Abstract
Cell nuclei are physically integrated with the cytoskeleton through the LINC complex (for LInker of Nucleoskeleton and Cytoskeleton), a structure that spans the nuclear envelope to link the nucleoskeleton and cytoskeleton. Outer nuclear membrane KASH domain proteins and inner nuclear membrane SUN domain proteins interact to form the core of the LINC complex. In this review we provide a comprehensive analysis of the reported protein-protein interactions for KASH and SUN domain proteins. This critical structure, directly connecting the genome with the rest of the cell, contributes to a myriad of cellular functions and, when perturbed, is associated with human disease.
Keywords: cytoskeleton, KASH, LINC complex, nuclear envelope, nucleus, SUN
Introduction
Mechanical integration of the nucleus and cytoskeleton was revealed by experiments that pulled on cell surface integrin receptors to induce concomitant deformation of the nucleus(Maniotis et al., 1997). Since those original observations, we have come to understand the primary structure by which the nucleus is physically integrated with the cytoskeleton and many of the various ways it influences cellular organization and function. Called the LINC-complex for LInker of the Nucleoskeleton and Cytoskeleton(Crisp et al., 2006), the structure spans the nuclear envelope (NE) to physically connect the cytoskeleton to the nucleoskeleton. The NE is a specialized extension of the endoplasmic reticulum (ER) that encases the genome during interphase, thus separating the nucleus from the cytoplasm. As a component of the ER, the NE has two membranes, an outer and inner nuclear membrane (ONM and INM, respectively) as well as a lumenal region called the perinuclear space (PNS). These two membranes are continuous with the rest of the ER and the PNS is continuous with the ER lumen. The INM and ONM are directly connected by numerous annulate junctions of high membrane curvature. Within these NE gaps reside large proteinaceous nuclear pore complexes (NPCs) that, among other functions, enable and regulate nucleo-cytoplasmic trafficking. Underlying the INM is a major constituent of the nucleoskeleton called the nuclear lamina, composed of type-V intermediate filaments called lamins. The LINC complex is fundamentally composed of ONM-resident KASH domain proteins that translumenally interact with INM-resident SUN domain proteins (hereafter simply called KASH and SUN proteins). In this way the KASH and SUN proteins create a structure that spans the NE. By association of KASH proteins with cytoskeletal proteins and SUN proteins with nucleoskeletal proteins, the LINC complex can integrate these mechanical networks. Although cytoskeletal and nucleoskeletal proteins contribute to the LINC complex, this review will focus on interacting partners of the core LINC complex, the KASH and SUN proteins.
Structure of the LINC complex
SUN proteins are type-II transmembrane proteins with nuclear-oriented N-termini and lumenal C-termini. Named for SUN domain family members (Sad1p, Unc-84) (Malone et al., 1999) they all share a conserved SUN domain at the C-terminus of the lumenal domain. This lumenal region of SUN proteins also includes an extended coiled-coil domain. The nucleoplasmic domain of SUN proteins is structurally less characterized, but at least in some mammalian family members, has been shown to be capable of nuclear localization independent of the rest of the protein(Haque et al., 2006; Hodzic et al., 2004; Liu et al., 2007). KASH domain proteins, named for Klarsicht, Anc-1, Syne-1 homology (Starr and Han, 2002), are also type-II transmembrane proteins that have a short luminal tail with a very loosely conserved PPPX motif at the C-terminus (this is most conserved in mammals where X is variable residue), and a far more diverse and sizeable cytoplasmic N-terminus.
Based on structural analysis of human Sun2 (Sosa et al., 2012; Wang et al., 2012; Zhou et al., 2012b), the coiled-coil domain of SUN proteins homo-typically associate to create an extended trimeric stalk. Within this complex, each of the three globular SUN domains are positioned proximate to the ONM where they can each bind to an individual KASH domain in the binding pocket the SUN domains form, in part via its PPPX motif. Conserved cysteine residues, one each in the KASH and SUN domain, may stabilize this association by disulfide bond formation. In this way the KASH and SUN proteins interact to create a stable tether between the INM and ONM. Although their exact stoichiometry remains unclear, many KASH proteins have also been shown to homotypically oligomerize, potentially contributing to the mechanical stability of these complexes. In mammals there appears to be general promiscuity of KASH-SUN protein interactions. Consequently, there is no clear preference for a specific SUN protein to bind a particular KASH protein (Stewart-Hutchinson et al., 2008).
Functions of an ancient LINC
The LINC complex has been studied in yeast, worms, flies, fish, mammals and plants. The fundamental constituents of the structure are well conserved throughout eukaryotic cells, although there is a diverse repertoire of KASH and SUN proteins that varies between species. The number of KASH and SUN domain proteins generally increases with organism complexity. In mammals there is clear evidence for cell-type specific members of the LINC complex. Many of the functions of the LINC complex are also well conserved. These functions include nuclear retention, nuclear migration, cell polarity, cytoskeletal organization, meiotic chromosome movement to facilitate recombination, protein recruitment to the ONM and potentially mechanotransduction. Not surprisingly there is growing evidence of LINC complex involvement in human disease. In humans, autosomal dominant mutations in KASH protein genes, SYNE1 and SYNE2, are reportedly associated with Emery-Dreifuss Muscular Dystrophy (EMD) (Wheeler et al., 2007; Zhang et al., 2007a). In addition, autosomal recessive mutations in SYNE1 are associated with cerebellar ataxia (Gros-Louis et al., 2007) and arthrogyrposis (Attali et al., 2009). A frameshift mutation of Nesprin4 was recently identified in hereditary hearing loss (Horn et al., 2013a). Mutations in genes encoding SUN proteins have recently been associated with muscular dystrophy (Meinke et al., 2014). Interestingly, loss of Sun1 ameliorates the pathology resulting from altered expression of A-type lamins in mouse models of EMD and Hutchinson-Gilford Progeria Syndrome (HGPS) (Chen et al., 2012).
Functional interactions of the LINC complex
Yeast
In yeast, the LINC complex has two primary functions: providing chromosome movement and embedding the spindle pole body (SPB) within the NE. The ONM proteins that bind to the SUN proteins function like KASH proteins but lack the conserved proline residue adjacent to the C-terminus, found in many other eukaryotes.
S. cerevisiae
- SUN proteins
Mps3 interacts with Mps2, a KASH protein coupled with the SPB (Conrad et al., 2008; Friederichs et al., 2011; Koszul et al., 2008). In this way, the interaction between Mps3 and Mps2 leads to recruitment of the SPB into the NE.
Mps3 also functions during meiosis. In S. cerevisiae, meiotic telomeres are tethered to the INM and transiently clustered at one pole of the NE, where the SPB locates. In meiotic telomere clustering, often called bouquet, Mps3 interacts with a telomere adaptor protein Ndj1 (Conrad et al., 2008; Conrad et al., 2007; Kosaka et al., 2008; Wanat et al., 2008). The interaction of Ndj1 with Mps3 is crucial for telomere tethering. Loss of Ndj1 leads to meiotic chromosome segregation defects (Conrad et al., 2007). The force translocating telomeres is delivered by Csm4, a meiotic specific KASH protein that appears to interact with the actin cytoskeleton (Conrad et al., 2008; Kosaka et al., 2008; Koszul et al., 2008; Wanat et al., 2008).
Functions of Mps3 extend beyond retention of KASH proteins on the ONM. In interphase, telomeres are tethered at the NE largely by two different mechanisms. In both cases, the interaction between telomere binding proteins and Mps3 mediates telomere tethering (Bupp et al., 2007; Schober et al., 2009). The association between telomeres and the NE during interphase is thought to protect telomeres from transcription and inappropriate recombination. One mechanism of telomere tethering is mediated by the interaction between telomere-bound ribosome biogenesis factors and Mps3, which also recruits Sir4, a gene silencing protein (Bupp et al., 2007; Horigome et al., 2011). The other mechanism requires the interaction between Mps3 and DNA double-strand break ends binding proteins Ku70/Ku80 hetero-dimer protein (Antoniacci et al., 2007; Schober et al., 2009). Interestingly, chromosome regions with DNA double stand breaks (DSB) are also tethered by Msp3, which is assisted by Ku70/Ku80 (Oza et al., 2009).
In addition to the roles of Mps3 in chromosome organization, Mps3 has been shown to bind directly to Replication Factor C (RFC) complex subunits and histone variant Htz1, which are linked with DNA replication and repair mechanism(Haas et al., 2012). Mps3 also directly binds to acetyltransferase Eco1/Ctf7 resulting in post-translational modification of Mps3. Acetylation of Mps3 is required for accurate sister chromatid cohesion and for chromosome recruitment to the nuclear membrane (Antoniacci et al., 2004; Ghosh et al., 2012). Although Csm4, a KASH protein of S. cerevisiae, has been implicated to participate in the Mps3-mediated interphase chromosome tethering (Chan et al., 2011), the role of KASH proteins still remains elusive.
- KASH proteins
Mps2 is required to embed SPB in the NE, much like a NPC. The N-terminus of Mps2 interacts with Bbp1, which interacts with the core SPB protein Spc29 (Schramm et al., 2000). In turn, the C-terminus of Mps2 interacts with Mps3. Together, these three proteins, Mps3, Mps2, and Bbp1, form the half-bridge, a protein complex that anchors the SPB within the NE (Schramm et al., 2000). Another binding partner of Mps2 is Spc24, a peripheral kinetochore component involved in chromosome segregation (Le Masson et al., 2002). However, the role of Mps2 in chromosome segregation remains unclear.
Csm4 is retained on the ONM via association with Mps3 (Conrad et al., 2008; Kosaka et al., 2008; Koszul et al., 2008; Wanat et al., 2008). There is no direct evidence regarding the cytoplasmic binding partners of Csm4. Perturbation of the actin cytoskeleton leads to failure of telomere movement during meiosis, which resembles the phenotype of Csm4 deletion mutants (Koszul et al., 2008; Scherthan et al., 2007; Trelles-Sticken et al., 2005; Wanat et al., 2008; Yamamoto et al., 1999). Given these observations, it has been proposed that Csm4 interacts with the actin cytoskeleton to mediate the movement of Mps3-tethered telomeres in meiosis.
S. pombe
- SUN proteins
Sad1, the only known SUN protein in S. pombe, is required for the SPB insertion to the NE via its KASH partner Kms2. On the nucleoplasmic side, Sad1 works cooperatively to connect chromatins to the SPB during interphase. In this complex, integral membrane proteins Ima1 and Ndc80 stabilize the complex of Sad1 and centromeres (King et al., 2008). Interaction of Sad1 with centromeres, assisted by Ima1 and Ndc80, suggests the role of these proteins in maintenance of nuclear organization. However, recent studies suggest a dispensable role of Ima1 in linking centromeres to the SPB (Hiraoka et al., 2011).
During interphase, centromeres of S. pombe are concentrated at the NE where the SPB is inserted. This centromere anchoring is mediated by Sad1 and Csi1 (Hou et al., 2012). Csi1 interacts both with Sad1 and kinetochore at the same time, thus bridging centromeres and Sad1. The current model suggests that the interaction between Sad1 and Csi1, which closely locates centromeres to the SPB, aids kinetochore capture microtubules as cells enter early mitosis. Csi1 mutants demonstrated defects in chromosome segregation and mitosis progression (Hou et al., 2012).
Sad1 also mediates a repetitive chromosome movement that stretches the NE during meiosis. This characteristic movement of chromosomes, often called “horse tail” movement, is led by the association between NE-tethered telomeres and dynein bound KASH protein Kms1 (Niwa et al., 2000; Shimanuki et al., 1997). Sad1 tethers telomeres to the NE via meiotic specific proteins, Bqt1 and Bqt2. The direct interaction between Sad1 and Bqt1 recruits Bqt2, which then allows interaction with a telomere binding protein Rap1 (Chikashige et al., 2006).
- KASH proteins
Kms1 mediates “horsetail” movement during meiosis via dynein. Although identified genetically (Niwa et al., 2000; Shimanuki et al., 1997), the physical interaction between dynein and Kms1 is not fully characterized.
Kms2 accompanies the SPB during interphase. Recently, core SPB components Cut12 and Pcp1 were identified as direct binding partners of Kms2 (Walde and King, 2014). Loss of Kms2 led to late mitotic entry, disruption of stable bipolar spindle formation, and abnormal insertion of the SPB. Interestingly, defects in recruitment of polo kinase, Plo1, to the SPB at mitotic entry were also observed in Kms2 deletion mutant cells (Walde and King, 2014), suggesting a role of Kms2-Sad1 in regulation of the SPB integration and mitotic entry.
Insects
The LINC complex in Drosophila melanogaster is involved in nuclear migration and positioning. Although highly conserved with the mammalian LINC complex, the mechanisms of these functions of the LINC complex in D. melanogaster remain unclear.
- SUN proteins
Spag4, also known as Giacomo, is a homolog of the mammalian SUN protein Spag4, which is specifically expressed in the male reproductive organ (Kennedy et al., 2004; Shao et al., 1999). Spag4 plays a role in nuclear and centriolar attachment during spermatogenesis. Knockout of Spag4 leads to sterility in males suggesting that it has a crucial role in spermatogenesis. A coiled-coil cytoplasmic protein Yuri Gagarin and dynein-dynactin are also involved during this process and have been found to colocalize with Spag4. Intriguingly, other known drosophila KASH proteins, Klarsicht and MSP-300, are dispensable for this process (Kracklauer et al., 2010).
Klaroid is essential to localize its KASH proteins Klarsicht and Msp-300 (Kracklauer et al., 2007; Malone et al., 2003; Technau and Roth, 2008). With these KASH protein partners, Klaroid is necessary for nuclear migration in differentiation of the eye disc and muscle cells. The B-type lamin, Lam Dm0, is required for proper targeting of Klaroid (Patterson et al., 2004).
- KASH proteins
Klarsicht (Klar) is an essential KASH-domain protein for proper migration of nuclei during the eye development (Fischer-Vize and Mosley, 1994; Kracklauer et al., 2007; Mosley-Bishop et al., 1999; Patterson et al., 2004). In either Klar or dynein mutants, the nuclei in the eye disc fail to migrate (Kracklauer et al., 2007; Patterson et al., 2004; Swan et al., 1999). Since Klar co-localizes with microtubules at the NE, it is speculated that microtubule-dependent motor protein complexes associated with Klar may mediate nuclear migration (Fischer et al., 2004; Mosley-Bishop et al., 1999; Patterson et al., 2004; Welte, 2004). However, a Klar-associated motor protein has not yet been defined.
Msp-300 interacts with the actin cytoskeleton via its N-terminal actin binding domain. The interaction between Msp-300 and the actin cytoskeleton was originally proposed as an essential KASH-domain protein for the positioning of nuclei during muscle and ovarian nurse cell development (Rosenberg-Hasson et al., 1996; Volk, 1992; Yu et al., 2006). More recently, however, several other groups contest that Msp-300 is dispensable in the previously mentioned tissues (Technau and Roth, 2008; Xie and Fischer, 2008). Msp-300 has been found to form a specialized ring structure at the periphery of larval myogenic NE. In this ring structure, Msp-300 associates with Klar, suggesting that the interactions between Mps-300 and Klar link microtubules to the NE (Elhanany-Tamir et al., 2012; Volk, 2013).
Worms
The LINC complex of Caenorhabditis elegans plays an important role in nuclear migration, positioning, and meiotic chromosome movement.
- SUN proteins
SUN1/MTF-1 and UNC-84 are the only identified SUN domain proteins in C. elegans. SUN1/MTF1 is required for localization of ZYG-12 and Kdp-1 on the ONM (Malone et al., 2003; McGee et al., 2009). SUN1/MTF1 is involved in chromosome movement during meiosis. C. elegans chromosomes harbor specific regions called pairing centers where meiosis specific zinc-finger proteins Him-8, Zim1, Zim2, and Zim3 mediate the attachment of meiotic chromosomes to the NE. In the pairing center, SUN1/MTF1 is also found co-localized with Him-8 (Sato et al., 2009). Interestingly, Sun1/MTF1 is involved in apoptosis. It binds to pro-apoptotic factor CED4 (Tzur et al., 2006a) and subsequently shifts the location of CED4 from mitochondria to the NE. The interaction between Sun1/MTF1 and CED4 is thought be required for apoptosis.
Unc-84 is essential to localize Unc-83 and Anc-1 on the ONM (Starr and Han, 2002; Starr et al., 2001). The interaction with Ce-lamin is required for targeting of Unc-84 (Lee et al., 2002).
- KASH proteins
Anc-1 bridges the NE to the actin cytoskeleton via its N-terminal actin binding domain (Starr and Han, 2002). Anc-1 is known to contribute in tethering clustered nuclei to the actin cytoskeleton in hypodermal cells. Loss of function mutants of Anc-1 lead to unanchored nuclei that aggregate in the cytoplasm (Starr and Han, 2002).
A recent study revealed a novel interaction between Anc-1 and the PHR protein Rpm-1, a large signaling protein involved in numerous developmental events in neurons. This interaction positively regulates a β-catenin protein, Bar-1, which functions in development of axon termination in the mechanosensory neurons and synapse formation in the GABAergic motor neurons (Tulgren et al., 2014).
Unc-83 plays a role in nuclear migration, which is developmentally important in a wide variety of tissues. It was originally found to associate both with kinesin and dynein (Fridolfsson et al., 2010; Meyerzon et al., 2009). Mutations in kinesin-1 demonstrated disorganized nuclei in hypodermal cells, which lead to developmental defects in neurons and vulva cells (Meyerzon et al., 2009). The loss of dynein, however, caused minimal defects (Fridolfsson et al., 2010). A recent study revealed dynein induces a short backward movement of hypodermal nuclei migration, which contributes to efficient nuclear migration (Fridolfsson et al., 2010). These observations suggest that the association between Unc-83 and kinesin has a major role during nuclear migration of hypodermal cells, as assisted by dynein.
Zyg-12 plays an essential role in pronuclei fusion that requires the association of the NE and centrosome. During pronuclear fusion, Zyg-12 is retained by Sun1 at the NE where it interacts with dynein. In contrast, the KASH-less isoform of Zyg-12 (Zyg-12A) associates with the centrosome (Malone et al., 2003; Meyerzon et al., 2009). Centrosome attachment is completed by the dimerization of both membrane and centrosome bound Zyg-12 (Malone et al., 2003). Zyg-12 also functions in early meiosis when it accumulates at the pairing center (MacQueen et al., 2005; Phillips and Dernburg, 2006) and associates with dynein. The interaction between dynein and Zyg-12 drives the tethered chromosomes to form a meiotic bouquet and facilitates proper pairing of chromosomes (Sato et al., 2009).
Kdp-1 is a recently identified KASH-domain protein. The cytoplasmic binding partner of Kdp-1 has not yet been identified. Kdp-1 is essential for viability and development of C. elegans, suggesting its role in cell cycle progression (McGee et al., 2009).
Fish
LRMP is the only known KASH protein in Danio rerio. It is involved in pronuclear fusion and attachment of the centrosome to the NE (Lindeman and Pelegri, 2012). The cytoplasmic binding partners of LRMP, as well as the identity of cognate SUN proteins, are currently unknown.
Mammals
The mammalian LINC complex is structurally conserved with other organisms’ LINC complex. There are several KASH and SUN proteins, some with tissue-specific expression and functions. SUN proteins appear to share their functions to retain KASH proteins at the ONM. Evidence of unique roles for SUN protein variants remains limited.
- SUN proteins
There are five conventional SUN proteins in mammals. Sun1 and 2 are widely expressed whereas Sun3-5 are restricted to the testis(Crisp et al., 2006; Haque et al., 2006; Hodzic et al., 2004; Shao et al., 1999; Tzur et al., 2006b). Despite the interaction with A-type lamin, targeting of SUN1 and 2 is not primarily dependent on A-type lamins (Crisp et al., 2006; Haque et al., 2006). A sixth mammalian Sun-like protein is osteopotentia, which harbors an internal SUN domain. Whether osteopotentia has the capacity to bind KASH-domain proteins remains unclear (Sohaskey et al., 2010).
In mammalian meiosis, Sun1is required for tethering meiotic telomeres to the NE (Ding et al., 2007; Horn et al., 2013b; Morimoto et al., 2012). In this way, Sun1 and KASH5 mediate telomere movement and proper pairing of chromosomes during meiosis (Horn et al., 2013b). However, the nuclear proteins that mediate telomeres and Sun1 are currently unknown.
Sun1η is a newly identified splicing variant of Sun1 which interacts with Nesprin3 at the anterior of the outer acrosomal membrane of sperm (Gob et al., 2010). In addition, it was proposed that Sun3 and Nesprin-1contribute to formation of polarized sperm heads at the posterior of acrosomal membrane of sperm (Gob et al., 2010).
As for SUN proteins’ role within the NE, recent studies have revealed the involvement of Sun1 and Sun2 in the DNA damage response (DDR). Both were found to interact with DNA-dependent kinase (DNAPK), Ku70, and Ku80 to elicit DNA damage response. Sun1/Sun2 double knockout fibroblasts exhibit DNA damage and compromised DDR activation (Lei et al., 2012). In addition to lamin-A, Sun1 has been shown to associate with hALP (NAT10) and be involved in chromosome de-condensation following mitosis (Chi et al., 2007). Both Sun1 and Sun2 have been shown to bind to SAMP1 (TMEM201) (Borrego-Pinto et al., 2012; Jafferali et al., 2014), an INM protein that appears to regulate the role of the LINC complex in cell migration (Borrego-Pinto et al., 2012).
- KASH Proteins
Nesprin-1 and Nesprin-2 encompass many splice isoforms, the largest of which, called giant isoforms, are ~800–1,000 kDa (Zhang et al., 2002; Zhang et al., 2001). The largest isoforms feature an N-terminal actin binding domain followed by numerous spectrin repeats within their cytoplasmic domain. Given their size, functional domains, and splicing isoforms, Nesprin-1 and -2 can interact with diverse proteins. Indeed, they are involved in many important cellular processes as described below.
As expected, the ONM targeting ability of Nesprin-1 and -2 requires SUN proteins, but several smaller KASH-domain containing isoforms of Nepsrin-1 and -2 were found to reside within the nucleus (Rajgor et al., 2012; Zhang et al., 2005). There are also KASH-less isoforms of Nesprins-1 and-2 found in a variety of cellular locations which are reported to associate with diverse proteins. The role of KASH-less isoforms needs to be clarified (Gough et al., 2003; Mislow et al., 2002; Warren et al., 2010; Zhang et al., 2002; Zhang et al., 2005).
Likely via interactions with their binding partners, Nesprin-1 and -2 are also involved in mechanotransduction, a process that monitor physical forces and trigger biological responses (Anno et al., 2012; Lombardi and Lammerding, 2010; Stewart-Hutchinson et al., 2008). Recently, forces transmitted via Nesprin-1 in isolated nuclei led to changes in nuclear stiffness, resulting in phosphorylation of emerin (Guilluy et al., 2014). Interestingly, this study further illustrates that changes in nuclear stiffness affect stress fiber formation and SRF (serum response factor)-dependent gene expression (Guilluy et al., 2014). In cardiomyocytes, compared to wild type or single deletion of Nesprin-1 and-2, loss of both Nesprin-1 and -2 induced altered nuclei positioning, shape, and gene expression of biomechanical response genes upon mechanical stimulation (Banerjee et al., 2014). These observations suggest that Nesprin-1 and -2 play important roles in sensing mechanical force and regulating gene expression.
Actin: The actin binding ability of Nesprins-1 and -2 is the primary mechanism anchoring the clustered nuclei at the neuromuscular junction (NMJ) (Padmakumar et al., 2004; Zhang et al., 2002; Zhen et al., 2002). In Nesprin-1-deficient mice, the clustered nuclei at the NMJ are displaced; however, loss of Nesprin-2 has no similar effect (Zhang et al., 2007b), which suggests Nesprin-2 is functionally redundant. Yet the loss of both Nesprins leads to perinatal mortality due to malfunction of the diaphragm (Zhang et al., 2007b). Nesprin2 giant and Sun2 are involved in polarized nuclear movement, which mediated by the LINC complex perpendicularly aligned with the actin cytoskeleton at the nuclear periphery. (Gomes et al., 2005; Luxton et al., 2010). The specialized actin cytoskeleton and its aligned LINC-complex were subsequently termed transmembrane actin-associated nuclear (TAN) line. The role of TAN lines in vivo remains unclear.
Microtubule: Nesprin-1 and -2 are essential for nuclear migration during neuronal development. The neuronal nuclei move between the apical and basal surface according to the cell cycles. In the mouse embryonic brain and retinal cells, Nesprin-1 and -2 interact with dynein complex (Yu et al., 2011; Zhang et al., 2009). Nesprin-2, in turn, interacts with kinesin (Yu et al., 2011; Zhang et al., 2009). Given this observation, Nesprin-1 and -2 are speculated to participate in movement of neuronal nuclei toward the apical or basal surface in the developing brain or retina. Mice deficient in both Nesprin-1 and -2 suffer severe developmental defects in the brain and retina, likely due to the failure of neuronal nuclear migration (Yu et al., 2011; Zhang et al., 2009). This abnormal neuronal development may also contribute to the neonatal lethality of the mice deficient in Nesprin-1 and -2.
Nesprin3: Giant isoforms of Nesprin-1 and -2 were shown to interact with Nesprin3 via a direct interaction (Taranum et al., 2012). Based on this observation, a new model for the architecture of nuclear-cytoskeletal interactions has been proposed. In this model, Giant Nepsrin-1, and -2 are drawn down to the periphery of the NE and form interchains with Nepsrin-3. In this manner, the interchain potentially serves as a “filamentous cage” that, among other things, can regulate nuclear size (Taranum et al., 2012).
Nuclear proteins: In mammals, Nesprin-1 and -2 were shown to interact with various proteins at the inner nuclear membrane. Small isoforms of Nesprin-1 and -2 bind to the nucleoplasmic domain of Sun proteins (Haque et al., 2010), emerin (Mislow et al., 2002; Wheeler et al., 2007; Zhang et al., 2005), and lamin A/C (Libotte et al., 2005; Mislow et al., 2002; Zhang et al., 2005). However, the role beyond the association between Nesprins and inner nuclear membrane proteins remains unclear.
TorsinA: The lumenal AAA+ protein torsinA was shown to associate with the KASH domains of Nesprins 1 and -2 (Nery et al., 2008). Interestingly, a dystonia-associated mutant of torsinA requires Sun1 for its aberrant localization to the NE (Jungwirth et al., 2011).
Nesprin3 localizes to the NE in a Sun1 and Sun2 dependent manner and is rather ubiquitously expressed. Considerably smaller than Nesprin-1 and Nesprin-2, Nesprin3can form a large protein complex by which it binds to cyto-linker protein plectin (Ketema et al., 2007; Wilhelmsen et al., 2005). Plectin is a multi-functional protein that binds the intermediate filament system, actin cytoskeleton, and the cytoplasmic domain of integrin β4 (Geerts et al., 1999; Ketema et al., 2007; Postel et al., 2011; Wilhelmsen et al., 2005). Given that plectin can form extended oligomers, the interaction between Nesprin3 and plectin has been proposed to potentially connect the nucleus to the plasma membrane (Ketema et al., 2013). The loss of Nesprin3 in zebra fish and mice demonstrates a reduced association between the intermediate filament system and the NE; however, this loss revealed at most a minimal effect on embryonic development, viability, and fertility (Ketema et al., 2013; Postel et al., 2011). Nesprin3 and Sun1η, a spermiogenesis specific Sun1 isoform, have been proposed to function in the formation of sperm heads (Gob et al., 2010).
Nesprin4 was originally detected in secretory epithelial cells (Roux et al., 2009). By interacting with kinesin-1, Nesprin4 was proposed to have a role in positioning the nucleus at the basal membrane of secretory epithelial cells (Roux et al., 2009). Subsequently, patients with hereditary hearing loss were shown to have predicted loss-of-function mutations in the gene that encodes Nesprin4. Furthermore, Nesprin4-deficient mice also exhibit hearing loss with defective basal nuclear positioning and maintenance of outer hair cells (Horn et al., 2013a). Interestingly, Sun1-deficient mice have similar deficits in hearing and outer hair cell polarity (Horn et al., 2013a). It appears that outer hair cells lack Nesprin-2 (another kinesin-binding KASH protein), suggesting functional redundancy of Nesprin-2 and -4 in secretory epithelial cells.
KASH5, unlike Nesprins, does not contain cytoplasmic spectrin repeats and its expression appears restricted to germ cells during and after meiosis (Horn et al., 2013b; Morimoto et al., 2012). During mammalian meiotic prophase I, dynein-bound KASH-5 colocalizes with NE-tethered telomeres and Sun1 (Horn et al., 2013b; Morimoto et al., 2012). Mice deficient in KASH5 are infertile and display an arrested telomere movement (Horn et al., 2013b), indicating that KASH5 is a mechanism conveying telomeres during meiosis prophase I. Recently, Link et al. reports that KASH5 can target to the NE in a Sun1 independent manner, presumably via interaction with Sun2 (Link et al., 2014).
LRMP, also called Jaw1, is an atypical mammalian KASH protein. The KASH domain of LRMP is sufficient to target the NE in a SUN-dependent manner (Horn et al., 2013b). However, posttranslational proteolytic processing can remove the luminal region, resulting in a potentially variable targeting of LRMP to the NE (Behrens et al., 1996; Horn et al., 2013b). Unlike other Nesprins, LRMP does not appear to associate with the cytoskeleton but instead binds inositol triphosphate receptor (IP3R), a Ca2+ channel located at the ER (Shindo et al., 2010). LRMP is highly expressed in immune cells and certain taste receptor cells though its function remains unclear.
6. Plant
The LINC complex in plants, like other organisms, plays a role in many cellular events including nuclear positioning and shape, yet little is known about the mechanism involved.
- SUN proteins
In Arabidopsis thaliana, two SUN proteins AtSun1 and AtSun2 have been identified (Zhou et al., 2012a). AtSun1 and AtSun2 are shown to associate with Little Nuclei Proteins (LINC) that contain structural homology with lamins (Graumann et al., 2010). In addition to C-terminal SUN proteins ZmSun1, ZmSun2, Zea mays has three mid-SUN domain containing proteins (ZmSun3-5) (Murphy et al., 2010). However, the role of these mid-SUN domain containing proteins remains uncertain.
-KASH proteins
AtWIPs (AtWIP1, AtWIP2, and AtWIP3) are KASH domain proteins in A. thaliana, which harbor relatively short KASH domains. Originally identified as proteins anchoring AtRanGAP1 to the NE (Meier et al., 2010; Xu et al., 2007), AtWIPs are indeed KASH-domain proteins contingent on AtSun1 and AtSun2 (Zhou et al., 2012a). AtWIPs also interact with WIT1 and 2 that seem to aid in recruitment of AtRanGAP1 to the NE (Zhao et al., 2008). Interestingly, WIT1 and 2 physically interact with a plant specific myosin XI, thus bridging the actin cytoskeleton to the NE (Tamura et al., 2013). Disruptions of the interaction between AtWIPs and AtSuns have no obvious defects except less elongated nuclear shapes in epidermal cells (Zhou et al., 2012a). The consequence of the less elongated nuclear shape is currently unknown.
Recently, a group of proteins named Sun interacting NE (SINE) proteins were reported as KASH proteins. SINE1 is involved in actin-dependent nuclear positioning in guard cells, suggesting that it associates with the actin cytoskeleton (Zhou et al., 2014). SINE2 contributes to innate immunity to oomycete pathogens (Zhou et al., 2014).
Conclusions
The LINC complex bridges the nucleoskeleton to the cytoskeleton. However, there is growing evidence demonstrating that the LINC complex also functions as more than a simple physical tether. In yeast and C. elegans, the LINC complex participates in transcription, DNA repair, and signaling pathways. These observations suggest that the mammalian LINC complex has the potential to regulate gene expression and signaling pathways. It is not clear if KASH proteins retained by SUN proteins at the NE also interact with the previously mentioned signaling proteins. In addition to studying the functional consequences of the LINC complex-mediated signal transduction, elucidating the interaction between the LINC complex and non-cytoskeleton proteins will shed light on our understanding about the role of the LINC complex.
Many LINC complexes have been characterized by their respective cytoplasmic binding partners. Nevertheless, the function of SUN proteins in the nucleoplasm remains unclear. Although yeast do not have nuclear lamina, yeast SUN proteins play important roles in chromosome organization, gene expression, and DNA repair. Given the findings that Sun1 and Sun2 interact with DNAPK, a protein complex responding to DNA damage in mammals (Lei et al., 2012), there is an implication about the role of the SUN proteins in the nucleoplasm. Studies to clarify the function of SUN proteins will provide more clues about the role of the LINC complex in the nucleus. It also remains to be shown whether Sun proteins have unique or conserved functions within organisms. In mammals, the SUN proteins demonstrate a considerable degree of structural redundancy. Mouse models lacking either Sun protein have defects in nuclear anchorage in muscle cells, while double knockout mice were perinatally lethal (Lei et al., 2009). However, Sun1 was found closely associated with the nuclear pore complex (NPC) while Sun2 is more clustered (Liu et al., 2007). These observations suggest that Sun1 and 2 may have discrete roles. Thus, dissecting the role of SUN proteins will greatly enhance our understanding about the LINC complex.
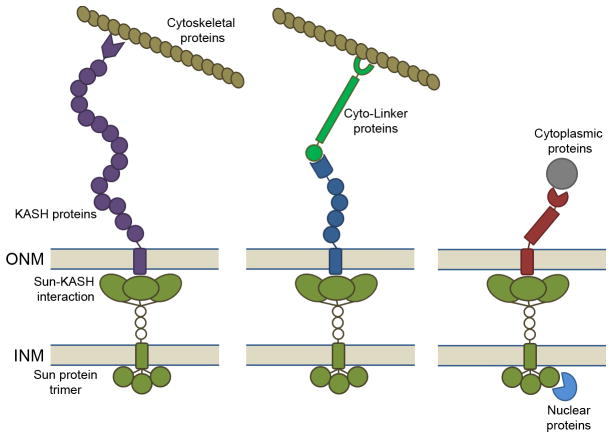
Figure 1. Schematic overview of SUN-KASH interactions.
SUN domain protein trimers form a binding pocket for KASH domain proteins in the perinuclear space. Given that KASH proteins homo-typically oligomerize in an unknown stoichiometry upon SUN domain trimer, KASH proteins are simplified as monomers in this model. Through SUN-KASH luminal coupling, KASH proteins can directly interact with cytoskeletal proteins such as actin (Left). The interactions of KASH proteins and cytoskeletal proteins can be mediated by cyto-linker proteins such as plectin (Middle) or microtubule motor proteins. In addition to the cytoskeletal proteins, KASH proteins are known to bind to diverse cytoplasmic proteins while SUN proteins interact with nuclear proteins likely via the nuclear domain of SUN proteins (Right). The structure of nuclear domain of SUN proteins is remains poorly characterized.
Table 1.
List of KASH and SUN protein associations
| Species | Cytoplasmic Binding proteins | KASH proteins | SUN proteins | Nuclear Binding proteins | Functions | Methods |
|---|---|---|---|---|---|---|
| S. cerevisiae | Bbp1 | Mps2 | Mps3 | SPB integrity | Mps2 & Bbp1: coIP, GST pull down (Schramm et al., 2000) Mps2 & Mps3: IF, Y2H (Friederichs et al., 2011) |
|
| Spc24 | Mps2 | Chromosome segregation | Mps2 & Spc24: coIP (Le Masson et al., 2002) | |||
| Actin | Csm4 | Mps3 | Ndj1 | Meiotic telomere movement Meiotic telomere tethering |
Actin & Csm4: mutant animal study (Conrad et al., 2008; Kosaka et al., 2008; Wanat et al., 2008) Csm4 & Mps3: coIP (Conrad et al., 2008) Mps3 & Ndj1: coIP (Conrad et al., 2008; Kosaka et al., 2008) |
|
| Mps3 | Sir4, ribosome biogenesis factors | Telomere tethering Protection of telomeres from transcription |
Mps3 & Sir4: coIP (Bupp et al., 2007) Mps3 & Ribosome biogenesis factors: coIP (Horigome et al., 2011), Y2H (Horigome et al., 2011) |
|||
| Mps3 | Telomerase, Ku70, Ku80 | Telomere tethering Protection of telomeres from transcription |
Mps3 & telomerase: IF of reporter (Schober et al., 2009), GST pull down (Antoniacci et al., 2007) Mps4 & K70/Ku80: IF of reporter (Antoniacci et al., 2004), mutant animal study (Oza et al., 2009) |
|||
| Mps3 | Replication factor C, Htz1 | DNA damage response | Mps3 & Replication factor C, Htz1: CoIP, in vitro coupling (Haas et al., 2012) | |||
| Mps3 | Eco1/Ctf7 | Chromosome tethering Sister chromatin cohesion |
Mps3 & Eco1/Ctf7: GST pull down, coIP (Antoniacci et al., 2004) | |||
| S. pombe | Dynein (Dlc1) | Kms1 | Sad1 | Bqt1, Bqt2 | Meiotic telomere movement Meiotic telomere tethering |
Dlc1 & Kms1: Y2H (Miki et al., 2004), IF (Yoshida et al., 2013) Kms1 & Sad1: Y2H (Miki et al., 2004) Sad1 & Bqt1/2: IF, coIP, mutant animal study (Chikashige et al., 2006) |
| Cut12, Pcp1 | Kms2 | Sad1 | SPB integrity and tethering | Cut12 & Kms2: IF, GST pull down (Walde and King, 2014) Kms2 & Sad1: IF (King et al., 2008) |
||
| Sad1 | Csi | Centromere tethering | Sad1 & Csi1: coIP (Hou et al., 2012) | |||
| D. melanogaster | Yuri Gagarin, dynein, dynactin | Spag4 | Centriolar coupling during spermatogenesis | Yuri Gagarin, dynein, dynactin & Spag4: IF (Kracklauer et al., 2010) | ||
| Microtubule | Klarsicht | Klaroid | Lam Dm0 | Nuclear migration during eye development | Microtubule & Klarsich: IF (Fischer et al., 2004; Patterson et al., 2004) Klarisicht & Klaroid: mutant animal study (Kracklauer et al., 2007) Klaroid & Lam Dm0: IF (Patterson et al., 2004) |
|
| Actin | MSP-300 | Klaroid | Lam Dm0 | Nuclear anchorage in muscle fiber | Actin & MSP-300: IF, in vitro coupling (Volk, 1992) MSP-300 & Klaroid: mutant animal study (Technau and Roth, 2008) |
|
| Klar | MSP-300 | Klaroid | Lam Dm0 | Microtubule coupling | MSP-300 & Klar: coIP (Elhanany-Tamir et al., 2012) | |
| C. elegans | Actin | Anc-1 | Unc-84 | Ce-Lamin | Nuclear anchorage | Actin & Anc-1: in vitro coupling (Starr and Han, 2002) Anc-1 & Unc-84: mutant animal study (Starr and Han, 2002) Unc-84 & Ce Lamin: mutant animal study (Lee et al., 2002) |
| Rpm1 | Anc-1 | Positive regulation of Bar-1 during neuronal development | Rpm1 &Anc1: coIP (Tulgren et al., 2014) | |||
| Dynein (DLC-1/BICD-1/Nud-2) | Unc-83 | Unc-84 | Ce-Lamin | Nuclear migration of hypodermal cells | Dynein & Unc-83: GST pull down, coIP (Fridolfsson et al., 2010) Unc-83 & Unc-84: mutant animal study (Starr et al., 2001) |
|
| Kinesin (KLC-2) | Unc-83 | Unc-84 | Ce-Lamin | Nuclear migration of hypodermal cells | Kinesin & Unc83: GST pull down, coIP, IF (Meyerzon et al., 2009) | |
| Zyg-12A | Zyg-12 | Sun1/MTF1 | Centrosome nucleus coupling | Zyg-12A & Zyg12: coIP (Malone et al., 2003) Zyg12 & Sun1/MTF: mutant animal study (Malone et al., 2003) |
||
| Dynein (DLI-1/Lis-1/Arp-1/DHC1) | Zyg-12 | Sun1/MTF1 | Him-8, Zim1, Zim2, Zim3 | Meiotic chromosome movement Meiotic chromosome tethering |
Dynein & Zyg12: IF, mutant animal study (Malone et al., 2003; Sato et al., 2009; Zhou et al., 2009) Sun1 & Him-8, Zim-1,-2,-3: IF (Sato et al., 2009) |
|
| KDP-1 | Sun1/MTF1 | Cell cycle progression | Kdp1 & Sun1/MTF1: Y2H, mutant animal study (McGee et al., 2009) | |||
| Sun1/MTF1 | CED | Recruit CED to nucleus | Sun1 & CED: in vitro coupling (Tzur et al., 2006a) | |||
| D. rerio | LRMP | Pronuclear fusion Centrosome nucleus coupling |
Binding partners unknown (Lindeman and Pelegri, 2012) | |||
| Vertebrate | Actin | Nesprin-1 | Sun1/Sun2 | Lamin A/C | Nuclear anchorage at the NMJ | Actin & Nesprin-1: in vitro coupling(Padmakumar et al., 2004), IP (Nikolova-Krstevski et al., 2011) Nesprins & Sun1,2: coIP (Crisp et al., 2006; Haque et al., 2006) Sun1,2 & Atype lamins: GST pull down (Crisp et al., 2006), coIP (Haque et al., 2006) |
| Dynein, dynactin | Nesprin-1 | Sun1/Sun2 | Lamin A/C | Nuclear migration during neuronal development | Dynein & Nesprin-1: IF, IP (Yu et al., 2011; Zhang et al., 2009) | |
| Nesprin3 | Nesprin-1 | Sun1/Sun2 | Lamin A/C | Regulation of nuclear size | Nepsrin1 & Nesprin3: GST, His-tag pull down, Y2H (Taranum et al., 2012) | |
| Nesprin-1 | Sun3 | Formation of acrosome | Nesprin-1 & Sun3: IF (Gob et al., 2010) | |||
| Actin | Nesprin-2 | Sun1/Sun2 | Lamin A/C | Nuclear migration during neuronal development | Actin & Nesprin-2: in vitro coupling (Zhen et al., 2002) Nesprin-2 & Sun1/2: coIP (Crisp et al., 2006; Haque et al., 2006) |
|
| Dynein, dynactin | Nesprin-2 | Sun1/Sun2 | Lamin A/C | Nuclear migration during neuronal development | Dynein & Nesprin-2: IP, IF (Yu et al., 2011; Zhang et al., 2009) | |
| Kinesin | Nesprin-2 | Sun1/Sun2 | Lamin A/C | Nuclear migration during retinal development | Kinesin & Nesprin-2: GST pull down (Schneider et al., 2011), IP (Yu et al., 2011) | |
| Plectin | Nesprin-3 | Sun1/Sun2 | Lamin A/C | Intermediate filament coupling | Plectin & Nesprin3: coIP, IF (Ketema et al., 2007; Wilhelmsen et al., 2005) Nesprin3 & Sun1/2: coIP, IF(Ketema et al., 2007) |
|
| Nesprin-3 | Sun1η | Formation of sperm heard | Nesprin3 & Sun1η: IF (Gob et al., 2010) | |||
| KLC-1/kif5b | Nepsrin-4 | Sun1/Sun2 | Lamin A/C | Nuclear positioning of secretory epithelia cells Nuclear positioning of inner ear cells |
KLC-1 & Nesprin4: coIP, IF (Roux et al., 2009), GST pull down (Wang et al., 2013) Nesprin4 & Sun1/2: IF (Roux et al., 2009) |
|
| Dynein, Dynactin | KASH5 | Sun1 | Meiotic telomere movement | Dynein & KASH5: IP (Morimoto et al., 2012), coIP, IF (Horn et al., 2013b) KASH5 & Sun1: IF (Horn et al., 2013b) |
||
| IP3R | LRMP | Sun1/Sun2 | Lamin A/C | IP3R recruitment to the NE | IP3R & LRMP: coIP, IF (Shindo et al., 2010) LRMP & Sun1: IF (Horn et al., 2013b) |
|
| Nesprin-1/-2 | LaminA/C Emerin | Nesprin-1/Nesprin-2 & LaminA/C: GST pull down (Mislow et al., 2002), coIP, IP (Zhang et al., 2005) Nesprin-1/Nesprin-2 & Emerin: in vitro coupling (Mislow et al., 2002), GST pull down (Wheeler et al., 2007), coIP, IP (Zhang et al., 2005) |
||||
| TorsinA | Nesprin-1/-2/-3 | TorsinA & Nesprins: coIP (Nery et al., 2008) | ||||
| Sun1 | DNAPK, Ku70, Ku80, NAT10 | DNA damage response | Sun1 & DNPK: coIP (Lei et al., 2012) Sun1 & NAT10:coIP (Chi et al., 2007) |
|||
| Sun1/Sun2 | SAMP1 | Cell migration | Sun2 & SAMP1: IF, coIP (Borrego-Pinto et al., 2012) Sun1 & SAMP1: MCLIP(Jafferali et al., 2014) |
|||
| RanGAP | AtWips | AtSun1/2 | LINC | Recruitment of RanGAP | RanGAP & AtWips: IP, IF (Meier et al., 2010; Xu et al., 2007) AtWips & AtSuns: coIP, FRAP (Zhou et al., 2012a) AtSun1/2 & LINC: FRET (Graumann, 2014) |
|
| A. thaliana | WIT1, 2 | AtWips | AtSun1/2 | Actin coupling | AtWit1,2 & AtWips: IP (Zhao et al., 2008) | |
| WIT1, 2 | AtWips | AtSun1/2 | Actin coupling | AtWit1,2 & AtWips: IP (Zhao et al., 2008) |
coIP: co-immunoprecipitation, IF: immunofluorescence, Y2H: yeasttwo-hybrid, IP: immunoprecipitation, MCLIP: Membrane protein cross-link immunoprecipitation
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award numbers RO1GM102203, RO1GM102486 and RO1EB014869. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We apologize to the researchers whose works could not be cited due to practical space limits.
References
- Anno T, Sakamoto N, Sato M. Role of nesprin-1 in nuclear deformation in endothelial cells under static and uniaxial stretching conditions. Biochem Biophys Res Commun. 2012;424:94–99. doi: 10.1016/j.bbrc.2012.06.073. [DOI] [PubMed] [Google Scholar]
- Antoniacci LM, Kenna MA, Skibbens RV. The nuclear envelope and spindle pole body-associated Mps3 protein bind telomere regulators and function in telomere clustering. Cell Cycle. 2007;6:75–79. doi: 10.4161/cc.6.1.3647. [DOI] [PubMed] [Google Scholar]
- Antoniacci LM, Kenna MA, Uetz P, Fields S, Skibbens RV. The spindle pole body assembly component mps3p/nep98p functions in sister chromatid cohesion. J Biol Chem. 2004;279:49542–49550. doi: 10.1074/jbc.M404324200. [DOI] [PubMed] [Google Scholar]
- Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J. Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum Mol Genet. 2009;18:3462–3469. doi: 10.1093/hmg/ddp290. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Zhang J, Moore-Morris T, Pfeiffer E, Buchholz KS, Liu A, Ouyang K, Stroud MJ, Gerace L, Evans SM, McCulloch A, Chen J. Targeted ablation of nesprin 1 and nesprin 2 from murine myocardium results in cardiomyopathy, altered nuclear morphology and inhibition of the biomechanical gene response. PLoS Genet. 2014;10:e1004114. doi: 10.1371/journal.pgen.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TW, Kearns GM, Rivard JJ, Bernstein HD, Yewdell JW, Staudt LM. Carboxyl-terminal targeting and novel post-translational processing of JAW1, a lymphoid protein of the endoplasmic reticulum. J Biol Chem. 1996;271:23528–23534. doi: 10.1074/jbc.271.38.23528. [DOI] [PubMed] [Google Scholar]
- Borrego-Pinto J, Jegou T, Osorio DS, Aurade F, Gorjanacz M, Koch B, Mattaj IW, Gomes ER. Samp1 is a component of TAN lines and is required for nuclear movement. J Cell Sci. 2012;125:1099–1105. doi: 10.1242/jcs.087049. [DOI] [PubMed] [Google Scholar]
- Bupp JM, Martin AE, Stensrud ES, Jaspersen SL. Telomere anchoring at the nuclear periphery requires the budding yeast Sad1-UNC-84 domain protein Mps3. J Cell Biol. 2007;179:845–854. doi: 10.1083/jcb.200706040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JN, Poon BP, Salvi J, Olsen JB, Emili A, Mekhail K. Perinuclear cohibin complexes maintain replicative life span via roles at distinct silent chromatin domains. Dev Cell. 2011;20:867–879. doi: 10.1016/j.devcel.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Chen CY, Chi YH, Mutalif RA, Starost MF, Myers TG, Anderson SA, Stewart CL, Jeang KT. Accumulation of the inner nuclear envelope protein Sun1 is pathogenic in progeric and dystrophic laminopathies. Cell. 2012;149:565–577. doi: 10.1016/j.cell.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi YH, Haller K, Peloponese JM, Jr, Jeang KT. Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J Biol Chem. 2007;282:27447–27458. doi: 10.1074/jbc.M703098200. [DOI] [PubMed] [Google Scholar]
- Chikashige Y, Tsutsumi C, Yamane M, Okamasa K, Haraguchi T, Hiraoka Y. Meiotic proteins bqt1 and bqt2 tether telomeres to form the bouquet arrangement of chromosomes. Cell. 2006;125:59–69. doi: 10.1016/j.cell.2006.01.048. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Lee CY, Chao G, Shinohara M, Kosaka H, Shinohara A, Conchello JA, Dresser ME. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell. 2008;133:1175–1187. doi: 10.1016/j.cell.2008.04.047. [DOI] [PubMed] [Google Scholar]
- Conrad MN, Lee CY, Wilkerson JL, Dresser ME. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007;104:8863–8868. doi: 10.1073/pnas.0606165104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisp M, Liu Q, Roux K, Rattner JB, Shanahan C, Burke B, Stahl PD, Hodzic D. Coupling of the nucleus and cytoplasm: role of the LINC complex. J Cell Biol. 2006;172:41–53. doi: 10.1083/jcb.200509124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Xu R, Yu J, Xu T, Zhuang Y, Han M. SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev Cell. 2007;12:863–872. doi: 10.1016/j.devcel.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Elhanany-Tamir H, Yu YV, Shnayder M, Jain A, Welte M, Volk T. Organelle positioning in muscles requires cooperation between two KASH proteins and microtubules. J Cell Biol. 2012;198:833–846. doi: 10.1083/jcb.201204102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Vize JA, Mosley KL. Marbles mutants: uncoupling cell determination and nuclear migration in the developing Drosophila eye. Development. 1994;120:2609–2618. doi: 10.1242/dev.120.9.2609. [DOI] [PubMed] [Google Scholar]
- Fischer JA, Acosta S, Kenny A, Cater C, Robinson C, Hook J. Drosophila klarsicht has distinct subcellular localization domains for nuclear envelope and microtubule localization in the eye. Genetics. 2004;168:1385–1393. doi: 10.1534/genetics.104.028662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridolfsson HN, Ly N, Meyerzon M, Starr DA. UNC-83 coordinates kinesin-1 and dynein activities at the nuclear envelope during nuclear migration. Dev Biol. 2010;338:237–250. doi: 10.1016/j.ydbio.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederichs JM, Ghosh S, Smoyer CJ, McCroskey S, Miller BD, Weaver KJ, Delventhal KM, Unruh J, Slaughter BD, Jaspersen SL. The SUN protein Mps3 is required for spindle pole body insertion into the nuclear membrane and nuclear envelope homeostasis. PLoS Genet. 2011;7:e1002365. doi: 10.1371/journal.pgen.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A. Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Gardner JM, Smoyer CJ, Friederichs JM, Unruh JR, Slaughter BD, Alexander R, Chisholm RD, Lee KK, Workman JL, Jaspersen SL. Acetylation of the SUN protein Mps3 by Eco1 regulates its function in nuclear organization. Mol Biol Cell. 2012;23:2546–2559. doi: 10.1091/mbc.E11-07-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gob E, Schmitt J, Benavente R, Alsheimer M. Mammalian sperm head formation involves different polarization of two novel LINC complexes. PLoS One. 2010;5:e12072. doi: 10.1371/journal.pone.0012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gough LL, Fan J, Chu S, Winnick S, Beck KA. Golgi localization of Syne-1. Mol Biol Cell. 2003;14:2410–2424. doi: 10.1091/mbc.E02-07-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann K. Evidence for LINC1-SUN associations at the plant nuclear periphery. PLoS One. 2014;9:e93406. doi: 10.1371/journal.pone.0093406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graumann K, Runions J, Evans DE. Characterization of SUN-domain proteins at the higher plant nuclear envelope. Plant J. 2010;61:134–144. doi: 10.1111/j.1365-313X.2009.04038.x. [DOI] [PubMed] [Google Scholar]
- Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA. Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet. 2007;39:80–85. doi: 10.1038/ng1927. [DOI] [PubMed] [Google Scholar]
- Guilluy C, Osborne LD, Van Landeghem L, Sharek L, Superfine R, Garcia-Mata R, Burridge K. Isolated nuclei adapt to force and reveal a mechanotransduction pathway in the nucleus. Nat Cell Biol. 2014;16:376–381. doi: 10.1038/ncb2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas J, Lemoncelli A, Morozov C, Franke K, Dominder J, Antoniacci LM. Physical links between the nuclear envelope protein Mps3, three alternate replication factor C complexes, and a variant histone in Saccharomyces cerevisiae. DNA Cell Biol. 2012;31:917–924. doi: 10.1089/dna.2011.1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Lloyd DJ, Smallwood DT, Dent CL, Shanahan CM, Fry AM, Trembath RC, Shackleton S. SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol Cell Biol. 2006;26:3738–3751. doi: 10.1128/MCB.26.10.3738-3751.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque F, Mazzeo D, Patel JT, Smallwood DT, Ellis JA, Shanahan CM, Shackleton S. Mammalian SUN protein interaction networks at the inner nuclear membrane and their role in laminopathy disease processes. J Biol Chem. 2010;285:3487–3498. doi: 10.1074/jbc.M109.071910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka Y, Maekawa H, Asakawa H, Chikashige Y, Kojidani T, Osakada H, Matsuda A, Haraguchi T. Inner nuclear membrane protein Ima1 is dispensable for intranuclear positioning of centromeres. Genes Cells. 2011;16:1000–1011. doi: 10.1111/j.1365-2443.2011.01544.x. [DOI] [PubMed] [Google Scholar]
- Hodzic DM, Yeater DB, Bengtsson L, Otto H, Stahl PD. Sun2 is a novel mammalian inner nuclear membrane protein. J Biol Chem. 2004;279:25805–25812. doi: 10.1074/jbc.M313157200. [DOI] [PubMed] [Google Scholar]
- Horigome C, Okada T, Shimazu K, Gasser SM, Mizuta K. Ribosome biogenesis factors bind a nuclear envelope SUN domain protein to cluster yeast telomeres. EMBO J. 2011;30:3799–3811. doi: 10.1038/emboj.2011.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn HF, Brownstein Z, Lenz DR, Shivatzki S, Dror AA, Dagan-Rosenfeld O, Friedman LM, Roux KJ, Kozlov S, Jeang KT, Frydman M, Burke B, Stewart CL, Avraham KB. The LINC complex is essential for hearing. J Clin Invest. 2013a;123:740–750. doi: 10.1172/JCI66911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn HF, Kim DI, Wright GD, Wong ES, Stewart CL, Burke B, Roux KJ. A mammalian KASH domain protein coupling meiotic chromosomes to the cytoskeleton. J Cell Biol. 2013b;202:1023–1039. doi: 10.1083/jcb.201304004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou H, Zhou Z, Wang Y, Wang J, Kallgren SP, Kurchuk T, Miller EA, Chang F, Jia S. Csi1 links centromeres to the nuclear envelope for centromere clustering. J Cell Biol. 2012;199:735–744. doi: 10.1083/jcb.201208001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jafferali MH, Vijayaraghavan B, Figueroa RA, Crafoord E, Gudise S, Larsson VJ, Hallberg E. MCLIP, an effective method to detect interactions of transmembrane proteins of the nuclear envelope in live cells. Biochimica et biophysica acta. 2014;1838:2399–2403. doi: 10.1016/j.bbamem.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Jungwirth MT, Kumar D, Jeong DY, Goodchild RE. The nuclear envelope localization of DYT1 dystonia torsinA-DeltaE requires the SUN1 LINC complex component. BMC cell biology. 2011;12:24. doi: 10.1186/1471-2121-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Sebire K, de Kretser DM, O’Bryan MK. Human sperm associated antigen 4 (SPAG4) is a potential cancer marker. Cell Tissue Res. 2004;315:279–283. doi: 10.1007/s00441-003-0821-2. [DOI] [PubMed] [Google Scholar]
- Ketema M, Kreft M, Secades P, Janssen H, Sonnenberg A. Nesprin-3 connects plectin and vimentin to the nuclear envelope of Sertoli cells but is not required for Sertoli cell function in spermatogenesis. Mol Biol Cell. 2013;24:2454–2466. doi: 10.1091/mbc.E13-02-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketema M, Wilhelmsen K, Kuikman I, Janssen H, Hodzic D, Sonnenberg A. Requirements for the localization of nesprin-3 at the nuclear envelope and its interaction with plectin. J Cell Sci. 2007;120:3384–3394. doi: 10.1242/jcs.014191. [DOI] [PubMed] [Google Scholar]
- King MC, Drivas TG, Blobel G. A network of nuclear envelope membrane proteins linking centromeres to microtubules. Cell. 2008;134:427–438. doi: 10.1016/j.cell.2008.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H, Shinohara M, Shinohara A. Csm4-dependent telomere movement on nuclear envelope promotes meiotic recombination. PLoS Genet. 2008;4:e1000196. doi: 10.1371/journal.pgen.1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszul R, Kim KP, Prentiss M, Kleckner N, Kameoka S. Meiotic chromosomes move by linkage to dynamic actin cables with transduction of force through the nuclear envelope. Cell. 2008;133:1188–1201. doi: 10.1016/j.cell.2008.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracklauer MP, Banks SM, Xie X, Wu Y, Fischer JA. Drosophila klaroid encodes a SUN domain protein required for Klarsicht localization to the nuclear envelope and nuclear migration in the eye. Fly (Austin) 2007;1:75–85. doi: 10.4161/fly.4254. [DOI] [PubMed] [Google Scholar]
- Kracklauer MP, Wiora HM, Deery WJ, Chen X, Bolival B, Jr, Romanowicz D, Simonette RA, Fuller MT, Fischer JA, Beckingham KM. The Drosophila SUN protein Spag4 cooperates with the coiled-coil protein Yuri Gagarin to maintain association of the basal body and spermatid nucleus. J Cell Sci. 2010;123:2763–2772. doi: 10.1242/jcs.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Masson I, Saveanu C, Chevalier A, Namane A, Gobin R, Fromont-Racine M, Jacquier A, Mann C. Spc24 interacts with Mps2 and is required for chromosome segregation, but is not implicated in spindle pole body duplication. Mol Microbiol. 2002;43:1431–1443. doi: 10.1046/j.1365-2958.2002.02844.x. [DOI] [PubMed] [Google Scholar]
- Lee KK, Starr D, Cohen M, Liu J, Han M, Wilson KL, Gruenbaum Y. Lamin-dependent localization of UNC-84, a protein required for nuclear migration in Caenorhabditis elegans. Mol Biol Cell. 2002;13:892–901. doi: 10.1091/mbc.01-06-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Zhang X, Ding X, Guo X, Chen M, Zhu B, Xu T, Zhuang Y, Xu R, Han M. SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc Natl Acad Sci U S A. 2009;106:10207–10212. doi: 10.1073/pnas.0812037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K, Zhu X, Xu R, Shao C, Xu T, Zhuang Y, Han M. Inner nuclear envelope proteins SUN1 and SUN2 play a prominent role in the DNA damage response. Curr Biol. 2012;22:1609–1615. doi: 10.1016/j.cub.2012.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libotte T, Zaim H, Abraham S, Padmakumar VC, Schneider M, Lu W, Munck M, Hutchison C, Wehnert M, Fahrenkrog B, Sauder U, Aebi U, Noegel AA, Karakesisoglou I. Lamin A/C-dependent localization of Nesprin-2, a giant scaffolder at the nuclear envelope. Mol Biol Cell. 2005;16:3411–3424. doi: 10.1091/mbc.E04-11-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeman RE, Pelegri F. Localized products of futile cycle/lrmp promote centrosome-nucleus attachment in the zebrafish zygote. Curr Biol. 2012;22:843–851. doi: 10.1016/j.cub.2012.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link J, Leubner M, Schmitt J, Gob E, Benavente R, Jeang KT, Xu R, Alsheimer M. Analysis of meiosis in SUN1 deficient mice reveals a distinct role of SUN2 in mammalian meiotic LINC complex formation and function. PLoS Genet. 2014;10:e1004099. doi: 10.1371/journal.pgen.1004099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Pante N, Misteli T, Elsagga M, Crisp M, Hodzic D, Burke B, Roux KJ. Functional association of Sun1 with nuclear pore complexes. J Cell Biol. 2007;178:785–798. doi: 10.1083/jcb.200704108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi ML, Lammerding J. Altered mechanical properties of the nucleus in disease. Methods Cell Biol. 2010;98:121–141. doi: 10.1016/S0091-679X(10)98006-0. [DOI] [PubMed] [Google Scholar]
- Luxton GW, Gomes ER, Folker ES, Vintinner E, Gundersen GG. Linear arrays of nuclear envelope proteins harness retrograde actin flow for nuclear movement. Science. 2010;329:956–959. doi: 10.1126/science.1189072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen AJ, Phillips CM, Bhalla N, Weiser P, Villeneuve AM, Dernburg AF. Chromosome sites play dual roles to establish homologous synapsis during meiosis in C. elegans. Cell. 2005;123:1037–1050. doi: 10.1016/j.cell.2005.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone CJ, Fixsen WD, Horvitz HR, Han M. UNC-84 localizes to the nuclear envelope and is required for nuclear migration and anchoring during C. elegans development. Development. 1999;126:3171–3181. doi: 10.1242/dev.126.14.3171. [DOI] [PubMed] [Google Scholar]
- Malone CJ, Misner L, Le Bot N, Tsai MC, Campbell JM, Ahringer J, White JG. The C. elegans hook protein, ZYG-12, mediates the essential attachment between the centrosome and nucleus. Cell. 2003;115:825–836. doi: 10.1016/s0092-8674(03)00985-1. [DOI] [PubMed] [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee MD, Stagljar I, Starr DA. KDP-1 is a nuclear envelope KASH protein required for cell-cycle progression. J Cell Sci. 2009;122:2895–2905. doi: 10.1242/jcs.051607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier I, Zhou X, Brkljacic J, Rose A, Zhao Q, Xu XM. Targeting proteins to the plant nuclear envelope. Biochem Soc Trans. 2010;38:733–740. doi: 10.1042/BST0380733. [DOI] [PubMed] [Google Scholar]
- Meinke P, Mattioli E, Haque F, Antoku S, Columbaro M, Straatman KR, Worman HJ, Gundersen GG, Lattanzi G, Wehnert M, Shackleton S. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014;10:e1004605. doi: 10.1371/journal.pgen.1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerzon M, Fridolfsson HN, Ly N, McNally FJ, Starr DA. UNC-83 is a nuclear-specific cargo adaptor for kinesin-1-mediated nuclear migration. Development. 2009;136:2725–2733. doi: 10.1242/dev.038596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics. 2004;270:449–461. doi: 10.1007/s00438-003-0938-8. [DOI] [PubMed] [Google Scholar]
- Mislow JM, Holaska JM, Kim MS, Lee KK, Segura-Totten M, Wilson KL, McNally EM. Nesprin-1alpha self-associates and binds directly to emerin and lamin A in vitro. FEBS Lett. 2002;525:135–140. doi: 10.1016/s0014-5793(02)03105-8. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Shibuya H, Zhu X, Kim J, Ishiguro K, Han M, Watanabe Y. A conserved KASH domain protein associates with telomeres, SUN1, and dynactin during mammalian meiosis. J Cell Biol. 2012;198:165–172. doi: 10.1083/jcb.201204085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosley-Bishop KL, Li Q, Patterson L, Fischer JA. Molecular analysis of the klarsicht gene and its role in nuclear migration within differentiating cells of the Drosophila eye. Curr Biol. 1999;9:1211–1220. doi: 10.1016/s0960-9822(99)80501-6. [DOI] [PubMed] [Google Scholar]
- Murphy SP, Simmons CR, Bass HW. Structure and expression of the maize (Zea mays L.) SUN-domain protein gene family: evidence for the existence of two divergent classes of SUN proteins in plants. BMC Plant Biol. 2010;10:269. doi: 10.1186/1471-2229-10-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FC, Zeng J, Niland BP, Hewett J, Farley J, Irimia D, Li Y, Wiche G, Sonnenberg A, Breakefield XO. TorsinA binds the KASH domain of nesprins and participates in linkage between nuclear envelope and cytoskeleton. J Cell Sci. 2008;121:3476–3486. doi: 10.1242/jcs.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolova-Krstevski V, Leimena C, Xiao XH, Kesteven S, Tan JC, Yeo LS, Yu ZY, Zhang Q, Carlton A, Head S, Shanahan C, Feneley MP, Fatkin D. Nesprin-1 and actin contribute to nuclear and cytoskeletal defects in lamin A/C-deficient cardiomyopathy. J Mol Cell Cardiol. 2011;50:479–486. doi: 10.1016/j.yjmcc.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Niwa O, Shimanuki M, Miki F. Telomere-led bouquet formation facilitates homologous chromosome pairing and restricts ectopic interaction in fission yeast meiosis. EMBO J. 2000;19:3831–3840. doi: 10.1093/emboj/19.14.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev. 2009;23:912–927. doi: 10.1101/gad.1782209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmakumar VC, Abraham S, Braune S, Noegel AA, Tunggal B, Karakesisoglou I, Korenbaum E. Enaptin, a giant actin-binding protein, is an element of the nuclear membrane and the actin cytoskeleton. Exp Cell Res. 2004;295:330–339. doi: 10.1016/j.yexcr.2004.01.014. [DOI] [PubMed] [Google Scholar]
- Patterson K, Molofsky AB, Robinson C, Acosta S, Cater C, Fischer JA. The functions of Klarsicht and nuclear lamin in developmentally regulated nuclear migrations of photoreceptor cells in the Drosophila eye. Mol Biol Cell. 2004;15:600–610. doi: 10.1091/mbc.E03-06-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips CM, Dernburg AF. A family of zinc-finger proteins is required for chromosome-specific pairing and synapsis during meiosis in C. elegans. Dev Cell. 2006;11:817–829. doi: 10.1016/j.devcel.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Postel R, Ketema M, Kuikman I, de Pereda JM, Sonnenberg A. Nesprin-3 augments peripheral nuclear localization of intermediate filaments in zebrafish. J Cell Sci. 2011;124:755–764. doi: 10.1242/jcs.081174. [DOI] [PubMed] [Google Scholar]
- Rajgor D, Mellad JA, Autore F, Zhang Q, Shanahan CM. Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS One. 2012;7:e40098. doi: 10.1371/journal.pone.0040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg-Hasson Y, Renert-Pasca M, Volk T. A Drosophila dystrophin-related protein, MSP-300, is required for embryonic muscle morphogenesis. Mech Dev. 1996;60:83–94. doi: 10.1016/s0925-4773(96)00602-8. [DOI] [PubMed] [Google Scholar]
- Roux KJ, Crisp ML, Liu Q, Kim D, Kozlov S, Stewart CL, Burke B. Nesprin 4 is an outer nuclear membrane protein that can induce kinesin-mediated cell polarization. Proc Natl Acad Sci U S A. 2009;106:2194–2199. doi: 10.1073/pnas.0808602106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Isaac B, Phillips CM, Rillo R, Carlton PM, Wynne DJ, Kasad RA, Dernburg AF. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell. 2009;139:907–919. doi: 10.1016/j.cell.2009.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherthan H, Wang H, Adelfalk C, White EJ, Cowan C, Cande WZ, Kaback DB. Chromosome mobility during meiotic prophase in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 2007;104:16934–16939. doi: 10.1073/pnas.0704860104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M, Lu W, Neumann S, Brachner A, Gotzmann J, Noegel AA, Karakesisoglou I. Molecular mechanisms of centrosome and cytoskeleton anchorage at the nuclear envelope. Cell Mol Life Sci. 2011;68:1593–1610. doi: 10.1007/s00018-010-0535-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev. 2009;23:928–938. doi: 10.1101/gad.1787509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm C, Elliott S, Shevchenko A, Schiebel E. The Bbp1p-Mps2p complex connects the SPB to the nuclear envelope and is essential for SPB duplication. EMBO J. 2000;19:421–433. doi: 10.1093/emboj/19.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Tarnasky HA, Lee JP, Oko R, van der Hoorn FA. Spag4, a novel sperm protein, binds outer dense-fiber protein Odf1 and localizes to microtubules of manchette and axoneme. Dev Biol. 1999;211:109–123. doi: 10.1006/dbio.1999.9297. [DOI] [PubMed] [Google Scholar]
- Shimanuki M, Miki F, Ding DQ, Chikashige Y, Hiraoka Y, Horio T, Niwa O. A novel fission yeast gene, kms1+, is required for the formation of meiotic prophase-specific nuclear architecture. Mol Gen Genet. 1997;254:238–249. doi: 10.1007/s004380050412. [DOI] [PubMed] [Google Scholar]
- Shindo Y, Kim MR, Miura H, Yuuki T, Kanda T, Hino A, Kusakabe Y. Lrmp/Jaw1 is expressed in sweet, bitter, and umami receptor-expressing cells. Chem Senses. 2010;35:171–177. doi: 10.1093/chemse/bjp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohaskey ML, Jiang Y, Zhao JJ, Mohr A, Roemer F, Harland RM. Osteopotentia regulates osteoblast maturation, bone formation, and skeletal integrity in mice. J Cell Biol. 2010;189:511–525. doi: 10.1083/jcb.201003006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa BA, Rothballer A, Kutay U, Schwartz TU. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 2012;149:1035–1047. doi: 10.1016/j.cell.2012.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr DA, Han M. Role of ANC-1 in tethering nuclei to the actin cytoskeleton. Science. 2002;298:406–409. doi: 10.1126/science.1075119. [DOI] [PubMed] [Google Scholar]
- Starr DA, Hermann GJ, Malone CJ, Fixsen W, Priess JR, Horvitz HR, Han M. unc-83 encodes a novel component of the nuclear envelope and is essential for proper nuclear migration. Development. 2001;128:5039–5050. doi: 10.1242/dev.128.24.5039. [DOI] [PubMed] [Google Scholar]
- Stewart-Hutchinson PJ, Hale CM, Wirtz D, Hodzic D. Structural requirements for the assembly of LINC complexes and their function in cellular mechanical stiffness. Exp Cell Res. 2008;314:1892–1905. doi: 10.1016/j.yexcr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan A, Nguyen T, Suter B. Drosophila Lissencephaly-1 functions with Bic-D and dynein in oocyte determination and nuclear positioning. Nat Cell Biol. 1999;1:444–449. doi: 10.1038/15680. [DOI] [PubMed] [Google Scholar]
- Tamura K, Iwabuchi K, Fukao Y, Kondo M, Okamoto K, Ueda H, Nishimura M, Hara-Nishimura I. Myosin XI-i links the nuclear membrane to the cytoskeleton to control nuclear movement and shape in Arabidopsis. Curr Biol. 2013;23:1776–1781. doi: 10.1016/j.cub.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Taranum S, Sur I, Muller R, Lu W, Rashmi RN, Munck M, Neumann S, Karakesisoglou I, Noegel AA. Cytoskeletal interactions at the nuclear envelope mediated by nesprins. Int J Cell Biol. 2012;2012:736524. doi: 10.1155/2012/736524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Technau M, Roth S. The Drosophila KASH domain proteins Msp-300 and Klarsicht and the SUN domain protein Klaroid have no essential function during oogenesis. Fly (Austin) 2008;2:82–91. doi: 10.4161/fly.6288. [DOI] [PubMed] [Google Scholar]
- Trelles-Sticken E, Adelfalk C, Loidl J, Scherthan H. Meiotic telomere clustering requires actin for its formation and cohesin for its resolution. J Cell Biol. 2005;170:213–223. doi: 10.1083/jcb.200501042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulgren ED, Turgeon SM, Opperman KJ, Grill B. The Nesprin family member ANC-1 regulates synapse formation and axon termination by functioning in a pathway with RPM-1 and beta-Catenin. PLoS Genet. 2014;10:e1004481. doi: 10.1371/journal.pgen.1004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur YB, Margalit A, Melamed-Book N, Gruenbaum Y. Matefin/SUN-1 is a nuclear envelope receptor for CED-4 during Caenorhabditis elegans apoptosis. Proc Natl Acad Sci U S A. 2006a;103:13397–13402. doi: 10.1073/pnas.0604224103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzur YB, Wilson KL, Gruenbaum Y. SUN-domain proteins: ‘Velcro’ that links the nucleoskeleton to the cytoskeleton. Nat Rev Mol Cell Biol. 2006b;7:782–788. doi: 10.1038/nrm2003. [DOI] [PubMed] [Google Scholar]
- Volk T. A new member of the spectrin superfamily may participate in the formation of embryonic muscle attachments in Drosophila. Development. 1992;116:721–730. doi: 10.1242/dev.116.3.721. [DOI] [PubMed] [Google Scholar]
- Volk T. Positioning nuclei within the cytoplasm of striated muscle fiber: cooperation between microtubules and KASH proteins. Nucleus. 2013;4:18–22. doi: 10.4161/nucl.23086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walde S, King MC. The KASH protein Kms2 coordinates mitotic remodeling of the spindle pole body. J Cell Sci. 2014;127:3625–3640. doi: 10.1242/jcs.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat JJ, Kim KP, Koszul R, Zanders S, Weiner B, Kleckner N, Alani E. Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet. 2008;4:e1000188. doi: 10.1371/journal.pgen.1000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Shi Z, Jiao S, Chen C, Wang H, Liu G, Wang Q, Zhao Y, Greene MI, Zhou Z. Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 2012;22:1440–1452. doi: 10.1038/cr.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xue W, Li X, Lin R, Cui J, Huang JD. Dissect Kif5b in nuclear positioning during myogenesis: the light chain binding domain and the autoinhibitory peptide are both indispensable. Biochem Biophys Res Commun. 2013;432:242–247. doi: 10.1016/j.bbrc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- Warren DT, Tajsic T, Mellad JA, Searles R, Zhang Q, Shanahan CM. Novel nuclear nesprin-2 variants tether active extracellular signal-regulated MAPK1 and MAPK2 at promyelocytic leukemia protein nuclear bodies and act to regulate smooth muscle cell proliferation. J Biol Chem. 2010;285:1311–1320. doi: 10.1074/jbc.M109.032557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Davies JD, Zhang Q, Emerson LJ, Hunt J, Shanahan CM, Ellis JA. Distinct functional domains in nesprin-1alpha and nesprin-2beta bind directly to emerin and both interactions are disrupted in X-linked Emery-Dreifuss muscular dystrophy. Exp Cell Res. 2007;313:2845–2857. doi: 10.1016/j.yexcr.2007.03.025. [DOI] [PubMed] [Google Scholar]
- Wilhelmsen K, Litjens SH, Kuikman I, Tshimbalanga N, Janssen H, van den Bout I, Raymond K, Sonnenberg A. Nesprin-3, a novel outer nuclear membrane protein, associates with the cytoskeletal linker protein plectin. J Cell Biol. 2005;171:799–810. doi: 10.1083/jcb.200506083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Fischer JA. On the roles of the Drosophila KASH domain proteins Msp-300 and Klarsicht. Fly (Austin) 2008;2:74–81. doi: 10.4161/fly.6108. [DOI] [PubMed] [Google Scholar]
- Xu XM, Meulia T, Meier I. Anchorage of plant RanGAP to the nuclear envelope involves novel nuclear-pore-associated proteins. Curr Biol. 2007;17:1157–1163. doi: 10.1016/j.cub.2007.05.076. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, West RR, McIntosh JR, Hiraoka Y. A cytoplasmic dynein heavy chain is required for oscillatory nuclear movement of meiotic prophase and efficient meiotic recombination in fission yeast. J Cell Biol. 1999;145:1233–1249. doi: 10.1083/jcb.145.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Katsuyama S, Tateho K, Nakamura H, Miyoshi J, Ohba T, Matsuhara H, Miki F, Okazaki K, Haraguchi T, Niwa O, Hiraoka Y, Yamamoto A. Microtubule-organizing center formation at telomeres induces meiotic telomere clustering. J Cell Biol. 2013;200:385–395. doi: 10.1083/jcb.201207168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Lei K, Zhou M, Craft CM, Xu G, Xu T, Zhuang Y, Xu R, Han M. KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum Mol Genet. 2011;20:1061–1073. doi: 10.1093/hmg/ddq549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Starr DA, Wu X, Parkhurst SM, Zhuang Y, Xu T, Xu R, Han M. The KASH domain protein MSP-300 plays an essential role in nuclear anchoring during Drosophila oogenesis. Dev Biol. 2006;289:336–345. doi: 10.1016/j.ydbio.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM. Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet. 2007a;16:2816–2833. doi: 10.1093/hmg/ddm238. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG. The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics. 2002;80:473–481. [PubMed] [Google Scholar]
- Zhang Q, Ragnauth CD, Skepper JN, Worth NF, Warren DT, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprin-2 is a multi-isomeric protein that binds lamin and emerin at the nuclear envelope and forms a subcellular network in skeletal muscle. J Cell Sci. 2005;118:673–687. doi: 10.1242/jcs.01642. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM. Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci. 2001;114:4485–4498. doi: 10.1242/jcs.114.24.4485. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M. SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron. 2009;64:173–187. doi: 10.1016/j.neuron.2009.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Xu R, Zhu B, Yang X, Ding X, Duan S, Xu T, Zhuang Y, Han M. Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development. 2007b;134:901–908. doi: 10.1242/dev.02783. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Brkljacic J, Meier I. Two distinct interacting classes of nuclear envelope-associated coiled-coil proteins are required for the tissue-specific nuclear envelope targeting of Arabidopsis RanGAP. Plant Cell. 2008;20:1639–1651. doi: 10.1105/tpc.108.059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen YY, Libotte T, Munck M, Noegel AA, Korenbaum E. NUANCE, a giant protein connecting the nucleus and actin cytoskeleton. J Cell Sci. 2002;115:3207–3222. doi: 10.1242/jcs.115.15.3207. [DOI] [PubMed] [Google Scholar]
- Zhou K, Rolls MM, Hall DH, Malone CJ, Hanna-Rose W. A ZYG-12-dynein interaction at the nuclear envelope defines cytoskeletal architecture in the C. elegans gonad. J Cell Biol. 2009;186:229–241. doi: 10.1083/jcb.200902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Graumann K, Evans DE, Meier I. Novel plant SUN-KASH bridges are involved in RanGAP anchoring and nuclear shape determination. J Cell Biol. 2012a;196:203–211. doi: 10.1083/jcb.201108098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Graumann K, Wirthmueller L, Jones JD, Meier I. Identification of unique SUN-interacting nuclear envelope proteins with diverse functions in plants. J Cell Biol. 2014;205:677–692. doi: 10.1083/jcb.201401138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Du X, Cai Z, Song X, Zhang H, Mizuno T, Suzuki E, Yee MR, Berezov A, Murali R, Wu SL, Karger BL, Greene MI, Wang Q. Structure of Sad1-UNC84 homology (SUN) domain defines features of molecular bridge in nuclear envelope. J Biol Chem. 2012b;287:5317–5326. doi: 10.1074/jbc.M111.304543. [DOI] [PMC free article] [PubMed] [Google Scholar]