Abstract
Background
Rather than the absolute dose of diuretic or urine output, the primary signal of interest when evaluating diuretic responsiveness is the efficiency with which the kidneys can produce urine after a given dose of diuretic. As a result, we hypothesized that a metric of diuretic efficiency (DE) would capture distinct prognostic information beyond that of raw fluid output or diuretic dose.
Methods and Results
We independently analyzed two cohorts: 1) consecutive admissions at the University of Pennsylvania (Penn) with a primary discharge diagnosis of HF (n=657) and 2) patients in the ESCAPE dataset (n=390). DE was estimated as the net fluid output produced per 40 mg of furosemide equivalents, then dichotomized into high vs. low DE based on the median value. There was only a moderate correlation between DE and both the IV diuretic dose and net fluid output (r2 ≤ 0.26 for all comparisons), indicating that the diuretic efficiency was describing unique information. With the exception of metrics of renal function and pre-admission diuretic therapy, traditional baseline characteristics including right heart catheterization variables were not consistently associated with DE. Low DE was associated with worsened survival even after adjusting for in-hospital diuretic dose, fluid output, in addition to baseline characteristics (Penn HR=1.36, 95% CI 1.04–1.78, p=0.02; ESCAPE HR= 2.86, 95% CI 1.53–5.36, p=0.001.
Conclusions
Although in need of validation in less selected populations, low diuretic efficiency during decongestive therapy portends poorer long-term outcomes above and beyond traditional prognostic factors in patients hospitalized with decompensated heart failure.
Keywords: diuretic efficiency, diuretic resistance, diuretics, acute heart failure
Acute decompensated heart failure (ADHF) is predominantly a disease of fluid overload.1, 2 As such, the primary therapeutic objective of most ADHF hospitalizations is fluid removal, with the mainstay of therapy being intravenous loop diuretics.1, 3 It has been suggested that resistance to loop diuretics is an adverse prognostic indicator, and several authors have described a steep dose-response relationship between the amount of loop diuretic administered and adverse outcomes.3–5 However, the dose of loop diuretic prescribed captures much more than simply the amount of diuretic resistance since dose selection is influenced by factors such as perceived disease severity, degree of congestion, and the physician’s individual practices regarding diuretic dosing. In fact, some studies have actually found a lack of survival disadvantage or even a survival benefit associated with higher loop diuretic doses after accounting for these potential confounding factors; illustrating that diuretic dose is not an ideal surrogate for diuretic resistance.6–9
Fundamental to the assessment of diuretic responsiveness is determining how well the diuretic can actually facilitate augmentation of urine production. As such, the amount of urine produced is really only valid in context of the dose of diuretic given. For example, the loop diuretic dose in a patient who has a goal fluid loss of 500 mL will often be significantly less than a patient who has a goal fluid loss of >3L. However, if both patients required 200 mg of intravenous furosemide to reach their goal, the patient that produced only 500 mL of urine would have much greater diuretic resistance than the patient that produced 3L despite the identical diuretic dose. The reciprocal analogy can be drawn with patients producing similar fluid output with different doses of diuretic. As a result, the primary signal of interest in diuretic responsiveness is really the efficiency with which the diuretic can facilitate urine production, not the absolute dose of diuretic or the absolute production of urine. As such, we hypothesized that a metric of diuretic efficiency, defined as the net fluid lost per mg of loop diuretic during an ADHF hospitalization, would capture distinct prognostic and potentially mechanistic information from that of raw fluid output or diuretic dose. Accordingly, we sought to investigate the association between diuretic efficiency and clinical variables and outcomes in two independent cohorts of ADHF.
Methods
Given that decongestion strategies vary substantially across institutions and by the composition of the patient population, two distinct cohorts of ADHF patients were analyzed separately. The first represents a single center retrospective cohort of consecutively admitted patients to the Hospital of the University of Pennsylvania (Penn Cohort) with which detailed information about diuretic administration was collected. The concept was subsequently validated in a second independent cohort, the prospective multicenter Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial. Details of both cohorts are as follows:
Penn Cohort
We reviewed the charts of all patients with a primary discharge diagnosis of congestive heart failure who had been admitted to non-interventional cardiology and internal medicine services at the Hospital of the University of Pennsylvania within the years of 2004 to 2009. Inclusion required a B-type natriuretic peptide (BNP) level of > 100 pg/mL within 24 hours of admission, receipt of intravenous loop diuretics, and availability of data on fluid intake and output during the hospitalization. In order to focus primarily on the physiology and timing of decongestion, patients with a length of stay ≤ 2 days (who likely underwent limited decongestion) and patients with length of stay > 14 days (who likely had either atypical degrees of congestion or non-diuresis-related problems driving the length of stay) were excluded from the cohort. Patients receiving renal replacement therapy were also excluded. In the event of multiple hospitalizations for a single patient, only the first admission meeting the above inclusion criteria was retained. Please see Supplementary Figure 1A for additional details on patient selection. All-cause mortality was determined via the Social Security Death Index and status was ascertained 2.5 years after discharge of the last patient in the dataset.10 The median time from discharge to ascertainment of all-cause mortality was 5.1 years (interquartile range 3.7–6.3 years).
ESCAPE Cohort
The ESCAPE Trial was a National Heart, Lung and Blood Institute sponsored, randomized, multicenter trial of therapy guided by pulmonary artery catheter vs. clinical assessment in hospitalized patients with ADHF. Methods and results have been published previously.11, 12 Briefly, 433 patients were enrolled at 26 sites from January 2000 to November 2003. Inclusion criteria included an ejection fraction of 30% or less, systolic blood pressure of 125 mmHg or less, hospitalization for HF within the preceding year, treatment during the preceding month with more than 160 mg of furosemide equivalents daily, and at least 1 sign and 1 symptom of congestion. Exclusion criteria included an admission creatinine level >3.5 mg/dL. Patients were randomized to therapy guided by clinical assessment alone vs. pulmonary artery catheter and clinical assessment. Treatment goals were resolution of the signs and symptoms of congestion and investigators were encouraged to “avoid progressive renal dysfunction or symptomatic systemic hypotension.” Patients in the ESCAPE population that did not have data available to calculate net urine output (n=19) and patients that did not have data available on peak loop diuretic dose (n=24) were not included in the current analysis. All-cause mortality was determined 180 days after randomization.
The relative diuretic efficiency in each patient was determined as the fluid output per mg of loop diuretic received (expressed as mL of net fluid output per 40 mg of furosemide equivalents). Forty milligrams of furosemide equivalents was chosen as a reference since this is a dose reported to produce near maximal rate of instantaneous natriuresis in a healthy volunteer naive to diuretics.13 For the Penn cohort, where detailed information on diuretic administration was available, diuretic efficiency was calculated using the cumulative in-hospital net fluid output divided by the cumulative in-hospital amount of intravenous (IV) loop diuretic received (Cumulative diuretic efficiency). For the ESCAPE cohort, only maximum loop diuretic dose received in a 24 hour period was available, thus diuretic efficiency was calculated using the average daily fluid output divided by the peak IV loop diuretic (Peak diuretic efficiency). Given the desire to compare effect sizes across variables and between cohorts, the median values for diuretic efficiency [Penn cohort median 480 (interquartile range 195–1024) mL net fluid output/40 mg furosemide equivalents; ESCAPE cohort median 148 (interquartile range 61–283) mL net fluid output/40 mg furosemide equivalents] was primarily employed. To allow direct comparison between the cohorts, the primary analyses were repeated using Peak diuretic efficiency in the Penn cohort calculated using the median from the ESCAPE cohort. Estimated glomerular filtration rate (eGFR) was calculated using the four variable Modified Diet and Renal Disease equation.14 Worsening renal function (WRF) was defined as a ≥ 20% decrease in eGFR at any time during the hospitalization, unless otherwise specified.15–20 Loop diuretic doses were converted to furosemide equivalents with 1 mg bumetanide = 20 mg torsemide = 80 mg furosemide for oral diuretics, and 1 mg bumetanide = 20 mg torsemide = 40 mg furosemide for intravenous diuretics.21, 22 The study was approved or determined to qualify as exempt from Institutional Review Board review by the Hospital of the University of Pennsylvania and Yale University Institutional Review Boards.
Statistical Analysis
Values reported are mean ± SD, median (quartile 1 - quartile 4) and percentile. Independent Student’s t test or the Wilcoxon Rank Sum test was used to compare continuous variables between two groups of patients. The chi-square test was used to evaluate associations between categorical variables. Correlation coefficients reported are Spearman’s rho and are reported as r2 values. The independent association between renal variables associated with diuretic efficiency was determined using logistic regression. Proportional hazards modeling was used to evaluate time-to-event associations with all-cause mortality. Candidate covariates entered in the model were baseline characteristics with less than 10% missing values and a univariate association with all-cause mortality at p ≤ 0.2. In the Penn cohort these variables consisted of age, race, diabetes, ischemic heart failure etiology, presence of edema, digoxin use, outpatient loop diuretic dose, thiazide diuretic use, heart rate, systolic blood pressure, B-type natriuretic peptide, serum sodium, hemoglobin, eGFR, and blood urea nitrogen. In the ESCAPE cohort these variables were age, hypertension, ischemic heart failure etiology, presence of edema, jugular venous distension, baseline beta blocker use, baseline angiotensin converting enzyme or receptor blocker use, pre-admission loop diuretic dose, thiazide diuretic use, systolic blood pressure, serum sodium, eGFR, blood urea nitrogen and hemoglobin. In-hospital or discharge variables with a theoretical basis for confounding were forced into subsequent models regardless of univariate association with mortality. Models were built using backward elimination (likelihood ratio test) where all covariates with a p<0.2 were retained.23 Survival curves were plotted for patients with the 4 combinations of diuretic efficiency above or below the median and diuretic dose above or below the median for both cohorts. The x axis was terminated when the number at risk was <10% and statistical significance was determined using the log rank test. Statistical analysis was performed with IBM SPSS Statistics version 20.0 (IBM Corp., Armonk, NY) and statistical significance was defined as 2-tailed p<0.05 for all analyses except for tests for interaction, where p<0.1 was considered significant.
Results
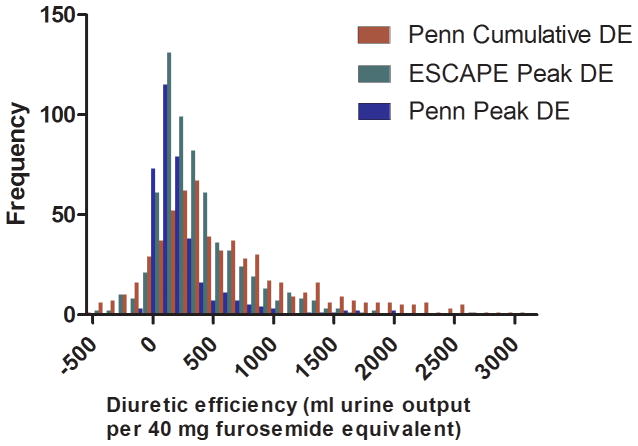
Baseline characteristics of the two cohorts are presented in Table 1. There was a broad range of diuretic efficiency represented in the cohorts (Figure 1). There was only a moderate correlation between diuretic efficiency and the IV diuretic dose (Penn r2=0.12, ESCAPE r2= 0.21) or net fluid output (Penn r2= 0.26, ESCAPE r2= 0.21, Supplementary Figure 2). The direct correlation between diuretic dose and net fluid output was also moderate (Penn r2=0.27, ESCAPE r2= 0.14). In line with the requirement for baseline high dose loop diuretic use for enrollment into the ESCAPE trial, loop diuretic doses were generally higher and diuretic efficiency was lower in the ESCAPE population (Table 1).
Table 1.
Patient characteristics of the Penn and ESCAPE cohorts grouped by diuretic efficiency
| Characteristic | Penn Cohort | ESCAPE Cohort | ||||
|---|---|---|---|---|---|---|
| Diuretic Efficiency | p-value | Diuretic Efficiency | value | |||
| Low (n=329) | High (n=328) | Low (n=195) | High (n=195) | |||
| Demographics | ||||||
| Age (y) | 64.3 ± 15.0 | 61.3 ± 15.8 | 0.014* | 56.8 ± 14.6 | 55.2 ± 13.0 | 0.26 |
| Black race | 65% | 64% | 0.78 | 36% | 46% | 0.040* |
| Male | 51% | 62% | 0.008* | 77% | 73% | 0.29 |
| Medical History | ||||||
| Hypertension | 75% | 71% | 0.16 | 48% | 45% | 0.54 |
| Diabetes | 49% | 36% | 0.002* | 34% | 34% | 0.94 |
| Ischemic etiology Ejection fraction | 26% | 26% | 0.91 | 54% | 47% | 0.16 |
| ≥40% | 36% | 31% | 0.19 | 0% | 0% | N/A |
| Admission Physical Exam | ||||||
| Heart rate (beats/min) | 88.6 ± 20.6 | 90.2 ± 19.4 | 0.31 | 81.5 ± 14.9 | 82.4 ± 15.0 | 0.54 |
| Systolic blood pressure (mmHg) | 135 ± 34 | 136 ± 33 | 0.82 | 104 ± 17 | 108 ± 16 | 0.005* |
| Jugular venous distention (≥12 cm water) | 61% | 61% | 0.87 | 53% | 53% | 0.96 |
| Edema > 1+ | 47% | 46% | 0.79 | 38% | 34% | 0.36 |
| Hepatojugular reflux | 21% | 24% | 0.36 | 78% | 82% | 0.24 |
| Cardiac Function | ||||||
| Ejection fraction (%) | 30 (15–45) | 23 (15–42) | 0.028* | 20 (15–21) | 20 (15–25) | 0.088 |
| Laboratory Values | ||||||
| Serum sodium (mEq/L) | 138 ± 5 | 139 ± 4 | 0.028* | 136 ± 5 | 137 ± 4 | 0.15 |
| B-type natriuretic peptide (pg/mL) | 1454 (748–2771) | 1285 (692–2312) | 0.049* | 593 (264–1285) | 557 (209–1142) | 0.421 |
| eGFR (mL/min/1.73m2) | 55.4 ± 29.3 | 61.9 ± 26.2 | 0.003* | 55.6 ± 27.4 | 59.1 ± 23.4 | 0.028* |
| BUN (mg/dL) | 33.7 ± 24.2 | 26.8 ± 20.5 | <0.001* | 38.6 ± 25.4 | 31.6 ± 18.9 | 0.002* |
| Hemoglobin (g/dL) | 11.8 ± 2.1 | 12.4 ± 2.1 | <0.001* | 12.9 ± 5.3 | 12.5 ± 1.8 | 0.41 |
| Right Heart Catheterization Variables † | ||||||
| Right atrial pressure (mmHg) | 13.4 ± 7.8 | 10.2 ± 5.8 | 0.010* | 14.1 ± 9.0 | 13.6 ± 10.4 | 0.69 |
| Pulmonary capillary wedge pressure (mmHg) | 23.8 ± 8.5 | 23.5 ± 8.3 | 0.85 | 26.0 ± 9.4 | 23.8 ± 8.8 | 0.19 |
| Cardiac index (L/min/m2) | 2.1 ± 0.7 | 2.1 ± 0.6 | 0.95 | 2.0 ± 0.6 | 2.0 ± 0.6 | 0.54 |
| Systemic vascular resistance (dyn·s/cm5) | 1538 ± 582 | 1622 ± 618 | 0.43 | 1428 ± 753 | 1474 ± 897 | 0.71 |
| Medications (Admission) | ||||||
| Beta blocker | 69% | 74% | 0.18 | 63% | 60% | 0.51 |
| ACE inhibitor or ARB | 60% | 68% | 0.025* | 90% | 90% | 0.87 |
| Digoxin | 24% | 28% | 0.25 | 73% | 71% | 0.65 |
| Aldosterone antagonist | 17% | 18% | 0.68 | 33% | 34% | 0.91 |
| Loop diuretic dose (mg) | 80 (20–160) | 40 (0–80) | <0.001* | 320) | 160 (80–320) | 0.003* |
| Thiazide diuretic | 15% | 10% | 0.046* | 13% | 13% | 0.98 |
| Medications (Discharge) | ||||||
| Beta blocker | 82% | 87% | 0.055 | 52% | 60% | 0.15 |
| ACE inhibitor or ARB | 70% | 87% | <0.001* | 83% | 88% | 0.14 |
| Digoxin | 25% | 26% | 0.77 | 72% | 80% | 0.097 |
| Aldosterone antagonist | 23% | 23% | 0.90 | 49% | 54% | 0.27 |
| Loop diuretic dose (mg) | 80 (40–160) | 80 (40–100) | <0.001* | 120 (60–240) | 100 (60–160) | 0.035* |
| Thiazide diuretic | 15% | 5% | <0.001* | 14% | 10% | 0.27 |
eGFR: Estimated glomerular filtration rate, BUN: Blood urea nitrogen, ACE: Angiotensin converting enzyme inhibitor, ARB: Angiotensin receptor blocker. Diuretic efficiency was calculated as the cumulative mL of net fluid output divided by the cumulative loop diuretic dose (per 40 mg of furosemide equivalent) during the hospitalization in the Penn cohort. In the ESCAPE cohort where cumulative diuretic dose was unavailable, diuretic efficiency was estimated using the peak dose of loop diuretic administered in 24 hours (per 40 mg furosemide equivalents). Diuretic efficiency was then dichotomized about the median to define high vs. low diuretic efficiency.
Significant p value.
Data available in n=139 patients in the Penn cohort and in n=192 patients in the ESCAPE cohort.
Figure 1.
Distribution of diuretic efficiency in the Penn and ESCAPE cohorts
Baseline and in-hospital factors associated with low diuretic efficiency
Characteristics of patients with diuretic efficiency above or below the median value (below the median hence forth referred to as “low diuretic efficiency”) are reported in Table 1. As expected from the nature of the dichotomization, the net urine output was significantly less and doses of loop diuretics greater in patients with low diuretic efficiency (Table 2). However, patients with low diuretic efficiency were not universally given high doses of loop diuretics (32.5% had diuretic doses below the median in the Penn cohort and 32.9% in the ESCAPE cohort). Both the baseline and discharge loop diuretic doses were greater in patients with low in-hospital diuretic efficiency (Table 1).
Table 2.
In hospital variables of the Penn and ESCAPE cohorts grouped by diuretic efficiency
| Characteristic | Penn Cohort | p-value | ESCAPE Cohort | p-value | ||
|---|---|---|---|---|---|---|
| Diuretic Efficiency | Diuretic Efficiency | |||||
| Low (n=329) | High (n=328) | Low (n=195) | High (n=195) | |||
| Diuresis Related Variables | ||||||
| Cumulative IV loop diuretic dose (mg) | 460 (200–950) | 200 (80–340) | <0.001* | -- | -- | -- |
| Average daily IV loop dose (mg/day) | 80 (40–138) | 35 (18–63) | <0.001* | -- | -- | -- |
| Peak IV loop diuretic dose in 24 hours (mg) | 160 (80–260) | 80 (40–124) | <0.001* | 480) | 160 (80–280) | <0.001* |
| Continuous diuretic infusion | 8% | 2% | 0.001* | -- | -- | -- |
| Adjuvant thiazide diuretic | 22% | 9% | <0.001* | 34% | 25% | 0.034* |
| Time receiving IV diuretic (% of hospitalization) | 69.6 ± 23.1 | 61.0 ± 25.7 | <0.001* | -- | -- | -- |
| Net fluid loss (L) | 2.7 (−0.04–5.9) | 5.7 (3.2–9.0) | <0.001* | 3.6 (1.0–6.9) | 5.6 (3.1–10.4) | <0.001* |
| Fluid intake (L) | 7.2 (4.7–11.8) | 6.6 (4.2–10.0) | 0.090 | -- | -- | -- |
| Fluid output (L) | 10.0 (5.9–16.2) | 12.5 (7.8–20.2) | <0.001* | -- | -- | -- |
| Average net daily fluid loss (L) | 0.5 (−0.1–0.9) | 1.0 (0.7–1.5) | <0.001* | 0.5 (0.2–0.9) | 1.4 (0.9–1.9) | <0.001* |
| Diuretic efficiency (mL fluid output/40 mg furosemide equivalents) | 198 (−8.9–347) | 1024 (698–1760) | N/A | -- | -- | -- |
| Estimated peak dose diuretic efficiency (mL fluid output/40 mg furosemide equivalents) | 92.3 (−3.4–174) | 148456 (296–724) | <0.001* | 60 (22–104) | 281 (194–552) | <0.001* |
| In-hospital inotropes | ||||||
| Milrinone | 14% | 16% | 0.69 | 20% | 14% | 0.13 |
| Dobutamine | 2% | 1% | 0.47 | 36% | 21% | 0.001* |
| In-Hospital Maximum Change in Laboratory Variables | ||||||
| eGFR (%) | −18.8 ± 15.8 | −13.0 ± 13.3 | <0.001* | -- | -- | -- |
| Worsening renal function | 42% | 28% | <0.001* | -- | -- | -- |
| Blood urea nitrogen (%) | 49.4 ± 55.6 | 33.5 ± 44.0 | <0.001* | -- | -- | -- |
| Bicarbonate (%) | 20.7 ± 17.2 | 23.2 ± 51.3 | 0.86 | -- | -- | -- |
| Admission to Discharge Change in Laboratory Variables | ||||||
| eGFR (%) | −1.7 ± 26.6 | 4.9 ± 25.2 | <0.001* | 0.2 ± 33.2 | 0.1 ± 25.8 | 0.97 |
| Worsening renal function | 22% | 11% | <0.001* | 24% | 20% | 0.29 |
| Blood urea nitrogen (%) | 29.9 ± 54.5 | 15.9 ± 48.1 | <0.001* | 22.3 ± 61.4 | 23.0 ± 55.3 | 0.91 |
| Bicarbonate (%) | 11.1 ± 17.7 | 14.1 ± 52.7 | 0.95 | -- | -- | -- |
| Sodium (%) | −0.6 ± 3.3 | −1.1 ± 4.8 | 0.43 | −1.1 ± 3.2 | −1.0 ± 2.8 | 0.69 |
| Hospital Course | . | |||||
| Length of stay (days) | 6 (4–9) | 5 (4–8) | 0.099 | 9 (6–14) | 6 (4–8) | <0.001* |
| Discharge Physical Examination | ||||||
| Jugular venous distention (≥8 cm H2O) | 19% | 21% | 0.563 | 39% | 29% | 0.035* |
| Edema > 1+ | 17% | 15% | 0.38 | 7% | 2% | 0.032* |
| Hepatojugular reflux | 4% | 4% | 0.97 | 22% | 16% | 0.11 |
| Rales | -- | -- | -- | 14% | 4% | <0.001* |
| Ascites | -- | -- | -- | 6% | 2% | 0.034* |
| Right Heart Catheterization Variables at Removal † | ||||||
| Right atrial pressure (mmHg) | -- | -- | -- | 11.5 ± 8.5 | 7.4 ± 4.1 | <0.001* |
| Pulmonary capillary wedge pressure (mmHg) | -- | -- | -- | 18.7 ± 7.6 | 15.5 ± 7.0 | 0.027* |
| Cardiac index (L/min/m2) | -- | -- | -- | 2.3 ± 0.7 | 2.4 ± 0.6 | 0.52 |
| Systemic vascular resistance (dyn·s/cm5) | -- | -- | -- | 1081 ± 489 | 1135 ± 446 | 0.48 |
eGFR: Estimated glomerular filtration rate.
Significant p value.
Data available in n=166.
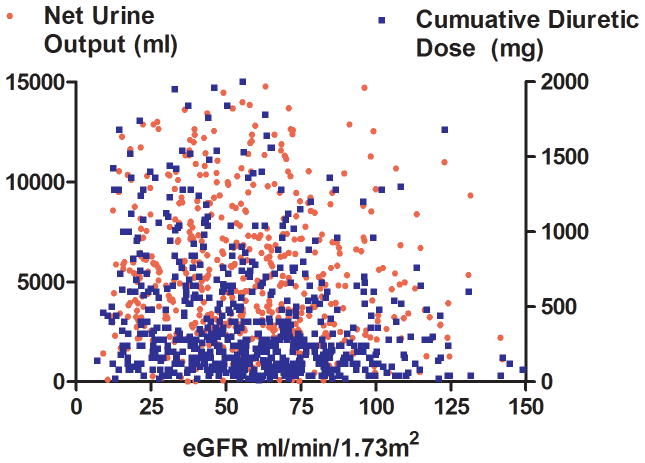
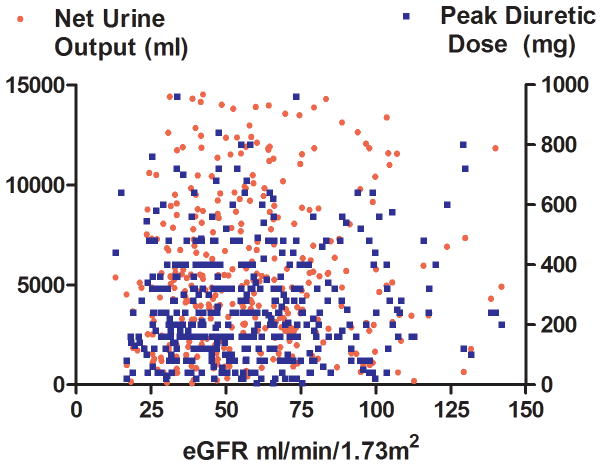
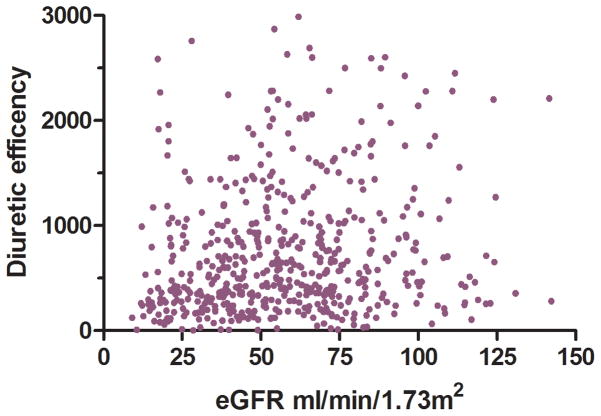
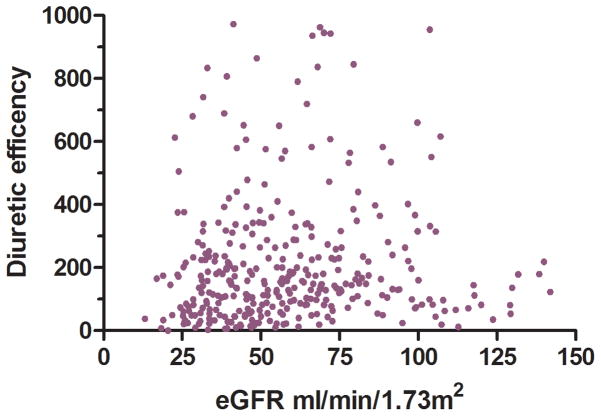
Non-diuretic baseline differences between patients with and without low diuretic efficiency, including right heart catheterization variables and physical examination findings, were small and generally not statistically significant across both cohorts (Table 1). The only non-diuretic baseline characteristics associated with diuretic efficiency in both ESCAPE and Penn cohorts were blood urea nitrogen and eGFR (Table 1). Interestingly, the correlation was small between eGFR and diuretic dose (Penn r2=0.05, p<0.001; ESCAPE r2=0.0, p=0.76), net fluid output (Penn r2=0.0, p=0.35; ESCAPE r2=0.03, p=0.002), and diuretic efficiency (Penn r2= 0.02, p<0.001; ESCAPE r2=0.04, p<0.001) (Figure 2). In a multivariable model incorporating both eGFR and baseline BUN, only BUN remained significantly associated with low diuretic efficiency (Penn cohort OR=1.14 per 10 increase in BUN, p=0.009; ESCAPE cohort OR=1.19 per 10 increase in BUN, p=0.005). In ESCAPE there was no difference in diuretic efficiency based on randomization to a pulmonary artery catheter (PAC) guided treatment strategy (p=0.72) or treatment with a pulmonary artery catheter (p=0.19). However, amongst patients randomized to care guided by clinical assessment alone, there was a higher rate of crossover to PAC use in patients with low diuretic efficiency (OR=3.5, p=0.014). The use of dobutamine and length of stay was greater with low diuretic efficiency in the ESCAPE cohort but not the Penn cohort (Table 2). Similar findings were noted in the Penn cohort when using the Peak diuretic efficiency definition from the ESCAE cohort (Supplementary Tables 1 and 2).
Figure 2.
Scatterplots of eGFR and net fluid output, diuretic dose (Panel A) and diuretic efficiency (Panel B) in the Penn Cohort (top panels) and ESCAPE cohort (bottom panels) eGFR: Estimated glomerular filtration rate. Diuretic efficiency expressed as mL of net fluid output per 40 mg of furosemide equivalent.
Diuretic strategy, relief of congestion, and worsening renal function
Overall, the in-hospital treatment approach/outcomes for patients with or without low diuretic efficiency appeared to differ somewhat between cohorts. In both cohorts, low diuretic efficiency resulted in escalation of diuretic strategies such as higher doses of loop diuretics, greater use of adjuvant thiazide diuretics, and initiation of a loop diuretic infusion (diuretic infusion usage available only in the Penn cohort). In patients with low diuretic efficiency the maximum 24 hour loop diuretic dose was 4.0 times the baseline dose in the Penn cohort whereas in the ESCAPE cohort there was a 2.6 fold increase in maximum furosemide equivalents over the pre-admission dose. In ESCAPE, low diuretic efficiency was associated with what appeared to be less complete decongestion. This was evidenced by higher right atrial and pulmonary capillary wedge pressure and discharge physical examination findings consistent with continued volume overload compared to patients with preserved diuretic efficiency (Table 2). Interestingly, despite discharge with persistent congestion, the rate of deterioration in renal function was no different between patients with or without low diuretic efficiency (Table 2). In the Penn cohort, significant differences in the degree of decongestion were not apparent as discharge physical examination findings were not different between groups (Table 2). However, the rate of worsening renal function was substantially greater in the low diuretic efficiency group (Table 2). Overall the above findings were also noted in the Penn cohort when using the Peak diuretic efficiency definition from the ESCAPE cohort (Supplementary Tables 1 and 2).
Diuretic efficiency and prognosis
In the Penn cohort, a total of 346 patients (52.7%) died over a median follow up of 3.2 years (1.2–5.2 years) and in the ESCAPE cohort, a total of 75 patients (19.2%) died over a median follow up of 179 days (129–180). Consistent with prior literature, a loop diuretic dose above the median was associated with significantly reduced survival in both Penn and ESCAPE cohorts (Table 3 and Supplementary Table 3). However, net fluid output below the median was not associated with survival in either cohort (Table 3). The same lack of association was found for the continuous net fluid output variables, even after accounting for length of stay (p ≥0.18 for both cohorts). Despite the lack of mortality information related to net fluid output, low diuretic efficiency was associated with substantially worsened survival (Table 3). In line with the limited correlation between net fluid output, IV loop diuretic dose, and diuretic efficiency, this mortality disadvantage with low diuretic efficiency appeared to be relatively independent from the absolute dose of diuretic or amount of fluid lost (Table 3). After adjustment for baseline characteristics, only diuretic efficiency remained significantly associated with mortality in both cohorts (Table 3). These findings were also noted in the Penn cohort when using the Peak diuretic efficiency definition from the ESCAPE cohort (Table 3). There was a trend toward a stronger association between low diuretic resistance and mortality in patients with reduced compared to preserved ejection fraction, however this did not reach statistical significance (Supplementary Table 4). The unadjusted risk for mortality in patients with low diuretic efficiency receiving doses of loop diuretics above or below the median is depicted in Figure 3A and 3B. The risk of death associated with low diuretic efficiency was also present in patients who did not receive high doses of IV loop diuretic (dose below the median) in both Penn (adjusted for baseline characteristics HR=1.85 95% CI 1.28–2.27, p=0.001, p interaction=0.053, Figure 3A) and ESCAPE cohorts (adjusted for baseline characteristics HR=4.08, 95% CI 1.70–9.82, p=0.002, p interaction=0.53, Figure 3B). In both cohorts, patients that received low doses of loop diuretic and had preserved diuretic efficiency had substantially better survival than the remainder of the groups (Figure 3). After extensive adjustment for baseline characteristics in addition to in-hospital and discharge related variables (length of stay, use of milrinone, dobutamine, adjuvant thiazide use, worsening renal function, hemoconcentration, discharge physical examination findings and discharge medications) the association between diuretic efficiency and mortality remained in both the Penn cohort (HR=1.46, 95% CI 1.15–1.86, p=0.002) and the ESCAPE cohort (HR 3.57, 95% CI 1.46–8.73, p=0.005)
Table 3.
Risk of death associated with loop diuretic dose, net fluid output, and diuretic efficiency in the Penn and ESCAPE cohorts
| Univariable | Multivariable | Multivariable with Adjustment for Baseline Characteristics | |||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| Penn Cohort (Cumulative diuretic efficiency) | Loop diuretic† | 1.56 (1.26–1.93) | <0.001* | 1.36 (1.05–1.76) | 0.021* | 1.24 (0.94–1.64) | 0.13 |
| Net fluid output† | 1.01 (0.82–1.25) | 0.93 | 1.04 (0.80–1.34) | 0.78 | 0.96 (0.74–1.26) | 0.78 | |
| Diuretic efficiency† | 1.64 (1.32–2.03) | <0.001* | 1.51 (1.17–1.95) | 0.002* | 1.36 (1.04–1.78) | 0.023* | |
| Penn Cohort (Peak diuretic efficiency) | Loop diuretic† | 1.56 (1.26–1.93) | <0.001* | 1.41 (1.11–1.79) | 0.005* | 1.25 (0.97–1.62) | 0.073 |
| Net fluid output† | 1.01 (0.82–1.25) | 0.93 | 1.08 (0.84–1.39) | 0.54 | 0.96 (0.74–1.25) | 0.78 | |
| Diuretic efficiency† | 1.70 (1.38–2.11) | <0.001* | 1.67 (1.31–2.18) | <0.001* | 1.39 (1.08–1.77) | 0.007* | |
| ESCAPE Cohort (Peak diuretic efficiency) | Loop diuretic† | 2.46 (1.54–3.94) | <0.001* | 1.68 (0.98–2.88) | 0.059 | 1.32 (0.72–2.40) | 0.37 |
| Net fluid output† | 0.96 (0.61–1.51) | 0.86 | 0.91 (0.54–1.51) | 0.71 | 1.17 (0.66–2.09) | 0.59 | |
| Diuretic efficiency† | 3.98 (2.32–6.84) | <0.001* | 3.47 (1.97–6.24) | <0.001* | 2.86 (1.53–5.36) | 0.001* | |
HR: Hazard ratio, CI: Confidence interval.
To facilitate comparison of effect sizes, hazard ratios represent the risk for all-cause mortality associated with a value above or below the median with the HR reflecting an exposure of diuretic dose above the median, efficiency below the median or fluid output below the median.
In the Penn cohort, variables entered in the multivariable model consisted of: age, race, diabetes, ischemic heart failure etiology, presence of edema, digoxin use, outpatient loop diuretic dose, thiazide diuretic use, heart rate, B-type natriuretic peptide, systolic blood pressure, serum sodium, hemoglobin, eGFR, and blood urea nitrogen. In the ESCAPE cohort these variables were: age, hypertension, ischemic heart failure etiology, presence of edema, jugular venous distension, baseline beta blocker use, baseline angiotensin converting enzyme or receptor blocker use, outpatient loop diuretic dose, thiazide diuretic use, systolic blood pressure, serum sodium, eGFR, blood urea nitrogen and hemoglobin.
Figure 3.
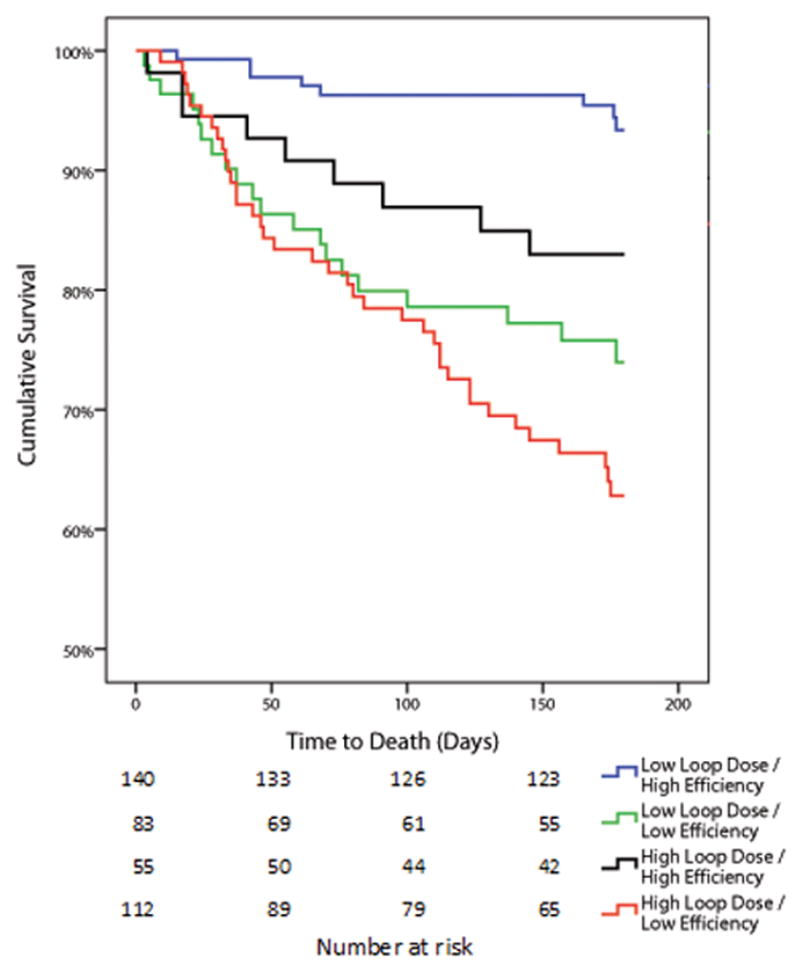
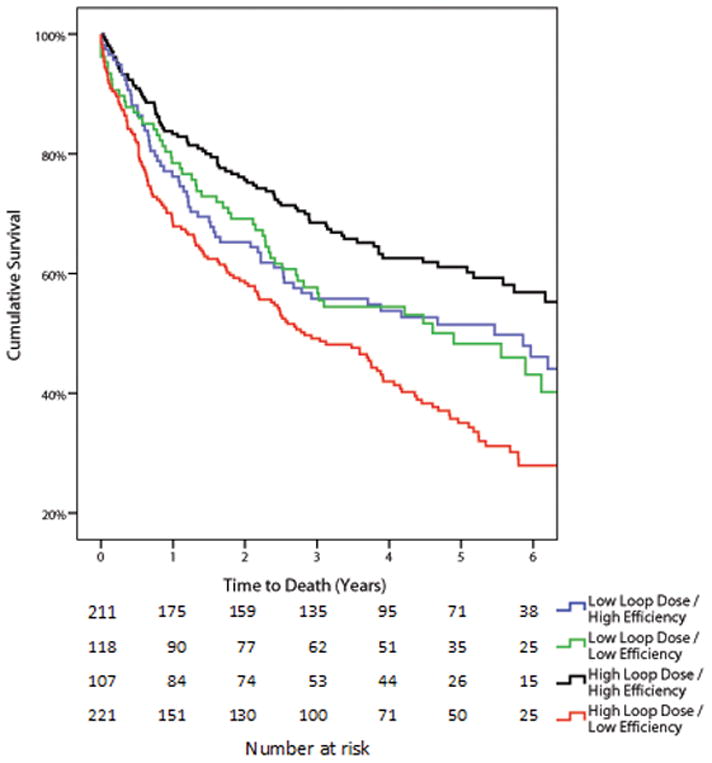
Kaplan-Meier survival curves grouped by diuretic efficiency and diuretic dose in the Penn cohort (Panel A) and ESCAPE cohort (Panel B) Loop diuretic dose and diuretic efficiency were dichotomized into high and low by the median value in each cohort. “Low loop dose” was also defined as a loop diuretic dose above or below the median value which was 280 mg (120–600) in the Penn cohort and 240 mg in 24 hours (120–400) in the ESCAPE cohort.
Discussion
In the current analysis we found that in the setting of ADHF the efficiency with which loop diuretics induce diuresis is strongly and independently associated with survival. Notably, diuretic efficiency was only modestly correlated with diuretic dose and net urine output, but provided distinct prognostic information to either parameter. Furthermore, despite the strong association between diuretic efficiency and mortality, baseline disease severity indicators such as right heart catheterization variables, vital signs, and physical examination findings were remarkably similar between groups. Although the simple metric of diuretic efficiency presented in this analysis is not without shortcomings, it does provide an easily calculated metric that strongly associates with mortality and may have advantage over diuretic dose or fluid output in describing diuretic resistance.
The previously reported association between high doses of loop diuretics and mortality, in conjunction with known direct adverse cardio-renal effects of loop diuretics, raised the possibility that high dose diuretics were directly causing adverse outcomes.24–26 However, a confounding factor is that sicker patients generally receive higher doses of diuretics. Supporting this notion, previous studies have reported that a wide range of disease severity indicators are generally worse in patients receiving high doses of diuretics.4, 6, 7, 27, 28 Furthermore, after extensive multivariable adjustment or propensity matching, several investigators found no association or even a survival advantage associated with the use of high doses of loop diuretics.6–9 Although not formally testing high vs. low absolute doses, the Diuretic Optimization Strategies Evaluation (DOSE) trial randomized patients to high vs. low intensification of their home diuretics (although the low intensification group still received >120 mg/day of IV furosemide) and found no difference in outcomes between groups.29 Moreover, in the recent Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial, patients treated with aggressive dosing of diuretics had similar outcomes to those treated primarily with ultrafiltration.30 In the current analysis we found that: 1) diuretic dose did not retain independent prognostic information in fully adjusted multivariable models incorporating both loop diuretic dose and diuretic efficiency and 2) low diuretic efficiency had an equal, if not worse, prognosis in patients receiving lower doses of loop diuretics. These data add to a growing literature arguing that the bulk of the association between in-hospital high dose loop diuretics and mortality is unlikely to be cause and effect.
Although the long term mortality associated with diuretic efficiency was consistent across cohorts, the short-term renal and decongestive outcomes were not. In the Penn cohort, there was a strong signal for worsening in renal function in patients with low diuretic efficiency, but a limited signal for differences in the degree of decongestion achieved as suggested by similar discharge physical examination findings. In ESCAPE, the reciprocal was found with no differences in renal outcomes but substantial persistent volume overload by right heart catheterization variables and multiple physical examination findings. Interestingly, the relative augmentation in diuretic dose over the baseline dose was larger in the Penn cohort (4 fold) than the ESCAPE cohort (2.6 fold). A common clinical dilemma when diuresing a diuretic resistant patient is that additional decongestion often comes at the expense of deterioration in renal function. Notably, the ESCAPE trial protocol specifically called for therapy to be adjusted to achieve stable or improving renal function by discharge. Although interesting observations, the clinical profile of patients enrolled in ESCAPE was substantially different from Penn and the fidelity of the available data is not sufficient to allow speculation that differences in treatment decisions caused the discordant renal and decongestive outcomes between cohorts. However, the discordant findings between the Penn and ESCAPE cohorts, with respect to degree of discharge congestion, does indicate that low diuretic efficiency may not always translate into an inevitable inability to decongest a patient.
It is widely known that renal function is an important determinate of diuretic responsiveness. Thus, it was somewhat surprising that a strong relationship between eGFR and diuretic dose, net fluid output, or diuretic efficiency was not identified. However, multiple facets of renal physiology contribute to diuretic resistance, only one of which is captured by GFR. In the setting of a severely depressed GFR, it has been well described that diuretic responsiveness is impaired.13 However, this relationship is primarily pharmacokinetic and the result of reduced tubular drug delivery rather than true resistance since the relationship between tubular diuretic concentrations and natriuresis is unchanged in chronic kidney disease.13, 31 Primarily a result of the fact that loop diuretics are avidly bound to albumin, glomerular filtration is actually a minor pathway in which loop diuretics are delivered to their tubular sight of action.13 Rather, active secretion by the proximal tubular cells, a process which is dependent on renal blood flow, is primarily responsible for diuretic delivery to the site of action. However, in the setting of heart failure the normal relationship between GFR and renal blood flow can be uncoupled by a substantial increase in filtration fraction, weakening the pharmacokinetic relationship between GFR and drug delivery in heart failure. Perhaps more importantly, it is thought that the primary source of variability in response to a loop diuretic in patients with heart failure is actually pharmacodynamics.31 Notably, multiple processes which are distinct from GFR or drug delivery such as diuretic breaking, distal tubular structural remodeling, and renal neurohormonal activation can all cause increased tubular sodium reabsorption even in the setting of normal diuretic delivery.13 Although diuretic efficiency appeared to correlate slightly better than diuretic dose or net fluid output, the relationship remained weak. Notably, a very low eGFR did not exclude the possibility of preserved diuretic efficiency nor did a normal eGFR exclude the possibility of poor diuretic efficiency. Although there are clearly substantial limitations in the assessment of renal function estimated with creatinine based metrics in the setting of acute heart failure, but these results provide further support that renal function is not the dominant factor driving diuretic efficiency in heart failure. Rather, it is the complex interplay between cardiac function, renal function, and volume status that ultimately determines diuretic responsiveness.
Limitations
Given the post hoc nature of this study, the limitations of retrospective analyses apply, residual confounding cannot be excluded, and causality is impossible to determine. Although the definition of diuretic efficiency used in the current analyses provides substantially more physiologic information than use of diuretic dose or net fluid output alone, it is not a perfect measure of diuretic resistance. The sigmoidal shape of the dose response relationship of loop diuretics with both threshold and plateau natriuretic portion limits any linear parameter of diuretic resistance unless it is assured that all patients are on the same portion of the dose response curve. Given that only 33 patients in the Penn cohort and 17 patients in the ESCAPE cohort received peak loop diuretic doses less than 40 mg (a dose which produces maximal instantaneous natriuresis in a healthy volunteer) suggests that, in the majority of patients, only through reduced diuretic efficiency was the renal threshold not met. However, patients that received diuretic doses that put them on the plateau portion of the dose response curve likely had an underestimation of their diuretic efficiency. Additionally, patients that received an adjuvant thiazide diuretic likely had a significant alteration in their loop diuretic efficiency. However, these changes in loop diuretic efficiency induced by non-loop diuretics is not accounted for in these analyses potentially biasing the results. Furthermore, diuretic resistance is really only relevant in patients with volume overload and the fidelity with which volume overload can be defined on a population level is limited. As such, it is probable that some patients may have had low diuretic efficiency in response to a dose of diuretic which was in fact appropriate given that they were not volume overloaded (i.e., a patient admitted primarily due to fluid redistribution rather than total body overload). However, the majority of these limitations would be expected to influence associations with loop diuretic dose or raw fluid output as a metric of diuretic resistance more so than diuretic efficiency. The use of creatinine based metrics for assessment of renal function in the likely non-steady state scenario of acute decompensated heart failure is a significant limitation and thus descriptions of static and dynamic associations with renal function should be considered hypothesis generating. Although validating the diuretic resistance concept in the multicenter ESCAPE population adds value, the ESCAPE trial was not designed to study diuretic efficiency, the trial required high doses of baseline loop diuretic for inclusion, and cumulative loop diuretic exposure was not collected requiring the use of 24 hour peak diuretic dose to estimate diuretic efficiency. Furthermore, both the ESCAPE and Penn cohorts employed relatively selective inclusion criteria. As a result, the reported observations may not apply to less selected populations and thus are in need of validation. Additionally, patients with a length of stay of one or two days were excluded from the Penn cohort. This is potentially an important source of bias as these patients may have responded particularly briskly to diuretics permitting early discharge. These factors significantly limit the certainty of generalizability of our findings and as a result the observations reported in this manuscript should be interpreted as hypothesis generating and require validation in unselected cohorts.
Conclusion
Low diuretic efficiency during decongestive therapy portends poorer long-term outcomes above and beyond traditional prognostic factors in patients hospitalized with decompensated heart failure. In light of the central role for loop diuretics in the management of volume overload in HF, additional research is required to validate these findings in less selected populations, to develop more precise metrics of diuretic efficiency, and to determine if therapeutic strategies which improve diuretic efficiency can positively impact outcomes.
Supplementary Material
Acknowledgments
This manuscript was prepared using ESCAPE research materials obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the ESCAPE study investigators or the NHLBI.
Sources of Funding
NIH Grants, 1K23HL114868, L30HL115790 (JT) and K24DK090203 (CP): The funding source had no role in study design, data collection, analysis or interpretation.
References
- 1.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP. Characteristics and outcomes of patients hospitalized for heart failure in the united states: Rationale, design, and preliminary observations from the first 100,000 cases in the acute decompensated heart failure national registry (adhere) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: An essential target of evaluation and treatment. The American Journal of Medicine. 2006;119:S3–S10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Felker GM, Mentz RJ. Diuretics and ultrafiltration in acute decompensated heart failure. J Am Coll Cardiol. 2012;59:2145–2153. doi: 10.1016/j.jacc.2011.10.910. [DOI] [PubMed] [Google Scholar]
- 4.Neuberg GW, Miller AB, O'Connor CM, Belkin RN, Carson PE, Cropp AB, Frid DJ, Nye RG, Pressler ML, Wertheimer JH, Packer M. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144:31–38. doi: 10.1067/mhj.2002.123144. [DOI] [PubMed] [Google Scholar]
- 5.Eshaghian S, Horwich TB, Fonarow GC. Relation of loop diuretic dose to mortality in advanced heart failure. The American Journal of Cardiology. 2006;97:1759–1764. doi: 10.1016/j.amjcard.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 6.Yilmaz MB, Gayat E, Salem R, Lassus J, Nikolaou M, Laribi S, Parissis J, Follath F, Peacock WF, Mebazaa A. Impact of diuretic dosing on mortality in acute heart failure using a propensity-matched analysis. European journal of heart failure. 2011;13:1244–1252. doi: 10.1093/eurjhf/hfr121. [DOI] [PubMed] [Google Scholar]
- 7.Mielniczuk LM, Tsang SW, Desai AS, Nohria A, Lewis EF, Fang JC, Baughman KL, Stevenson LW, Givertz MM. The association between high-dose diuretics and clinical stability in ambulatory chronic heart failure patients. J Card Fail. 2008;14:388–393. doi: 10.1016/j.cardfail.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 8.Testani JM, Cappola TP, Brensinger CM, Shannon RP, Kimmel SE. Interaction between loop diuretic-associated mortality and blood urea nitrogen concentration in chronic heart failure. J Am Coll Cardiol. 2011;58:375–382. doi: 10.1016/j.jacc.2011.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez J, Nunez E, Minana G, Bodi V, Fonarow GC, Bertomeu-Gonzalez V, Palau P, Merlos P, Ventura S, Chorro FJ, Llacer P, Sanchis J. Differential mortality association of loop diuretic dosage according to blood urea nitrogen and carbohydrate antigen 125 following a hospitalization for acute heart failure. European journal of heart failure. 2012;14:974–984. doi: 10.1093/eurjhf/hfs090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinn J, Kramer N, McDermott D. Validation of the social security death index (ssdi): An important readily-available outcomes database for researchers. West J Emerg Med. 2008;9:6–8. [PMC free article] [PubMed] [Google Scholar]
- 11.Shah MR, O'Connor CM, Sopko G, Hasselblad V, Califf RM, Stevenson LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness (escape): Design and rationale. Am Heart J. 2001;141:528–535. doi: 10.1067/mhj.2001.113995. [DOI] [PubMed] [Google Scholar]
- 12.Binanay C, Califf RM, Hasselblad V, O'Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW, Investigators E, Coordinators ES. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: The escape trial. JAMA : the journal of the American Medical Association. 2005;294:1625–1633. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 13.Brenner BM, Rector FC. Brenner & rector's the kidney. Philadelphia: Saunders Elsevier; 2008. [Google Scholar]
- 14.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology C. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 15.Testani JM, McCauley BD, Kimmel SE, Shannon RP. Characteristics of patients with improvement or worsening in renal function during treatment of acute decompensated heart failure. Am J Cardiol. 2010;106:1763–1769. doi: 10.1016/j.amjcard.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Testani JM, McCauley BD, Chen J, Shumski M, Shannon RP. Worsening renal function defined as an absolute increase in serum creatinine is a biased metric for the study of cardio-renal interactions. Cardiology. 2010;116:206–212. doi: 10.1159/000316038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testani JM, Kimmel SE, Dries DL, Coca SG. Prognostic importance of early worsening renal function after initiation of angiotensin-converting enzyme inhibitor therapy in patients with cardiac dysfunction. Circulation Heart failure. 2011;4:685–691. doi: 10.1161/CIRCHEARTFAILURE.111.963256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Testani JM, Coca SG, McCauley BD, Shannon RP, Kimmel SE. Impact of changes in blood pressure during the treatment of acute decompensated heart failure on renal and clinical outcomes. European journal of heart failure. 2011;13:877–884. doi: 10.1093/eurjhf/hfr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Testani JM, Chen J, McCauley BD, Kimmel SE, Shannon RP. Potential effects of aggressive decongestion during the treatment of decompensated heart failure on renal function and survival. Circulation. 2010;122:265–272. doi: 10.1161/CIRCULATIONAHA.109.933275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Testani JM, Cappola TP, McCauley BD, Chen J, Shen J, Shannon RP, Kimmel SE. Impact of worsening renal function during the treatment of decompensated heart failure on changes in renal function during subsequent hospitalization. Am Heart J. 2011;161:944–949. doi: 10.1016/j.ahj.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brater DC, Day B, Burdette A, Anderson S. Bumetanide and furosemide in heart failure. Kidney Int. 1984;26:183–189. doi: 10.1038/ki.1984.153. [DOI] [PubMed] [Google Scholar]
- 22.Vargo DL, Kramer WG, Black PK, Smith WB, Serpas T, Brater DC. Bioavailability, pharmacokinetics, and pharmacodynamics of torsemide and furosemide in patients with congestive heart failure. Clin Pharmacol Ther. 1995;57:601–609. doi: 10.1016/0009-9236(95)90222-8. [DOI] [PubMed] [Google Scholar]
- 23.Mickey RM, Greenland S. The impact of confounder selection criteria on effect estimation. Am J Epidemiol. 1989;129:125–137. doi: 10.1093/oxfordjournals.aje.a115101. [DOI] [PubMed] [Google Scholar]
- 24.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (solvd) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 25.Francis GS, Siegel RM, Goldsmith SR, Olivari MT, Levine TB, Cohn JN. Acute vasoconstrictor response to intravenous furosemide in patients with chronic congestive heart failure. Activation of the neurohumoral axis. Ann Intern Med. 1985;103:1–6. doi: 10.7326/0003-4819-103-1-1. [DOI] [PubMed] [Google Scholar]
- 26.McCurley JM, Hanlon SU, Wei SK, Wedam EF, Michalski M, Haigney MC. Furosemide and the progression of left ventricular dysfunction in experimental heart failure. J Am Coll Cardiol. 2004;44:1301–1307. doi: 10.1016/j.jacc.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 27.Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, Emerman CL, Committee ASA, Investigators Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: Insights from the adhere registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 28.Hasselblad V, Gattis Stough W, Shah MR, Lokhnygina Y, O'Connor CM, Califf RM, Adams KF., Jr Relation between dose of loop diuretics and outcomes in a heart failure population: Results of the escape trial. European journal of heart failure : journal of the Working Group on Heart Failure of the European Society of Cardiology. 2007;9:1064–1069. doi: 10.1016/j.ejheart.2007.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, LeWinter MM, Deswal A, Rouleau JL, Ofili EO, Anstrom KJ, Hernandez AF, McNulty SE, Velazquez EJ, Kfoury AG, Chen HH, Givertz MM, Semigran MJ, Bart BA, Mascette AM, Braunwald E, O'Connor CM. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med. 2011;364:797–805. doi: 10.1056/NEJMoa1005419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bart BA, Goldsmith SR, Lee KL, Givertz MM, O'Connor CM, Bull DA, Redfield MM, Deswal A, Rouleau JL, LeWinter MM, Ofili EO, Stevenson LW, Semigran MJ, Felker GM, Chen HH, Hernandez AF, Anstrom KJ, McNulty SE, Velazquez EJ, Ibarra JC, Mascette AM, Braunwald E. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med. 2012;367:2296–2304. doi: 10.1056/NEJMoa1210357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brater DC, Seiwell R, Anderson S, Burdette A, Dehmer GJ, Chennavasin P. Absorption and disposition of furosemide in congestive heart failure. Kidney Int. 1982;22:171–176. doi: 10.1038/ki.1982.149. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.