Abstract
BACKGROUND
The incidence, dynamics, and management of cytopenias were investigated in patients with chronic myeloid leukemia in chronic phase (CP CML) who received dasatinib therapy after imatinib failure.
METHODS
Data were analyzed from 130 patients with CP CML who were treated with dasatinib from November 2003 to March 2006 in phase 1 (n = 22) or phase 2 or 3 (n = 108) studies for the development of grade 2 to 4 cytopenia (according to the National Cancer Institute Common Terminology Criteria [version 3.0]).
RESULTS
Grade 2 to 4 neutropenia and/or thrombocytopenia occurred in 94 (72%) patients during dasatinib therapy and grade 3 to 4 occurred in 67 (52%) patients. Of the 94 patients who developed grade 2 to 4 neutropenia and/or thrombocytopenia, 64 (68%) also developed at least grade 2 anemia, and 16 (17%) developed grade 3 to 4 anemia. Management of cytopenias included transient dasatinib interruption in 35 (37%) patients, filgrastim in 12 (14%) patients, recombinant erythropoietin in 29 (45%) patients, and interleukin-11 in 3 (5%) patients. Factors associated with an increased risk for developing grade 2 to 4 cytopenias were longer time from diagnosis to treatment, prior interferon or imatinib therapy, and a lower white blood cell count at the initiation of dasatinib therapy.
CONCLUSIONS
Hematologic toxicity was frequent during dasatinib therapy in patients with CP CML, particularly at doses >100 mg daily. Treatment interruption and/or dose reduction as well as growth factor support were found to be safe and efficacious strategies to facilitate the continuous administration of dasatinib.
Keywords: cytopenias, chronic myeloid leukemia, dasatinib, imatinib, chronic phase, toxicity, treatment interruption
Dasatinib is a tyrosine kinase inhibitor (TKI) with potent activity against BCR-ABL1 kinase (IC50 < 1 nM).1 Therapy with this agent is associated with remarkable response rates and tolerability in patients with chronic myeloid leukemia (CML).2 In phase 2 studies, dasatinib administered at a dose of 70 mg twice daily to patients in chronic phase (CP) with resistance or intolerance of imatinib therapy rendered a complete hematologic response (CHR) in >90% of patients, whereas a major (MCyR) and a complete cytogenetic response (CCyR) was achieved by 55% and 44% of patients, respectively, after 24 months of follow-up.3 Nonhematologic adverse effects, although relatively frequent during dasatinib therapy, are usually mild and manageable. In a dose-finding study of dasatinib in patients with CML, the most frequent nonhematologic toxicities were diarrhea (23%), peripheral edema (19%), pleural effusion (18%), and headache (10%).2 By contrast, grade ≥3 neutropenia (absolute neutrophil count [ANC] <109/L) or thrombocytopenia (platelet count <50 × 109/L) have been observed in 45% and 35% of patients with CP CML, respectively.2 The incidence of grade 3 to 4 hematologic toxicity is remarkably higher in patients with more advanced stages of the disease. Hematologic toxicity requires transient interruptions of dasatinib therapy in approximately 60% of patients, and this toxicity frequently resolves within 3 months, in many cases accompanied by cytogenetic response.2 The current management of patients with CML who develop grade ≥3 neutropenia or thrombocytopenia during dasatinib therapy involves transient drug interruption, followed by dose reduction when the recovery of cytopenias requires >2 weeks. However, dose interruptions and reductions may jeopardize the achievement of MCyR because they reduce dasatinib exposure/dose intensity.
A strategy to overcome the development of hematologic toxicity and to maximize imatinib exposure in patients with CML consists of the use of growth factors tailored to specific cytopenias.4–6 For example, granulocyte–colony-stimulating factor (G-CSF) reduces the severity of chemotherapy-associated neutropenia in patients with solid tumors and lymphomas,7 and accelerates neutrophil regeneration in patients with acute myeloid leukemia.8 G-CSF has also been shown to overcome imatinib-induced neutropenia in patients with CP CML.4 Interleukin-11 (IL-11) is a megakaryopoietic cytokine that reduces the incidence and severity of thrombocytopenia associated with chemotherapy in solid tumors and CML.5 Finally, recombinant human erythropoietin (rh-Epo) has been administered to patients with CML who developed anemia while receiving imatinib therapy.6
In the current study, we determined the incidence of hematologic toxicity among patients with CP CML who received therapy with dasatinib in phase 1 and 2 studies at our institution. More importantly, we evaluated the dynamics, duration, and recurrence of cytopenias during dasatinib therapy as well as the outcome of patients who received supportive therapy with growth factors to ameliorate dasatinib-induced cytopenias.
MATERIALS AND METHODS
Study Design and Dasatinib Therapy
We investigated adult patients with a diagnosis of Philadelphia (Ph)-positive CP CML who were either newly diagnosed or resistant or intolerant to imatinib therapy and were receiving treatment in open-label phase 1, 2, or 3 studies of dasatinib conducted at The University of Texas M. D. Anderson Cancer Center (MDACC). Studies were approved by the institutional review board and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent before study entry. Patients aged ≥18 years were eligible if they had imatinib-intolerant or imatinib-resistant CP CML. Patients enrolled in the phase 1 study received dasatinib at doses ranging from 15 mg to 180 mg, which were administered as a single or divided dose on either a 5-days-on/2-days-off, 6-days-on/1-day-off, or continuous schedule. In phase 2 studies, dasatinib was administered on 4 oral schedules: 50 mg twice daily, 70 mg twice daily, 100 mg daily, and 140 mg daily (Table 1).
Table 1.
Dose Escalation/Reduction of Dasatinib in Phase 1 and 2 Studies in Patients With CML
| Phase | Dose Level | CML Phase | |
|---|---|---|---|
| 1 | CP, AP, BP* | ||
| 1 | 15 | ||
| 2 | 30 | ||
| 3 | 50 | ||
| 4 | 75 | ||
| 5 | 105 | ||
| 6 | 140 | ||
| 7 | 180 | ||
| 2 | CP, mg bid | AP and BP, mg bid | |
| Escalation 1 | 90 | 100 | |
| Starting dose | 70 | 70 | |
| Reduction 1 | 50 | 50 | |
| Reduction 2 | 40 | 40 |
CML indicates chronic myeloid leukemia; CP, chronic phase; AP, accelerated phase; BP, blastic phase; bid, twice daily.
Total daily dose (in milligrams) administered in 1 or 2 doses.
Dasatinib Dose Modifications
Therapy with dasatinib could be interrupted and/or reduced in response to the development of grade 3 to 4 hematologic toxicity. Dasatinib was discontinued in the event of grade 3 to 4 neutropenia until an ANC >1.0 × 109/L was reached, and reinitiated at the same dose if the recovery occurred within 7 days or at the next lower dose level if this recovery occurred after 7 days. If a second episode of grade 3 to 4 neutropenia occurred, a second dose reduction was allowed. If grade 4 thrombocytopenia occurred during the first 2 months of therapy, dasatinib was interrupted until a platelet count >50 × 109/L was reached and then resumed at the same dose. If a second episode of grade 4 thrombocytopenia occurred, dasatinib dose was reduced at the next lower dose level. A second dose level reduction was allowed in the event of a second episode of grade 4 thrombocytopenia. When grade 3 to 4 thrombocytopenia occurred past the second month of therapy, dasatinib was interrupted until a platelet count >50 × 109/L was reached. Dose escalation was allowed in patients who failed to achieve a CHR after 1 month, a CCyR after 3 months of therapy, or whenever loss of response was documented. Dasatinib was interrupted if the toxicity was deemed unacceptable or in the event of disease progression despite dose escalation. Other anti-leukemic therapies were not permitted except for anagrelide and hydroxyurea for the treatment of elevated platelet (>700 × 109/L) and white blood cell (WBC) counts (>50 × 109/L), respectively, for a maximum of 2 weeks. Administration of colony-stimulating factors and/or rh-Epo and/or IL-11 was left to the discretion of the investigator.
Patient Monitoring
Complete blood counts were performed weekly for the first 12 weeks and every 3 months thereafter. Cytogenetic and hematologic responses to dasatinib were assessed by bone marrow biopsies and aspirates every 12 weeks. Assessment of dasatinib toxicities included a physical examination conducted weekly for the first month and every 4 weeks thereafter. Response criteria have been detailed in previous studies.9 Briefly, a CHR required normalization for at least 4 weeks of the bone marrow (<5% blasts) and peripheral blood with a leukocyte count <10 × 109/L without blasts, promyelocytes, or myelocytes and a platelet count <450 × 109/L, in addition to the disappearance of all signs and symptoms of CML. Patients who achieved a CHR were further categorized according to cytogenetic response as follows: no cytogenetic response (Ph-positive 100%), minor cytogenetic response (Ph-positive 36%–90%), partial cytogenetic response (PCyR; Ph-positive 1%–35%), and CCyR (Ph-positive 0%). An MCyR encompassed CCyR and PCyR (ie, Ph-positive <35%). To determine molecular responses to dasatinib, BCR-ABL1 transcripts in peripheral blood were evaluated using quantitative reverse transcriptase–polymerase chain reaction at baseline and every month while the patient was on the study and compared with levels of total ABL1 transcripts. Adverse affects were evaluated at each visit and graded according to the National Cancer Institute Common Terminology Criteria (version 3.0).
Statistical Analysis
Univariate and multivariate analyses were performed to identify potential prognostic factors associated with the development of cytopenia. The chi-square test and the Mann-Whitney U test were used to identify prognostic factors, which were subsequently included as variables in a multivariate regression model for the development of cytopen. Multivariate analysis used the multivariate logistic regression model.
RESULTS
Study Group
We evaluated 130 patients with CP CML who were treated with dasatinib from November, 2003 to March, 2006 at the University of Texas M. D. Anderson Cancer Center, including 22 patients who were treated in a phase 1 study and 108 who treated in phase 2 or 3 studies (42 of them in a phase 2 frontline study for newly diagnosed patients). The median age of the patients was 54 years (range, 19 years–81 years), and the median time from CML diagnosis to the initiation of dasatinib therapy was 39 months (range, 0 months–207 months). Eighty-eight patients (68%) had received prior therapy with imatinib for a median of 31 months (range, 1 month–77 months). Imatinib therapy was withdrawn in 88% of patients due to imatinib resistance, whereas 12% of patients exhibited imatinib intolerance. Other prior therapies included interferon-α in 42 (48%) patients, homoharringtonine in 11 (13%) patients, and farnesyl transferase inhibitors or nilotinib in 5 (6%) patients each. Dasatinib therapy was administered for a median of 57 weeks (range, 4 weeks–204 weeks).
Dasatinib Therapy and Incidence of Neutropenia and Thrombocytopenia
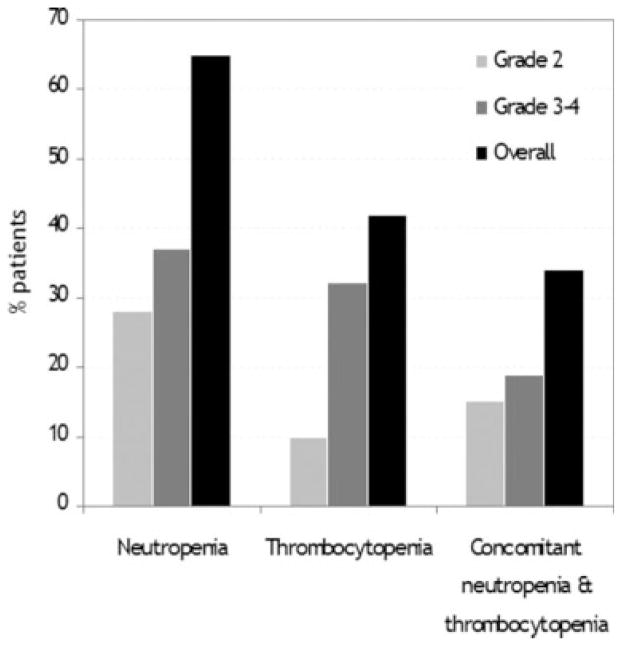
Clinical characteristics of the patients who developed cytopenia during dasatinib therapy are summarized in Table 2. Ninety-four (72%) patients developed at least 1 episode of grade 2 to 4 neutropenia and/or thrombocytopenia during dasatinib therapy, including 67 (52%) patients in whom the toxicities were grade 3 or 4. The initial dasatinib dose in patients who developed grade 3 to 4 cytopenia was 70 mg twice daily in 23 (34%) patients, 140 mg once daily in 8 (12%) patients, 50 mg twice daily in 8 (12%) patients, 100 mg once daily in 16 (24%) patients, >140 mg daily in 3 (4%) patients, and <100 mg daily in 9 (13%) patients. Grade 2 to 4 neutropenia was reported in 83 (65%) patients, and was grade 2 in 35 (28%) patients, grade 3 in 32 (25%) patients, and grade 4 in 18 (14%) patients (Figure 1). Grade 2 to 4 thrombocytopenia occurred in 54 (42%) patients, being grade 2 in 13 (10%) patients, grade 3 in 27 (21%) patients, and grade 4 in 14 (11%) patients. Forty-four (34%) patients developed concomitant grade 2 to 4 neutropenia and thrombocytopenia, being simultaneously grade 3 to 4 in 25 (19%) patients (Fig. 1). The median time from dasatinib therapy to the development of neutropenia was 42 days (range, 2 days–415 days), whereas the development of the first episode of thrombocytopenia occurred after a median of 31 days (range, 4 days–176 days) from the initiation of dasatinib. Grade 2 to 4 neutropenia or thrombocytopenia was recurrent in all but 11 (12%) patients. The development of a first episode of grade 2 to 4 neutropenia or thrombocytopenia occurred within the first 90 days of therapy in 82 (87%) patients, including 50 (53%) within the first 30 days. Among those with a first occurrence before Day 90, 10 (12%) patients had no further myelosuppression after Day 90, whereas the remainder had recurrent episodes. Notably, despite the relatively high incidence of grade 2 to 4 neutropenia, only 1 patient was hospitalized due to neutropenic fever resulting from pneumonia. The administration of intravenous antibiotics and G-CSF resulted in prompt recovery of a normal ANC, which facilitated continuation of dasatinib therapy.
Table 2.
Characteristics of Patients Who Developed Cytopenia During Dasatinib Therapy*
| All Patients | Grade 2–4 Neutropenia |
Grade 2–4 Thrombocytopenia |
Grade 2–4 Anemia |
|
|---|---|---|---|---|
| Total no. of patients | 94 | 83 | 54 | 64 |
| Sex, no. (%) | ||||
| Male | 39 (41) | 35 (37) | 24 (26) | 24 (25) |
| Female | 55 (59) | 48 (51) | 30 (32) | 40 (43) |
| Age, y | ||||
| Median | 54 | 54 | 54 | 57 |
| Range | 19–81 | 19–81 | 19–81 | 19–81 |
| Prior therapies, no. (range) | 1 (0–6) | 1 (0–6) | 2 (0–4) | 2 (0–4) |
| Frontline therapy, no. (%) | 31 (33) | 27 (33) | 11 (20) | 14 (23) |
| Imatinib, no. (%) | 63 (67) | 56 (67) | 43 (80) | 47 (77) |
| IFN-α, no. (%) | 40 (43) | 35 (42) | 28 (52) | 29 (48) |
| SCT, no. (%) | 2 (2.1) | 2 (2.4) | 2 (3.7) | 2 (3.2) |
| FTI, no. (%) | 5 (5.3) | 5 (6) | 2 (3.7) | 4 (5.9) |
| Months on imatinib | ||||
| Median | 38 | 36 | 38 | 40 |
| Range | 1–77 | 1–77 | 1–77 | 1–77 |
| Reason off imatinib, no. (%) | ||||
| Resistance | 52 (82) | 46 (83) | 38 (88) | 38 (81) |
| Intolerance | 11 (18) | 10 (17) | 5 (12) | 9 (19) |
| Weeks on dasatinib | ||||
| Median | 109 | 111 | 102 | 106 |
| Range | 4–204 | 4–204 | 4–192 | 4–204 |
| Time from dasatinib to cytopenias, d | ||||
| Median | 27 | 42 | 31 | 16 |
| Range | 2–415 | 2–415 | 4–176 | 3–182 |
IFN-α indicates interferon-α; SCT, stem cell transplantation; FTI, farnesyl transferase inhibitors.
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria (version 3.0).
FIGURE 1.
Incidence of grade 2 to 4 neutropenia and thrombocytopenia in patients with chronic myeloid leukemia in chronic phase who were receiving dasatinib therapy is shown.
Management of Dasatinib-Induced Neutropenia and Thrombocytopenia
The management of neutropenia and/or thrombocytopenia associated with dasatinib therapy involved transient treatment interruptions as well as the use of growth factors. Dasatinib interruptions were required during the course of therapy due to grade 3 to 4 neutropenia or thrombocytopenia in 35 (37%) patients. Of these, 16 (46%) patients required dasatinib dose reductions a median of 2 times (range, 1 time–4 times). Among patients who required treatment discontinuation, the median time off dasatinib was 24 days (range, 4 days–217 days). Twelve patients received therapy with G-CSF (filgrastim) at doses of 300 μg or 480 μg daily administered for 2 to 7 days per week. G-CSF therapy was initiated after a median of 80 days (range, 11 days–495 days) from the initiation of dasatinib. The G-CSF dose was adjusted to maintain an ANC >1.0 × 109/L. The median ANC at the time G-CSF was initiated was 0.75 × 109/L (range, 0.3 × 109/L –0.9 × 109/L). G-CSF was administered concomitantly with dasatinib therapy in 6 (50%) of 12 patients. All patients responded to G-CSF therapy, reaching an ANC >2.0 × 109/L after a median of 12 days. From the initiation of dasatinib therapy to the start of G-CSF, patients had been off dasatinib a median of 41% of the total treatment time compared with 21% after the initiation of G-CSF (P = .08). The combination of dasatinib and G-CSF was well tolerated in all cases. Before the administration of G-CSF, none of the 12 patients treated had achieved a MCyR. In contrast, after the initiation of G-CSF therapy, 5 of 9 evaluable patients improved their cytogenetic response, including 1 patient with MCyR and 1 with CCyR.
Three patients who developed grade 3 to 4 dasatinib-induced thrombocytopenia received growth factor support with IL-11. Initially, IL-11 was administered at a dose of 10 μg/kg 3 times per week. In 2 of these patients, IL-11 was administered concomitantly with dasatinib therapy. None of these patients had required platelet transfusion. After the initiation of IL-11 therapy, 2 patients acheived a platelet count >100 × 109/L, which occurred 94 days and 125 days, respectively, after IL-11 therapy was initiated. One other patient developed protracted thrombocytopenia in spite of the administration of IL-11, which led to the termination of dasatinib therapy.
Incidence and Management of Dasatinib-Induced Anemia
Of the 94 patients who developed grade 2 to 4 neutropenia or thrombocytopenia, 64 (68%) developed grade 2 to 4 anemia, and 15 (16%) developed grade 3 to 4 anemia. In these patients, the median time from the initiation of dasatinib therapy to the development of grade 3 anemia was 16 days (range, 3 days–182 days). Twenty-nine (45%) patients received growth factor support with rh-Epo, including both epoetin-α (n = 22) and darbepoetin-α (n = 7). Therapy with rh-Epo was titrated to maintain a hemoglobin level >10 g/dL. Of the 29 patients who received rh-Epo therapy, 23 (79%) experienced increments of their hemoglobin levels >2 g/dL. The median time to achieve a hemoglobin increment ≥2 g/dL was 17 days (range, 7 days–84 days). However, 7 of the patients receiving rh-Epo support required concomitant transfusions of packed red blood cells (PRBC). In this subset of patients, the median number of transfused PRBC units was 4 (range, 2 units–10 units).
Relation Between the Development of Cytopenia and Dasatinib Dose Schedule
Because different dasatinib dose schedules were used in the phase 1, 2, and 3 studies, we investigated the impact of these variables on the development of cytopenia (Table 3). Of 90 patients who received an initial daily dose of ≤100 mg, 57 (63%) developed grade 2 to 4 cytopenia, compared with 37 (92%) of 40 patients who were given dasatinib at an initial dose of >100 mg (P = .001). Of the 42 newly diagnosed patients who received single-agent dasatinib therapy as frontline therapy, 31 (74%) developed grade 2 to 4 cytopenia. Of these 31, 16 patients received dasatinib at a dose of 50 mg twice daily and 15 received dasatinib at a dose of 100 mg daily.
Table 3.
Incidence of Grade 3–4 Cytopenia During Dasatinib Therapy by Dasatinib Dose and Daily Schedule*
| Type of Cytopenia, No. (%) | |||
|---|---|---|---|
| Total (n=67) | Grade 3–4 Neutropenia |
Grade 3–4 Thrombocytopenia |
Grade 3–4 Anemia |
| Dasatinib daily dose | |||
| >140 mg/d | 2 (3) | 3 (4) | 0 (0) |
| 140 mg/d | 22 (33) | 20 (30) | 5 (7) |
| 100 mg/d | 19 (28) | 13 (19) | 5 (7) |
| <100 mg/d | 7 (10) | 5 (7) | 5 (7) |
| Schedule | |||
| Once daily | 23 (34) | 17 (25) | 7 (10) |
| Twice daily | 27 (40) | 24 (36) | 8 (12) |
Toxicities were graded according to the National Cancer Institute Common Terminology Criteria (version 3.0).
On univariate analysis, variables associated with a higher risk of the development of cytopenia included longer time from diagnosis to treatment, prior interferon or imatinib therapy, and a lower WBC (Table 4). A multivariate analysis did not identify any independent risk factors, most likely because of the high incidence of events.
Table 4.
Multivariate Logistic Regression Model: Estimate of the Association Between Patient Characteristics and Risk of Development of Cytopenia, Considering Other Covariates in the Model
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | Effect | P* | Effect | P* | |
| Dose schedule | bid vs qd | .46 | |||
| Splenomegaly | Yes vs no | .13 | |||
| CE | Yes vs no | .75 | |||
| Ph>90% | Yes vs no | .65 | |||
| Prior SCT | Yes vs no | .38 | |||
| Prior IFN | Yes vs no | Worse | .03 | NS | .13 |
| Prior imatinib | Yes vs no | Worse | .05 | NS | .72 |
| Cytogenetic response on imatinib | Yes vs no | .71 | |||
| CML stage | CP vs AP | .72 | |||
| Age | Older | .15 | |||
| CML duration | Longer | Worse | .05 | NS | .25 |
| WBC | Lower | Worse | .04 | NS | .11 |
| PB basophil, % | Higher | .6 | |||
| PB blast, % | Higher | .55 | |||
| BM blast, % | Higher | .75 | |||
| BM basophil, % | Higher | .89 | |||
| Platelets | Lower | .13 | |||
| Hemoglobin | Lower | .17 | |||
| Dasatinib dose | Higher | .47 | |||
bid indicates twice daily; qd, daily; CE, clonal evolution; Ph, Philadelphia chromosome; SCT, stem cell transplantation; IFN, interferon; NS, not significant, CML, chronic myeloid leukemia; CP, chronic phase; AP, accelerated phase; WBC, white blood cell; PB, peripheral blood; BM, bone marrow.
Bold type indicates statistical significance.
DISCUSSION
Dose-dependent hematologic toxicity, frequently affecting several hematopoietic lineages, is a common adverse effect of imatinib therapy in patients with CML.10 The main objective of the current study was to study the dynamics of cytopenias associated with dasatinib therapy and the efficacy of different management strategies to overcome this adverse event. Because the majority of patients with advanced phase CML (accelerated phase or blastic phase) have cytopenias even before the initiation of therapy, we focused our investigation on 130 consecutive patients with CP CML who had failed imatinib and subsequently received dasatinib either on an open-label phase 1 study (n = 22) or on phase 2 or 3 studies (n = 108). Hematologic toxicity was relatively frequent, but this was generally manageable with transient dasatinib interruptions, dose reductions, growth factor support, or combinations thereof.
Hematologic toxicity is the most common grade 3 to 4 adverse event reported with imatinib therapy, particularly in patients with advanced phase CML. Interestingly, the development of new-onset grade 3 to 4 hematologic toxicity decreased drastically after 2 years of imatinib therapy.10 Grade 3 to 4 hematologic toxicity is also the most frequently reported adverse event in patients treated with dasatinib after failure of imatinib therapy. In a multinational phase 2 study of dasatinib that included 387 patients with CP CML after failure of imatinib therapy, grade 3 to 4 neutropenia and thrombocytopenia were reported in 50% and 49%, respectively, after 24 months of follow-up.11 In the current study, the development of hematologic toxicity during dasatinib therapy was also frequently observed, but this was reversible and manageable with dose adjustments and/or transient treatment interruptions. In our analysis, the dose and schedule of dasatinib was not identified as a risk factor for grade 2 to 4 myelosuppression. This was most likely due to the high percentage of patients treated with dasatinib as frontline therapy, all of them with a daily dose of 100 mg. These patients had a lower incidence of myelosuppression. When using dasatinib after imatinib failure, a recent phase 3 study randomized patients to receive dasatinib at either 50 mg or 70 mg twice daily or 100 mg or 140 mg once daily.12 In this study, the durations of cytogenetic response and progression-free survival were found to be similar across all 4 treatment arms but there were significantly fewer cases of grade 3 to 4 neutropenia, thrombocytopenia, and anemia in the 100-mg-daily arm compared with the other 3 arms combined.12
To our knowledge, the exact mechanism whereby ABL1 kinase inhibitors induce hematologic toxicity has not been completely ascertained. In patients with untreated CML, normal hematopoiesis relies mostly on the Ph-positive clones. The brisk clearance of the malignant progenitors from the bone marrow by ABL1 kinase inhibitors is believed to lead to a scenario in which the remainder of the Ph-negative progenitors would be rendered insufficient to sustain normal blood production. Similar to imatinib,13 dasatinib is highly selective against Ph-positive bone marrow progenitors with little effect on normal Ph-negative progenitors. Dasatinib at a concentration of 5 nM was not reported to inhibit the growth of bone marrow progenitors isolated from healthy individuals, but inhibited by 60% to 80% the growth of bone marrow progenitors isolated from patients with CML, both those expressing wild-type BCR-ABL1 and those expressing the mutant M351T isoform.14
The development of dasatinib-induced hematologic toxicity can potentially compromise the achievement of clinical responses. Among patients with CP CML who were treated with imatinib, the development of grade 3 to 4 neutropenia resulted in a significantly lower probability of achieving a CCyR compared with patients who did not have this complication (44% vs 62%; P = .03).15 This risk has also been documented for patients who develop grade 3 to 4 thrombocytopenia.15 The occurrence of these toxicities within 45 days to 90 days after the initiation of imatinib therapy was associated with poor survival.16 Both the prognostic implications and the decreased probability of the achievement of CCyR secondary to the development of hematologic toxicity during imatinib therapy are likely to translate to patients receiving dasatinib and, in all likelihood, are a consequence of a lower dose intensity secondary to frequent interruptions and/or dose reductions. A potential means to overcome dasatinib-induced hematologic toxicity is the use of growth factors. In the current study, 12 patients received therapy with G-CSF that was adjusted to maintain an ANC >1.0 × 109/L. All patients responded to G-CSF, including 6 who achieved an ANC >2.0 × 109/L while receiving uninterrupted dasatinib therapy. The time off dasatinib before and after G-CSF therapy was 41% and 21%, respectively (P = .08). In addition, G-CSF therapy was associated with the achievement of MCyR in 2 of 9 evaluable patients. These results are in accord with previously reported data derived from patients with CML receiving growth factor support for imatinib-induced hematologic toxicity.4,5,17–19 Finally, given that potentially serious infections may ensue in the context of grade 3 to 4 neutropenia, it is remarkable that only 1 patient in the current study required hospitalization for neutropenic fever associated with pneumonia. This low rate of infectious complications could be due to the prompt administration of G-CSF.
Neither disease progression nor thromboembolic events were observed in any patient receiving rh-Epo in the current study. This is particularly important in view of the recent public health advisory issued by the US Food and Drug Administration regarding the use of erythropoiesis-stimulating agents, based on an increased rate of adverse outcomes in a series of trials involving patients with squamous cell carcinoma of the head and neck and metastatic non-small cell lung cancer.20,21 Far from promoting disease progression, the use of growth factors in the current study was found to hasten the recovery of peripheral blood counts in most patients, which resulted in the administration of dasatinib in a more continuous manner. In addition, therapy with filgrastim was found to be associated in some cases with the achievement of cytogenetic responses (MCyR in 1 patient and CCyR in 1 patient). However, because G-CSF was initiated soon after the beginning of therapy with dasatinib (median, 80 days), we cannot rule out the possibility that these responses might have occurred later in the course of treatment regardless of the use of growth factor support. The lack of adverse events reported in our trial may be due to the finding that the dosages of rh-Epo and G-CSF were adjusted to maintain the lowest hemoglobin and ANC levels needed to avoid blood transfusions or infections, respectively.
In conclusion, hematologic toxicity is a frequent occurrence in patients with CP CML who are receiving dasatinib. This complication can be readily managed with transient dasatinib interruptions and dose adjustments. Most importantly, this complication can be frequently overcome by using growth factor support, which in this context, is safe and allows for a more continuous dasatinib administration that may result in improved clinical outcomes.
Footnotes
Conflict of Interest Disclosures
The authors made no disclosures
References
- 1.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl-phenyl)-2-(6-(4-(2-hydroxyethyl)-piperazin-1-yl)-2-methylpyrimidin-4-ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47:6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 2.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 3.Baccarani M, Rosti G, Saglio G, et al. Dasatinib time to and durability of major and complete cytogenetic response (MCyR and CCyR) in patients with chronic myeloid leukemia in chronic phase (CML-CP) [abstract] Blood. 2008;112:Abstract 450. [Google Scholar]
- 4.Quintas-Cardama A, Kantarjian H, O’Brien S, et al. Granulocyte-colony-stimulating factor (filgrastim) may overcome imatinib-induced neutropenia in patients with chronic-phase chronic myelogenous leukemia. Cancer. 2004;100:2592–2597. doi: 10.1002/cncr.20285. [DOI] [PubMed] [Google Scholar]
- 5.Ault P, Kantarjian H, Welch MA, Giles F, Rios MB, Cortes J. Interleukin 11 may improve thrombocytopenia associated with imatinib mesylate therapy in chronic myelogenous leukemia. Leuk Res. 2004;28:613–618. doi: 10.1016/j.leukres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Cortes J, O’Brien S, Quintas A, et al. Erythropoietin is effective in improving the anemia induced by imatinib mesylate therapy in patients with chronic myeloid leukemia in chronic phase. Cancer. 2004;100:2396–2402. doi: 10.1002/cncr.20292. [DOI] [PubMed] [Google Scholar]
- 7.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 8.Godwin JE, Kopecky KJ, Head DR, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study. Blood. 1998;91:3607–3615. [PubMed] [Google Scholar]
- 9.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 11.Mauro M, Baccarani M, Cervantes F, et al. Dasatinib 2-year efficacy in patients with chronic-phase chronic myelogenous leukemia (CML-CP) with resistance or intolerance to imatinib (START-C). [abstract] Proc Am Soc Clin Oncol. 2008;26(May 20 suppl):Abstract 7009. [Google Scholar]
- 12.Shah N, Kim DW, Kantarjian H, et al. Dasatinib dose-optimization in chronic phase chronic myeloid leukemia (CML-CP): 2-year data from CA180–034 show equivalent long-term efficacy and improved safety with 100 mg once daily dose [abstract] Blood. 2008;112:Abstract 3225. [Google Scholar]
- 13.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 15.Sneed TB, Kantarjian HM, Talpaz M, et al. The significance of myelosuppression during therapy with imatinib mesylate in patients with chronic myelogenous leukemia in chronic phase. Cancer. 2004;100:116–121. doi: 10.1002/cncr.11863. [DOI] [PubMed] [Google Scholar]
- 16.Marin D, Marktel S, Bua M, et al. Prognostic factors for patients with chronic myeloid leukaemia in chronic phase treated with imatinib mesylate after failure of interferon alfa. Leukemia. 2003;17:1448–1453. doi: 10.1038/sj.leu.2402996. [DOI] [PubMed] [Google Scholar]
- 17.Marin D, Marktel S, Foot N, Bua M, Goldman JM, Apperley JF. Granulocyte colony-stimulating factor reverses cytopenia and may permit cytogenetic responses in patients with chronic myeloid leukemia treated with imatinib mesylate. Haematologica. 2003;88:227–229. [PubMed] [Google Scholar]
- 18.Zaucha JM, Wyrowinska E, Prejzner W, Calbecka M, Hellmann A. Imatinib-associated neutropenia may not be overcome by filgrastim treatment in patients with blastic phase of chronic myeloid leukaemia. Clin Lab Haematol. 2006;28:208–210. doi: 10.1111/j.1365-2257.2006.00772.x. [DOI] [PubMed] [Google Scholar]
- 19.Heim D, Ebnother M, Meyer-Monard S, et al. G-CSF for imatinib-induced neutropenia. Leukemia. 2003;17:805–807. doi: 10.1038/sj.leu.2402869. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg E, Catley L, Wright RD, et al. Beneficial effects of combining nilotinib and imatinib in preclinical models of BCR-ABL+ leukemias. Blood. 2007;109:2112–2120. doi: 10.1182/blood-2006-06-026377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khuri FR. Weighing the hazards of erythropoiesis stimulation in patients with cancer. N Engl J Med. 2007;356:2445–2448. doi: 10.1056/NEJMp078101. [DOI] [PubMed] [Google Scholar]