Abstract
Up to 50% of patients with chronic rhinosinusitis (CRS) have comorbid asthma, and we have reported that a subset of CRS patients who have nasal polyps (CRSwNP) have elevated autoantigen-specific antibodies within their nasal polyps (NP). While increases in the prevalence and/or severity of both asthma and autoimmunity in women are well characterized, it is not known whether CRSwNP is more severe or frequent in women than men. We sought to determine whether CRSwNP demonstrated sex-specific differences in frequency and/or severity. Using a retrospectively collected database of tertiary care patients (n = 1393), we evaluated the distribution of sex in patients with CRSwNP with or without comorbid asthma or aspirin hypersensitivity. We further compared the severity of sinus disease between men and women with CRSwNP. Although women comprised 55% of CRS patients without NP (CRSsNP), a significantly smaller proportion of CRSwNP patients were female (38%, P < 0.001). Interestingly, women with CRSwNP were significantly more likely than men to have comorbid asthma (P < 0.001), and 61% of patients with the most severe form of disease (aspirin-exacerbated respiratory disease (CRSwNP plus asthma plus aspirin sensitivity)) were women (P < 0.05). Women with CRSwNP were significantly more likely to have taken oral steroids, and were more likely to have a history of revision surgeries (P < 0.05) compared to men. These data suggest that women with CRSwNP have more severe disease than men in a tertiary care setting. Future studies are needed to elucidate the mechanisms that drive disease severity in men and women, paving the way for the development of personalized treatment strategies for CRSwNP based on sex.
Keywords: AERD, asthma, chronic inflammation, chronic rhinosinusitis, oral steroids
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the upper airways that affects up to 30 million people in the United States. It is associated with a significant impairment in quality of life and places a large financial burden on the health care system, with over $6 billion spent annually on clinical and surgical management 1–4. A specific subgroup of patients with CRS also has nasal polyps (CRSwNP), and up to 50% of this group has comorbid asthma 5. Despite the high prevalence of CRS, the mechanisms that underlie its pathogenesis and its association with asthma remain unclear.
Dysregulation of both the innate and adaptive immune responses has been hypothesized to promote the chronic inflammation observed in CRSwNP. We have previously demonstrated that B cell activating factor of the TNF family (BAFF), a key B cell survival factor from the TNF family, as well as B cell attracting chemokines, CXCL12 and CXCL13, are highly elevated in nasal polyp tissue from patients with CRSwNP 6,7. B lineage cells (B cells, plasmablasts, and plasma cells) and their antibody products, in particular autoantibodies, are also highly elevated in nasal polyps 5,8–11. Together, these data suggest that B cell responses may be critical components in CRSwNP pathogenesis, and additional studies are needed to further investigate how they impact disease.
B cell activation and antibody production can be induced by the female sex hormone, estrogen 12–14. Extensive studies in a murine model of systemic lupus erythematous (SLE), an autoimmune disease in which B cells play an important role, have shown this model for disease to be more strongly manifested in females, as is also found in human patients with SLE. It has been further demonstrated that female sex hormones, especially estrogen, are capable of driving this gender bias toward females 14–16. Additionally, human epidemiological studies have shown that several autoimmune diseases, including SLE, are more prevalent and/or severe in women 14. Although asthma is not viewed as an autoimmune disease, it is also more prevalent, and it can be more severe, in women 15–17. Despite the fact that CRSwNP has features of asthma and autoimmunity, along with elevations of B lineage cells and autoantibodies in nasal polyps, no studies have investigated whether CRSwNP affects females disproportionally. As a result, we sought to determine whether the frequency or severity of CRSwNP varied by sex in our study population.
Methods
Patients
Demographic and clinical history data were collected from all non-CRS controls and patients with CRS (both with (CRSwNP) and without nasal polyps (CRSsNP)) who were treated in the Allergy-Immunology and the Otolaryngology Clinics of the Northwestern Medical Faculty Foundation (NMFF) or the Northwestern Sinus Center at NMFF and recruited to participate in studies on CRS between 2003–2013 (n = 1393)(Table1). Control patients were undergoing surgery for non-CRS indicated procedures, such as cranial tumor resection and septoplasty (Table2). Some control patients provided nasal epithelial cells or nasal lavage samples, which were obtained in the clinic, but did not undergo surgery (27.2%, Table2). All CRS subjects met the criteria for CRS as defined by the American Academy of Otolaryngology-Head and Neck Surgery Chronic Rhinosinusitis Task Force 18, such that the diagnosis of CRS was based upon the presence of clinical symptoms (i.e., nasal congestion, rhinorrhea, facial pressure, hyposmia) persisting for more than 12 weeks in addition to having objective evidence of chronic inflammatory disease on sinus CT imaging or nasal endoscopy. Patients with Aspirin Exacerbated Respiratory Disease (AERD) had the clinical triad of CRSwNP, asthma, and a documented history of developing respiratory symptoms following ingestion of either aspirin or a non-steroidal anti-inflammatory drug (NSAID)19,20. Patients with AERD were considered a separate subgroup from those with CRSwNP, and they were not included in the data analyses of patients with CRSwNP unless indicated. Patients were considered to have asthma if they had an asthma diagnosis documented by an allergist, pulmonologist or otolaryngologist. For atopy, patients needed to have a positive skin prick test to at least one of the following allergens: tree pollens, grass pollens, ragweed pollen, dust mite, cat, dog, molds or cockroaches. Severity of sinus inflammation was determined by clinical radiologists’ interpretation of sinus mucosal thickening on sinus Computed Tomography (CT) imaging as being mild, mild-moderate, moderate, moderate-severe, or severe. Additionally, sinus mucosal thickening was assessed by two independent reviewers using the Lund-Mackay (LM) scoring system 21. Patients with an established immunodeficiency, pregnancy, coagulation disorder, solid organ transplant, classic allergic fungal sinusitis, or cystic fibrosis were excluded from the study. All subjects provided informed consent, and the study was approved by the Institutional Review Board of Northwestern University Feinberg School of Medicine.
Table 1.
Patient demographics
| Control | CRSsNP | CRSwNP | AERD | |
|---|---|---|---|---|
| Number | 363 | 490 | 488 | 44 |
| Age, median (range) | 36 (16–87) | 38 (18–73) | 46 (20–81)*# | 43 (19–72) |
| % Caucasian (95% CI) | 73.1 (68.1–78.0) | 79.7 (75.9–83.4) | 75.2 (71.1–79.4) | 68.2 (54.4–81.9) |
| % Women (95% CI) | 45.7 (40.6–50.9) | 55.3 (50.9–59.7) | 38.1 (33.8–42.4)#$ | 61.4 (47.0–75.8) |
| % Asthmatic (95% CI) | 4.2 (1.9–6.5) | 24.3 (20.3–28.3)*@ | 53.6 (49.0–58.2)*# | 100*#@ |
| % Oral Steroids (95% CI) | 1.3 (0.0–2.5) | 8.9 (6.3–11.5)* | 23.4 (19.4–27.4)*# | 47.7 (33.0–62.5)*#@ |
| % Atopic (95% CI) | 17.4 (13.0–21.8) | 56.9 (52.1–61.8)* | 66.4 (61.8–71.1)* | 82.4 (69.5–95.2)*# |
P < 0.05 compared to control
P < 0.05 compared to CRSsNP
P < 0.05 compared to CRSwNP
P < 0.05 compared to AERD.
Table 2.
Control patient surgical procedures
| Procedure | Percentage of control patients |
|---|---|
| No Surgery | 27.2 |
| Septoplasty | 36.1 |
| Tumor Resection | 14.8 |
| CSF Leak Repair | 3.6 |
| Uvulopalatopharyngoplasty | 3.6 |
| Mucocele Decompression | 1.9 |
| Dacryocystorhinostomy | 1.7 |
| Other | 11.1 |
Statistical analysis
All calculations were done using Graphpad Prism v5.0b. The Chi-squared test was used for comparisons of prevalence among different groups. The Mann–Whitney U test was used to compare median values between 2 groups, and the Kruskal–Wallis test with Dunn's correction was used to compare medians among more than 2 groups. A P-value of less than 0.05 was considered significant.
Results
Sex-specific differences in the prevalence of CRS
We first wanted to determine whether there were any sex-specific differences in the prevalence of CRS among our patient population. We examined patient records from control (n = 367), CRSsNP (n = 490), CRSwNP (n = 492), and AERD (n = 44) patients who had previously been recruited from tertiary care centers at Northwestern for our studies on CRS between 2003–2013. We found that women comprised approximately half of control (45%) and CRSsNP patients (55%), but women made up a significantly smaller proportion of CRSwNP patients compared to CRSsNP (38%, P < 0.001; Fig. 1A). In contrast, 61% of patients with AERD, the most severe form of CRSwNP, were women, and this was significantly higher than the proportion of women with CRSwNP (P = 0.032). These data suggest that women with CRSwNP may have more severe disease than men. In support of this, we found that if we combined the AERD patients with the CRSwNP patients, women were more than 2.5 times as likely to have an AERD diagnosis compared to men (P = 0.003; Fig. 1B). However, for the remainder of our analyses, AERD patients were excluded from the CRSwNP group. Interestingly, while we found that patients with CRSwNP were slightly older than control and CRSsNP patients (Table1, and Fig. S1A), we did not find any differences in age between men and women of any patient group (Fig. S1B).
Figure 1.
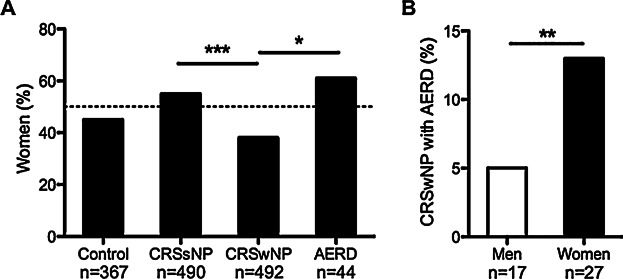
Proportion of patients that were women in each group. The frequency of women was determined among all patients recruited for study on CRS at Northwestern University between 2003–2013 (n = 1393). (A). The proportion of women was not different between control and CRSsNP patients. However, women were much less likely than men to have CRSwNP compared to CRSsNP and AERD patients. (B). Among all patients with CRSwNP, women were 2.5 times more likely to have AERD. *P < 0.05, **P < 0.01, ***P < 0.001 by Chi-squared test.
Sex-specific differences in the frequency of asthma and atopy
Patients with more severe forms of CRSwNP are also more likely to have comorbid asthma 22, and asthma is known to be more prevalent in women 15–17. We also found that the prevalence of asthma was significantly higher in CRSwNP patients compared to CRSsNP and control patients in our study group (Table1). However, no studies to date have investigated whether asthma is more common in women with CRSwNP compared to men. In order to address this, we assessed the frequency of comorbid asthma among men and women in our study population. We found that women with CRSwNP were significantly more likely to have comorbid asthma than men with CRSwNP (66% vs. 46%; P < 0.001, Fig. 2A). We also assessed the frequency of asthma in CRSsNP and control patients. Although there was a trend toward higher asthma frequency, there was no significant difference in asthma frequency between the men and women of these groups (Fig. 2A). These data suggest that the increased prevalence of asthma in women with CRSwNP may not simply be due to the overall increase in asthma among women, but may be linked to airway disease severity. We also assessed the frequency of atopy among men and women in each patient group but found no sex-specific differences (Fig. 2B).
Figure 2.
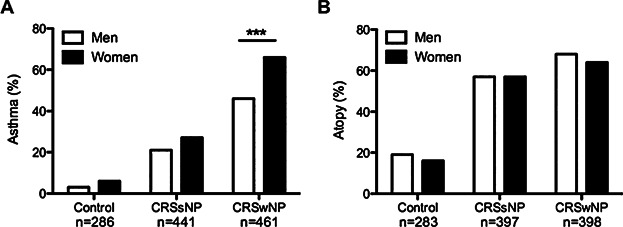
Frequency of asthma and atopy among men and women. (A). CRSwNP women were more likely to have comorbid asthma (64%) compared to CRSwNP men (46%). Asthma frequency was not different between men and women in the control or CRSsNP groups. (B). There was no difference in the frequency of atopy between men and women in any group. ***P < 0.001 by Chi-squared test.
Sex-specific differences in race and oral steroid use among CRSwNP patients
It has been well documented that asthma is more prevalent among women, but it is also known that African-Americans have a higher prevalence of asthma than Caucasians 16. Although we found no differences in the proportion of Caucasian patients among our study groups (Table1), we wanted to determine whether women with CRSwNP were more likely to be African-American and whether they were more likely to have taken oral steroids, a common feature of patients with severe asthma 17. We found that women with CRSwNP were significantly more likely to be African-American, compared to men (18% vs. 10%, respectively, P = 0.027; Fig. 3A), but this was not affected by the asthmatic status of the men or women (data not shown). In addition, we found that CRSwNP women were significantly more likely to have been prescribed perioperative oral steroids compared to men (29% vs. 20%, respectively, P = 0.034; Fig. 3B). Moreover, the frequency of oral steroid use was highest in asthmatic CRSwNP women compared to non-asthmatic women and men with and without asthma (P < 0.001, P = 0.015, and P < 0.001, respectively; Fig. 3C). Interestingly, asthma status did not have an effect on oral steroid use in men with CRSwNP (Fig. 3C). Together, these data further support the notion that CRSwNP women have more severe disease than men.
Figure 3.
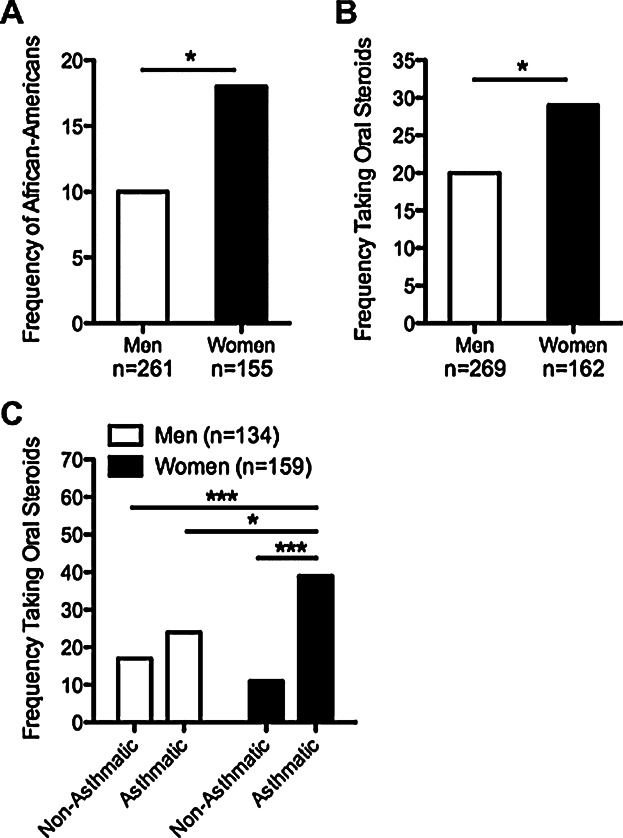
Race and oral steroid use in men and women with CRSwNP. (A). Women with CRSwNP were more likely to be African-American compared to men (18% vs. 10%). (B). Women with CRSwNP were more likely to be prescribed perioperative oral steroids than men (29% vs. 20%). (C). Women with CRSwNP with asthma had the highest frequency of oral steroid use (39%). *P < 0.05, ***P < 0.001 by Chi-squared test.
Sex-specific differences in sinus disease severity in CRSwNP patients
Because patient chart data were more complete for those patients recruited for study from 2010 and on, including data on CT scans and number of surgeries performed, we used the charts from the subset of CRSwNP patients from our study group recruited in those years to determine sinus disease severity (n = 226 out of 492 CRSwNP patients). We first examined whether women with CRSwNP had more severe sinus disease compared to men with CRSwNP by using the traditional Lund–Mackay (LM) scoring system, but we found no differences in the overall average LM score between the sexes (Fig. 4A). In contrast, based upon radiologists’ unbiased interpretation of overall sinus mucosal thickening on diagnostic sinus CT scans, 36% of women with CRSwNP were classified as having severe disease compared to only 17% of men (P = 0.002; Fig. 4B). In addition, when we converted the radiologists' interpretations to a scale of 1–5 (1 being mild and 5 being severe), we found that women with CRSwNP had significantly higher scores than men (P = 0.013; Fig. S2). This suggests that despite the fact that CRSwNP was more common among men, women with CRSwNP on average had more severe sinus disease than men.
Figure 4.
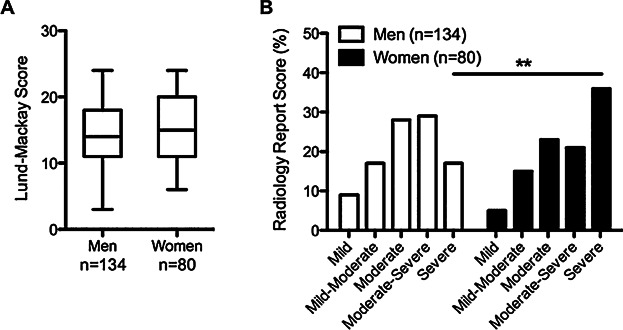
Sinus disease severity in men and women with CRSwNP. (A). There was no difference in the Lund–Mackay scores for men and women with CRSwNP. Boxes represent medians with 25th and 75th percentiles, whiskers represent the min and max values. (B). Women with CRSwNP were two times more likely to be diagnosed with severe sinus disease, compared to men (36% vs. 17%). **P < 0.01 by Chi-squared test.
Another indicator of CRSwNP disease severity is the need for revision surgeries. We assessed the total number of surgeries for men and women with CRSwNP, as well as the percentage of men and women that ever required revision surgery. We found that CRSwNP women were significantly more likely to require revision surgery than men (P = 0.039; Fig. 5A), and they had a history of more total revision surgeries than men (P = 0.018, Fig. 5B). Interestingly, CRSwNP women with comorbid asthma were significantly more likely to require revision surgery compared to non-asthmatic women (50% vs. 26%, respectively, P = 0.024, Fig. 5C), but revision surgery frequency was not different based upon asthma status in men (Fig. 5C). Together, these data further support the hypothesis that women with CRSwNP have significantly more severe disease than men.
Figure 5.
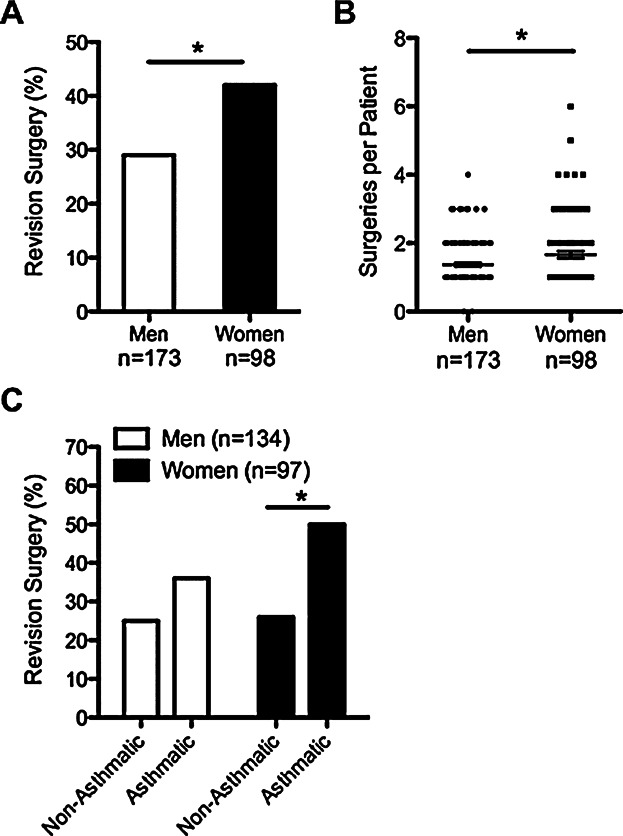
Revision surgeries in men and women with CRSwNP. (A). CRSwNP women were more likely to require revision surgery than men (42% vs. 29%). (B). Women with CRSwNP required more surgeries on average than men. Line represents the mean. (C). CRSwNP women with asthma had the highest frequency of revision surgeries (50%). *P < 0.05 by Chi-squared test (A and C) or by Mann–Whitney U test (B).
Discussion
It is well established that asthma as well as many autoimmune diseases are more prevalent and/or severe in women 14,15. B cells are known to play a key role in these diseases, and estrogen is known to promote B cell activation and antibody production in murine models of autoimmune disease 12–14. We have previously demonstrated that B cells and antibodies likely play a key role in the pathogenesis of CRSwNP, but the mechanisms responsible for the activation of B cells and production of antibodies in NP are unclear 5,6,10,23. Despite the fact that CRSwNP has features of both asthma and autoimmunity, it has not been determined whether there are sex-specific differences in the prevalence and/or severity of this disease.
In the current study, we assessed the frequency and severity of CRSwNP among men and women previously recruited from our tertiary care clinics at Northwestern to study of CRS. Within this selected population, the frequency of CRSsNP between the sexes was similar, while men more commonly had CRSwNP than women. Interestingly, however, it was women with CRSwNP who had more severe sinus disease, were more likely to require revision surgeries, who were more likely to have comorbid asthma, and who were more likely to have been prescribed perioperative oral steroids (Figs. 5). Also, we found that the most severe form of CRSwNP, AERD, was much more common in women than men, which is in agreement with other published studies 24,25 (Fig. 1).
The increased frequency of asthma among CRSwNP women could simply be due to the overall increase in asthma prevalence among adult women compared to men in the general population. According to the National Center for Health Statistics 2014 National Health Interview Survey, the prevalence of asthma among women 35 and older in the USA was 8.8%, compared to 5.2% for men in the same age range 30. It is also well established that patients with CRS have an increased prevalence of asthma compared to the general population 31, and the data in this work confirm that finding. Importantly, however, we found that the frequency of asthma was 46% in CRSwNP men and 66% in CRSwNP women. This increased frequency of 20% is far higher than the 3.6% increase in asthma prevalence documented in the general population of women compared to men. We also found that the frequency of asthma was 6% in control women, and 3% in control men, which is in line with the estimates for the general population above. Moreover, we found that the frequency of asthma was 27% in CRSsNP women, and 21% in CRSsNP men, which highlights the fact that CRSwNP women have a greater increase in asthma frequency than would be explained by the increased prevalence of asthma in patients with upper airway disease.
The severity of CRS disease can be measured using a variety of different techniques, ranging from assessment of patients' clinical symptoms (e.g., SNOT-22) 26, to evaluation of patients’ sinus thickening on CT scan. For the latter, several different radiological scoring systems are available for quantifying CRS disease, with the Lund–Mackay (LM) scoring method being one more commonly utilized 21. In this system, individual sinuses are assigned a number based on sinus mucosal thickening: 1) absent; 2) partial opacification of the sinus; or 3) complete opacification of the sinus. The scores for each individual sinus are then added to scores assessing patency of the ostiomeatal units to generate a final total value. In our study, we found no differences in the averaged total LM score between men and women with CRSwNP (Fig. 4A). One of the drawbacks in using this scoring system, however, is that there is no delineation between degrees of partial sinus opacification, such that a sinus with 75% opacification receives the same score as one with only 10% opacification. Such a limited scoring system is not able to account for varying degrees of sinus inflammation observed in clinical populations. In contrast, the radiology-based scoring system used in our study has a more expanded classification for sinus opacification, as interpreted by clinical radiologists, who were not affiliated with this study. This method allows for the spectrum of sinus disease to be more fully characterized in our study population, making differences between men and women more evident. The clinical radiology scores showed that severity is unequivocally worse in women with CRSwNP than in men with CRSwNP (Fig. 4).
While our results strongly suggest that women with CRSwNP have more severe disease than men, the mechanisms responsible for this difference are not clear. As mentioned previously, estrogen has been shown to play a role in the sex bias seen in asthma and autoimmunity 14,15; thus it is possible that estrogen plays a role in CRSwNP disease severity as well. In addition, our preliminary studies indicate that nasal polyp tissues from CRSwNP women have higher levels of inflammatory markers, such as eosinophil cationic protein (ECP), and increased levels of autoantibodies compared to polyp tissue from men (data not shown), suggesting that regulation of some inflammatory processes may be altered in women. Interestingly, estrogen has been shown to directly activate eosinophils in vitro, and eosinophils can support the survival of antibody-secreting cells in the bone marrow 27–29. Furthermore, estrogen has been shown to promote production of autoantibodies by allowing autoreactive B cells to escape tolerance 14. Thus, we speculate that estrogen may be a critical sex-specific factor that promotes the accumulation of ECP and autoantibodies in nasal polyps of women. Our ongoing studies are focused on elucidating the roles of estrogens and the mechanisms that may be responsible for the sex-specific differences in CRSwNP disease severity seen in this study.
Finally, it is important to note the limitations of this work. First, these results are based on a selective population of patients, those who actively sought specialized care for their CRS disease and often required surgical intervention. Although it is a weakness of our study that they may not represent the general population of people with CRSwNP, it may be a strength of the study that the patients in our cohort are likely to be those patients with the most severe forms of the disease. It is reasonable to expect that patients with the most severe disease disproportionately utilize medical care and drive CRS health care costs. In addition, we examined records from a large number of patients, over a 10-year time span, which strengthens our analyses. There are myriad reasons why women in our study could have more severe disease; it may be that women with CRSwNP only seek out care once their disease passes a certain threshold of morbidity, or that socio-economic factors have a greater influence on their decisions to seek care. However, it has been documented that women with asthma visit all heath care providers more than men, and women are more likely to seek care sooner than men 32,33. Thus the above explanations seem unlikely to account for the differences we have described in our study. Importantly, the epidemiologic studies required to elucidate the possible factors for these differences in disease severity have yet to be performed in CRS. As such, this work represents an important initial analysis that raises awareness of marked sex-specific differences in CRS, and hopefully will stimulate mechanistic studies and larger epidemiological studies in the field.
In summary, we have found that CRSwNP is more frequent in men in a large tertiary care population, but that women suffer from more severe disease. These findings suggest that the mechanisms that underlie the pathogenesis of CRSwNP in men and women may be different, and they may provide novel insights for the development of improved therapeutic strategies for men and women with CRSwNP.
Author Contributions
K. E. Hulse wrote the manuscript, analyzed the data, and designed the study; W. W. Stevens assisted with writing the manuscript, analyzing data, and collecting patient information; A. T. Peters assisted with recruiting patients and collecting patient information; L. Suh, J. E. Norton, and R. Carter assisted with collection of patient samples; R. C. Kern, D. B. Conley, R. K. Chandra, B. K. Tan, L. C. Grammer, and K. E. Harris assisted with patient recruitment and sample collection; A. Kato, M. Urbanek and R. P. Schleimer assisted with study design and writing the manuscript.
Conflict of Interest
There are no conflicts of interest to declare by any of the authors of this work.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's web-site.
Figure S1: Median age of the patients in each group at the time of study participation. (A). CRSwNP patients were older than control and CRSsNP patients. There was no difference in age between CRSwNP and AERD patients. (B). There was no difference in age between men and women in any patient group. Boxes represent medians with 25th and 75th percentiles, whiskers represent the min and max values. ***P < 0.001 by Kruskal–Wallis test with Dunn's correction.
Figure S2: Radiology score for CRSwNP in men and women. Women with CRSwNP had higher median radiologic scores (scale 1–5) based on clinical radiologist CT scoring. Boxes represent medians with 25th and 75th percentiles, whiskers represent the min and max values. *P < 0.05 by Mann–Whitney U test.
References
- Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple, BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J. Allergy Clin. Immunol. 2004;114(6 Suppl):155–212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykewicz MS,, Hamilos DL. Rhinitis and sinusitis. J. Allergy Clin. Immunol. 2010;125(2 Suppl 2):S103–S115. doi: 10.1016/j.jaci.2009.12.989. [DOI] [PubMed] [Google Scholar]
- Tan BK, Schleimer, RP, Kern RC. Perspectives on the etiology of chronic rhinosinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2010;18(1):21–26. doi: 10.1097/MOO.0b013e3283350053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya N, Orlandi RR, Grebner, J, Martinson M. Cost burden of chronic rhinosinusitis: a claims-based study. Otolaryngol. Head Neck Surg. 2011;144(3):440–445. doi: 10.1177/0194599810391852. [DOI] [PubMed] [Google Scholar]
- Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M. Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J. Allergy Clin. Immunol. 2013;131(4):1075–1083. doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Peters A, Suh L, Carter R, Harris, KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2008;121(6):1385–1392. doi: 10.1016/j.jaci.2008.03.002. 1392 e 1381–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patadia M, Dixon J, Conley D, Chandra R, Peters A. Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am. J. Rhinol. Allergy. 2010;24(1):11–16. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachert C, Zhang N, Holtappels G, De Lobel L, van Cauwenberge, P, Liu S, et al. Presence of IL-5 protein and IgE antibodies to staphylococcal enterotoxins in nasal polyps is associated with comorbid asthma. J. Allergy Clin. Immunol. 2010;126(5):962–968. doi: 10.1016/j.jaci.2010.07.007. 968 e961–966. [DOI] [PubMed] [Google Scholar]
- Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar, P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68(1):55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- Tan BK, Li QZ, Suh L, Kato A, Conley, DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2011;128(6):1198–1206. doi: 10.1016/j.jaci.2011.08.037. e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter, R, Suh L, et al. A Role for Anti-BP180 Autoantibodies in Chronic Rhinosinusitis. Laryngoscope. 2013;123:2104–2111. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi CM, Cleary J, Dagtas AS, Moussai, D, Diamond B. Estrogen alters thresholds for B cell apoptosis and activation. J. Clin. Invest. 2002;109(12):1625–1633. doi: 10.1172/JCI14873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verthelyi DI,, Ahmed SA. Estrogen increases the number of plasma cells and enhances their autoantibody production in nonautoimmune C57BL/6 mice. Cell Immunol. 1998;189(2):125–134. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- Lee TP,, Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun. Rev. 2012;11(6–7):A422–A429. doi: 10.1016/j.autrev.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Townsend EA, Miller, VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr. Rev. 2012;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma-Summary report 2007. J Allergy Clin Immunol. 120(5 Suppl):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro, M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am. J. Respir. Crit. Care Med. 2012;185(4):356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer EO,, Hamilos DL. Rhinosinusitis diagnosis and management for the clinician: a synopsis of recent consensus guidelines. Mayo Clin. Proc. 2011;86(5):427–443. doi: 10.4065/mcp.2010.0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AA,, Stevenson DD. Aspirin-exacerbated respiratory disease: update on pathogenesis and desensitization. Semin. Respir. Crit. Care Med. 2012;33(6):588–594. doi: 10.1055/s-0032-1325618. [DOI] [PubMed] [Google Scholar]
- Laidlaw TM,, Boyce JA. Pathogenesis of aspirin-exacerbated respiratory disease and reactions. Immunol. Allergy Clin. North Am. 2013;33(2):195–210. doi: 10.1016/j.iac.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oluwole M, Russell N, Tan L, Gardiner, Q, White P. A comparison of computerized tomographic staging systems in chronic sinusitis. Clin. Otolaryngol. Allied Sci. 1996;21(1):91–95. [PubMed] [Google Scholar]
- Bachert C,, Zhang N. Chronic rhinosinusitis and asthma: novel understanding of the role of IgE 'above atopy'. J. Intern. Med. 2012;272(2):133–143. doi: 10.1111/j.1365-2796.2012.02559.x. [DOI] [PubMed] [Google Scholar]
- Kato A, Hulse KE, Tan, BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J. Allergy Clin. Immunol. 2013;131(4):933–957. doi: 10.1016/j.jaci.2013.02.023. quiz 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samter M,, Beers RF., Jr Intolerance to aspirin. Clinical studies and consideration of its pathogenesis. Ann. Intern. Med. 1968;68(5):975–983. doi: 10.7326/0003-4819-68-5-975. [DOI] [PubMed] [Google Scholar]
- Szczeklik A, Nizankowska, E, Duplaga M. Natural history of aspirin-induced asthma. AIANE Investigators. European Network on Aspirin-Induced Asthma. Eur. Respir. J. 2000;16(3):432–436. doi: 10.1034/j.1399-3003.2000.016003432.x. [DOI] [PubMed] [Google Scholar]
- Hopkins C, Gillett S, Slack R, Lund, VJ, Browne JP. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Hamano N, Terada N, Maesako K, Numata, T, Konno A. Effect of sex hormones on eosinophilic inflammation in nasal mucosa. Allergy Asthma Proc. 1998;19(5):263–269. doi: 10.2500/108854198778557773. [DOI] [PubMed] [Google Scholar]
- Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch, T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nat. Immunol. 2011;12(2):151–159. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- Chu VT,, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol. Rev. 2013;251(1):177–188. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- Ward BW. Schiller JS. Freeman G. Clarke TC. Early release of selected estimates based on data from the January–March 2014 National Health Interview Survey. National Center for Health Statistics. September 2014. Available from http://www.cdc.gov/nchs/nhis.htm.
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid, I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol. Suppl. 2012;23:1–298. [PubMed] [Google Scholar]
- Kynyk JA, Mastronarde, JG, McCallister JW. Asthma, the sex difference. Curr. Opin. Pulm. Med. 2011;17(1):6–11. doi: 10.1097/MCP.0b013e3283410038. [DOI] [PubMed] [Google Scholar]
- Sinclair AH,, Tolsma DD. Gender differences in asthma experience and disease care in a managed care organization. J. Asthma. 2006;43(5):363–367. doi: 10.1080/02770900600705334. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Median age of the patients in each group at the time of study participation. (A). CRSwNP patients were older than control and CRSsNP patients. There was no difference in age between CRSwNP and AERD patients. (B). There was no difference in age between men and women in any patient group. Boxes represent medians with 25th and 75th percentiles, whiskers represent the min and max values. ***P < 0.001 by Kruskal–Wallis test with Dunn's correction.
Figure S2: Radiology score for CRSwNP in men and women. Women with CRSwNP had higher median radiologic scores (scale 1–5) based on clinical radiologist CT scoring. Boxes represent medians with 25th and 75th percentiles, whiskers represent the min and max values. *P < 0.05 by Mann–Whitney U test.


