Abstract
In the past 15 years, major advances have been made in understanding the role of lipids in podocyte biology. First, susceptibility to focal segmental glomerulosclerosis (FSGS) and glomerular disease is associated with an APOL1 sequence variant, is expressed in podocytes and encodes apolipoprotein L1, an important component of HDL. Second, acid sphingomyelinase-like phosphodiesterase 3b encoded by SMPDL3b has a role in the conversion of sphingomyelin to ceramide and its levels are reduced in renal biopsy samples from patients with recurrent FSGS. Furthermore, decreased SMPDL3b expression is associated with increased susceptibility of podocytes to injury after exposure to sera from these patients. Third, in many individuals with membranous nephropathy, autoantibodies against the phospholipase A2 (PLA2) receptor, which is expressed in podocytes, have been identified. Whether these autoantibodies affect the activity of PLA2, which liberates arachidonic acid from glycerophospholipids and modulates podocyte function, is unknown. Fourth, clinical and experimental evidence support a role for ATP-binding cassette sub-family A member 1-dependent cholesterol efflux, free fatty acids and glycerophospolipids in the pathogenesis of diabetic kidney disease. An improved understanding of lipid biology in podocytes might provide insights to develop therapeutic targets for primary and secondary glomerulopathies.
Introduction
Clinical and experimental studies have provided insights into the roles of lipids and lipid-modulating proteins as key determinants of podocyte function in health and kidney disease. The podocyte slit diaphragm—which has a critical role in the formation and maintenance of the glomerular filtration barrier—is assembled in lipid rafts (Figure 1). These small (10–200 nm diameter) specialized plasma membrane domains are enriched with sphingolipids, cholesterol and protein complexes that have roles in signal transduction. Cholesterol is enriched 5–8-fold in lipid rafts compared with the rest of the plasma membrane and interacts with sphingolipids via its saturated hydrophobic side chains and different lipids have specific roles in maintaining cell structure and function (Table 1).1
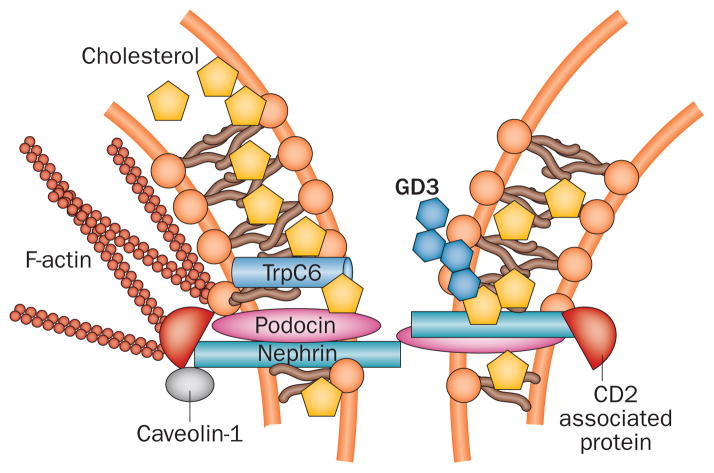
Figure 1.
Lipids in the slit diaphragm. Slit-diaphragm proteins such as podocin contain prohibitin-domains that enable binding to cholesterol and the formation of multiprotein complexes such as that between podocin and TrpC6. Lipid raft domains in podocytes contain cell-specific gangliosides such as GD3. Other slit-diaphragm proteins such as nephrin and CD2-associated protein are bound to caveolin-1, a protein specific to lipid rafts. Antibodies against GD3 can increase nephrin phosphorylation and its trafficking from the plasma membrane to the cytosol. Lipids are shown in different shades of orange. Abbreviations: GD3, disialosyllactosylceramide; TrpC6, short transient receptor potential cation channel 6.
Table 1.
Lipids that contribute to cell structure and function
| Class | Primary location | Function(s) |
|---|---|---|
|
Saturated fatty acids
| ||
| Palmitic acid (16:0)* | Component of complex lipids in plasma membrane or in lipid droplets when esterified | Energy source: β oxidation in mitochondria |
| Stearic acid (18:0)* | ||
| Arachidic acid (20:0)* | ||
|
| ||
|
Unsaturated fatty acids
| ||
| Oleic acid (18:1)* | Component of complex lipids in plasma membrane | Energy source: β oxidation in mitochondria |
| Arachidonic acid (20:4)* | ||
| Eicosapentaenoic acid (20:5)* | ||
|
| ||
|
Eicosanoids
| ||
| Prostaglandins | Intracellular | Signal transmission |
| Leukotrienes | ||
| Thromboxanes | ||
|
| ||
|
Glycerolipids
| ||
| Triglyceride‡ | Lipid droplets | Energy storage |
|
| ||
|
Phospholipids
| ||
| Phosphatidylcholine | Lipid bilayers | Structural |
| Phosphatidylserine | Signal transmission | |
| Phosphatidylethanolamine | ||
| Phosphatidylinositol | ||
| Phosphatidic acid | ||
|
| ||
|
Phosphosphingolipids
| ||
| Sphingomyelin | Cell membranes | Structural |
| Signal transmission | ||
|
| ||
|
Glycosphingolipids
| ||
| Cerebroside | Cell membranes | Structural |
| Ganglioside | Signal transmission | |
|
| ||
|
Sterol lipid§
| ||
| Cholesterol | Cell membranes (enriched in lipid rafts and caveolae but also present among the hydrophobic tails of amphipathic lipids) | Structural building block for other sterol lipids |
| Intracellular lipid droplets when esterified (containing cholesterol esters) | ||
Free fatty acids are incorporated into complex lipids such as glycerolipids, phospholipids and sphingolipids. The plasma membrane and membranes of cellular organelles (including the nucleus, endoplasmic reticulum, lysosomes and mitochondria) are composed of a bilayer of amphipathic lipids (phospholipids, glycolipids and sterols) arranged with the hydrophobic tails facing into the membrane and the polar head groups facing the cytoplasm.
Number of carbon atoms:number of C=C double bonds; the presence of the latter indicates an unsaturated fatty acid.
An ester derived from glycerol and three fatty acids.
This list is selective and does not include steroids (which have a four-ring structure and include many hormones) and prenol lipids.
The first disease of glomerular lipid accumulation to be recognized was minimal change disease (also known as lipoid nephrosis). Foam cells are reportedly more common in focal segmental glomerulosclerosis (FSGS) than in minimal change disease;2 however, the origin of foam cells in these diseases is unclear. Although foam cells are traditionally thought to be derived from macrophages, studies of renal biopsy samples from patients with FSGS indicate that lipids can also be taken up by mesangial cells and podocytes or deposited in the mesangial matrix.3 Further investigation is needed to define whether or not, and how, lipid accumulation within glomeruli differs among glomerular diseases in terms of particular lipids and specific glomerular and cellular compartments.
Several advances have generated new interest in the lipid biology of the podocyte. The susceptibility of African Americans to podocytopathies such as FSGS enabled the identification of APOL1 sequence variants that are associated with this disease.4,5 The APOL1 gene encodes alipoprotein L1, an integral component of HDL particles that might be involved in cholesterol efflux from the cell, oxidative stress, phospholipid transport and regulation of intracellular processes, including autophagy and vesicle transport.6 Another important finding is that cholesterol accumulates in the renal cortex in animal models of diabetic kidney disease (DKD).7,8 Strategies that reduce this accumulation (such as treatment with liver X receptor [LXR] agonists,9,10 farsenoid X receptor [FXR] agonists7 or cyclodextrin8) protect against kidney damage. A role for cholesterol in kidney disease is further supported by evidence that genes involved in the regulation of cholesterol homeostasis are differentially expressed in glomeruli isolated from patients with DKD and those from healthy living kidney donors.8,11
Complex lipids, including glycerophospholipids and glycolipids, can also negatively affect podocyte function. Glucosylceramide synthase inhibition in diabetic rats reduces glomerular glycerophospholipid accumulation and protects against kidney disease.12 In patients with Fabry disease, podocyte-specific accumulation of globotriaosylceramide (Gb3) is associated with proteinuria and effacement of foot processes (a manifestation of podocyte injury).13 Phospholipase A2 receptor (PLA2R), a transmembrane glycoprotein that binds phospholipase A2 (PLA2), is expressed in podocytes and functions as an autoantigen in individuals with primary membranous nephropathy.14 Whether or not, and how, PLA2R autoantibodies modulate the activity of PLA2 in metabolizing glycerophospholipids into arachidonic acids is unknown. Additionally sphingolipids might affect podocyte function. For example, we have demonstrated that expression of the SMPDL3b gene, which encodes acid sphingomyelinase (ASM)-like phosphodiesterase 3b is inversely associated with podocyte susceptibility to injury in patients with FSGS and might have an important role in the conversion of sphingomyelin to ceramide.15 The number of SMPDL3b-positive podocytes in biopsy samples of postreperfusion transplanted kidneys from patients with FSGS might represent a histological predictor of posttransplant recurrence of proteinuria.15
In this Review, we describe lipids and lipid-related proteins that are relevant to human podocyte biology and to the pathogenesis of glomerular diseases, focusing on FSGS and DKD. We also discuss the potential for therapeutic targeting of lipids in proteinuric glomerular disorders, with a particular emphasis on those that have been shown to be clinically relevant.
Podocyte cholesterol homeostasis
The content and distribution of cholesterol inside cells is maintained via regulation of cholesterol synthesis and intracellular trafficking. Circulating LDLs are the main carriers of cholesterol available for receptor-dependent uptake into lysosomes. The proteins Niemann-Pick C1 and Niemann-Pick C2 have major roles in cholesterol transport from lysosomes to the endoplasmic reticulum,16,17 whereas ATP-binding cassette transporters (for example ATP-binding cassette sub-family A member 1 [ABCA1] and ABC sub-family G member 1 [ABCG1]) have roles in cholesterol efflux to HDL acceptors.18 Each of these steps is connected by positive-feedback or negative-feedback loops that act on the enzymes involved in cholesterol synthesis, primarily HMG-CoA reductase and cholesterol acyl-transferase 1. The expression of genes that encode these enzymes is regulated by members of the sterol-regulatory element binding protein (SREBP) family of transcription factors.19,20 This complex system enables homeostatic control of cellular cholesterol levels despite physiological fluctuations in cholesterol requirements and exogenous supply (Figure 2).16
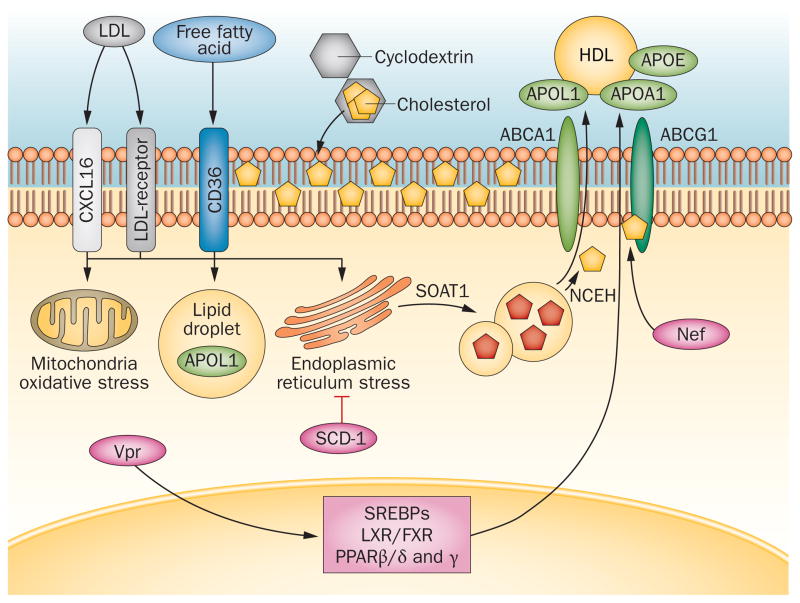
Figure 2.
Podocyte handling of cholesterol and free fatty acids. In podocytes, cholesterol uptake from circulating oxidized or unoxidized LDL occurs via the LDL-receptor or CXCL16. Cholesterol synthesis and metabolism is regulated by several nuclear receptors and transcription factors (LXR, FXR, SREBPs and PPARs). Neutral cholesterol accumulates in lipid droplets together with triglycerides that are derived from the uptake and metabolism of free fatty acids primarily via platelet glycoprotein 4 (also known as CD36). These free fatty acids can cause oxidative and endoplasmic reticulum stress based on the degree of saturation, which is regulated by SCD-1. Systemic or locally produced APOL1 might modulate oxidative stress and/or contribute to cholesterol efflux via ABCA1 and ABCG1 by serving as an HDL acceptor together with APOL1 and APOE. Cholesterol is converted by SOAT1 into esterified cholesterol (red pentagons) inside lipid droplets and is then converted by NCEH into free cholesterol and reaches the plasma membrane where it can be sequestered by cyclodextrin. HIV proteins such as Nef and Vpr can modulate PPAR transcriptional activity and ABCA1-dependent cholesterol efflux. Abbreviations: ABCA1, ATP-binding cassette sub-family A member 1; ABCG1, ABC sub-family G member 1; APOA1, apolipoprotein A-I; APOE, apolipoprotein E; APOL1; alipoprotein L1; CXCL16, CXC motif chemokine 16; GD3, O-acetylated disialosyllactosylceramide; FXR, farnesoid X-activated receptor; LXR, liver X receptor; NCEH, neutral cholesterol ester hydrolase 1; Nef, nef protein; PPAR, peroxisome proliferator-activated receptor; SCD-1, stearoyl-CoA desaturase 1; SOAT1, sterol-O-acyltransferase 1; SREBP, sterol-regulatory element binding protein; Vpr, viral protein R.
Cellular cholesterol homeostasis is compromised in various diseases of genetic21 and nongenetic origin such as Niemann Pick disease and atherosclerosis, and lead to lipid accumulation in target organs.22,23 These findings provide a rationale for pharmacological intervention to modulate cellular cholesterol content.24 Excessive intracellular cholesterol deposition can result from impaired cholesterol efflux due to downregulation of ABCA1 expression.25,26 Under normal physiological conditions, ABCA1 mediates the efflux of cholesterol and phospholipids via apolipoprotein A-1 and apolipoprotein E, which then form nascent HDLs.27
The importance of lipid rafts in the spatial organization of glomerular slit-diaphragm proteins was recognized several years ago when nephrin and podocin were found to be enriched in these rafts.28 The cholesterol component of lipid rafts is required for proper localization and function of slit-diaphragm proteins (Figure 1). Binding of podocin enables cholesterol to associate with short transient receptor potential cation channel 6, a step that is necessary for podocin-dependent activation of this protein.29 Many other proteins are probably also able to bind cholesterol, regulate the formation and function of large protein–cholesterol supercomplexes at the plasma membrane, and thus regulate the function of the slit diaphragm. However, excess accumulation of cellular cholesterol might adversely affect podocyte function8 as is the case in macrophages.30 Too much cholesterol might negatively affect the binding of podocyte slit-diaphragm proteins to each other, or interfere with the ability of podocyte slit-diaphragm proteins to bind caveolin-1, a lipid raft-associated protein that binds nephrin and CD2-associated protein.31 Pathological accumulation of cellular lipids occurs in in vitro models of podocyte injury (induced by exposure of podocytes to inflammatory cytokines32 or puromycin33) as well as in other experimental and clinical pathological conditions such as DKD and FSGS as discussed in more detail below.
Insights from APOL1 mutations in FSGS
Individuals of sub-Saharan African ethnicity have an increased risk of chronic glomerular diseases.34 Genetic analyses have shown that the locus associated with an increased risk of developing FSGS, HIV-associated nephropathy (HIVAN) and nondiabetic kidney disease is localized on chromosome 22.4,5 Two independent sequence variants in APOL1 are associated with an increased risk of kidney disease. Individuals with mutations that encode either two amino acid substitutions (Ser342Gly and Ile384Met) or a deletion (Asn388–Tyr389del) have no increased risk or a minimally increased risk of kidney disease. Individuals with two risk alleles have a substantially increased risk of FSGS, HIVAN, arterionephrosclerosis (hypertension-attributed kidney disease) and chronic kidney disease (CKD) with progression to end-stage renal disease (ESRD) of both nondiabetic4,5 and diabetic origin.35
Several lines of evidence suggest that apolipoprotein L1 and apolipoprotein A-1 have distinct roles in lipoprotein biology that might affect glomerular function (Figure 2). APOL1 is one member of a six gene family clustered on chromosome 22. Of the encoded proteins, apolipoprotein L1 is the only family member with a signal sequence for export from the cell. After export, apolipoprotein L1 colocalizes with the abundant plasma protein apolipoprotein A-1 in HDL particles.36 Apolipoprotein A-1 is involved in the formation of most cholesterol esters in plasma and promotes efflux of cholesterol from cells. The contribution of apolipoprotein L1 to cholesterol efflux remains to be established. Like all other members of the apolipoprotein family, apolipoprotein L1 has a BH3 domain, 37 suggesting a potential role in apoptosis. As apolipoprotein L1 also increases autophagy,38 a pathway relevant to podocyte function and survival,39 it would be interesting to determine whether or not APOL1 sequence variants are associated with alterations in this process.
Apolipoprotein L1 is present in HDL3 particles, which have antioxidative functions. Interestingly, the proteins that show the strongest correlations with the antioxidative functions of HDL3 include apolipoprotein L1, serum paraoxonase/arylesterase (PON) 1 and PON2.40 In support of these observations, fetal HDL, which does not have antioxidative functions, lacks apolipoprotein A-1, apolipoprotein L1 and PON1.41 Sequence variants of APOL1 and PON1 have been associated with an increased risk of CKD.42,43 Levels of HDL-bound cholesterol are positively associated with estimated glomerular filtration rate (eGFR) in healthy individuals of European and Asian ethnicity, but these levels are inversely correlated with eGFR (85–120 ml/min/1.73 m2) in healthy African Americans who carry two APOL1 risk alleles.44 The importance of this finding and the potential mechanisms that could explain the discrepancy in eGFR is unclear. Furthermore, individuals with two APOL1 risk alleles have an increased risk of cardiovascular events.45 A plausible hypothesis is that APOL1 genetic variants might result in dysfunctional HDL with altered antioxidative properties and/or altered ability to promote cholesterol efflux. Drugs that increase total levels of HDL do not result in cardiovascular protection,46 suggesting that interventions that target cholesterol efflux rather than HDL per se need to be developed.
How the function of apolipoproteins and other proteins involved in cholesterol efflux is altered in primary and secondary forms of FSGS is yet to be established. In support of a role for lipid metabolism in FSGS, the expression of SREBP-1 mRNA was increased in glomeruli from patients with FSGS when compared to living donors.8 Increased SREBP-1 levels in the presence of unchanged ABCA1 expression suggest that upregulation of lipid synthesis might occur in the glomeruli of patients with FSGS.8 The results of these studies differ from those in experimental models of LDL-receptor deficiency, in which increased SREBP-1 expression decreased ABCA1 promoter activity, ABCA1 expression and cholesterol efflux in macrophages.47 Intriguingly, HIV and obesity, which affect lipid metabolism and fat distribution, can cause histological lesions that resemble FSGS. 48,49 Rare genetic disorders associated with systemic lipid dysmetabolism also support an association between dysregulated lipid homeostasis and nephropathy. For example, renal histological lesions in familial hypercholesterolaemia resemble those in FSGS.50 Patients with FSGS have a higher frequency of the APOE*ε4 allele, which is associated with higher plasma cholesterol levels, than in healthy individuals (33.3% versus 10.8%).51
HIVAN is of particular relevance to podocyte cholesterol metabolism and APOL1. African Americans with one of the APOL1 risk alleles and untreated HIV infection have a 50% risk of developing HIVAN, suggesting that HIV infection might be a second hit in the induction of podocyte injury.52 In transgenic mice, overexpression of the HIV-1 accessory protein viral protein R (Vpr) in liver and adipose tissue dramatically decreased plasma triglyceride and cholesterol levels.53 Vpr overexpression in adipocytes induced expression of peroxisome proliferator-activated receptors (PPARs) β, δ and γ, nuclear receptor proteins that function as transcription factors, and affected the net energy expenditure of the cell.54 Another HIV-1 accessory protein, Nef, attenuated lipid efflux in macrophages by suppressing the activity of ABCA1.55 Nef-mediated transfer of cholesterol to lipid rafts competed with ABCA1-dependent cholesterol efflux through a reduction in cell-surface available ABCA1 and induction of ABCA1 catabolism in the lysosome. As patients with HIV often have atherosclerosis,56 the interplay between APOL1 risk alleles, HIV and the pathogenesis of atherosclerosis merits further study. Such investigations might indicate that atherosclerosis and glomerulosclerosis are mechanistically related. HIVAN might be an important disease in which to explore glomerular lipid biology, from which findings could be extended to patients with FSGS.
Sphingolipids and gangliosides
Since the first description of glycosphingolipid accumulation in the renal parenchyma resulting in glomerular hypertrophy in mice with streptozotocin-induced diabetes,8 several studies have highlighted a role for sphingolipids and gangliosides in podocyte biology. In Fabry disease, reduced activity of the lysosomal enzyme α-galactosidase A leads to lysosomal accumulation of Gb3, resulting in characteristic inclusions called zebra bodies in various organs and cell types (Figure 3).57 Analysis of kidney biopsy samples from 14 patients (median age 12 years) with Fabry disease using unbiased quantitative stereology demonstrated age-dependent accumulation of Gb3 in podocytes.13 Another substrate of α-galactosidase A, globotriaosylsphingosine (also known as lysoglobotriaosylceramide) is an active metabolite that acts as a profibrotic agent in cultured human podocytes.58 Enzyme replacement therapy with recombinant α-galactosidase A in patients with Fabry disease successfully cleared glycolipid accumulation from the kidney and vasculature.59 Interestingly, different kinds of substrate tend to accumulate in different cell types. In Gaucher disease, for example, proteinuria is associated with the accumulation of glucocerebroside and the formation of Gaucher bodies in glomerular mesangial and endothelial cells, as well as in interstitial cells, as a result of the absence of functional nonlysosomal glucosylceramidase.60
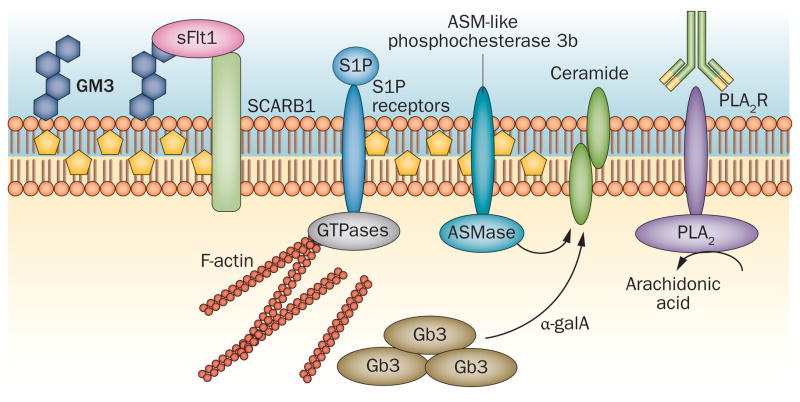
Figure 3.
Role of lipids other than cholesterol in podocytes. An important function of GM3 as a ligand for soluble Flt1 in podocytes might enable interaction between soluble Flt1 and the HDL receptor SCARB1. In Fabry disease, α-galactosidase A deficiency results in accumulation of Gb3 in zebra bodies. Circulating S1P can bind to various S1P receptors and modulate MAPK signalling and small GTPase activity. PLA2R binds PLA2 and liberates arachidonic acid from glycerophospholipids. This receptor is expressed in podocytes and represents an autoantigen in many individuals with membranous nephropathy. ASM-like phosphodiesterase 3b might have a role in the pathogenesis of proteinuria in focal segmental glomerulosclerosis. This protein is stabilized by rituximab and modulates the activity of ASMase and regulates the generation of ceramide. Abbreviations: α-galA, α-galactosidase A; ASMase, acid sphingomyelinase; Gb3, globotriaosylceramide; GM3, ganglioside GM3; MAPK, mitogen-activated protein kinase; PLA2, phospholipase A2; PLA2R, PLA2 receptor; S1P, sphingosine-1-phosphate; SCARB1, scavenger receptor class B member 1; sFlt1, soluble Flt1.
Glycosphingolipiduria occurs in the presence of proteinuria of various aetiologies61 but whether it contributes to disease progression is yet to be established. Some glycosphingolipids act as regulators of outside-in signalling. For example, ganglioside GM3 (GM3) located in lipid-raft domains in the slit diaphragm of podocytes is a receptor for soluble Flt162 (Figure 3). Binding of soluble Flt1 to GM3 is essential for autocrine preservation of the podocyte actin cytoskeleton and for prevention of proteinuria.62 These findings have implications for pathogenesis as reduced glomerular expression of GM3 has been observed in experimental models of diabetes63 and increased expression of GM3 has been described in DKD.64 Mice with mutations in UDP-GlcNAc-2-epimerase/ManAc kinase (an enzyme responsible for sialylation of GM3) manifest glomerular defects and proteinuria, supporting a role for GM3 in the maintenance of glomerular structure and function.65 Interestingly, soluble Flt1 also binds to scavenger receptor class B member (SCARB) 1, a HDL receptor that localizes to lipid rafts.62 Mutations in SCARB2, a member of the same gene family, are associated with structural damage to podocytes and with nephrotic syndrome, supporting the clinical relevance of these findings.66 Other gangliosides are also associated with kidney damage, for example hexosaminidase deficiency results in Sandhoff disease, which is characterized by accumulation of the ganglioside GM2 (GM2) in several organs, including the kidney, in which deposits occur in podocytes and tubular cells.67 A sialic-acid-containing lipid, O-acetylated disialosyllactosylceramide (GD3), was identified as a cell-type-specific ganglioside in rat podocytes.68 Treatment of mice with an antibody against podocyte-specific O-acetylated GD3 caused nephrin phosphorylation and dislocation from the podocyte slit diaphragm69 (Figure 1). Whether or not O-acetylated GD3 deficiency, as observed in the aminonucleoside puromycin model of experimental FSGS,70 can cause podocyte injury and proteinuria per se is currently unknown. The discovery of gangliosides specific to podocytes is, however, important as it makes them an attractive cell surface molecule for targeted drug delivery.
A role for sphingolipids as modulators of podocyte function in FSGS and other glomerular diseases is an emerging concept. Sphingosine 1-phosphate receptors have, for example, gained much interest as potential therapeutic targets in DKD71 and acute kidney injury, and have been reviewed elsewhere.72 Patients with FSGS are at high risk of recurrence of proteinuria after kidney transplantation, and as mentioned, the number of SMPDL3b-positive podocytes is reduced in patients with recurrent proteinuria15 (Figure 3).15 Exposure to sera from patients with FSGS augments the response of podocytes with reduced SMPDL3b expression to outside-in signalling.15 Treatment with rituximab, which stabilizes ASM-like phosphodiesterase 3b expression and regulates the conversion of sphingomyelin to ceramide, reduced the incidence of posttransplant proteinuria in patients with FSGS15 and in pigs with xenotransplant-related proteinuria.73
If and how plasma membrane lipids might affect the susceptibility of podocytes to circulating factors in FSGS is unknown. Among several potential circulating factors, studies have shown that soluble urokinase receptor (suPAR) is elevated in two-thirds of patients with primary FSGS and that a high concentration of suPAR before transplantation underlies an increased risk of recurrence.74 Circulating suPAR is associated with lipid-dependent activation of podocyte β3 integrin, a phenomenon that might lead to foot process effacement and urinary protein loss.75 Sequestration of plasma membrane lipids with cyclodextrin abolished the suPAR-dependent activation of β3 integrin in podocytes in vitro. The identities of the lipids that regulate suPAR signalling in podocytes and the potential relationship between suPAR and ASM-like phosphodiesterase 3b have yet to be determined.
Cholesterol transport deficiency
The LCAT gene encodes phosphatidylcholine-sterol acyltransferase, which has an important role in reverse cholesterol transport—the process of moving excess cholesterol from tissues to circulating HDL.76 The enzyme is located on the surface of circulating HDL and LDL particles, where it transfers fatty acids (typically linoleic acid) to cholesterol, generating a hydrophobic cholesteryl ester that forms the core of a lipoprotein particle. In patients with phosphatidylcholine-sterol acyltransferase deficiency, HDL particles cannot mature and pre-β HDL particles accumulate and are cleared from the circulation via the kidney.77 Complete phosphatidylcholine-sterol acyltransferase deficiency is a recessive disorder that leads to ESRD in the fourth or fifth decade with a typical triad of diffuse corneal opacities, target cell haemolytic anaemia and proteinuria with renal failure.78 The mechanism underlying disease pathogenesis is not well understood. However, the presence of foam cells in the mesangium and microvasculature of the kidney and characteristic vacuoles in the glomerular basement membrane suggest lipid toxicity.79 Whether or not individuals with LCAT deficiency have an atherogenic lipoprotein phenotype remains unclear, as the number of affected individuals is low, the role of lifestyle factors in driving atherogenesis is difficult to adjust for, and imaging approaches vary between studies.78 Potential therapeutic strategies for LCAT-related renal disease that are being explored include gene therapy and recombinant phosphatidylcholine-sterol acyltransferase enzyme replacement therapy.79
Phospholipase A2 signalling
Most individuals with membranous nephropathy have autoantibodies against PLA2R, which is expressed in podocytes.14 Whether autoantibodies against PLA2R affect PLA2, which liberates arachidonic acid from glycerophospholipids and modulates podocyte function is unknown. Regulation of cell senescence by PLA2R through the p53 pathway has been suggested.80 However, whether cellular injury is the result of antibody deposition on the podocyte cell membrane and subsequent complement activation, or altered PLA2R signalling is unclear. Calcium-dependent and calcium-independent activation of PLA2R modulates synaptic plasticity and the function of glutamate receptors in neurons,81 probably through the modulation of glycerophospholipids by PLA2. This observation combined with evidence that podocytes express functional glutamate receptors82 may open new avenues of investigation into the biology of PLA2R and how autoantibodies against PLA2R might affect podocyte injury through lipid-dependent damage. A major obstacle to investigating the role of PLA2R autoantibodies in the pathogenesis of membranous nephropathy is the lack of PLA2R expression on podocytes in animal models. Translational studies are therefore required, including use of humanized experimental models to identify potential PLA2R signalling pathways that are modulated by autoantibodies and might affect podocyte function.
Cholesterol, free fatty acids and DKD
DKD is the most common cause of ESRD in the USA.83 Podocyte injury is a feature of DKD in patients with type 1 or type 2 diabetes mellitus (T1DM or T2DM)84–88 and podocytopenia is an independent predictor of DKD progression.84,85 However, the mechanisms underlying podocytopenia in DKD are not fully understood. Trials of multifactorial interventions, including glycaemic control, blood pressure control and lifestyle modifications have demonstrated that current treatments might slow but do not halt the progression of DKD.89,90 The identification of other determinants of disease progression might lead to novel drug targets.
The interplay of intracellular lipid metabolism, chronic inflammation, insulin resistance and innate immunity might be important in the pathogenesis of macrovascular complications.91,92 In diabetes, cholesterol accumulation in adipocytes and hepatocytes contributes to remodelling of the actin cytoskeleton, insulin resistance and cell death.93,94 Clinical studies and experimental models of DKD show that renal accumulation of cholesterol correlates with the development of glomerulosclerosis.26,95–97 This finding is similar to that described in patients with atherosclerosis, in whom the capacity of cholesterol efflux in macrophages is inversely correlated with the frequency of carotid and coronary atherosclerotic plaques.22 The accumulation of cholesterol in peripheral tissues in experimental models of diabetes, owing to diabetic complications, has also been described.98 Intracellular accumulation of cholesterol is one of the major determinants of cellular insulin signalling,99–103 and multiple studies show that insulin resistance correlates with microalbuminuria in patients with T1DM104,105 or T2DM,106,107 their siblings108,109 and nondiabetic individuals.110
In the nonobese diabetic mouse model of T1DM, ABCA1 expression is downregulated in the kidneys and circulating macrophages.25 This observation is consistent with our findings in glomeruli from 70 patients with T2DM and early nephropathy8 and in glomeruli from a separate cohort of 34 patients with T2DM.11 In this last cohort the presence of lipid droplets in podocytes (visualized using electron microscopy) was reported. This increase was associated with downregulation of genes that encode proteins involved in cholesterol efflux (ABCA1, ABCG1 and APOE), upregulation of LDL receptors, impaired fatty-acid β-oxidation and increased expression of angiopoietin-related protein 4.11 ABCA1 sequence variants are associated with an increased risk of coronary artery disease111 and the ability of serum to induce ABCA1-mediated cholesterol efflux in macrophages is impaired in patients with T2DM and incipient or overt nephropathy.112 However, the cause of decreased ABCA1 expression in organs affected by diabetes, and whether cholesterol efflux and VEGF signalling are linked in podocytes (as has been described in endothelial cells113) is unclear. Levels of tumour necrosis factor (TNF), circulating soluble TNF receptor 1 and TNF receptor 2 are associated with DKD progression in T1DM and T2DM.114,115 TNF and/or any of its soluble receptors might downregulate ABCA1 mRNA expression in podocytes, similar to that which occurs in hepatocytes116 and intestinal cells.117 TNF has also been shown to increase cholesterol uptake in macrophages,118 and increased uptake and metabolism of lipoproteins has been described in glomerular epithelial cells from patients with T2DM.119 Podocyte survival and the integrity of the actin cytoskeleton are impaired after CXC motif chemokine 16-dependent uptake of oxidized LDL120,121 and further studies are needed to investigate cholesterol influx in podocytes in DKD. Another interesting observation is that lipid phosphatases, such as phosphatidylinositol 3,4,5-trisphosphate 5-phosphatase 2, downregulate insulin signalling via interaction with CD2-associated protein with subsequent effects on Akt activity and cell survival.122
Other lipids might affect podocyte function in DKD. For example, saturated free fatty acids (FFAs) involved in the pathogenesis of T2DM are thought to induce endoplasmic reticulum stress and apoptosis of podocytes.123,124 Loss of podocytes is a hallmark of DKD and these cells are highly susceptible to damage from saturated but not monounsaturated FFAs.124 Both endoplasmic reticulum stress and podocyte cell death could be ameliorated by inducing stearoyl-CoA desaturase 1, which converts saturated FFAs to monounsaturated FFAs and is upregulated in podocytes in biopsy samples from patients with DKD (Figure 2).125
Disturbed transport and oxidation of FFAs, paralleled by an impaired antioxidant response, damages podocyte structure and leads to glomerulopathy during the early stages of DKD.126 Enhanced FFA uptake by podocytes is mediated by increased expression of the scavenger receptor platelet glycoprotein 4 (also known as CD36) and a decrease in fatty acid β-oxidation leading to intracellular lipid accumulation. Accumulated FFAs are trapped in the mitochondrial matrix, leading to production of reactive oxygen species, lipid peroxidation and mitochondrial damage and dysfunction.127 The association between renal accumulation of triglycerides and reduced expression of the ultrasensitive energy sensor AMPKα1128 strongly suggests that energy-generating and energy-consuming pathways might link lipid accumulation to podocyte dysfunction in DKD and other disorders that result in CKD. Podocyte-specific expression of fatty-acid-binding proteins correlates with proteinuria in patients with obesity-related glomerulopathy and in diabetic (db/db) mice.129 In mice fed a high-fat diet and in models of age-related kidney disease, increased renal expression of SREBP-1 has a critical role in renal lipid accumulation, and increases the activity of proinflammatory cytokines.118 Mice treated with an FXR-activating ligand show decreased triglyceride accumulation through modulation of fatty acid synthesis and oxidation, which is associated with decreased proteinuria and prevention of podocyte loss.7 Intracellular lipid overload is particularly severe in podocytes in patients with CKD, in whom binding and/or uptake of triglyceride-rich LDL by glomerular cells leads to increased endocytic accumulation of triglycerides that might have a role in lipotoxicity.128,130
Therapeutic implications
A meta-analysis showed that treatment of patients with CKD using statins reduces albuminuria, with an effect size of ~50%.131 Whether statins also slow disease progression is unknown132,133 and other therapies are needed to lower cellular cholesterol levels in DKD. Pharmacological interventions that increase ABCA1 expression (such as LXR agonists) might be beneficial in DKD but their application is limited by a high incidence of adverse events such as confusion and palpitation134 and their intrinsic lipogenic effects.135 One potential alternative is to use drugs that sequester plasma-membrane cholesterol, such as cyclodextrins,24,136 which reduce intracellular cholesterol and inflammation137 and increase insulin sensitivity in adipocytes in vitro.138–140 Long-term studies of experimental treatment with hydroxy-propyl-β cyclodextrin (HPBCD) for compassionate use in Niemann–Pick disease are ongoing.141 We reported a beneficial effect of HPBCD on DKD in the leptin-deficient BTBR ob/ob murine model of T2DM.8 These preclinical studies might lead to the clinical use of HPBCD in DKD. However, the efficacy of HPBCD in other glomerular disorders remains to be established.
Targeting triglycerides with fibrates partially protects against microvascular complications in T2DM.142 In addition, selective agonists of bile acid receptors, such as FXR, are effective in preventing DKD in experimental models of T1DM with DKD.26,143 The use of other bile-acid-receptor agonists such as G protein-coupled receptor (for example TGR5) agonists alone or in combination with FXR agonists might represent a new strategy to treat DKD.
Several inhibitors of the cholesteryl ester transfer protein effectively increase HDL cholesterol levels and have been developed for the treatment of dyslipidemia. However, many trials of these inhibitors have been discontinued due to lack of efficacy or safety concerns.46 These issues highlight the importance of developing therapeutic strategies to target specific subclasses of HDL and/or other small molecules that might increase reverse cholesterol transport by reducing cholesterol sequestered in target organs. Ongoing clinical trials of apolipoprotein A-I mimetic peptides for the treatment of Tangier disease (also known as familial α-lipoprotein deficiency), which is caused by mutations in ABCA1,144 might prove that these therapies are efficacious in enhancing cholesterol efflux and thus enable broader clinical applications for apolipoprotein A-I-mimetic peptides.
Conclusions
Lipids and lipid-related enzymes have a major role in modulating podocyte function in glomerular disorders of diabetic and nondiabetic origin and individuals carrying sequence variants in genes important in lipid metabolism often present with kidney disease. Targeting lipid dysmetabolism might, therefore, be an important strategy to treat proteinuric kidney diseases. Identification of clinically relevant targets, such as ABCA1, intracellular cholesterol, LXR and FXR, have led to the development of new drugs that are being tested for their therapeutic efficacy in kidney disease. PLA2R and apolipoprotein L1 are also potential therapeutic targets but further preclinical studies are required to elucidate the mechanisms by which autoantibodies against PLA2R and APOL1 genetic variants contribute to disease pathogenesis.
Key points.
Lipids and lipid-related enzymes might modulate podocyte function
Lipid biology is important in understanding the pathogenesis of glomerular diseases of metabolic and nonmetabolic origin
Plasma membrane and intracellular lipids can modulate podocyte function irrespective of circulating lipids
Therapeutic strategies that target cellular lipids, such as cholesterol and sphingolipids, might be protective in kidney disease
Review criteria.
We searched PubMed without limits on year of publication, but with a focus on studies from the past 10 years. We included only English-language full text papers. The search terms used were “podocyte”, “proteinuria”, “nephrotic syndrome”, “FSGS”, “diabetic nephropathy”, “ApoL1”. Each of these terms was searched alone or in combination with “lipid”, “cholesterol”, “fatty acids”, “sphingolipids”, “ganglioside”, and “lypotoxicity”.
Acknowledgments
A.F. and S.M. are supported by the NIH and NIDDK (grant numbers DK090316 and 5U24DX076169), the National Center for Advancing Translational Sciences (grant number 1UL1TR000460), the Diabetes Research Institute Foundation, the Nephcure Foundation and the Peggy and Harold Katz Family Foundation. S.M. is supported by the Stanley J. Glaser Foundation Research Award. J.B.K. is supported by the NIDDK Intramural Research Program.
Footnotes
Competing interests
A.F. and S.M. hold patent application numbers US13/879,892, PCT/US11/56272: ‘Assays, methods and kits for predicting renal disease and personalized treatment strategies’; PCT/US2012/062594, ‘Soluble urokinase receptor (suPAR) in diabetic kidney disease’; PCT/US13/36484, ‘Method of using cyclodextrin’. A.F. is a consultant for Hoffman-La Roche and Mesoblast. J.B.K. declares no competing interests.
Author contributions
J.B.K. researched the data and A.F. wrote the article. S.M. and J.B.K. provided substantial contributions to discussion of the content and reviewed or edited the manuscript before submission.
Contributor Information
Alessia Fornoni, Peggy and Harold Katz Family Drug Discovery Center, Division of Nephrology and Hypertension, University of Miami Miller School of Medicine, 1580 North West 10th Avenue, Miami, FL 33136, USA.
Sandra Merscher, Peggy and Harold Katz Family Drug Discovery Center, Division of Nephrology and Hypertension, University of Miami Miller School of Medicine, 1580 North West 10th Avenue, Miami, FL 33136, USA.
Jeffrey B. Kopp, Kidney Disease Section, Kidney Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, NIH, 10 Center Drive, 3N116 Bethesda, MD 20892-1268, USA
References
- 1.Fessler MB, Parks JS. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signalling. J Immunol. 2011;187:1529–1535. doi: 10.4049/jimmunol.1100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schonholzer KW, Waldron M, Magil AB. Intraglomerular foam cells and human focal glomerulosclerosis. Nephron. 1992;62:130–136. doi: 10.1159/000187020. [DOI] [PubMed] [Google Scholar]
- 3.Lee HS, Kruth HS. Accumulation of cholesterol in the lesions of focal segmental glomerular sclerosis. Nephrology (Carlton) 2003;8:224–223. doi: 10.1046/j.1440-1797.2003.00160.x. [DOI] [PubMed] [Google Scholar]
- 4.Kao WH, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kopp JB, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pays E, et al. The trypanolytic factor of human serum. Nat Rev Microbiol. 2006;4:477–486. doi: 10.1038/nrmicro1428. [DOI] [PubMed] [Google Scholar]
- 7.Wang XX, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59:2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merscher-Gomez S, et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes. 2013;62:3817–3827. doi: 10.2337/db13-0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tachibana H, et al. Activation of liver X receptor inhibits osteopontin and ameliorates diabetic nephropathy. J Am Soc Nephrol. 2012;23:1835–1846. doi: 10.1681/ASN.2012010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kiss E, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am J Pathol. 2013;182:727–741. doi: 10.1016/j.ajpath.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 11.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2013;55:561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zador IZ, et al. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest. 1993;91:797–803. doi: 10.1172/JCI116299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Najafian B, et al. Progressive podocyte injury and globotriaosylceramide (GL-3) accumulation in young patients with Fabry disease. Kidney Int. 2011;79:663–670. doi: 10.1038/ki.2010.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck LH, Jr, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornoni A, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85ra46. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abi-Mosleh L, Infante RE, Radhakrishnan A, Goldstein JL, Brown MS. Cyclodextrin overcomes deficient lysosome-to-endoplasmic reticulum transport of cholesterol in Niemann-Pick type C cells. Proc Natl Acad Sci USA. 2009;106:19316–19321. doi: 10.1073/pnas.0910916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maxfield FR, Wustner D. Intracellular cholesterol transport. J Clin Invest. 2002;110:891–898. doi: 10.1172/JCI16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fielding CJ, Fielding PE. Cellular cholesterol efflux. Biochim Biophys Acta. 2001;1533:175–189. doi: 10.1016/s1388-1981(01)00162-7. [DOI] [PubMed] [Google Scholar]
- 19.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35–46. doi: 10.1016/j.cell.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Peake KB, Vance JE. Defective cholesterol trafficking in Niemann-Pick C-deficient cells. FEBS Lett. 2010;584:2731–2739. doi: 10.1016/j.febslet.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 22.Khera AV, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Lay S, et al. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J Biol Chem. 2001;276:16904–16910. doi: 10.1074/jbc.M010955200. [DOI] [PubMed] [Google Scholar]
- 24.Christian AE, Haynes MP, Phillips MC, Rothblat GH. Use of cyclodextrins for manipulating cellular cholesterol content. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 25.Tang C, Kanter JE, Bornfeldt KE, Leboeuf RC, Oram JF. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J Lipid Res. 2010;51:1719–1728. doi: 10.1194/jlr.M003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Proctor G, et al. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 27.Mulya A, et al. Initial interaction of apoA-I with ABCA1 impacts in vivo metabolic fate of nascent HDL. J Lipid Res. 2008;49:2390–2401. doi: 10.1194/jlr.M800241-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwarz K, et al. Podocin, a raft-associated component of the glomerular slit diaphragm, interacts with CD2AP and nephrin. J Clin Invest. 2001;108:1621–1629. doi: 10.1172/JCI12849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huber TB, et al. Podocin and MEC-2 bind cholesterol to regulate the activity of associated ion channels. Proc Natl Acad Sci USA. 2006;103:17079–17086. doi: 10.1073/pnas.0607465103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bie J, Zhao B, Song J, Ghosh S. Improved insulin sensitivity in high fat- and high cholesterol-fed Ldlr−/− mice with macrophage-specific transgenic expression of cholesteryl ester hydrolase: role of macrophage inflammation and infiltration into adipose tissue. J Biol Chem. 2010;285:13630–13637. doi: 10.1074/jbc.M109.069781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sorensson J, et al. Glomerular endothelial fenestrae in vivo are not formed from caveolae. J Am Soc Nephrol. 2002;13:2639–2647. doi: 10.1097/01.asn.0000033277.32822.23. [DOI] [PubMed] [Google Scholar]
- 32.Zhang G, Li Q, Wang L, Chen Y, Zhang W. Interleukin-1β enhances the intracellular accumulation of cholesterol by up-regulating the expression of low-density lipoprotein receptor and 3-hydroxy-3-methylglutaryl coenzyme A reductase in podocytes. Mol Cell Biochem. 2011;346:197–204. doi: 10.1007/s11010-010-0605-4. [DOI] [PubMed] [Google Scholar]
- 33.Mayrhofer C, et al. Alterations in fatty acid utilization and an impaired antioxidant defense mechanism are early events in podocyte injury: a proteomic analysis. Am J Pathol. 2009;174:1191–1202. doi: 10.2353/ajpath.2009.080654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins AJ, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63(Suppl):A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Parsa A, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duchateau PN, et al. Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem. 1997;272:25576–25582. doi: 10.1074/jbc.272.41.25576. [DOI] [PubMed] [Google Scholar]
- 37.Zhaorigetu S, Wan G, Kaini R, Jiang Z, Hu C. ApoL1, a BH3-only lipid-binding protein, induces autophagic cell death. Autophagy. 2008;4:1079–1082. doi: 10.4161/auto.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan G, et al. Apolipoprotein L1, a novel Bcl-2 homology domain 3-only lipid-binding protein, induces autophagic cell death. J Biol Chem. 2008;283:21540–21549. doi: 10.1074/jbc.M800214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartleben B, Wanner N, Huber TB. Autophagy in glomerular health and disease. Semin Nephrol. 2014;34:42–52. doi: 10.1016/j.semnephrol.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Précourt LP, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosism. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- 41.Sreckovic I, et al. Distinct composition of human fetal HDL attenuates its anti-oxidative capacity. Biochim Biophys Acta. 2013;1831:737–746. doi: 10.1016/j.bbalip.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 42.Araki S, et al. Polymorphisms of human paraoxonase 1 gene (PON1) and susceptibility to diabetic nephropathy in type I diabetes mellitus. Diabetologia. 2000;43:1540–1543. doi: 10.1007/s001250051566. [DOI] [PubMed] [Google Scholar]
- 43.Genovese G, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bentley AR, et al. Variation in APOL1 contributes to ancestry-level differences in HDLc-kidney function association. Int J Nephrol. 2012;2012:748984. doi: 10.1155/2012/748984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ito K, et al. Increased burden of cardiovascular disease in carriers of APOL1 genetic variants. Circ Res. 2014;114:845–850. doi: 10.1161/CIRCRESAHA.114.302347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz GG, et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, et al. Genetic deletion of low density lipoprotein receptor impairs sterol-induced mouse macrophage ABCA1 expression. A new SREBP1-dependent mechanism. J Biol Chem. 2008;283:2129–2138. doi: 10.1074/jbc.M706636200. [DOI] [PubMed] [Google Scholar]
- 48.Medapalli RK, He JC, Klotman PE. HIV-associated nephropathy: pathogenesis. Curr Opin Nephrol Hypertens. 2011;20:306–311. doi: 10.1097/MNH.0b013e328345359a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–1509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 50.Elmaci AM, et al. A case of homozygous familial hypercholesterolemia with focal segmental glomerulosclerosis. Pediatr Nephrol. 2007;22:1803–1805. doi: 10.1007/s00467-007-0534-y. [DOI] [PubMed] [Google Scholar]
- 51.Asami T, Ciomartan T, Hayakawa H, Uchiyama M, Tomisawa S. Apolipoprotein Eε4 allele and nephrotic glomerular diseases in children. Pediatr Nephrol. 1999;13:233–236. doi: 10.1007/s004670050599. [DOI] [PubMed] [Google Scholar]
- 52.Kopp JB, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balasubramanyam A, et al. Effects of transgenic expression of HIV-1 Vpr on lipid and energy metabolism in mice. Am J Physiol Endocrinol Metab. 2007;292:E40–E48. doi: 10.1152/ajpendo.00163.2006. [DOI] [PubMed] [Google Scholar]
- 54.Shrivastav S, et al. HIV-1 Vpr enhances PPARβ/δ-mediated transcription, increases PDK4 expression, and reduces PDC activity. Mol Endocrinol. 2013;27:1564–1576. doi: 10.1210/me.2012-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cui HL, et al. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J Lipid Res. 2012;53:696–708. doi: 10.1194/jlr.M023119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gibellini D, et al. HIV-related mechanisms in atherosclerosis and cardiovascular diseases. J Cardiovasc Med (Hagerstown) 2013;14:780–790. doi: 10.2459/JCM.0b013e3283619331. [DOI] [PubMed] [Google Scholar]
- 57.Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol. 2002;13(Suppl 2):S134–S138. [PubMed] [Google Scholar]
- 58.Sanchez-Nino MD, et al. Globotriaosylsphingosine actions on human glomerular podocytes: implications for Fabry nephropathy. Nephrol Dial Transplant. 2011;26:1797–1802. doi: 10.1093/ndt/gfq306. [DOI] [PubMed] [Google Scholar]
- 59.Thurberg BL, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 60.Chander PN, Nurse HM, Pirani CL. Renal involvement in adult Gaucher’s disease after splenectomy. Arch Pathol Lab Med. 1979;103:440–445. [PubMed] [Google Scholar]
- 61.Townsend RR, Orth RM, Clawson CM, Li SC, Li YT. Increased glycosphingolipid excretion associated with proteinuria. J Clin Invest. 1978;62:119–123. doi: 10.1172/JCI109095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin J, et al. Soluble FLT1 binds lipid microdomains in podocytes to control cell morphology and glomerular barrier function. Cell. 2012;151:384–399. doi: 10.1016/j.cell.2012.08.037. [DOI] [PubMed] [Google Scholar]
- 63.Kwak DH, et al. Decreases of ganglioside GM3 in streptozotocin-induced diabetic glomeruli of rats. Life Sci. 2003;72:1997–2006. doi: 10.1016/s0024-3205(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 64.Novak A, et al. Renal distribution of ganglioside GM3 in rat models of types 1 and 2 diabetes. J Physiol Biochem. 2013;69:727–735. doi: 10.1007/s13105-013-0249-4. [DOI] [PubMed] [Google Scholar]
- 65.Galeano B, et al. Mutation in the key enzyme of sialic acid biosynthesis causes severe glomerular proteinuria and is rescued by N-acetylmannosamine. J Clin Invest. 2007;117:1585–1594. doi: 10.1172/JCI30954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Berkovic SF, et al. Array-based gene discovery with three unrelated subjects shows SCARB2/LIMP-2 deficiency causes myoclonus epilepsy and glomerulosclerosis. Am J Hum Genet. 2008;82:673–684. doi: 10.1016/j.ajhg.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tatematsu M, et al. Sandhoff disease. Acta Pathol Jpn. 1981;31:503–512. doi: 10.1111/j.1440-1827.1981.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 68.Reivinen J, Holthofer H, Miettinen A. A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int. 1992;42:624–631. doi: 10.1038/ki.1992.327. [DOI] [PubMed] [Google Scholar]
- 69.Simons M, et al. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol. 2001;159:1069–1077. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Holthofer H, Reivinen J, Solin ML, Haltia A, Miettinen A. Decrease of glomerular disialogangliosides in puromycin nephrosis of the rat. Am J Pathol. 1996;149:1009–1015. [PMC free article] [PubMed] [Google Scholar]
- 71.Awad AS, et al. Chronic sphingosine 1-phosphate 1 receptor activation attenuates early-stage diabetic nephropathy independent of lymphocytes. Kidney Int. 2011;79:1090–1098. doi: 10.1038/ki.2010.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jo SK, Bajwa A, Awad AS, Lynch KR, Okusa MD. Sphingosine-1-phosphate receptors: biology and therapeutic potential in kidney disease. Kidney Int. 2008;73:1220–1230. doi: 10.1038/ki.2008.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tasaki M, et al. Rituximab treatment prevents the early development of proteinuria following pig-to-babon xeno-kidney transplantation. J Am Soc Nephrol. 2014;25:737–744. doi: 10.1681/ASN.2013040363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei C, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei C, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 76.Kunnen S, Van Eck M. Lecithin:cholesterol acyltransferase: old friend or foe in atherosclerosis? J Lipid Res. 2012;53:1783–1799. doi: 10.1194/jlr.R024513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Calabresi L, Franceschini G. Lecithin: cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med. 2010;20:50–53. doi: 10.1016/j.tcm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 78.Jimi S, et al. Possible induction of renal dysfunction in patients with lecithin:cholesterol acyltransferase deficiency by oxidized phosphatidylcholine in glomeruli. Arterioscler Thromb Vasc Biol. 1999;19:794–801. doi: 10.1161/01.atv.19.3.794. [DOI] [PubMed] [Google Scholar]
- 79.Rousset X, Shamburek R, Vaisman B, Amar M, Remaley AT. Lecithin cholesterol acyltransferase: an anti- or pro-atherogenic factor? Curr Atheroscler Rep. 2011;13:249–256. doi: 10.1007/s11883-011-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Augert A, et al. The M-type receptor PLA2R regulates senescence through the p53 pathway. EMBO Rep. 2009;10:271–277. doi: 10.1038/embor.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Allyson J, Bi X, Baudry M, Massicotte G. Maintenance of synaptic stability requires calcium-independent phospholipase A2 activity. Neural Plast. 2012;2012:569149. doi: 10.1155/2012/569149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giardino L, et al. Podocyte glutamatergic signaling contributes to the function of the glomerular filtration barrier. J Am Soc Nephrol. 2009;20:1929–1940. doi: 10.1681/ASN.2008121286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Collins AJ, et al. United States Renal Data System 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2011;59:e1–e420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 84.Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with Type II diabetes and microalbuminuria. Diabetologia. 1999;42:1341–1344. doi: 10.1007/s001250051447. [DOI] [PubMed] [Google Scholar]
- 85.Pagtalunan ME, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Steffes MW, Schmidt D, McCrery R, Basgen JM. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–2113. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 87.Verzola D, et al. Apoptosis in the kidneys of patients with type II diabetic nephropathy. Kidney Int. 2007;72:1262–1272. doi: 10.1038/sj.ki.5002531. [DOI] [PubMed] [Google Scholar]
- 88.White KE, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–3089. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 89.Hovind P, et al. Decreasing incidence of severe diabetic microangiopathy in type 1 diabetes. Diabetes Care. 2003;26:1258–1264. doi: 10.2337/diacare.26.4.1258. [DOI] [PubMed] [Google Scholar]
- 90.Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 91.Miller YI, Choi SH, Fang L, Harkewicz R. Toll-like receptor-4 and lipoprotein accumulation in macrophages. Trends Cardiovasc Med. 2009;19:227–232. doi: 10.1016/j.tcm.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shi H, et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qi M, Liu Y, Freeman MR, Solomon KR. Cholesterol-regulated stress fiber reduction. J Cell Biochem. 2009;106:1031–1040. doi: 10.1002/jcb.22081. [DOI] [PubMed] [Google Scholar]
- 94.Yu BL, Zhao SP, Hu JR. Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity. Obes Rev. 2010;11:560–567. doi: 10.1111/j.1467-789X.2009.00699.x. [DOI] [PubMed] [Google Scholar]
- 95.Jiang T, et al. Diet-induced obesity in C57BL/56J mice causes increased renal lipid accumulation and glomerulosclerosis via a sterol regulatory element-binding protein-1c-dependent pathway. J Biol Chem. 2005;280:32317–32325. doi: 10.1074/jbc.M500801200. [DOI] [PubMed] [Google Scholar]
- 96.Wang Z, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 97.Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:79–85. doi: 10.1016/j.numecd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 98.Tam J, et al. Peripheral cannabinoid-1 receptor inverse agonism reduces obesity by reversing leptin resistance. Cell Metab. 2012;16:167–179. doi: 10.1016/j.cmet.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 100.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4:711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 101.Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 102.Uhles S, Moede T, Leibiger B, Berggren PO, Leibiger IB. Isoform-specific insulin receptor signaling involves different plasma membrane domains. J Cell Biol. 2003;163:1327–1337. doi: 10.1083/jcb.200306093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.White MF, Kahn CR. The insulin signaling system. J Biol Chem. 1994;269:1–4. [PubMed] [Google Scholar]
- 104.Ekstrand AV, Groop PH, Gronhagen-Riska C. Insulin resistance precedes microalbuminuria in patients with insulin-dependent diabetes mellitus. Nephrol Dial Transplant. 1998;13:3079–3083. doi: 10.1093/ndt/13.12.3079. [DOI] [PubMed] [Google Scholar]
- 105.Yip J, et al. Insulin resistance in insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;342:883–887. doi: 10.1016/0140-6736(93)91943-g. [DOI] [PubMed] [Google Scholar]
- 106.Groop L, et al. Insulin resistance, hypertension and microalbuminuria in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1993;36:642–647. doi: 10.1007/BF00404074. [DOI] [PubMed] [Google Scholar]
- 107.Parvanova AI, et al. Insulin resistance and microalbuminuria: a cross-sectional, case-control study of 158 patients with type 2 diabetes and different degrees of urinary albumin excretion. Diabetes. 2006;55:1456–1462. doi: 10.2337/db05-1484. [DOI] [PubMed] [Google Scholar]
- 108.Forsblom CM, et al. Insulin resistance and abnormal albumin excretion in non-diabetic first-degree relatives of patients with NIDDM. Diabetologia. 1995;38:363–369. doi: 10.1007/BF00400643. [DOI] [PubMed] [Google Scholar]
- 109.Yip J, Mattock M, Sethi M, Morocutti A, Viberti G. Insulin resistance in family members of insulin-dependent diabetic patients with microalbuminuria. Lancet. 1993;341:369–370. doi: 10.1016/0140-6736(93)90167-f. [DOI] [PubMed] [Google Scholar]
- 110.Mykkanen L, et al. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 111.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–169. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou H, Tan KC, Shiu SW, Wong Y. Cellular cholesterol efflux to serum is impaired in diabetic nephropathy. Diabetes Metab Res Rev. 2008;24:617–623. doi: 10.1002/dmrr.895. [DOI] [PubMed] [Google Scholar]
- 113.Fang L, et al. Control of angiogenesis by AIBP-mediated cholesterol efflux. Nature. 2013;498:118–122. doi: 10.1038/nature12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gohda T, et al. Circulating TNF receptors 1 and 2 predict stage 3 CKD in type 1 diabetes. J Am Soc Nephrol. 2012;23:516–524. doi: 10.1681/ASN.2011060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Niewczas MA, et al. Circulating TNF receptors 1 and 2 predict ESRD in type 2 diabetes. J Am Soc Nephrol. 2012;23:507–515. doi: 10.1681/ASN.2011060627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen Y, et al. Inflammatory stress exacerbates hepatic cholesterol accumulation via disrupting cellular cholesterol export. J Gastroenterol Hepatol. 2012;27:974–984. doi: 10.1111/j.1440-1746.2011.06986.x. [DOI] [PubMed] [Google Scholar]
- 117.Field FJ, Watt K, Mathur SN. TNF-α decreases ABCA1 expression and attenuates HDL cholesterol efflux in the human intestinal cell line Caco-2. J Lipid Res. 2010;51:1407–1415. doi: 10.1194/jlr.M002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hashizume M, Mihara M. Atherogenic effects of TNF-α and IL-6 via up-regulation of scavenger receptors. Cytokine. 2012;58:424–430. doi: 10.1016/j.cyto.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 119.Kramer-Guth A, et al. Uptake and metabolism of lipoproteins from patients with diabetes mellitus type II by glomerular epithelial cells. Nephrol Dial Transplant. 1997;12:1336–1343. doi: 10.1093/ndt/12.7.1336. [DOI] [PubMed] [Google Scholar]
- 120.Gutwein P, et al. CXCL16 is expressed in podocytes and acts as a scavenger receptor for oxidized low-density lipoprotein. Am J Pathol. 2009;174:2061–2072. doi: 10.2353/ajpath.2009.080960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bussolati B, et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–1947. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 122.Hyvonen ME, et al. Lipid phosphatase SHIP2 downregulates insulin signalling in podocytes. Mol Cell Endocrinol. 2010;328:70–79. doi: 10.1016/j.mce.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 123.Lennon R, et al. Saturated fatty acids induce insulin resistance in human podocytes: implications for diabetic nephropathy. Nephrol Dial Transplant. 2009;24:3288–3296. doi: 10.1093/ndt/gfp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sieber J, et al. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am J Physiol Renal Physiol. 2010;299:F821–F829. doi: 10.1152/ajprenal.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sieber J, et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol. 2013;183:735–744. doi: 10.1016/j.ajpath.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nosadini R, Tonolo G. Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes. Nutr Metab Cardiovasc Dis. 2011;21:79–85. doi: 10.1016/j.numecd.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 127.Soetikno V, et al. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J Nutr Biochem. 2013;24:796–802. doi: 10.1016/j.jnutbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 128.Lee HS. Mechanisms and consequences of hypertriglyceridemia and cellular lipid accumulation in chronic kidney disease and metabolic syndrome. Histol Histopathol. 2011;26:1599–1610. doi: 10.14670/HH-26.1599. [DOI] [PubMed] [Google Scholar]
- 129.Chen HM, Zhen CX, Gao Q, Ge YC, Liu ZH. Heart-type fatty acid binding protein is associated with proteinuria in obesity. PLoS ONE. 2011;7:e45691. doi: 10.1371/journal.pone.0045691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee HS, Lee SK. Intraglomerular lipid deposition in renal disease. Miner Electrolyte Metab. 1993;19:144–148. [PubMed] [Google Scholar]
- 131.Douglas K, O’Malley PG, Jackson JL. Meta-analysis: the effect of statins on albuminuria. Ann Intern Med. 2006;145:117–124. doi: 10.7326/0003-4819-145-2-200607180-00009. [DOI] [PubMed] [Google Scholar]
- 132.Agarwal R. Effects of statins on renal function. Am J Cardiol. 2006;97:748–755. doi: 10.1016/j.amjcard.2005.09.110. [DOI] [PubMed] [Google Scholar]
- 133.Colhoun HM, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am J Kidney Dis. 2009;54:810–819. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 134.Katz A, et al. Safety, pharmacokinetics, and pharmacodynamics of single doses of LXR-623, a novel liver X-receptor agonist, in healthy participants. J Clin Pharmacol. 2009;49:643–649. doi: 10.1177/0091270009335768. [DOI] [PubMed] [Google Scholar]
- 135.Grefhorst A, et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J Biol Chem. 2002;277:34182–34190. doi: 10.1074/jbc.M204887200. [DOI] [PubMed] [Google Scholar]
- 136.Stella VJ, He Q. Cyclodextrins. Toxicol Pathol. 2008;36:30–42. doi: 10.1177/0192623307310945. [DOI] [PubMed] [Google Scholar]
- 137.Arima H, et al. Inhibitory effects of dimethylacetyl-beta-cyclodextrin on lipopolysaccharide-induced macrophage activation and endotoxin shock in mice. Biochem Pharmacol. 2005;70:1506–1517. doi: 10.1016/j.bcp.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 138.Liu P, et al. Sphingomyelinase activates GLUT4 translocation via a cholesterol-dependent mechanism. Am J Physiol Cell Physiol. 2004;286:C317–C329. doi: 10.1152/ajpcell.00073.2003. [DOI] [PubMed] [Google Scholar]
- 139.Horvath EM, Tackett L, Elmendorf JS. A novel membrane-based anti-diabetic action of atorvastatin. Biochem Biophys Res Commun. 2008;372:639–643. doi: 10.1016/j.bbrc.2008.05.095. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 140.Horvath EM, et al. Antidiabetogenic effects of chromium mitigate hyperinsulinemia-induced cellular insulin resistance via correction of plasma membrane cholesterol imbalance. Mol Endocrinol. 2008;22:937–950. doi: 10.1210/me.2007-0410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 141.Addi and Cassi Fund. addiandcassicom. 2014 [online], http://addiandcassi.com/walgreens-support-twins-niemann-pick-type-receive-cyclodextrin-treatments-home/
- 142.Keech A, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 143.Wang XX, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.US National Library of Medicine. ClinicalTrials.gov. 2013 [online], http://clinicaltrials.gov/ct2/show/NCT01782027?term=Tangier&rank=1.