ, Vetter JR, Pickhardt PJ. MDCT artifact related to the intra-scan gravitational flow of opacified luminal fluid (the “dense waterfall” sign). Abdom Imaging 2012;37(2):292–296. [DOI] [PubMed] [Google Scholar]
- 55.Bruneton JN, Quoy AM, Dageville X, Lecomte P. Lipomas of the digestive tract: review of the literature apropos of 5 cases [in French]. Ann Gastroenterol Hepatol (Paris) 1984;20(1):27–32. [PubMed] [Google Scholar]
- 56.Berk RN, Sadowsky NL, Levenson SS. Carcinoma of the cecum and ascending colon: an analysis of ninety-one cases. Am J Surg 1962;104:27–36. [DOI] [PubMed] [Google Scholar]
- 57.Yörük G, Aksöz K, Buyraç Z, Unsal B, Nazli O, Ekinci N. Adenocarcinoma of the ileocecal valve: report of a case. Turk J Gastroenterol 2004;15(4): 268–269. [PubMed] [Google Scholar]
- 58.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112(1):24–28. [DOI] [PubMed] [Google Scholar]
- 59.Ahn SB, Han DS, Bae JH, Byun TJ, Kim JP, Eun CS. The miss rate for colorectal adenoma determined by quality-adjusted, back-to-back colonoscopies. Gut Liver 2012;6(1):64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Postic G, Lewin D, Bickerstaff C, Wallace MB. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol 2002;97(12): 3182–3185. [DOI] [PubMed] [Google Scholar]
- 61.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy 2008;40(4):284–290. [DOI] [PubMed] [Google Scholar]