Abstract
The prognosis and treatment of bladder cancer have hardly improved in the last 20 years. Bladder cancer remains a debilitating and often fatal disease, and among the most costly cancers to treat. The generation of informative mouse models has the potential to improve our understanding of bladder cancer progression, as well as impact its diagnosis and treatment. However, relatively few mouse models of bladder cancer have been described and particularly few that develop invasive cancer phenotypes. This review focuses on opportunities for improving the landscape of mouse models of bladder cancer.
Introduction
Bladder cancer is one of the leading causes of cancer related deaths in Western countries. It is more common than in Western than developing countries and, for reasons that are still not well-understood, three to four times more prevalent in males than in females. Although characterized by heterogeneous subtypes that have a range of disease outcomes, the broad subgroups are non-muscle invasive bladder cancer, which is more common and usually associated with a favorable prognosis, and muscle invasive bladder cancer, which is less prevalent but typically associated with a relatively poor prognosis (for general reviews on bladder cancer see 1–3). Notably, bladder cancer is one of the most costly cancers to treat, primarily due to the considerable costs associated with life-long clinical management of patients with non-muscle invasive disease, as well as those associated with the cost of caring for patients after surgical removal of the bladder 4.
However, despite its prevalence and adverse impact on human health, bladder cancer has been remarkably understudied relative to other cancers and remains significantly underrepresented by informative in vivo models, particularly genetically-engineered mouse (GEM) models. However, the tide is now changing with the recent the generation of new mouse models of bladder cancer, as well as the recent elucidation of molecular alterations that are prevalent in bladder cancer, which provide new avenues for developing models of disease relevant genes and/or pathways. Here we introduce key concepts that are essential for the generation of informative mouse models and their effective translation to human bladder cancer. In addition, we review the status of currently available mouse models of bladder cancer, and discuss prospects for their future development.
Biology of the bladder and lineage relationships of its epithelium
The bladder is comprised of a specialized epithelium, called the urothelium, which is encapsulated by the lamina propria and surrounded by a thick layer of smooth muscle (the detrusor muscle or muscularis propria), which forms the bladder wall (Figure 1) 5, 6. The urothelium includes three cell types: (i) basal cells, which are relatively small cuboidal cells that express p63 and high molecular weight cytokeratins, such as 5 and 14; (ii) intermediate cells, which also express p63 and high molecular weight cytokeratins, although at lower levels than the basal layer; and (iii) superficial cells, also called “umbrella cells”, which express uroplakin proteins and low molecular weight cytokeratins 18 and 207–11. Among these, the superficial cells are the most highly specialized, relatively large, and often polynucleate. They have polarized membranes that are insoluble, and specialized structures on their apical surface, called asymmetric unit membrane (AUM), comprised of uroplakin proteins that provide a barrier against re-absorption of urine (thus the term “umbrella cells”) 12.
Figure 1.
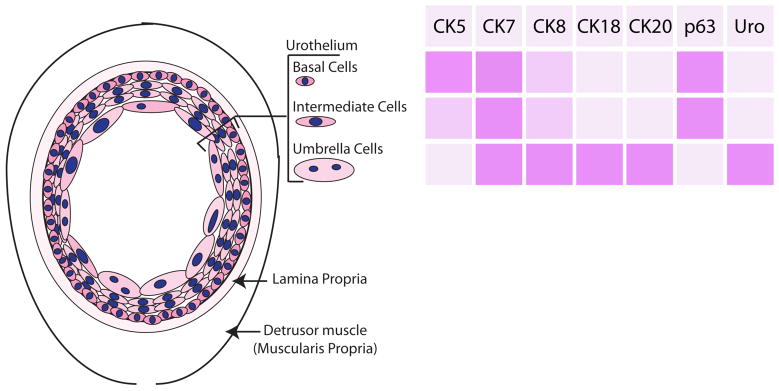
A. Diagram of bladder anatomy and cell types B. Summary of expression of cytokeratins (CK), p63, and Uroplakin (Uro) in bladder urothelial cells.
The bladder urothelium has among the slowest turn-over rates of any adult tissue 13, 14. However, in response to injury, for example, as a consequence of bacterial infection or exposure to toxins, the urothelium undergoes rapid proliferation and ultimately regenerates an intact urothelium 15, 16, although the actual response may depend upon the specific inducing agent (see 17 and below). The implication of these observations is that the adult urothelium contains stem or progenitor cells that are capable of its regeneration. Such stem or progenitor cells have long been thought to reside in the basal cell layer. In particular, lineage tracing of mouse bladder following pathogen-induced regeneration demonstrated that basal cells give rise to all urothelial cell types, supporting their progenitor role 18.
However, several lines of evidence, based on analyses of both human and mouse bladder, suggest that the urothelium may have independent lineages generated by distinct progenitors 19. Such a multiple progenitor model has been supported by an alternative lineage tracing study following chemically-induced regeneration, which concluded that umbrella cells are derived from intermediate rather than basal cells 20. In addition, analyses of label-retaining cells in mouse bladder during development as well as following pathogen-induced regeneration also supports a multiple lineage model 11, 21; notably, during development the progenitors are concentrated in the trigone 11, a specialized structure at the bladder. Furthermore, analyses of mice harboring a germline deletion of p63, which is expressed in basal but not umbrella cells, lack basal and intermediate cells but have a superficial cell layer 22, 23. In addition to these studies of mouse bladder, analyses of clonal relationships in human bladder, inferred from analyses of mitochondrial DNA, also support the model that that the bladder has multiple progenitors 24. Clearly, lineage relationships within the bladder urothelium are far from resolved.
Bladder cancer: a primer for the mouse modeler
The term “bladder cancer” actually refers to a heterogeneous set of diseases with a spectrum of pathologies and expected prognoses. Most (~90%) are urothelial carcinomas, which are the subject of this review and referred to simply as “bladder cancers” throughout (Figure 2); the remainder (~10%) include primary squamous cell carcinoma, adenocarcinoma, small cell carcinoma, or sarcomatoid carcinoma 1, 3, 25, which are not discussed further in this review.
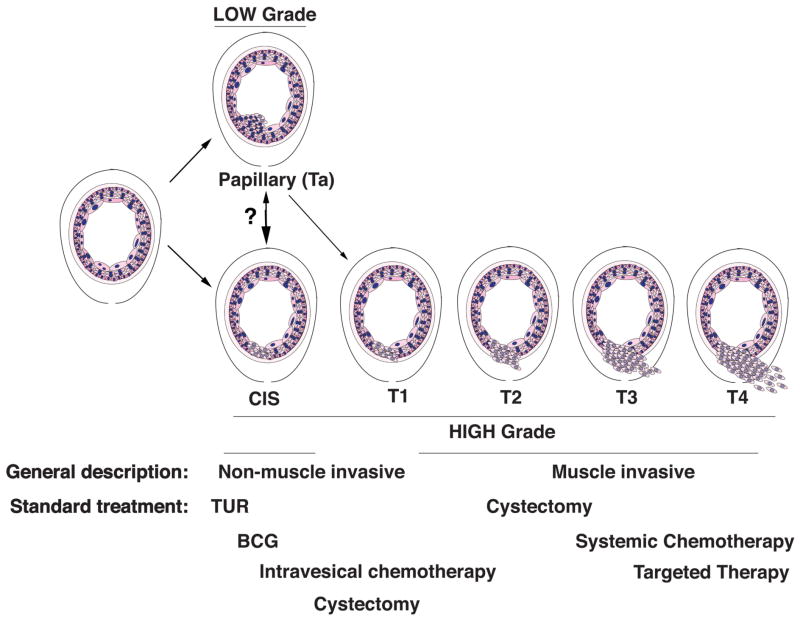
Figure 2. Clinical stages of bladder cancer.
Schematic representation of the clinical stages and grades of bladder cancer and standard treatments. TUR, transurethral resection; CIS, carcinoma in situ.
As introduced above, non-muscle invasive tumors account for vast majority of bladder cancers (~75%), most of which have a relatively favorable prognosis 1, 2. These can be further sub-grouped into low-grade, which are mainly superficial (or papillary) tumors, and high-grade, which include a subset of superficial tumors as well as carcinoma in situ (CIS) (Figure 2). As their names implies, superficial or papillary tumors grow into the bladder lumen but do not invade the muscle layer, while carcinoma in situ (CIS) is a characterized by a flattened layer of dysplastic cells that is the presumed major precursor of invasive bladder cancer 26, 27. In contrast to the non-muscle invasive disease, muscle invasive bladder cancer accounts for ~25% of cases and has a relatively poor prognosis; in particular, muscle invasive tumors have a 5-year survival of ~50%, and for those that have metastasized, the expected 5-year survival is only ~15% 1, 3. Below we discuss key clinical aspects of bladder cancer that impact the generation of informative mouse models.
Cells of origin of bladder cancer and their relationship to bladder cancer subtypes
Various studies in humans and mice have implicated basal cells as cells of origin of bladder cancer 19. In the mouse, for example, analyses of lineage-tracing in a carcinogen-based model concluded that basal cells can serve as cells of origin of bladder cancer 28. In humans, isolation of putative stem cells based on expression of cell surface markers followed by growth in xenograft models has shown that such stem cells are enriched for basal cells, particularly the most aggressive tumor subtypes 29–31. Furthermore, gene expression profiling analyses of invasive bladder cancer have categorized a basal-like subtype, with a more aggressive phenotype, and a luminal-like subtype, with a less aggressive phenotype 32–35. While it is not necessarily the case that the basal-subtype originates from basal cells, this observation is certainly consistent with the concept that basal cells can serve as cells of origin of bladder cancer, particularly for more aggressive subtypes.
However, studies in both humans and mice have demonstrated alternative cells of origin, which may give rise to distinct bladder cancer subtypes. In mice for example, an alternative analysis of lineage-tracing of a carcinogen-based model has shown that heterogeneous subtypes of bladder cancer can be attributed to distinct cells of origin 36. In humans, analyses of gene signatures from sub-populations of normal urothelial cells supports the concept that distinct subtypes of bladder cancer arise from distinct cells of origin 37. Moreover, the gene expression profiling studies discussed above, which identified molecular subtypes of bladder cancer categorized distinct basal-like and luminal-like subtypes 32–35; although these are necessarily indicative of multiple cells of origin, this is also not inconsistent with this concept. A precise understanding of the relationships cells of origin of bladder cancer and their relationship to specific clinical subtypes is of paramount importance for generating informative mouse models of bladder cancer.
Treating bladder cancer
Treatment of bladder cancer as well as the efficacy of such treatment varies profoundly depending on the clinical stage and associated risk factors 1, 3, 38, 39 (Figure 2). The front-line treatment for non-muscle invasive bladder cancer is transurethral resection (TUR) 39, which has a high disease free survival for low-grade cases although a high rate of relapse for high-grade disease. Because of the unique biology and tissue organization of the bladder, non-muscle invasive bladder cancers can be treated locally (rather than systemically) by what is called intravesical therapy. In particular, the front line treatment for patients with non-muscle invasive bladder cancer who are at high-risk or recur following TUR is intravesical delivery of bacillus Calmette-Guerin (BCG) 40, 41, which is actually a vaccine against tuberculosis that promotes immunoreaction against cancer cells 42. Patients that fail BCG treatment are candidates for cystectomy (surgical removal of the bladder), or alternatively for salvage intravesical therapy using chemotherapy regimens or targeted agents in an effort to preserve bladder function 43 (e.g., Clinical Trial.gov; NCT02202772).
Cystectomy, with or without neoadjuvant chemotherapy 44, 45, is also the front line treatment for muscle invasive bladder cancer 39, 46. Cystectomy has a 5-year survival ranging from 30–70% depending on the stage of the tumor, and the inevitable requirement of urinary tract diversion results in a profound impairment in quality of life 47, 48. Notably, cystectomy is not a viable option for all patients, and is generally not recommended for patients with metastatic bladder cancer, since it has very little chance of being curative. Rather, the standard of care for metastatic bladder cancer is a multidrug chemotherapy regimen consisting of methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) or, alternatively, gemcitabine plus cisplatin (GC). Both of these regimens have a low response rate (~40–50%) and limited improvement on overall survival (~12–15 months) 39. Moreover, cisplatin-based chemotherapy is not an option for many elderly patients, which are a large subset of those with advanced bladder cancer, due to the potential for kidney failure. Thus, treatment options for muscle invasive bladder cancer are limited and, in striking contrast with many other cancers, have not significantly improved in recent years.
What causes bladder cancer?
Although genome-wide association studies have identified various low-penetrance susceptibility loci associated with increased cancer risk 49, bladder cancer is thought to arise primarily as a consequence of environmental exposures 50–54. Indeed, the major risk factor for bladder cancer is smoking 50, 54 and the relatively high incidence of bladder cancer in Western versus developing countries is thought to reflect the prevalence of smoking in the former. Carcinogens from tobacco smoke, as well as those associated with occupational hazards 51–53, are presumed to promote bladder cancer by virtue of their concentrated in urine, essentially bathing the urothelium with carcinogens.
Interestingly, these associated risk factors cannot fully account for the approximately three to four fold difference in the incidence of bladder cancer in men versus women, particularly with respect to smoking 50. The implication is that there may be fundamental differences in bladder physiology and/or its development that underlie the striking prevalence of bladder cancer in men. Notably, the epithelium of the bladder and prostate, although highly specialized and distinct, share a common embryological origin, namely the urogenital sinus 55. Thus, it has been proposed that the increased prevalence of bladder cancer in males versus females reflects, at least in part, androgen receptor function in bladder cancer 56, as supported by recent analyses of genetically-engineered mouse models 57, 58.
Molecular subtypes and molecular alterations in bladder cancer
Non-muscle invasive versus muscle invasive bladder cancer
Several lines of evidence support the general concept that the distinct clinical outcomes of low-grade non-muscle invasive versus high-grade muscle invasive bladder tumors reflects their distinct molecular causes and, as discussed above, potentially also distinct cells of origin. Indeed, certain molecular alterations, such as gain of function mutations of FGFR3, are prevalent in low-grade non-muscle invasive bladder cancers whereas other alterations, such as p53 loss or mutation, are prevalent in high-grade muscle invasive bladder cancers 53, 59–62. Although analyses of gene expression profiling 63–68 and/or genomic alterations 65, 69–73 have supported the general concept that low-grade non-invasive versus high-grade invasive bladder tumors are molecularly distinct, it is difficult to fully reconcile a mutual-exclusivity model considering that some superficial bladder tumors can progress to invasive disease. Furthermore, meta-analysis of expression profiling data from non-invasive and invasive bladder cancers failed to identify molecular subtypes that are clearly associated with pathological stage 74. Furthermore, recent whole genome sequencing and transcriptome analyses comparing low-grade and high-grade bladder cancers supports the concept that these are evolve in parallel rather than mutually exclusively 75.
Thus, low-grade non-muscle invasive and high-grade muscle invasive bladder cancer may be more appropriately viewed as broadly distinct entities along a continuum of disease progression. In this framework, the actual phenotype and outcome may reflect the culmination of molecular alterations that tend to drive more or less aggressive phenotypes, distinct cells of origin, which may contribute to tumor aggressiveness, and potential interactions with environmental exposures, such as smoking, carcinogens or inflammation.
Molecular alterations in muscle invasive bladder cancer
The cancer genome atlas (TCGA) has recently reported a comprehensive molecular analysis of muscle invasive bladder cancer 32, which together with several additional whole exome or whole genome analyses 76–80 have both confirmed and extended the contribution of known genes/molecular pathways, as well as identified interesting new ones. In particular, as anticipated from many earlier studies, the TCGA study found that TP53 (which encodes p53) is deleted and/or mutated in ~49% of muscle invasive bladder cancers and, more generally, that genes encoding members of the p53-RB pathway are altered in a majority of muscle-invasive bladder cancers; however, surprisingly, FGFR3 mutations, which had long been associated almost exclusively with non-muscle invasive bladder cancer, were found to be relatively common (~12%) in muscle invasive disease 32.
Furthermore, the TCGA study, together with integrative analyses of high grade bladder tumors 81 as well as analyses of patients who are ‘exceptional responders’ to targeted therapy 82, 83, have demonstrated the relevance of the PI3K-mTOR signaling and RTK-RAS-MAPK signaling pathways, and support the rationale for therapeutic targeting of these ‘actionable’ signaling pathways for treatment of advanced bladder cancer. In particular, 42% or 44% of muscle invasive tumors were found to have alterations of genes associated with PI3K-mTOR signaling or RAS-RTK-MAPK pathways, respectively, including genes such as PIK3CA, tuberous sclerosis 1 (TSC1), TSC2, AKT3, FGFR3, epidermal growth factor receptor (EGFR) and ERBB2 (Her2) 32.
Indeed, there has been a long standing rationale for therapeutic targeting of RTK-RAS-MAPK signaling, since HRAS was originally identified in bladder cancer cells 84–86, although RAS itself has proven difficult to target. Thus, clinical efforts have been focused on targeting relevant downstream pathways, as such FGFR3 87 and EGFR 88 (eg., Clinical Trial.gov; NCT01732107; NCT01953926). Furthermore, current clinical trials targeting PI3K-mTOR pathway with various mTOR inhibitors such as temsirolimus or everolimus (also known as RAD001) are now underway (eg., Clinical Trial.gov; NCT01827943, NCT00805129 NCT02009332).
Additionally, the TCGA study identified several genes that are frequently (>10%) altered in bladder cancer but that had not been previously implicated in bladder and in some cases in any cancer, including mixed-lineage leukemia 2 (MLL2; also known as KMT2D), cyclin-dependent kinase inhibitor 1A (CDKN1A), ERCC2, and stromal antigen 2 (STAG2) 32. Also notable is the prevalence of alterations of epigenetic regulatory genes, including UTX (also known as KDM6A), MLL2, CREB binding protein (CREBBP), and AT rich interactive domain 1A (ARID1A) 32, 76, thus providing new avenues for therapeutic targeting of invasive bladder cancer. Interestingly, the TCGA study, along with meta-analyses of TCGA datasets representing 12 distinct cancers 89, 90, found that bladder cancer has among the highest number of mutations per DNA megabase of any cancer. Consistent with this observation, whole genome sequencing of five muscle-invasive bladder tumors that have mutated TP53 found a profound level of nucleotide alterations as well as chromothripsis 77, which refers to the shattering and reassembly of chromosomes as a consequence of genomic instability; potentially, this is a reflection of the unusual susceptibility of the urothelium to environmental carcinogens (discussed above).
Modeling bladder cancer in mice
Although recent studies have advanced our conceptual understanding of the biological, molecular, and environmental factors associated with bladder cancer, this knowledge has not yet advanced to the point of impacting patient care. In fact, the field is still grappling with major uncertainties regarding the nature and complexity of bladder cancer subtypes, how they are related to each other, and how they can best be treated to improve patient outcomes. Our understanding of these issues would greatly benefit from the availability of mouse models that accurately represent specific bladder cancer phenotypes or subtypes and are based on relevant genes/pathways/processes that are associated with bladder cancer (Table 1). However, in contrast to many other cancer types, which have experienced a veritable explosion of in the generation of mouse models over the last two decades, bladder cancer is relatively underrepresented by mouse models, particularly genetically-engineered mouse (GEM) models.
Table 1.
Major concepts in bladder cancer research that can be addressed using mouse models
| Key concepts | Opportunities | Challenges |
|---|---|---|
| Lineage relationships and cell of origin | ||
| Cell of origin |
|
|
| Molecular causes of bladder cancer | ||
| Non-muscle invasive vs muscle invasive bladder cancer |
|
|
| Muscle invasive bladder cancer |
|
|
| Metastatic bladder cancer |
|
|
| Risk Factors | ||
| Smoking |
|
|
| Genetic risk factors |
|
|
| Preclinical analyses of novel therapies | ||
| Intravesical treatment |
|
|
| Intravesical versus systemic treatment |
|
|
| Systemic treatments |
|
|
| Targeted therapy |
|
|
Presently, mouse models of bladder cancer include carcinogen-based models, in which tumors arise following treatment of mice (or rats) with carcinogens, various types of engraftment models, in which cells or tissues are grown in recipient hosts, and genetically-engineered mouse (GEM) models, based on activation or inactivation of gene function in the bladder (Table 2). An important distinction between these types of models is that carcinogen-based and GEM models are autochthonous, which means that tumors originate in the bladder, whereas graft models are non-autochthonous, since they are implanted into recipient hosts. Notably graft recipient mice are usually immunodeficient, which is of relevance given the known importance of the immune system for cancer progression and metastasis 91. However, engraftment models have the considerable advantage of their relative ease and rapidity of generation and use for analyses of the functional relevance of candidate genes. Furthermore, although they are both autochthonous, tumors in carcinogen-based models are, by definition, induced by carcinogens, whereas those in GEM models arise following manipulation of specific genes. Thus, these different approaches to modeling bladder cancer in mice are highly complementary (Table 2). In addition to the discussion that follows, we refer the reader to recent reviews of bladder cancer cell lines and in vivo models of bladder cancer found in 92–94.
Table 2.
Mouse models of bladder cancer
| Type of model | Description | Advantages | Disadvantages |
|---|---|---|---|
| Non-autochthonous models | |||
| Orthotopic engraftment models | Primary urothelial or bladder cancer cells implanted into the bladder wall of recipient hosts such that tumors arise in the bladder |
|
|
| Renal grafting models | Urothelial or bladder cells are combined with embryonic bladder mesenchyme and grown under the kidney capsule of recipient hosts |
|
|
| Patient derived Xenografts (PDX) | Engraftment of patient tumors into recipient mice |
|
|
| Autochthonous Carcinogen-based models | |||
| Carcinogen-based | Treatment of mice with carcinogens in the drinking water, the most common is BBN |
|
|
| Autochthonous GEM models | |||
| Transgenic models | Expression of oncogenes in the urothelium (such as expression of SV40 Large T antigen) |
|
|
| Germline models | Loss of function of tumor suppressor genes in the germline (such as p53 null mice) |
|
|
| Conditional alleles | Conditional or inducible gene recombination of oncogenes or tumor suppressor genes in the urothelium (such as deletion of Pten and p53) |
|
|
Carcinogen models
The classic model of bladder cancer is based on chemical carcinogenesis of the urothelium, which conceptually mimics environmental exposures that are known to be a leading cause of bladder cancer. First introduced for induction of bladder cancer in rats in the 1960’s 95–97, numerous applications have used several carcinogens in various species, including mice, rats, and dogs 98. Notably, carcinogen models were among the first preclinical models to evaluate chemotherapy for bladder cancer 99, and they continue to provide informative models for understanding processes involved in cancer progression and for elucidating cancer subtypes.
Currently, the majority of carcinogen-induced mouse models of bladder cancer utilize N-butyl-N-(4-hydroxybutyl) nitrosamine (BBN), which is delivered in the drinking water. BBN is highly relevant to human bladder cancer, since it is very similar to the major carcinogen associated with tobacco smoke 100. Although delivered systemically (rather than directly to bladder), the urothelium is particularly susceptible to BBN treatment, as evident from analyses of the mutagenesis spectrum across various tissues 101. BBN-treated mice develop a range of bladder cancer phenotypes, including hyperplasia, dysplasia, CIS, and muscle-invasive bladder cancer, as well as metastases in certain strain backgrounds 102. Notably, mutation of relevant genes such as Trp53 102, as well as the molecular profiles of tumors from BBN-treated mice 103, 104 share similarity with those of human invasive bladder cancer. Furthermore, treatment of genetically engineered mouse models with BBN has the advantage of exacerbating the consequences of loss or gain of function phenotypes (discussed below). In particular, a recent study of bladder tumors arising following BBN-treatment of mice with or without heterozygous deletion of Trp53 showed that the range of bladder cancer phenotypes is influenced by the status of Trp53 36.
Among their major advantages, carcinogen treatment simulates actual events that are known to give rise to bladder cancer in humans. In addition, these models are relatively straightforward to implement and autochthonous tumors arise in immune-competent mice. Among their disadvantages, despite the fact that the urothelium may be most susceptible, since BBN treatment is systemic, it is difficult to rule out the contribution of other tissues. Furthermore, tumor phenotypes and their temporal progression are highly heterogeneous in BBN-treated mice, and vary depending on species and strain background. Although this inherent heterogeneity may capture key elements of human bladder, it makes it difficult to implement preclinical studies or to model specific disease subtypes.
Engraftment models
Orthotopic and renal engraftment
Urothelial cancer cells can be engrafted orthotopically in mice or rats such that tumors arise within the bladder of recipient hosts. First introduced in the 1970’s 105, delivery of cancer cells into the bladder lumen has been widely used for modeling bladder cancer 106–110. The recent introduction of using ultrasound-guided implantation of cells between the urothelium and lamina propria/muscle layer 107, 108 has the benefit of being accurate in terms of cell delivery as well as minimally invasive. An alternative renal grafting approach involves recombination of urothelial cells with embryonic urogenital sinus mesenchyme (UGM) in vitro followed by engraftment under the kidney capsule of recipient hosts 111, 112.
The orthotopic and renal engraftment models are complementary, in that the former enables evaluation of tumor behavior in an organ-specific microenvironment, whereas the latter is particularly beneficial for investigating the role of epithelial-stromal interactions for tumor growth. Both have the advantage of their relative ease of manipulating gene expression in cell culture to introduce gain or loss of function alterations prior to engraftment, such that the consequences of such alterations for tumor growth can be evaluated in vivo 113. Notably, engraftment models are not limited to tumor cells; they are adaptable to primary and/or non-transformed cells, which can be particularly beneficial for evaluating gain of function mutations, while inclusion of fluorescent or luciferase reporter genes can enable in vivo imaging of tumors and metastases 114, 115. Orthotopic engraftment models have been used for preclinical evaluation of potential new treatment options, such as the targeting RTK inhibitor sunitinib and plasminogen activator inhibitor type-1 (PAI–1) 116, 117, although these agents have not yet been adapted to clinical practice.
Among their major limitations, however, engrafted tumors (as well as the patient derived xenograft tumors discussed below) are non-autochthonous, and therefore they do not model the de novo evolution of tumor phenotypes. Additionally, since recipient hosts are usually immunodeficient, the lack of an intact immune system may impact tumorigenesis as well as metastasis. Nonetheless, engraftment approaches are an excellent starting point to rapidly evaluate the functional significance of candidate genes and for prioritizing the generation of genetically-engineered mouse (GEM) models.
Patient derived xenografts
Engraftment of patient-derived tumor tissues into immunodeficient mouse hosts (called patient derived xenograft — PDX — models), which have become increasingly utilized for many types of cancers 118, have been described for bladder cancer 119. Since PDX models are derived from individual patient tumors, the expectation is that the resulting tumors capture the unique genomic and molecular properties of the individual patient from which they are derived; the further expectation is that PDX models should enable analyses of clinical responses based on the unique characteristics of a given tumor. Indeed, preclinical studies using PDX models of bladder cancer have supported the concept that co-targeting PI3K and MAP signaling may be beneficial for certain types of bladder cancers 120.
However, to date few reports have described the generation of PDX models for bladder cancer; thus, it is not clear whether such models can be generated efficiently or whether they will indeed capture all or most bladder cancer subtypes. On the other hand, whereas the generation of PDX models for certain types of cancers (such as prostate cancer for example) may be limited by tissue availability, in principal this should not be a consideration for bladder cancer because primary tissue is readily available from TUR as well as cystectomy. Thus, if indeed bladder cancer has a reasonable ‘take-rate’ in the recipient hosts, it should be feasible to generate a range of PDX models, ideally representative of the various subtypes of bladder cancer.
GEM models of bladder cancer
GEM models are now widely used for many applications in cancer biology, including analyses of tumor phenotypes, modeling disease subtypes, mechanistic investigations of candidate genes and signaling pathways, and preclinical evaluation of potential therapeutic agents 121–123. Notably, GEM models complement non-autochthonous mouse models since tumors arise de novo in the native tissue microenvironment, and they also complement carcinogen-based models, since they are based on defined genetic alterations. However, relatively few GEM models of bladder cancer have been described, particularly those that display muscle invasive and/or metastatic phenotypes (Table 3), which we believe reflects several major challenges in their design and generation (discussed further below). In particular, relatively few promoters display bladder-specific expression and can be used to generate GEM models. Additionally, bladder tumors appear to be unusually recalcitrant to developing invasive tumors, since most single gene alterations and even many combined alterations have relatively mild phenotypes (Table 3). However, given the recent description of molecular alterations found in bladder cancer that can be modeled in mice (e.g., 124), we envision that GEM models are likely to play an increasingly prominent role in the future.
Table 3.
GEM models of bladder cancer
| Allele name | Method for targeting to bladderb | Description | Phenotype | Ref. |
|---|---|---|---|---|
| Transgenic models | ||||
| UPII-SV40Ta | Uroplakin II Promoter | Expression of SV40 large T antigen | CIS | 126, 127 |
| UPII-SV40T high | CIS with progression to invasion, in some cases metastasis | 126 | ||
| CK19-SV40T | Cytokeratin 19 | CIS with progression to invasion, in some cases metastasis | 129 | |
| UPII-Ha-RasQ61L | Uroplakin II | Expression of mutant (active) H-Ras | Papillary non-invasive cancer | 131 |
| UPII-EGFR | Expression of EGFR | Hyperplasia | 132 | |
| UPII-SV40T low; EGFR | Expression of SV40 large T combined with EGFR | High-grade non-invasive cancer | 132 | |
| UPII-p53DN | Expression of mutant (active) p53 | Hyperplasia | 135 | |
| UPII-Ha-rasQ61L; p53DN | Mutant (active) H-Ras combined with mutant (active) allele of p53 | Hyperplasia with localized dysplasia | 135 | |
| UPII-Ha-rasQ61L; p53-null | Mutant H-Ras combined with a germline null allele of p53 | Papillary non-invasive cancer | 135 | |
| Models using Cre Drivers expressed in the urothelium | ||||
| β-cateninexon3/exon3 | UroII-Cre allele | Mutant (active) allele of β-catenin | Hyperplasia | 136 |
| β-cateninexon3/exon3; Ptenflox/flox | Mutant (active) allele of β-catenin combined with loss of function of Pten | Papillary non-invasive cancer | 136 | |
| β-cateninexon3/exon3; K-rasG12D | Mutant (active) allele of β-catenin combined with mutant (active) Kras | Papillary non-invasive cancer | 137 | |
| β-cateninexon3/exon3; H-rasQ61L | Mutant (active) allele of β-catenin combined with mutant (active) Ha-ras | Papillary non-invasive cancer | 137 | |
| β-cateninexon3/exon3; p21−/− | Mutant (active) allele of β-catenin combined with germline null allele of p21 | Papillary non-invasive cancer | 137 | |
| Fgfr3+/K644E; Ptenflox /flox | Mutant (active) allele of Fgfr3 combined with loss of function of Pten | Hyperplasia with localized dysplasia | 140 | |
| UPII-Ha-rasQ61L; CDKN2A null | Mutant H-Ras combined with a germline null allele of INK4a/Arf | Hyperplasia | 134 | |
| Ncstnflox/flox | UPII-Cre-GFP | Bladder-restricted deletion of Nicastrin, which abrogates Notch function | Hyperplasia and CIS | 139 |
| Models using Cre Drivers that are not specific for bladder | ||||
| β-cateninexon3/+ | Msx2rtTA;tetO-Cre | Mutant (active) allele of β-catenin | Papillary non-invasive cancer | 58 |
| Ptenflox /flox | Fabp-Cre | Conditional loss of function Pten | Papillary non-invasive cancer | 150 |
| Lkb1flox/flox; Ptenflox/flox | AhCreER | Conditional loss of function of Lkb and Pten | Papillary non-invasive cancer | 141 |
| Ncstnflox/flox | Rosa26rtTA;tet O-Cre | Systemic deletion of Nicastrin, which abrogates Notch function | Muscle-invasive bladder cancer | 139 |
| Models using delivery of Adeno-Cre to the bladder | ||||
| p53flox/flox; Ptenflox/flox | AdenoCre delivery (via surgery) | Conditional loss of function of p53 and Pten | Muscle-invasive bladder cancer with frequent metastasis | 112 |
| p53flox/flox; K-rasG12D | AdenoCre delivery (via instillation) | Mutant (active) allele of β-catenin combined with mutant (active) Kras | Hyperplasia | 143 |
| Rbflox/flox; p130flox/flox p107−/− | AdenoCre delivery (via surgery) | Loss of function of all retinoblastoma genes | Papillary non-invasive cancer | 159 |
Transgenic models of bladder cancer
Similar to many of the original “oncomice” 125, the earliest GEM models of bladder cancer were transgenic mice in which SV40 large T antigen is expressed in the urothelium under the control of the Upk2 promoter 126. The resulting transgenic mice develop CIS and invasive bladder cancer, some progressing to metastasis 126, 127, and their molecular profiles are conserved with human bladder cancer 128. A similar phenotype was observed when SV40 large T antigen was expressed under the control of the Krt19 promoter 129. Interestingly, although SV40 large T antigen inactivates Trp53 and Rb1, combined loss of function of Trp53 and Rb1 is not sufficient for bladder tumors to arise in GEM models 112, 130. Besides SV40 large T antigen, other oncogenes have been expressed in the urothelium, including Hras, Egfr, and Cyclin D1, with the latter two resulting in urothelial hyperplasia; these have also been combined with other alleles, such as mutant p53, resulting in progression to dysplasia or non-invasive bladder cancer 131–135.
Conditional models of bladder cancer
The majority of recent GEM models of cancer involve tissue-specific conditional or inducible gene targeting. However, the generation of such models for bladder cancer has been challenged by the paucity of Cre alleles that restrict gene targeting specifically to the urothelium and particularly to selected cell types (discussed below) (Table 3). Moreover, most GEM models of bladder cancer described thus far display non-invasive phenotypes. Interesting, in several cases GEM models generated using “bladder-specific” Cre drivers have less aggressive phenotypes than those made using other (non-bladder specific) Cre drivers (Table 3).
In particular, conditional activation of β-catenin in the bladder using a Cre driver based on expression of the Upk2 promoter (called UroII-Cre) results in hyperplasia, and together with activation of Hras or Kras or loss of function of Pten, in papillary non-invasive cancer 136–138 However, with an alternative, non-bladder-specific Cre driver, activation of β-catenin alone results in papillary non-invasive cancer 58 (Table 3). Similarly, abrogation of Notch function by expression of nicastrin (Ncstn) using a bladder specific promoter results in hyperplasia and CIS, whereas expression of Ncstn using a ubiquitously expressed promoter results in muscle invasive bladder cancer 139; of course this difference may be due to the actions of Notch outside of the urothelium. Lastly, while loss of function of Pten together with Fgfr3 activation using a UroII-Cre allele results in hyperplasia and localized dysplasia, using alternative non bladder specific Cre drivers, Pten loss alone or together with Lbk1 result in papillary non-invasive tumors 140–142.
Conditional models using Adeno-Cre delivery
An alternative to using tissue-specific Cre alleles to target gene recombination in bladder, delivery of Adeno-Cre directly into the bladder lumen has been used to inactivate Trp53 and Pten in the urothelium, resulting in invasive bladder cancer with prevalent metastasis 112. This approach has also been used to delete all three members of the retinoblastoma family, resulting in papillary non-invasive cancer 85. Interestingly, conditional activation of Kras and inactivation of Trp53 via instillation of Adeno-Cre (rather than its surgical into the bladder lumen) results in sarcomas outside the bladder, while the urothelial phenotype is modest 143.
Notably, the Adeno-Cre driven Pten; Trp53 mice display temporal progression from CIS to invasive disease and ultimately develop distant metastasis with high penetrance 112. Thus, these mice have enabled preclinical investigations comparing intravesical therapy, evaluated at the CIS stage, with systemic therapy, evaluated at more advanced stages 112, 144, 145. In particular, comparing intravesical versus systemic treatment of inhibition of mTOR signaling using rapamycin has demonstrated the efficacy of intravesical therapy 144; these findings formed the basis for a clinical trial to evaluate intravesical treatment with rapamycin for high-risk early stage bladder cancer (Clinical trials.gov, NCT02009332). Similarly, other preclinical studies in this model demonstrated the efficacy of intravesical administration of multi-chemotherapy regime, which has led to new clinical trials to evaluate intravesical delivery of this treatment for high-risk early stage bladder cancer (Clinical trials.gov, NCT02202772). These examples suggest that preclinical studies in GEM models having progressive phenotypes may be advantageous to test the effectiveness of promising drugs as well as to optimize the route of their administration.
Opportunities and challenges for GEM models of bladder cancer
Compared with other cancer types, bladder cancer is largely underrepresented by GEM models; moreover, the phenotypes of most existing GEM models are primarily non-invasive. Here we discuss major challenges that have impeded the generation of GEM models of bladder cancer and suggest various approaches to overcome these challenges.
Challenges in targeting gene expression to bladder
A key consideration for the generation of informative GEM models is the ability to restrict gene targeting to appropriate tissue layers, relevant cell(s) of origin, and at the appropriate stage of tissue development. For bladder, few promoters exist that meet these criteria. In fact, the most widely used is the Upk2 promoter 146, which can be expressed in other tissues besides bladder and even within the bladder urothelium is not uniformly expressed but rather primarily limited to the superficial cells. Of particular concern is that this Upk2 promoter, which used widely for the generation of transgenic mouse models as well as the development of Cre alleles, has been reported to have been cloned in the wrong orientation 94, 127, which has likely compromised its activity and specificity. Recently the Upk3a promoter has been used to express a tamoxifen-inducible Cre recombinase in the bladder urothelium 20. Furthermore, since uroplakin is primarily expressed in superficial cells, ideally Cre drivers using these promoters would be complemented by promoters that direct expression to other urothelial cell layers; thus far, promoters that restrict gene targeting specifically to the bladder but preferentially to basal or intermediate cells have not been described. Other promoters that have been used to direct gene expression and/or to express Cre recombinase in the urothelium, although they are not specific for bladder, include as the fatty acid binding protein 1 (Fabp1), cytokeratin 19 (Krt19), and the msh homeobox 2 (Msx2) promoter 58, 129, 147. Additionally, it has been reported that gene recombination specifically in the bladder can be achieved by delivery of tamoxifen directly into the bladder lumen of mice that have tamoxifen-inducible Cre alleles 148; however, this approach has not been used extensively since this initial report.
In lieu of suitable Cre-drivers, an alternative approach to achieve bladder-restricted gene targeting is to introduce an adenovirus expressing Cre-recombinase (Adeno-Cre) into the bladder lumen 112, 149. Adeno-Cre can be delivered intravesically rather than surgically 143; however, these mice develop tumors outside the bladder and intravesical delivery is only feasible for female mice, which is a considerable limitation since bladder cancer is more prevalent in men. Although gene recombination via adeno-Cre has the benefit of being efficient and selective for the urothelium and, because it does not require the generation of mice with an additional Cre allele, can be used to ‘screen’ the consequences of gene recombination in the bladder 112, since Adeno-Cre enables recombination in all the cell layers, it is difficult to evaluate the contribution of specific urothelial cell types and thus this approach is not ideal for cell of origin analyses.
Challenges for modeling invasive bladder cancer in mice
A striking difference of modeling bladder cancer in mice compared with other cancers is that relatively few GEM models described thus far display overtly invasive or metastatic phenotypes (Table 3). More generally, with few exceptions, dysregulation in the urothelium of individual tumor suppressor genes, such as Rb1, Cdkn1a (which encodes p21), Pten, Trp53, liver kinase B1 (Lkb1; also known as Stk11), or oncogenes, such as Hras, Kras, Egfr, or Fgfr3, have not resulted in invasive bladder cancer, irrespective of the strategy used to direct their expression or induce recombination, although in some cases these dysregulated genes collaborate with others to accelerate bladder cancer phenotypes112, 127, 130–132, 134–137, 140–142, 150.
Although it is conceivable that the apparent difficulties in generating invasive bladder cancer phenotypes may reflect a lack of ‘optimal’ targeting approaches or that the models thus far have not been based on ‘optimal’ combinations of genes, considering the numerous examples described thus far (Table 3), it seems likely that the urothelium may be inherently refractory to developing cancer, or at least in mice. It is plausible that this reflects the characteristic slow turnover of the urothelium, such that its very limited proliferation renders it resistant to genetic assaults. Indeed, as initially demonstrated for germline loss of function of Trp53151, carcinogen-treatment, even at sub-carcinogenic doses, exacerbates the bladder tumor phenotypes associated with several genes, including loss of function of patched homologue 1 (Ptch1), Rb1, secreted acidic cysteine rich glycoprotein (Sparc), Cdkn1b (which encodes p27), and gain of function of signal transducer and activator of transcription 3 (Stat3) and insulin growth factor1 (IGF1) 130, 152–158.
Why might the urothelium be inherently resistant to developing cancer? One possibility is that the current mouse models do not effectively model genomic instability, which is apparently a distinguishing feature of human bladder cancers (discussed above). Alternatively or additionally, the current models may not incorporate epigenetic modifications that are prevalent in human bladder cancer, or the “right” gene combinations to model specific cancer subtypes (discussed above). These are issues that will need to be addressed in future model development.
The future of modeling bladder cancer in mice
Historically, bladder cancer research has lagged significantly behind other cancers. This is particularly the case for the generation of mouse models and especially those that represent a spectrum of bladder cancer phenotypes and provide informative preclinical models. As discussed above, the generation of mouse models of bladder cancer has been fraught with inherent difficulties; however, we envision that these challenges are not insurmountable. Considering recent insights regarding the molecular alterations associated with bladder cancer and the description of disease subtypes associated with clinical relevance, the opportunity is now ripe for the exploration of new mouse models and particularly those that can have translatable impact to improve the therapeutic landscape for patients with bladder cancer.
At a glance summary.
Bladder cancers arise in the urothelium, a specialized epithelium comprised of basal, intermediate and superficial (umbrella) cells.
Basal cells can serve as urothelial progenitors as well as cells of origin of bladder cancer, particularly of more aggressive subtypes; however, other urothelial cell types can also serve as progenitors in normal bladder as well as cells of origin of bladder cancer, potentially of it different subtypes.
Bladder cancer represents a heterogeneous set of tumors that vary in histopathology, molecular alterations, and potentially cells of origin; the vast majority (>90%) are urothelial carcinomas, which are the subject of this review.
Urothelial carcinomas fall into two major categories: most (~75%) are non-muscle invasive, which include low-grade superficial (or papillary) and high-grade carcinoma in situ; the remainder (~25%) are muscle invasive.
Most non-muscle invasive bladder cancers, particularly low-grade tumors, have favorable prognosis; these can be clinically-managed with bladder-sparing treatments, which are generally effective but very costly.
Muscle invasive bladder cancers have relatively poor prognosis; those that have not metastasized are often treated by cystectomy (surgical removal of the bladder), which is reasonably effective (5-year survival of ~50%) but associated with high morbidity.
Metastatic bladder is treated using chemotherapy, which is neither well-tolerated nor highly effective; metastatic bladder cancer has a very poor prognosis (5-year survival of ~15%).
Unlike many other cancers, neither the prognosis nor treatment of bladder cancer has significantly improved in the past 20 years; bladder cancer remains a major cause of cancer mortality.
The molecular pathways that give rise to low-grade non-muscle invasive versus high-grade muscle invasive bladder cancer are distinct but not mutually exclusive.
The recent elucidation of genetic/genomic alterations prevalent in muscle invasive bladder cancer provides new avenues for understanding the underlying molecular mechanisms, as well as new targets for therapeutic intervention.
Currently available in vivo models of bladder cancer include carcinogen-based and genetically-engineered mouse (GEM) models, as well as orthotopic and renal grafting, each of which has advantages and limitations.
Bladder cancer is relatively underrepresented by GEM models, particularly those that model more aggressive phenotypes.
The mouse bladder may be relatively recalcitrant to developing invasive tumors, which has made it challenging to develop GEM models.
Other challenges to developing GEM models include inadequate approaches for restricting gene targeting to the urothelium and particularly to selected cells of origin.
Improved GEM models will lead to opportunities for preclinical evaluation of new treatment options for bladder cancer.
Acknowledgments
The authors would like to thank Drs. Hikmat Al-Ahmadie, Mireia Castillo-Martin, Gopa Iyer, Cathy Mendelsohn, and Michael Shen for helpful comments on the manuscript. Work in the authors’ laboratories is funded by a Grant-in-Aid for Young Scientists (A) from the Japan Society for the Promotion of Science (to TK), a grant from the Urology Care Foundation Research Scholars Program and Dornier MedTech (to TBO), and funding from the National Cancer Institute and the T.J. Martell Foundation for Leukemia, Cancer and AIDS research (to CAS). CAS is an American Cancer Society Research Professor supported in part by a generous gift from the F.M. Kirby Foundation.
Glossary of terms
- Non-muscle invasive bladder cancer
Urothelial carcinomas that invade the bladder muscle
- Carcinoma in situ (CIS)
A flattened malignant transformation of the urothelium that is presumed to be a precursor of muscle invasive bladder cancer
- Muscle invasive bladder cancer
Urothelial carcinomas that invade the muscle layer
- Papillary (superficial) tumor
An extrusion of the urothelium into the bladder lumen without invasion of the muscle layer
- Urothelium
The epithelium that lines the bladder, which is comprised of three cell types: basal, intermediate and umbrella cells
- Lamina propria
The layer of connective tissue that underlies the urothelium
- Detrusor muscle
The layer of smooth muscle that lines the bladder and controls the elimination of urine
- Uroplakins
Transmembrane proteins that that comprise the asymmetric unit membrane (AUM) on the lumen-facing side of the umbrella cells, which provide a barrier against entry of urine
- Transurethral resection (TUR)
Endoscopic surgical removal of bladder tumors or lesions
- Intravesical therapy
Delivery of interventional or therapeutic agents directly to the bladder lumen
- Bacillus Calmette-Guerin (BCG)
A form of immunotherapy that is a front line intravesical treatment for high-risk non-muscle invasive bladder cancer
- Cystectomy
Surgical removal of the bladder, which is the front line treatment for muscle invasive bladder cancer
- Autochthonous
Arising within the individual, usually refers to tumors arising de novo in genetically-engineered mouse models
Biographies
Cory Abate-Shen, Ph.D., is the Michael and Stella Chernow Professor of Urologic Sciences, a Professor in the Departments of Urology, Pathology and Cell Biology, and Systems Biology, and the Associate Director for Translational Research at the Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, College of Physicians and Surgeons, New York, NY. Her research interests are in the generation and analyses of genetically-engineered mouse models of genitourological cancers for investigating the molecular mechanisms as well as preclinical models for evaluating new therapies.
Takashi Kobayashi, M.D., Ph.D. obtained his MD and Ph.D. degrees at Kyoto University, Kyoto, Japan and then pursued post-doctoral training with Dr. Abate-Shen at the Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center, where he was and a Urology Care Foundation Research Scholar. He is an Assistant Professor in the Department of Urology, Kyoto University Graduate School of Medicine, where he maintains a research laboratory as well as a clinical practice in urological surgery. His research interests are in urogenital cancer, particularly in bladder and prostate cancer, and in his clinical practice specializes in renal transplantation.
Tomasz B. Owczarek, Ph.D. obtained his Masters and Ph.D. at the Wroclaw University of Environmental and Life Sciences, Wroclaw, Poland. He is a postdoctoral research scientist with Dr. Abate-Shen at the Herbert Irving Comprehensive Cancer Center, Columbia University Medical Center and a Urology Care Foundation Research Scholar. His research interests are in understanding mechanism of bladder cancer progression.
James McKiernan, MD is the John K. Lattimer Professor and Chair of the Department of Urology, Columbia University Medical Center. He specializes in urologic oncology, patients with high-risk bladder and kidney cancers. His research focuses on novel drug and biomarker development in urologic oncology. Dr. McKiernan has served as the Vice Chairman for the American Joint Committee on Staging of Cancer, as well as on the American Board of Urology and Society of Urologic Oncology Examination Committees. In addition, he is the principal investigator of an NIH-funded clinical trials program for experimental therapeutics in bladder cancer, which is investigating new agents for bladder preservation in high-risk patients.
References
- 1•.Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239–249. doi: 10.1016/S0140-6736(09)60491-8. Comprehensive overview of bladder cancer. [DOI] [PubMed] [Google Scholar]
- 2.Dinney CP, et al. Focus on bladder cancer. Cancer cell. 2004;6:111–116. doi: 10.1016/j.ccr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Prasad SM, Decastro GJ, Steinberg GD Medscape. Urothelial carcinoma of the bladder: definition, treatment and future efforts. Nature reviews. Urology. 2011;8:631–642. doi: 10.1038/nrurol.2011.144. [DOI] [PubMed] [Google Scholar]
- 4.Botteman MF, Pashos CL, Redaelli A, Laskin B, Hauser R. The health economics of bladder cancer: a comprehensive review of the published literature. PharmacoEconomics. 2003;21:1315–1330. doi: 10.1007/BF03262330. [DOI] [PubMed] [Google Scholar]
- 5•.Hicks RM. The mammalian urinary bladder: an accommodating organ. Biological reviews of the Cambridge Philosophical Society. 1975;50:215–246. doi: 10.1111/j.1469-185x.1975.tb01057.x. Classic review on the anatomy of the bladder. [DOI] [PubMed] [Google Scholar]
- 6.Bradley WE, Long DM. Morphology of the developing mammalian bladder. Investigative urology. 1969;7:66–73. [PubMed] [Google Scholar]
- 7•.Baskin LS, Hayward SW, Young PF, Cunha GR. Ontogeny of the rat bladder: smooth muscle and epithelial differentiation. Acta anatomica. 1996;155:163–171. doi: 10.1159/000147801. Classic review on the development of the bladder. [DOI] [PubMed] [Google Scholar]
- 8.De La Rosette J, Smedts F, Schoots C, Hoek H, Laguna P. Changing patterns of keratin expression could be associated with functional maturation of the developing human bladder. The Journal of urology. 2002;168:709–717. doi: 10.1097/00005392-200208000-00085. [DOI] [PubMed] [Google Scholar]
- 9.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. American journal of physiology. Renal physiology. 2009;297:F1477–1501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Castillo-Martin M, Domingo-Domenech J, Karni-Schmidt O, Matos T, Cordon-Cardo C. Molecular pathways of urothelial development and bladder tumorigenesis. Urologic oncology. 2010;28:401–408. doi: 10.1016/j.urolonc.2009.04.019. Reviews the relationship of bladder development and bladder cancer. [DOI] [PubMed] [Google Scholar]
- 11•.Sun W, Wilhelmina Aalders T, Oosterwijk E. Identification of potential bladder progenitor cells in the trigone. Developmental biology. 2014;393:84–92. doi: 10.1016/j.ydbio.2014.06.018. Description of bladder progenitors showing their prevalence in the trigone. [DOI] [PubMed] [Google Scholar]
- 12.Wu XR, Kong XP, Pellicer A, Kreibich G, Sun TT. Uroplakins in urothelial biology, function, and disease. Kidney international. 2009;75:1153–1165. doi: 10.1038/ki.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. The American journal of anatomy. 1960;106:247–285. doi: 10.1002/aja.1001060305. [DOI] [PubMed] [Google Scholar]
- 14•.Jost SP, Potten CS. Urothelial proliferation in growing mice. Cell and tissue kinetics. 1986;19:155–160. doi: 10.1111/j.1365-2184.1986.tb00725.x. One of the first studies to show that the urothelium has a slow turnover. [DOI] [PubMed] [Google Scholar]
- 15.Cooper EH, Cowen DM, Knowles JC. The recovery of mouse bladder epithelium after injury by 4-ethylsulphonylnaphthalene-1-sulphonamide. The Journal of pathology. 1972;108:151–156. doi: 10.1002/path.1711080209. [DOI] [PubMed] [Google Scholar]
- 16.Lavelle J, et al. Bladder permeability barrier: recovery from selective injury of surface epithelial cells. American journal of physiology. Renal physiology. 2002;283:F242–253. doi: 10.1152/ajprenal.00307.2001. [DOI] [PubMed] [Google Scholar]
- 17.Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell host & microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin K, et al. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110–114. doi: 10.1038/nature09851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nature reviews. Urology. 2012;9:583–594. doi: 10.1038/nrurol.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi D, et al. Retinoid signaling in progenitors controls specification and regeneration of the urothelium. Developmental cell. 2013;26:469–482. doi: 10.1016/j.devcel.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colopy SA, Bjorling DE, Mulligan WA, Bushman W. A population of progenitor cells in the basal and intermediate layers of the murine bladder urothelium contributes to urothelial development and regeneration. Developmental dynamics : an official publication of the American Association of Anatomists. 2014;243:988–998. doi: 10.1002/dvdy.24143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karni-Schmidt O, et al. Distinct expression profiles of p63 variants during urothelial development and bladder cancer progression. The American journal of pathology. 2011;178:1350–1360. doi: 10.1016/j.ajpath.2010.11.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Signoretti S, et al. p63 regulates commitment to the prostate cell lineage. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:11355–11360. doi: 10.1073/pnas.0500165102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Gaisa NT, et al. The human urothelium consists of multiple clonal units, each maintained by a stem cell. The Journal of pathology. 2011;225:163–171. doi: 10.1002/path.2945. Clonal analyses of human bladder progenitors by analysis of mitochondrial DNA. [DOI] [PubMed] [Google Scholar]
- 25.Dahm P, Gschwend JE. Malignant non-urothelial neoplasms of the urinary bladder: a review. European urology. 2003;44:672–681. doi: 10.1016/s0302-2838(03)00416-0. [DOI] [PubMed] [Google Scholar]
- 26.Williamson SR, et al. Diagnosis, evaluation and treatment of carcinoma in situ of the urinary bladder: the state of the art. Critical reviews in oncology/hematology. 2010;76:112–126. doi: 10.1016/j.critrevonc.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Nese N, Gupta R, Bui MH, Amin MB. Carcinoma in situ of the urinary bladder: review of clinicopathologic characteristics with an emphasis on aspects related to molecular diagnostic techniques and prognosis. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7:48–57. doi: 10.6004/jnccn.2009.0004. [DOI] [PubMed] [Google Scholar]
- 28.Shin K, et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nature cell biology. 2014;16:469–478. doi: 10.1038/ncb2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He X, et al. Differentiation of a highly tumorigenic basal cell compartment in urothelial carcinoma. Stem Cells. 2009;27:1487–1495. doi: 10.1002/stem.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Chan KS, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. One of the first studies to isolate bladder cancer stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Volkmer JP, et al. Three differentiation states risk-stratify bladder cancer into distinct subtypes. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:2078–2083. doi: 10.1073/pnas.1120605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32•.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014 doi: 10.1038/nature12965. Results of the TCGA analyses of invasive bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33•.Choi W, et al. Identification of distinct Basal and luminal subtypes of muscle-invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer cell. 2014;25:152–165. doi: 10.1016/j.ccr.2014.01.009. Description of molecular subtypes of bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Damrauer JS, et al. Intrinsic subtypes of high-grade bladder cancer reflect the hallmarks of breast cancer biology. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3110–3115. doi: 10.1073/pnas.1318376111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi W, et al. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nature Reviews Urology. 2014 doi: 10.1038/nrurol.2014.129. in press. [DOI] [PubMed] [Google Scholar]
- 36.Van Batavia J, et al. Bladder cancers arise from distinct urothelial sub-populations. Nature cell biology. 2014;16:982–991. doi: 10.1038/ncb3038. [DOI] [PubMed] [Google Scholar]
- 37.Dancik GM, Owens CR, Iczkowski KA, Theodorescu D. A cell of origin gene signature indicates human bladder cancer has distinct cellular progenitors. Stem Cells. 2014;32:974–982. doi: 10.1002/stem.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Resnick MJ, Bassett JC, Clark PE. Management of superficial and muscle-invasive urothelial cancers of the bladder. Current opinion in oncology. 2013;25:281–288. doi: 10.1097/CCO.0b013e32835eb583. [DOI] [PubMed] [Google Scholar]
- 39•.Clark PE, et al. Bladder cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2013;11:446–475. doi: 10.6004/jnccn.2013.0059. Summary of treatment options for bladder cancer. [DOI] [PubMed] [Google Scholar]
- 40.Redelman-Sidi G, Glickman MS, Bochner BH. The mechanism of action of BCG therapy for bladder cancer--a current perspective. Nature reviews. Urology. 2014;11:153–162. doi: 10.1038/nrurol.2014.15. [DOI] [PubMed] [Google Scholar]
- 41.Gontero P, et al. The role of bacillus Calmette-Guerin in the treatment of non-muscle-invasive bladder cancer. European urology. 2010;57:410–429. doi: 10.1016/j.eururo.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Herr HW, Morales A. History of bacillus Calmette-Guerin and bladder cancer: an immunotherapy success story. The Journal of urology. 2008;179:53–56. doi: 10.1016/j.juro.2007.08.122. [DOI] [PubMed] [Google Scholar]
- 43.Barlow LJ, Seager CM, Benson MC, McKiernan JM. Novel intravesical therapies for non-muscle-invasive bladder cancer refractory to BCG. Urologic oncology. 2010;28:108–111. doi: 10.1016/j.urolonc.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 44.Dash A, et al. A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer. 2008;113:2471–2477. doi: 10.1002/cncr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zargar H, et al. Multicenter Assessment of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer. European urology. 2014 doi: 10.1016/j.eururo.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stein JP, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 47.Singer S, et al. Quality of life in patients with muscle invasive and non-muscle invasive bladder cancer. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2013;21:1383–1393. doi: 10.1007/s00520-012-1680-8. [DOI] [PubMed] [Google Scholar]
- 48.Somani BK, Gimlin D, Fayers P, N’Dow J. Quality of life and body image for bladder cancer patients undergoing radical cystectomy and urinary diversion--a prospective cohort study with a systematic review of literature. Urology. 2009;74:1138–1143. doi: 10.1016/j.urology.2009.05.087. [DOI] [PubMed] [Google Scholar]
- 49.Wu X, Hildebrandt MA, Chang DW. Genome-wide association studies of bladder cancer risk: a field synopsis of progress and potential applications. Cancer metastasis reviews. 2009;28:269–280. doi: 10.1007/s10555-009-9190-y. [DOI] [PubMed] [Google Scholar]
- 50•.Freedman ND, Silverman DT, Hollenbeck AR, Schatzkin A, Abnet CC. Association between smoking and risk of bladder cancer among men and women. JAMA : the journal of the American Medical Association. 2011;306:737–745. doi: 10.1001/jama.2011.1142. Analyses of the link between smoking and risk of bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kiriluk KJ, Prasad SM, Patel AR, Steinberg GD, Smith ND. Bladder cancer risk from occupational and environmental exposures. Urologic oncology. 2012;30:199–211. doi: 10.1016/j.urolonc.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 52.Burger M, et al. Epidemiology and risk factors of urothelial bladder cancer. European urology. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 53•.Mitra AP, Cote RJ. Molecular pathogenesis and diagnostics of bladder cancer. Annual review of pathology. 2009;4:251–285. doi: 10.1146/annurev.pathol.4.110807.092230. Comprehensive review of the molecular biology, risk factors and subtypes of bladder cancer. [DOI] [PubMed] [Google Scholar]
- 54.Crivelli JJ, et al. Effect of smoking on outcomes of urothelial carcinoma: a systematic review of the literature. European urology. 2014;65:742–754. doi: 10.1016/j.eururo.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 55.Baskin LS, Hayward SW, Young P, Cunha GR. Role of mesenchymal-epithelial interactions in normal bladder development. The Journal of urology. 1996;156:1820–1827. [PubMed] [Google Scholar]
- 56.Marcinkiewicz K, et al. The androgen receptor and stem cell pathways in prostate and bladder cancers (review) International journal of oncology. 2012;40:5–12. doi: 10.3892/ijo.2011.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsu JW, et al. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. The American journal of pathology. 2013;182:1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Lin C, et al. Constitutive beta-catenin activation induces male-specific tumorigenesis in the bladder urothelium. Cancer research. 2013;73:5914–5925. doi: 10.1158/0008-5472.CAN-12-4198. Provides evidence that the prevalence of males to develop bladder cancer may be due to androgen receptor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Knowles MA. Molecular pathogenesis of bladder cancer. International journal of clinical oncology. 2008;13:287–297. doi: 10.1007/s10147-008-0812-0. [DOI] [PubMed] [Google Scholar]
- 60.Goebell PJ, Knowles MA. Bladder cancer or bladder cancers? Genetically distinct malignant conditions of the urothelium. Urologic oncology. 2010;28:409–428. doi: 10.1016/j.urolonc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 61.Wu XR. Urothelial tumorigenesis: a tale of divergent pathways. Nature reviews. Cancer. 2005;5:713–725. doi: 10.1038/nrc1697. [DOI] [PubMed] [Google Scholar]
- 62•.Esrig D, et al. Accumulation of nuclear p53 and tumor progression in bladder cancer. The New England journal of medicine. 1994;331:1259–1264. doi: 10.1056/NEJM199411103311903. One of the first studies to show the relevance of p53 expression for bladder cancer. [DOI] [PubMed] [Google Scholar]
- 63.Dyrskjot L, et al. Identifying distinct classes of bladder carcinoma using microarrays. Nature genetics. 2003;33:90–96. doi: 10.1038/ng1061. [DOI] [PubMed] [Google Scholar]
- 64.Sanchez-Carbayo M, et al. Gene discovery in bladder cancer progression using cDNA microarrays. The American journal of pathology. 2003;163:505–516. doi: 10.1016/S0002-9440(10)63679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blaveri E, et al. Bladder cancer stage and outcome by array-based comparative genomic hybridization. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11:7012–7022. doi: 10.1158/1078-0432.CCR-05-0177. [DOI] [PubMed] [Google Scholar]
- 66.Kim JH, et al. Alterations in transcription clusters underlie development of bladder cancer along papillary and nonpapillary pathways. Laboratory investigation; a journal of technical methods and pathology. 2005;85:532–549. doi: 10.1038/labinvest.3700250. [DOI] [PubMed] [Google Scholar]
- 67.Mitra AP, et al. Generation of a concise gene panel for outcome prediction in urinary bladder cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3929–3937. doi: 10.1200/JCO.2008.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Riester M, et al. Combination of a novel gene expression signature with a clinical nomogram improves the prediction of survival in high-risk bladder cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:1323–1333. doi: 10.1158/1078-0432.CCR-11-2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lauss M, Ringner M, Hoglund M. Prediction of stage, grade, and survival in bladder cancer using genome-wide expression data: a validation study. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4421–4433. doi: 10.1158/1078-0432.CCR-10-0606. [DOI] [PubMed] [Google Scholar]
- 70.Lindgren D, et al. Combined gene expression and genomic profiling define two intrinsic molecular subtypes of urothelial carcinoma and gene signatures for molecular grading and outcome. Cancer research. 2010;70:3463–3472. doi: 10.1158/0008-5472.CAN-09-4213. [DOI] [PubMed] [Google Scholar]
- 71.Zieger K, Marcussen N, Borre M, Orntoft TF, Dyrskjot L. Consistent genomic alterations in carcinoma in situ of the urinary bladder confirm the presence of two major pathways in bladder cancer development. International journal of cancer. Journal international du cancer. 2009;125:2095–2103. doi: 10.1002/ijc.24619. [DOI] [PubMed] [Google Scholar]
- 72.Hurst CD, Platt FM, Taylor CF, Knowles MA. Novel tumor subgroups of urothelial carcinoma of the bladder defined by integrated genomic analysis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:5865–5877. doi: 10.1158/1078-0432.CCR-12-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lindgren D, et al. Integrated genomic and gene expression profiling identifies two major genomic circuits in urothelial carcinoma. PloS one. 2012;7:e38863. doi: 10.1371/journal.pone.0038863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sjodahl G, et al. A molecular taxonomy for urothelial carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:3377–3386. doi: 10.1158/1078-0432.CCR-12-0077-T. [DOI] [PubMed] [Google Scholar]
- 75.Nordentoft I, et al. Mutational context and diverse clonal development in early and late bladder cancer. Cell reports. 2014;7:1649–1663. doi: 10.1016/j.celrep.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 76.Gui Y, et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nature genetics. 2011;43:875–878. doi: 10.1038/ng.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morrison CD, et al. Whole-genome sequencing identifies genomic heterogeneity at a nucleotide and chromosomal level in bladder cancer. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E672–681. doi: 10.1073/pnas.1313580111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ross JS, et al. Advanced urothelial carcinoma: next-generation sequencing reveals diverse genomic alterations and targets of therapy. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2014;27:271–280. doi: 10.1038/modpathol.2013.135. [DOI] [PubMed] [Google Scholar]
- 79.Guo G, et al. Whole-genome and whole-exome sequencing of bladder cancer identifies frequent alterations in genes involved in sister chromatid cohesion and segregation. Nature genetics. 2013;45:1459–1463. doi: 10.1038/ng.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cazier JB, et al. Whole-genome sequencing of bladder cancers reveals somatic CDKN1A mutations and clinicopathological associations with mutation burden. Nature communications. 2014;5:3756. doi: 10.1038/ncomms4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Iyer G, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3133–3140. doi: 10.1200/JCO.2012.46.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82•.Iyer G, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. Identification of TSC1 as a key “actionable” target for bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wagle N, et al. Activating mTOR Mutations in a Patient with an Extraordinary Response on a Phase I Trial of Everolimus and Pazopanib. Cancer discovery. 2014 doi: 10.1158/2159-8290.CD-13-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shih C, Weinberg RA. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982;29:161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- 85.Santos E, Tronick SR, Aaronson SA, Pulciani S, Barbacid M. T24 human bladder carcinoma oncogene is an activated form of the normal human homologue of BALB- and Harvey-MSV transforming genes. Nature. 1982;298:343–347. doi: 10.1038/298343a0. [DOI] [PubMed] [Google Scholar]
- 86.Reddy EP, Reynolds RK, Santos E, Barbacid M. A point mutation is responsible for the acquisition of transforming properties by the T24 human bladder carcinoma oncogene. Nature. 1982;300:149–152. doi: 10.1038/300149a0. [DOI] [PubMed] [Google Scholar]
- 87.Gust KM, et al. Fibroblast growth factor receptor 3 is a rational therapeutic target in bladder cancer. Molecular cancer therapeutics. 2013;12:1245–1254. doi: 10.1158/1535-7163.MCT-12-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rebouissou S, et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Science translational medicine. 2014;6:244ra291. doi: 10.1126/scitranslmed.3008970. [DOI] [PubMed] [Google Scholar]
- 89.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature reviews. Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 92.Degraff DJ, et al. Current preclinical models for the advancement of translational bladder cancer research. Molecular cancer therapeutics. 2013;12:121–130. doi: 10.1158/1535-7163.MCT-12-0508. [DOI] [PubMed] [Google Scholar]
- 93.Ahmad I, Sansom OJ, Leung HY. Exploring molecular genetics of bladder cancer: lessons learned from mouse models. Disease models & mechanisms. 2012;5:323–332. doi: 10.1242/dmm.008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ding J, et al. Current animal models of bladder cancer: Awareness of translatability (Review) Experimental and therapeutic medicine. 2014;8:691–699. doi: 10.3892/etm.2014.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95•.Druckrey H, et al. Selective Induction of Bladder Cancer in Rats by Dibutyl- and N-Butyl-N-Butanol(4)-Nitrosamine. Zeitschrift fur Krebsforschung. 1964;66:280–290. One of the first descriptions of carcinogenesis-induced bladder cancer. [PubMed] [Google Scholar]
- 96.Hicks RM, Wakefield JS. Rapid induction of bladder cancer in rats with N-methyl-N-nitrosourea. I. Histology. Chemico-biological interactions. 1972;5:139–152. doi: 10.1016/0009-2797(72)90040-3. [DOI] [PubMed] [Google Scholar]
- 97.Fukushima S, Hirose M, Tsuda H, Shirai T, Hirao K. Histological classification of urinary bladder cancers in rats induced by N-butyl-n-(4-hydroxybutyl)nitrosamine. Gann = Gan. 1976;67:81–90. [PubMed] [Google Scholar]
- 98.Cohen SM. Urinary bladder carcinogenesis. Toxicologic pathology. 1998;26:121–127. doi: 10.1177/019262339802600114. [DOI] [PubMed] [Google Scholar]
- 99.Soloway MS. Single and combination chemotherapy for primary murine bladder cancer. Cancer. 1975;36:333–340. doi: 10.1002/1097-0142(197508)36:2<333::aid-cncr2820360207>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 100.Vasconcelos-Nobrega C, Colaco A, Lopes C, Oliveira PA. Review: BBN as an urothelial carcinogen. In vivo. 2012;26:727–739. [PubMed] [Google Scholar]
- 101.He Z, Kosinska W, Zhao ZL, Wu XR, Guttenplan JB. Tissue-specific mutagenesis by N-butyl-N-(4-hydroxybutyl)nitrosamine as the basis for urothelial carcinogenesis. Mutation research. 2012;742:92–95. doi: 10.1016/j.mrgentox.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 102.Yamamoto S, et al. Frequent mutations of the p53 gene and infrequent H- and K-ras mutations in urinary bladder carcinomas of NON/Shi mice treated with N-butyl-N-(4-hydroxybutyl)nitrosamine. Carcinogenesis. 1995;16:2363–2368. doi: 10.1093/carcin/16.10.2363. [DOI] [PubMed] [Google Scholar]
- 103.Kim SK, et al. Identification of gene expression signature modulated by nicotinamide in a mouse bladder cancer model. PloS one. 2011;6:e26131. doi: 10.1371/journal.pone.0026131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Williams PD, Lee JK, Theodorescu D. Molecular credentialing of rodent bladder carcinogenesis models. Neoplasia. 2008;10:838–846. doi: 10.1593/neo.08432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Weldon TE, Soloway MS. Susceptibility of urothelium to neoplastic cellular implantation. Urology. 1975;5:824–827. doi: 10.1016/0090-4295(75)90367-2. [DOI] [PubMed] [Google Scholar]
- 106.Oshinsky GS, Chen Y, Jarrett T, Anderson AE, Weiss GH. A model of bladder tumor xenografts in the nude rat. The Journal of urology. 1995;154:1925–1929. [PubMed] [Google Scholar]
- 107.Gunther JH, et al. Optimizing syngeneic orthotopic murine bladder cancer (MB49) Cancer research. 1999;59:2834–2837. [PubMed] [Google Scholar]
- 108.Xiao Z, et al. Characterization of a novel transplantable orthotopic rat bladder transitional cell tumour model. British journal of cancer. 1999;81:638–646. doi: 10.1038/sj.bjc.6690741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chan ES, et al. Optimizing orthotopic bladder tumor implantation in a syngeneic mouse model. The Journal of urology. 2009;182:2926–2931. doi: 10.1016/j.juro.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 110.Dobek GL, Godbey WT. An orthotopic model of murine bladder cancer. Journal of visualized experiments : JoVE. 2011 doi: 10.3791/2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oottamasathien S, et al. Bladder tissue formation from cultured bladder urothelium. Developmental dynamics : an official publication of the American Association of Anatomists. 2006;235:2795–2801. doi: 10.1002/dvdy.20886. [DOI] [PubMed] [Google Scholar]
- 112•.Puzio-Kuter AM, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes & development. 2009;23:675–680. doi: 10.1101/gad.1772909. Describes a unique model of invasive, metasatic bladder cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kerbel RS, Cornil I, Theodorescu D. Importance of orthotopic transplantation procedures in assessing the effects of transfected genes on human tumor growth and metastasis. Cancer metastasis reviews. 1991;10:201–215. doi: 10.1007/BF00050792. [DOI] [PubMed] [Google Scholar]
- 114.Black PC, et al. Validating bladder cancer xenograft bioluminescence with magnetic resonance imaging: the significance of hypoxia and necrosis. BJU international. 2010;106:1799–1804. doi: 10.1111/j.1464-410X.2010.09424.x. [DOI] [PubMed] [Google Scholar]
- 115.van der Horst G, et al. Real-time cancer cell tracking by bioluminescence in a preclinical model of human bladder cancer growth and metastasis. European urology. 2011;60:337–343. doi: 10.1016/j.eururo.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 116.Chan ES, Patel AR, Hansel DE, Larchian WA, Heston WD. Sunitinib malate provides activity against murine bladder tumor growth and invasion in a preclinical orthotopic model. Urology. 2012;80:736 e731–735. doi: 10.1016/j.urology.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 117.Chen SC, Henry DO, Hicks DG, Reczek PR, Wong MK. Intravesical administration of plasminogen activator inhibitor type-1 inhibits in vivo bladder tumor invasion and progression. The Journal of urology. 2009;181:336–342. doi: 10.1016/j.juro.2008.08.123. [DOI] [PubMed] [Google Scholar]
- 118.Tentler JJ, et al. Patient-derived tumour xenografts as models for oncology drug development. Nature reviews. Clinical oncology. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Park B, et al. Development and characterization of a bladder cancer xenograft model using patient-derived tumor tissue. Cancer science. 2013 doi: 10.1111/cas.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cirone P, Andresen CJ, Eswaraka JR, Lappin PB, Bagi CM. Patient-derived xenografts reveal limits to PI3K/mTOR- and MEK-mediated inhibition of bladder cancer. Cancer chemotherapy and pharmacology. 2014;73:525–538. doi: 10.1007/s00280-014-2376-1. [DOI] [PubMed] [Google Scholar]
- 121.Sharpless NE, Depinho RA. The mighty mouse: genetically engineered mouse models in cancer drug development. Nature reviews. Drug discovery. 2006;5:741–754. doi: 10.1038/nrd2110. [DOI] [PubMed] [Google Scholar]
- 122.Politi K, Pao W. How genetically engineered mouse tumor models provide insights into human cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:2273–2281. doi: 10.1200/JCO.2010.30.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Abate-Shen C, Pandolfi PP. Effective utilization and appropriate selection of genetically engineered mouse models for translational integration of mouse and human trials. Cold Spring Harbor protocols. 2013;2013 doi: 10.1101/pdb.top078774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cancer Genome Atlas Research N. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hanahan D, Wagner EF, Palmiter RD. The origins of oncomice: a history of the first transgenic mice genetically engineered to develop cancer. Genes & development. 2007;21:2258–2270. doi: 10.1101/gad.1583307. [DOI] [PubMed] [Google Scholar]
- 126•.Zhang ZT, Pak J, Shapiro E, Sun TT, Wu XR. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer research. 1999;59:3512–3517. First transgenic “onco-mouse” model of bladder cancer. [PubMed] [Google Scholar]
- 127.Ayala de la Pena F, et al. Loss of p53 and acquisition of angiogenic microRNA profile are insufficient to facilitate progression of bladder urothelial carcinoma in situ to invasive carcinoma. The Journal of biological chemistry. 2011;286:20778–20787. doi: 10.1074/jbc.M110.198069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stone R, 2nd, et al. Identification of genes correlated with early-stage bladder cancer progression. Cancer Prev Res (Phila) 2010;3:776–786. doi: 10.1158/1940-6207.CAPR-09-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grippo PJ, Sandgren EP. Highly invasive transitional cell carcinoma of the bladder in a simian virus 40 T-antigen transgenic mouse model. The American journal of pathology. 2000;157:805–813. doi: 10.1016/S0002-9440(10)64594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.He F, et al. Deficiency of pRb family proteins and p53 in invasive urothelial tumorigenesis. Cancer research. 2009;69:9413–9421. doi: 10.1158/0008-5472.CAN-09-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang ZT, et al. Role of Ha-ras activation in superficial papillary pathway of urothelial tumor formation. Oncogene. 2001;20:1973–1980. doi: 10.1038/sj.onc.1204315. [DOI] [PubMed] [Google Scholar]
- 132.Cheng J, et al. Overexpression of epidermal growth factor receptor in urothelium elicits urothelial hyperplasia and promotes bladder tumor growth. Cancer research. 2002;62:4157–4163. [PubMed] [Google Scholar]
- 133.Garcia-Espana A, Salazar E, Sun TT, Wu XR, Pellicer A. Differential expression of cell cycle regulators in phenotypic variants of transgenically induced bladder tumors: implications for tumor behavior. Cancer research. 2005;65:1150–1157. doi: 10.1158/0008-5472.CAN-04-2074. [DOI] [PubMed] [Google Scholar]
- 134.Mo L, et al. Hyperactivation of Ha-ras oncogene, but not Ink4a/Arf deficiency, triggers bladder tumorigenesis. The Journal of clinical investigation. 2007;117:314–325. doi: 10.1172/JCI30062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gao J, et al. p53 deficiency provokes urothelial proliferation and synergizes with activated Ha-ras in promoting urothelial tumorigenesis. Oncogene. 2004;23:687–696. doi: 10.1038/sj.onc.1207169. [DOI] [PubMed] [Google Scholar]
- 136.Ahmad I, et al. beta-Catenin activation synergizes with PTEN loss to cause bladder cancer formation. Oncogene. 2011;30:178–189. doi: 10.1038/onc.2010.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ahmad I, et al. Ras mutation cooperates with beta-catenin activation to drive bladder tumourigenesis. Cell death & disease. 2011;2:e124. doi: 10.1038/cddis.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ahmad I, et al. K-Ras and beta-catenin mutations cooperate with Fgfr3 mutations in mice to promote tumorigenesis in the skin and lung, but not in the bladder. Disease models & mechanisms. 2011;4:548–555. doi: 10.1242/dmm.006874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rampias T, et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nature medicine. 2014;20:1199–1205. doi: 10.1038/nm.3678. [DOI] [PubMed] [Google Scholar]
- 140.Foth M, et al. Fibroblast Growth Factor Receptor 3 activation plays a causative role in urothelial cancer pathogenesis in cooperation with Pten loss in mice. The Journal of pathology. 2014 doi: 10.1002/path.4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PloS one. 2011;6:e16209. doi: 10.1371/journal.pone.0016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tsuruta H, et al. Hyperplasia and carcinomas in Pten-deficient mice and reduced PTEN protein in human bladder cancer patients. Cancer research. 2006;66:8389–8396. doi: 10.1158/0008-5472.CAN-05-4627. [DOI] [PubMed] [Google Scholar]
- 143.Yang X, et al. Simultaneous activation of Kras and inactivation of p53 induces soft tissue sarcoma and bladder urothelial hyperplasia. PloS one. 2013;8:e74809. doi: 10.1371/journal.pone.0074809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144•.Seager CM, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila) 2009;2:1008–1014. doi: 10.1158/1940-6207.CAPR-09-0169. Preclinical evaluation of rapamycin using intravesical therapy led to novel clinical trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delto JC, Kobayashi T, Benson M, McKiernan J, Abate-Shen C. Preclinical analyses of intravesical chemotherapy for prevention of bladder cancer progression. Oncotarget. 2013;4:269–276. doi: 10.18632/oncotarget.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146•.Lin JH, Zhao H, Sun TT. A tissue-specific promoter that can drive a foreign gene to express in the suprabasal urothelial cells of transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:679–683. doi: 10.1073/pnas.92.3.679. First description of the Uroplakin promoter expression in bladder. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. The Journal of biological chemistry. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- 148.Bex A, Vooijs M, Horenblas S, Berns A. Controlling gene expression in the urothelium using transgenic mice with inducible bladder specific Cre-lox recombination. The Journal of urology. 2002;168:2641–2644. doi: 10.1016/S0022-5347(05)64235-8. [DOI] [PubMed] [Google Scholar]
- 149.Seager C, Puzio-Kuter AM, Cordon-Cardo C, McKiernan J, Abate-Shen C. Mouse models of human bladder cancer as a tool for drug discovery. Curr Protoc Pharmacol. 2010;Chapter 14(Unit 14):14. doi: 10.1002/0471141755.ph1414s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yoo LI, et al. Pten deficiency activates distinct downstream signaling pathways in a tissue-specific manner. Cancer research. 2006;66:1929–1939. doi: 10.1158/0008-5472.CAN-05-1986. [DOI] [PubMed] [Google Scholar]
- 151•.Ozaki K, et al. High susceptibility of p53(+/−) knockout mice in N-butyl-N-(4-hydroxybutyl)nitrosamine urinary bladder carcinogenesis and lack of frequent mutation in residual allele. Cancer research. 1998;58:3806–3811. One of the first studies to combine carcinogenesis-induction with a GEM model. [PubMed] [Google Scholar]
- 152.Hamed S, et al. Accelerated induction of bladder cancer in patched heterozygous mutant mice. Cancer research. 2004;64:1938–1942. doi: 10.1158/0008-5472.can-03-2031. [DOI] [PubMed] [Google Scholar]
- 153.Hoogervorst EM, et al. p53 heterozygosity results in an increased 2-acetylaminofluorene-induced urinary bladder but not liver tumor response in DNA repair-deficient Xpa mice. Cancer research. 2004;64:5118–5126. doi: 10.1158/0008-5472.CAN-04-0350. [DOI] [PubMed] [Google Scholar]
- 154.Hikosaka A, et al. Susceptibility of p27 kip1 knockout mice to urinary bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl)nitrosamine may not simply be due to enhanced proliferation. International journal of cancer. Journal international du cancer. 2008;122:1222–1228. doi: 10.1002/ijc.23249. [DOI] [PubMed] [Google Scholar]
- 155.Ho PL, Lay EJ, Jian W, Parra D, Chan KS. Stat3 activation in urothelial stem cells leads to direct progression to invasive bladder cancer. Cancer research. 2012;72:3135–3142. doi: 10.1158/0008-5472.CAN-11-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Said N, Frierson HF, Sanchez-Carbayo M, Brekken RA, Theodorescu D. Loss of SPARC in bladder cancer enhances carcinogenesis and progression. The Journal of clinical investigation. 2013;123:751–766. doi: 10.1172/JCI64782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nishizawa K, et al. Thioredoxin-interacting protein suppresses bladder carcinogenesis. Carcinogenesis. 2011;32:1459–1466. doi: 10.1093/carcin/bgr137. [DOI] [PubMed] [Google Scholar]
- 158.Hursting S, et al. Urothelial Overexpression of Insulin-Like Growth Factor-1 Increases Susceptibility to p-Cresidine-Induced Bladder Carcinogenesis in Transgenic Mice. Molecular Carcinogenesis. 2009;48:671–677. doi: 10.1002/mc.20548. [DOI] [PubMed] [Google Scholar]
- 159.Santos M, et al. In vivo disruption of an Rb-E2F-Ezh2 signaling loop causes bladder cancer. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-14-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mo L, Cheng J, Lee EY, Sun TT, Wu XR. Gene deletion in urothelium by specific expression of Cre recombinase. American journal of physiology. Renal physiology. 2005;289:F562–568. doi: 10.1152/ajprenal.00368.2004. [DOI] [PubMed] [Google Scholar]
- 161.Shen TH, et al. A BAC-based transgenic mouse specifically expresses an inducible Cre in the urothelium. PloS one. 2012;7:e35243. doi: 10.1371/journal.pone.0035243. [DOI] [PMC free article] [PubMed] [Google Scholar]