Abstract
The Pacific Ocean contains approximately 25,000 islands, stretching from Papua New Guinea to Easter Island, populated by mixtures of Melanesians, Micronesians and Polynesians, as well as migrant groups from Asia and Europe. The region encompasses a third of the surface of the earth although it is sparsely populated at a total of around 9 million. With the exception of some of the more populated islands, such as New Zealand and Hawaii, few surveys of chronic diseases have been conducted, but it is increasingly recognized that obesity, diabetes and associated conditions are emerging public health problems and clearly there is a need for cooperation to optimize control. Here we focus on cancer registry and epidemiological findings for Papua New Guinea, the Solomons, Vanuatu, Samoa, New Caledonia, Fiji, Polynesia, French Polynesia, Maori in New Zealand, Native Hawaiians, Micronesia, including Guam, and Aboriginal populations in Australia as assessed by PubMed searches and perusal of the International Agency for Cancer Research descriptive epidemiology database. Overall, the major cancers in males are oral and liver in Papua New Guinea and the Solomon Islands, and lung and prostate elsewhere (Fiji being exceptional in demonstrating a predominance of esophageal cancer), whereas in females it is breast and either cervix or lung, depending largely on whether cervical cancer screening program is active. In certain locations thyroid cancer is also very prevalent in females. The similarities and variation point to advantages for collaborative research to provide the evidence-base for effective cancer control programs in the region.
Introduction
In a recent review of patterns of cancer incidence, focusing on mortality and survival in indigenous populations compared with populations of European origin in Polynesia, the limited nature of available findings for Pacific populations was highlighted (Dachs et al., 2008). The paucity of cancer data for Native Hawaiians and other Pacific Islanders has already been stressed (Hughes et al., 2000), although it had been noted that there are cancers of public health importance as well as a disproportionately high mortality rates compared to non-Maori, non-Pacific people in New Zealand (Foliaki et al., 2004b). The situation 30 years ago appeared far better when Henderson et al (1985) reported ‘The South Pacific Commission Cancer Registry has been operational since 1977, and reasonably complete cancer incidence rates are available for New Caledonia, Fiji, Micronesia, the Cook Islands, and Niue. In addition, less complete reporting is available from American Samoa, Papua New Guinea, and French Polynesia’. The earlier significant increase in the trend of cancer during 1952 to 1985 was concluded to not be due to improved registration alone (Finau and Tukuitonga, 2001). More recently, change with time has only been followed in Hawaii, Guam and French Polynesia.
Cancer Registration in the Pacific Islands
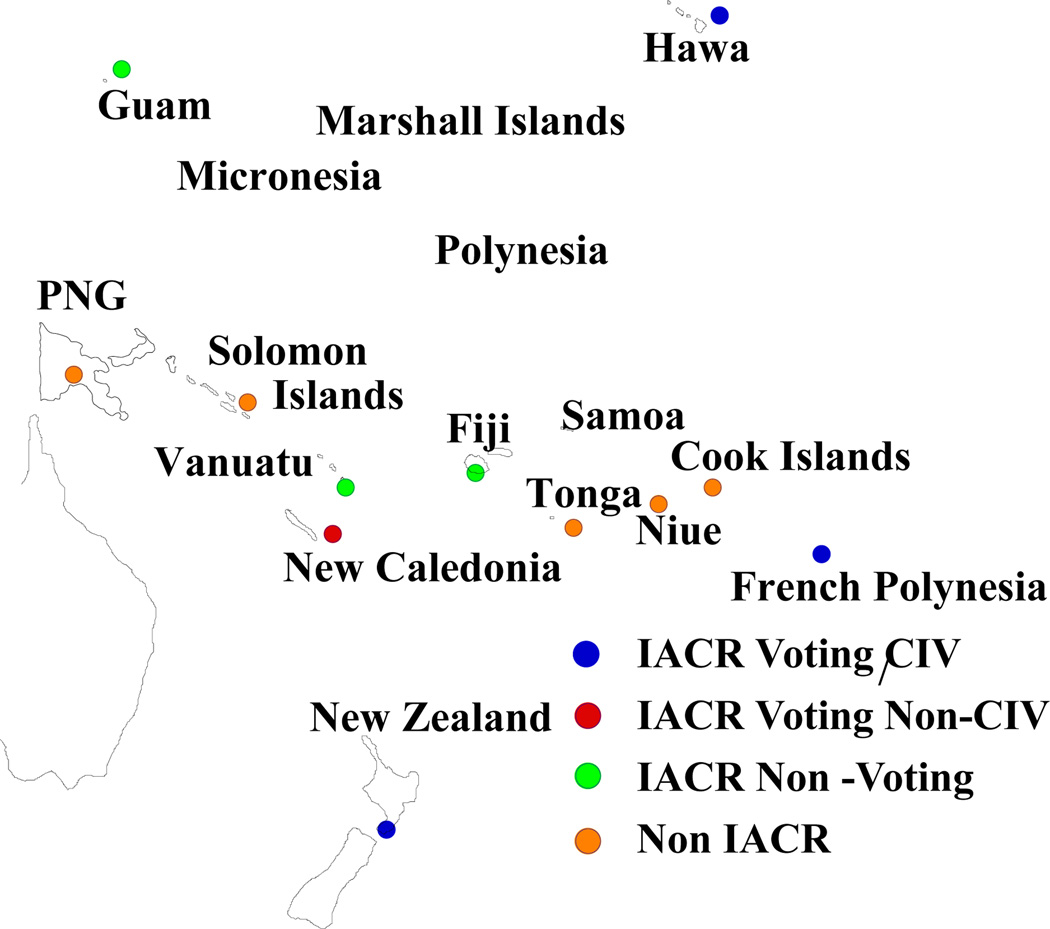
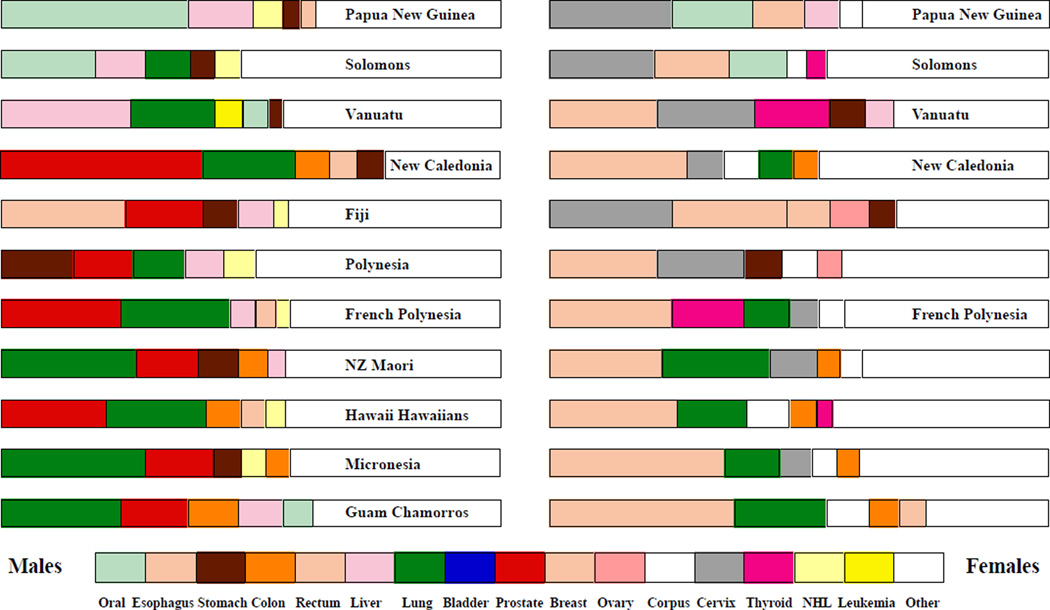
The registries which are now known to exist in the Pacific Islands are indicated in Figure 1 and relevant population based-data from the Cancer Incidence in Five Continents of the International Agency for Research on Cancer (IARC) (www-dep.iarc.fr) or from other sources are listed for Papua New Guinea, New Caledonians, Maoris, Native Hawaiians, Chamorros and French Polynesians in Tables 1 and 2 for males and females, respectively. For other populations, reference has been made to the Globocan 2002 datafile (Ferlay et al., 2004), with the percentages of total cancers accounted for by the five most common malignancies being indicated in Figure 2. The Centre for Public Health Research of Massey University has now installed the IARC cancer registry software CanReg4 in Tonga, the Cook Islands and Niue. The data are not yet regularly forwarded to IARC, pending clearance from the respective governments, but they have been included in the map.
Figure 1.
Cancer Registries in the Pacific Islands
Table 1.
Population-based Cancer Incidence Registry Data for Pacific Islanders - Males (100,000)
| French Polynesians | Hawaiians | Chamorros | Maori | New Caledonians | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1997* | 2007** | 1997* | 2007** | 1996–8# | 2005–7# | 1997* | 1997## | 2007## | |
| Buccal | 7.8 | 5.0 | 4.7 | 4.4 | 4.4 | 8.3 | 6.7 | 9.3 | 8.5 |
| Pharynx | 11.9 | 3.2 | 6.3 | 2.4 | 1.1 | 0.9 | 4.1 | 10.0 | 5.9 |
| Nasopharynx | - | 2.6 | - | 2.0 | 13.2 | 5.5 | - | 1.5 | 0.8 |
| Oesophagus | 7.2 | 9.3 | 7.6 | 7.1 | 3.3 | 5.5 | 9.2 | 4.4 | 6.6 |
| Stomach | 10.9 | 6.8 | 15.1 | 12.8 | 4.4 | 3.7 | 27.9 | 10.8 | 14.5 |
| Colon | 9.5 | 9.6 | 19.9 | 24.5 | 11.0 | 13.7 | 21.5 | 7.9 | 18.0 |
| Rectum | 8.6 | 7.2 | 13.9 | 16.5 | 4.4 | 12.8 | 12.8 | 10.7 | 14.9 |
| Liver | 11.8 | 13.4 | 6.8 | 11.0 | 7.7 | 20.1 | 12.8 | 8.2 | 4.5 |
| Gallbladder | 3.5 | 1.5 | 2.0 | 3.2 | 0.0 | 0.9 | 2.4 | 1.5 | 1.8 |
| Pancreas | 4.4 | 4.0 | 8.8 | 10.1 | 0.0 | 4.6 | 9.8 | 3.0 | 3.3 |
| Larynx | 8.3 | 4.7 | 3.8 | 4.2 | 3.3 | 0.9 | 3.1 | 8.7 | 6.0 |
| Trachea, lung | 73.7 | 62.3 | 72.3 | 68.4 | 49.6 | 48.5 | 99.7 | 71.9 | 50.3 |
| Prostate | 23.2 | 67.9 | 42.1 | 71.3 | 19.8 | 43.9 | 44.4 | 50.5 | 114.0 |
| Kidney | 5.5 | 5.7 | 8.6 | 10.7 | 1.1 | 2.8 | 7.6 | 2.1 | 4.0 |
| Bladder | 8.3 | 11.3 | 3.9 | 12.2 | 3.3 | 2.8 | 10.5 | 12.5 | 7.4 |
| Brain | 3.1 | 4.9 | 3.8 | 5.1 | 1.1 | 3.7 | 5.2 | 0.0 | 3.1 |
| Thyroid | 2.9 | 5.4 | 3.0 | 4.3 | 0.0 | 0.9 | 1.6 | 4.1 | 7.5 |
| Non-Hodgkin | 3.9 | 8.6 | 11.0 | 13.4 | 12.1 | 9.2 | 8.1 | 6.3 | 5.7 |
| Leukemia | 9.3 | 8.8 | 9.5 | 10.1 | 3.3 | 5.5 | 12.2 | 4.5 | 1.7 |
| Total | 249 | 284 | 276 | 345 | 143 | 194 | 360 | 267 | 351 |
Data from Parkin et al., 2002;
Haddock and Whippy (unpublished data);
Baumann et al (unpublished data)
Table 2.
Population-based Cancer Incidence Registry Data for Pacific Islanders - Females (/100,000)
| French Polynesians | Hawaiians | Chamorros | Maori | New Caledonians | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1997* | 2007** | 1997* | 2007** | 1996–8# | 2005–7# | 1997* | 1997## | 2007## | |
| Buccal | 3.7 | 1.9 | 2.1 | 2.7 | 4.1 | 0.0 | 2.2 | 6.1 | 0.9 |
| Pharynx | 2.2 | 0.3 | 1.2 | 0.4 | 1.0 | 0.0 | 0.7 | 1.5 | 1.4 |
| Nasopharynx | - | 0.9 | - | 0.2 | 5.1 | 4.5 | - | 0.0 | 0.7 |
| Oesophagus | 1.5 | 1.1 | 1.3 | 1.8 | 1.0 | 0.0 | 2.1 | 1.5 | 0.6 |
| Stomach | 7.5 | 5.0 | 10.4 | 8.3 | 5.1 | 0.9 | 13.7 | 6.7 | 3.0 |
| Colon | 4.8 | 5.6 | 16.6 | 18.9 | 8.1 | 14.5 | 16.0 | 13.4 | 13.1 |
| Rectum | 4.0 | 3.5 | 8.2 | 9.5 | 5.1 | 7.3 | 9.2 | 5.1 | 5.0 |
| Liver | 3.6 | 4.4 | 2.6 | 4.4 | 5.1 | 3.6 | 3.6 | 1.2 | 1.7 |
| Gallbladder | 1.6 | 2.2 | 1.4 | 1.2 | 1.0 | 0.9 | 1.2 | 1.5 | 1.4 |
| Pancreas | 3.5 | 4.5 | 7.2 | 11.5 | 2.0 | 4.5 | 6.7 | 0.0 | 4.4 |
| Larynx | 1.7 | 0.3 | 0.6 | 1.4 | 1.0 | 0.0 | 1.2 | 0.0 | 1.0 |
| Trachea, lung | 28.1 | 23.6 | 35.0 | 45.9 | 15.3 | 31.8 | 72.9 | 23.3 | 18.9 |
| Breast | 65.7 | 75.1 | 83.9 | 118.9 | 42.7 | 51.8 | 77.1 | 59.9 | 72.7 |
| Ovary | 11.0 | 8.2 | 8.3 | 8.8 | 7.1 | 0.0 | 12.2 | 14.5 | 3.1 |
| Corpus uteri | 9.7 | 12.9 | 20.6 | 28.0 | 13.2 | 22.6 | 15.8 | 10.2 | 19.0 |
| Cervix uteri | 27.7 | 15.6 | 8.6 | 9.3 | 6.1 | 4.5 | 32.2 | 20.0 | 19.7 |
| Kidney | 1.5 | 2.1 | 3.0 | 3.9 | 1.0 | 0.9 | 3.5 | 0.0 | 3.3 |
| Bladder | 1.9 | 3.3 | 3.2 | 3.7 | 0.0 | 0.0 | 2.2 | 4.9 | 3.5 |
| Brain | 4.6 | 4.0 | 2.9 | 4.5 | 0.0 | 0.9 | 4.0 | 1.0 | 0.0 |
| Thyroid | 15.9 | 37.4 | 9.1 | 10.4 | 3.1 | 10.9 | 6.5 | 60.4 | 27.7 |
| Non-Hodgkin | 4.4 | 6.4 | 5.0 | 8.0 | 3.1 | 4.5 | 6.7 | 2.5 | 3.9 |
| Leukemia | 7.1 | 7.2 | 6.6 | 7.7 | 2.0 | 5.5 | 7.8 | 9.0 | 6.0 |
| Total | 253 | 261 | 263 | 335 | 132 | 170 | 340 | 265 | 263 |
Data from Parkin et al., 2002;
Haddock and Whippy (unpublished data);
Baumann et al (unpublished data)
Figure 2.
Percentage Incidence Data for the Five Most Prevalent Cancers in Countries/Populations of the Pacific
There have also been a number of reports of general cancer incidence in the Pacific region over the years. For example, an analysis of the pathology reports of cancer at the Central Hospital, Honiara, Solomon Islands from 1970 to 1982 revealed that skin cancer, lympho-haematopoietic malignancies, cancer of the digestive organs and oral cancer were the most common cancers in males, with cancer of the genito-urinary organs, skin cancer, breast cancer and lympho-haematopoietic malignancies predominating in females (Taylor et al., 1983).
Registry data for the period 1958–1988 in Papua New Guinea demonstrated malignancies of the oral cavity, cervix, breast, and skin, a well as hepatomas, and lymphomas, to be the most common types of neoplasm, with oral cancer increasingly prevalent in the Highlands region, associated with the spread of betel nut chewing (Talonu, 1989). Earlier the incidence rates for oral cancer were significantly lower in the Highlands than in the Lowland regions of Papua New Guinea (Jamrozik, 1985). During the period 1980–88 data in Western Samoa, now Samoa, stomach, prostate and liver cancers were the most common malignancies in males and breast and cervix, followed by stomach, in females (Paksoy et al., 1991). Among American Samoan females, breast carcinoma was most frequent, followed by cancer of the corpus uteri, cervix uteri and thyroid, and leukemia (Mishra et al., 1996), along with relatively high rates for prostate in males (Ruidas et al., 2004). In Niue, lung, stomach and liver cancer in males, and ovarian, uterine and cervical cancer in females were reported to be most common for the 1962–1985 period (Finau and Tukuitonga, 2001).
Chamorros in Guam are reported to have higher rates for diabetes (Pinhey et al., 1997) and mortality from mouth and pharyngeal, nasopharyneal and prostate cancer, but lower rates for leukemia and Non-Hodgkin’s lymphoma compared to Caucasians (Haddock and Naval, 2002; Haddock et al., 2007). Recent data suggest that Chamorros experience higher incidence rates for cancers of the mouth and pharynx, nasopharynx, liver, and cervix than in the United States population, while rates for cancers of the prostate, female breast, ovary and colon-rectum-anus, as well as leukemia, and non-Hodgkin lymphoma, were all relatively low in comparison (Haddock et al., 2009). Considerable variation was also noted among the other ethnic groups.
In a first report based on data from the Cancer Registry of French Polynesia (Gleize et al., 2000), laryngeal cancer in men and cervix, corpus uteri, and thyroid cancer incidence rates in women were documented to be higher among populations born in French Polynesia than among immigrants, while rates for oral, colorectal and prostate cancers and melanomas were lower. There may be considerable variation between islands (Le Vu et al., 2000).
In New Zealand, cancer incidence from 1996–2000 was higher in Maori than in non-Maori females, but lower in Maori than in non-Maori males (Foliaki et al., 2004). However, more recent data appear to indicate that both male and female Maori are at higher cancer risk overall than non-Maori populations (Dachs et al., 2008). Furthermore, Maori and Pacific Islanders experience relatively poor survival overall, largely because they are more likely than other ethnic groups to be diagnosed at an advanced stage disease (Foliaki et al., 2004; Haynes et al., 2008). The same is the case for Aboriginal patients in Australia (Cottrell et al., 2007), basically independent of the cancer, with especially marked differences reported for lung (Supramaniam et al., 2006) and ovary (Laurvick et al., 2003).
As described in a recent review (Roder and Currow, 2009), Aboriginal and Torres Strait Islander Australians have a cancer incidence for all sites combined equivalent to or slightly lower than for other Australians. They have a higher incidence of cancers of the cervix, liver and gallbladder, oesophagus, unknown primary site, mouth and throat, lung and pancreas, but a lower incidence of cancers of the prostate, female breast, colon/rectum and skin (melanoma).
It was futher argued that case survivals are lower for Aboriginal and Torres Strait Islander patients, partly due to an excess of cancer types with a high case fatality, relatively low numbers with a low case fatality, and due to more advanced cancer stages at diagnosis (South Australian Cancer Registry, 1997; Condon et al., 2005a; 2005b; Valery et al., 2006; Cottrell et al., 2007; Cunningham et al., 2008). After accounting for these factors, Aboriginal and Torres Strait Islander Australians still fare worse, probably due to elevated co-morbidity and less complete care resulting from geographic remoteness, limited access to transport and accommodation services, and sometimes a cultural disconnect with mainstream services.
Organ Specific Epidemiology
Skin
Skin cancer is generally rare in Pacific Islanders, except among immigrants. Melanesians of the North Solomons are exposed to intense equatorial sunlight and yet have a very low incidence of skin cancer (Foster and Webb, 1988). As expected, cutaneous malignant melanomas have been reported to be frequent in whites but low in non-whites in New Caledonia (Di Schino et al., 1989; Baumann and Rougier, 2005).
Oral and Esophageal
Oral cancer is the most prevalent neoplasm in Papua New Guinea and the nearby Solomon Islands, with lower rates in Vanuatu. Combinations of tobacco smoking and chewing of betel quid are the main risk factors (Lumukana and King, 2003) and a recent study and systematic review provided evidence of the role of betel quid not containing tobacco in oral cancer development (Thomas et al., 2008). Interestingly, although the prevalence of betel nut use among Chamorro residents of Guam is higher than that of other Micronesians residing on the island, their incidence of oral cancer is lower (Haddock, 2005). However, it is difficult to draw conclusions from this observation because of the small populations and confounding by migration from other islands to Guam for cancer treatment.
Esophageal cancers are rare except in the Indian population of Fiji.
Gastric
Gastric cancer is common in Polynesia and to a certain extent also in Fiji and Vanuatu, as well as Maori in New Zealand and Native Hawaiians. Particularly high rates have been reported for Maori in Whangarei (Thompson, 2002). From the limited data available from CIV, the incidences are decreasing in almost all populations for which data are available (see Tables 1 and 2).
Colorectal
Incidence rates in the Pacific Islands other than Hawaii appear to be low. Increase over time has been found for Maori and other Pacific Islanders in New Zealand, catching up with the white population to some extent (Shaw et al., 2006). However, it was earlier argued that Maori consume more calires, eat more red meat, drink more alcohol, consume more saturated fat, have a higher prevalence of obesity and have a lower proportion of individuals consuming a given level of fruit and vegetables per day, so that they would be expected to have more rather than less colorectal cancer than people of European ancestry (Thomson and Shaw, 2002). Whether there may be protective chemical constituents present within their food plant supply is of obvious interest (Cambie and Ferguson, 2003).
Liver
Liver cancer is found at high frequency in Vanuatu, the Solomons and Papua New Guinea, with lower rates elsewhere in the Pacific. An early paper suggested the main risk factor at that time to be HBV (Paksoy et al., 1989) and in Maori and Pacific Islanders in New Zealand HBsAg carriage was found to explain most of the excess standardized rate compared to people of European stock (Blakely et al., 1999). Hepatitis B vaccination has been an effective means for reducing HBsAg carriers, as indicated by the results of an infant hepatitis B immunisation program in Fiji, Kiribati, Tonga and Vanuatu (Wilson et al., 2000). The effect of vaccination on HCC incidence has yet to be determined, however. The occurrence of liver cancer in Native Fijians was earlier found to be significantly higher than in the Indo-Fijian population (Lovelace and Aalbersberg, 1989). The same study showed contamination of food samples with aflatoxin to be low.
Larynx
Laryngeal cancer rates appear to be comparatively low among Pacific Islanders from teh data accessible through Globocan 2002.
Lung
Lung cancer is number one or two in most of the Pacific region, with Papua New Guinea and Fiji as interesting exceptions. Tobacco is the major risk factor, with gender and ethnicity predicting the likelihood of having ever smoked (Kaholokula et al., 2006). In Fiji, the low rate of lung cancer does not appear to be due to lower rates of tobacco smoking (Le Marchand et al., 1995). There may be some genetic influence. In this context it should be mentioned that among cigarette smokers, Native Hawaiians are more susceptible to lung cancer than other ethnic groups in Hawaii (Le Marchand et al., 1992).
Smoking levels were very low in PNG at one time but more recently tobacco use has become common in young people (Hiawalyer, 2002). There may be specific occupational or non-occupational exposures, for example to tremolite fibres, which increase the risk of lung cancer in New Caledonia (Menvielle et al., 2003). Rates of mesothelioma are exceedingly high in New Caledonia due to environmental asbestos (Baumann et al., 2007). However, there is no evidence that exposures specific to the nickel industry are important (Goldberg et al., 1994).
Kidney and Urinary Bladder
Kidney and bladder cancer are relatively infrequent, but appear to be increasing in those populations for whom data are available. New Zealand Maori have higher rates than non-Maori for all smoking-related cancers except in the bladder and larynx, pointing to a partial role for genetically determined or lifestyle-related protective effects (McCredie et al., 2000).
Prostate
Prostate cancer is very prevalent in all populations of the region except the Papua New Guineans, Solomon Islanders and inhabitants of Vanuatu (see Figure 2) and incidence rates appear to be increasing rapidly from the CIV data (see Table 1). Whether any role has been played by increased screening using the prostate-specific antigen (PSA) test is unclear. A study of the correlation of prostatic pathology and serum prostate-specific antigen (PSA) levels has been published for Papua New Guinea (Murthy et al., 1998).
Breast
Breast cancer is common in all of the communities of the Pacific, without exception. Apart from Guam (Balajadia et al., 2008) and Hawaii and New Zealand, there are no breast cancer screening programs in place. As in Asia, mammary cancer incidences appear to be rising, particularly among relatively young age groups. It is reported that native patients throughout the region tend to present at hospital with more advanced stage lesions (Kuska, 1999; Halder et al., 2001).
Endometrium
Endometrial cancer is the fourth or fifth most common malignancy in females in most Pacific Islander populations. In Chamorros on Guam, it now appears to be the third most common in women, after breast and lung cancers.
Cervix
Cervical cancer is the first or second most common neoplasm among women in five of the locations shown in Figure 2. Increase has been attributed to both social changes and improved registration (Martin et al., 1992). More recently, a collaborative mission by the World Health Organisation (WHO) and the Fiji Ministry of Health resulted in a report prepared by Irwin Law of the University of Melbourne on “Burden of cervical cancer and CIN in Fiji (2004–2007)”. Incidence rates were found to be over 65 /100,000 for Fijians and 38/100,000 for Indo-Fijians aged between 20 and 69 years, with respective mortality rates of 33/100,000 and 17/100,000. Human papillomavirus (HPV) types-16 and 18 appear to be the commonest high-risk types, with the highest infection rates in women under 25 years of age, although there are minimal data for many countries in Oceania (Garland et al., 2008). The regional status of cancer screening is unclear.
Thyroid
With the highest incidence rates ever reported, thyroid cancer is a major public health problem for Melanesians of New Caledonia (Ballivet et al., 1995; Baumann and Rougier, 2005). It is also relatively prevalent in Vanuatu (Paksoy et al., 1989). Data from French Polynesia do not point to a major role for radioiodine fallout (de Vathaire et al., 2000). Rather, menstrual and reproductive parameters with excess body weight, especially with onset during early adulthood, may be the most important risk factors (Brindel et al., 2008a; 2008b).
Perspectives
While cancer registration is not sophisticated in most of the region, the major cancers are nevertheless clear, allowing for priority targets for control. Unfortunately, it needs to be emphasized that the Pacific is experiencing a dual burden of the infection-related cancers of developing countries (like oral cavity and cervix) as well as emerging obesity-related cancers (including the colon, prostate, breast and endometrium) common in developed countries. In terms of recommendations for prevention: lifestyle improvement for oral and breast malignancies, and to a certain extent colorectal cancer; vaccination for liver and cervix cancers, if the costs become substantially reduced for the latter; tobacco control for lung cancer; and screening for cervical cancer; all need to be stressed. Such preventive measures could also reduce other chronic diseases.
Obesity and cardiovascular disease are documented problems in Samoa (Hodge et al., 1994; Hodge et al., 1997), along with hypertension (Wahi et al., 1997). The latest published prevalence of diabetes in Tonga is 15.1%, of which 80% is undiagnosed, the incidence having doubled over the past 25 years (Colagiuri et al., 2002). A similar experience has been reported for Melanesian and Indian Fijians, with excess mortality due to cardiovascular causes (Collins et al., 1996; Tomisaka et al., 2002). Tongans also appear prone to excessive obesity (Sawata et al., 1988), with affordability rather than “commonsense” as the key factor in food consumption (Evans et al., 2002).
Diabetes mellitus was earlier reported to be relatively rare in highland Papua New Guinea, very common in Micronesian Nauruans and the rural Wanigelas of coastal PNG and intermediate in Polynesian Western Samoans (Dowse, 1996). However, changes are taking place predominantly due to the adoption of a western lifestyle and diet (Natsuhara et al., 2000). In one report at the turn of the century, intake of Western food was described to be negligible and stroke and ischemic heart disease were absent or rare (Lindeberg et al., 1999). Betelnut chewing may also be independently associated with diabetes (Benjamin, 2001).
Given the relatively small populations and financial constraints, coordination of activity would be of great advantage for the region. In fact, the groundwork for the Pacific Islander cancer control network (PICCN) began in the early 1990s with a study of the cancer control needs of American Samoans (Hubbell et al., 2006). The project’s principal objectives were to increase cancer awareness and to enhance cancer control research among American Samoans, Tongans, and Chamorros and incorporated a training program to educated Pacific Islander students in the field and conducted pilot research projects (Hubbell et al., 2006). One focus of attention has been on building native Maori (Sporle and Koea, 2004) and Hawaiian responsiveness and capacity in cancer research (Braun et al., 2006) and a Pacific Cancer Initiative was launched to promote Pacific island partnerships (Palafox et al., 2006).
In addition, a “Regional Course for Cancer Registrars in Pacific Island Countries and Territories” was held in 1998 in Noumea, New Caledonia, this being organized by the Secretariat for the Pacific Community in collaboration with International Agency for Research on Cancer (IARC) and the Western Pacific Regional Office of WHO. Thirteen Pacific islands countries and territories attended. A further IARC “International Course on Cancer Epidemiology: Principles and Methods” was also held in Tonga in 2004 and a series of Japan International Cooperation Agency (JICA) funded courses on community-based cancer prevention included three participants from Fiji, one from Micronesia, three from Papua New Guinea, three from the Solomon Islands and one from Vanuatu (Wakai and Matsuo, 2007). Unfortunately, these activities do not appear to have been translated into many additions to the published literature. It has been stressed that interventions targeting the needs of the local populations in the Pacific are welcome but should not be another opportunity for “research colonialism” (Foliaki et al., 2004a). This should be borne firmly in mind in future efforts.
Whether a local research meeting can be organized with involvement of WHO, IARC and the International Union Against Cancer - Asian Regional Office (UICC-ARO) and the Asian Pacific Organization for Cancer Prevention should be explored. In addition to cancer registration and all aspects of epidemiology, through descriptive to clinical, tobacco control and screening needs to be covered. Effects of the unique local nutritional background and cultural interdictions against cancer screening might also be explored (Steiner, 2000; Cambie and Ferguson, 2003). If the recent Tongan finding of a very strong association of cancer with the idea of unavoidable death is typical (McMullin et al., 2008), then a major effort to educate and improve awareness at the community level must be encouraged.
Acknowledgment
Malcolm Moore thanks the Japanese Foundation for Promotion of Cancer Research and the Japanese National UICC committee for financial support for the drafting and publication of this review.
References
- Balajadia RG, Wenzel L, Huh J, Sweningson J, Hubbell FA. Cancer-related knowledge, attitudes, and behaviors among Chamorros on Guam. Cancer Detect Prev. 2008;32:S4–S15. doi: 10.1016/j.cdp.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballivet S, Salmi LR, Dubourdieu D, Bach F. Incidence of thyroid cancer in New Caledonia, South Pacific, during 1985–1992. Am J Epidemiol. 1995;141:741–746. doi: 10.1093/oxfordjournals.aje.a117496. [DOI] [PubMed] [Google Scholar]
- Baumann F, Rougier Y. Registre du cancer de Nouvelle-Caledonie, bilan de 15 annees de surveillance, 1989–2003. Bull Epidemiologique Hebdomadaire. 2005;33:169–171. [Google Scholar]
- Baumann F, Rougier Y, Ambrosi JP, Robineau BP. Pleural mesothelioma in New Caledonia: an acute environmental concern. Cancer Detect Prev. 2007;31:70–76. doi: 10.1016/j.cdp.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Benjamin AL. Community screening for diabetes in the National Capital District, Papua New Guinea: is betelnut chewing a risk factor for diabetes? PNG Med J. 2001;44:101–107. [PubMed] [Google Scholar]
- Blakely TA, Bates MN, Baker MG, Tobias M. Hepatitis B carriage explains the excess rate of hepatocellular carcinoma for Maori, Pacific Island and Asian people compared to Europeans in New Zealand. Int J Epidemiol. 1999;28:204–210. doi: 10.1093/ije/28.2.204. [DOI] [PubMed] [Google Scholar]
- Braun KL, Ichiho HM, Kuhaulua RL, et al. Empowerment through community building: Diabetes today in the Pacific. J Public Health Manag Pract. 2003;(Suppl):S19–S25. [PubMed] [Google Scholar]
- Braun KL, Tsark JU, Santos L, Aitaoto N, Chong C. Building native Hawaiian capacity in cancer research and programming. A legacy of ‘Imi Hale. Cancer. 2006;107(8 Suppl):2082–2090. doi: 10.1002/cncr.22157. [DOI] [PubMed] [Google Scholar]
- Brindel P, Doyon F, Rachédi F, et al. Menstrual and reproductive factors in the risk of differentiated thyroid carcinoma in native women in French Polynesia: a population-based case-control study. Am J Epidemiol. 2008a;167:219–229. doi: 10.1093/aje/kwm288. [DOI] [PubMed] [Google Scholar]
- Brindel P, Doyon F, Rachédi F, et al. Anthropometric factors in differentiated thyroid cancer in French Polynesia: a case-control study. Cancer Causes Control. 2008b;20:581–590. doi: 10.1007/s10552-008-9266-y. [DOI] [PubMed] [Google Scholar]
- Cambie RC, Ferguson LR. Potential functional foods in the traditional Maori diet. Mutat Res. 2003;523–524:109–117. doi: 10.1016/s0027-5107(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Colagiuri S, Colagiuri R, Na’ati S, et al. The prevalence of diabetes in the kingdom of Tonga. Diabetes Care. 2002;25:1378–1383. doi: 10.2337/diacare.25.8.1378. [DOI] [PubMed] [Google Scholar]
- Collins VR, Dowse GK, Ram P, Cabealawa S, Zimmet PZ. Non-insulin-dependent diabetes and 11-year mortality in Asian Indian and Melanesian Fijians. Diabet Med. 1996;13:125–132. doi: 10.1002/(SICI)1096-9136(199602)13:2<125::AID-DIA13>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Collins VR, Dowse GK, Cabealawa S, Ram P, Zimmet PZ. High mortality from cardiovascular disease and analysis of risk factors in Indian and Melanesian Fijians. Int J Epidemiol. 1996;25:59–69. doi: 10.1093/ije/25.1.59. [DOI] [PubMed] [Google Scholar]
- Condon JR, Armstrong BK, Barnes T, Zhao Y. Cancer incidence and survival for Indigenous Australians in the Northern Territory. Aust NZ J Public Health. 2005a;29:123–128. doi: 10.1111/j.1467-842x.2005.tb00061.x. [DOI] [PubMed] [Google Scholar]
- Condon JR, Armstrong BK, Barnes A, Selva-Nayagam S, Elwood M. Stage at diagnosis and cancer survival of Indigenous Australians in the Northern Territory. Med J Aust. 2005b;182:277–280. doi: 10.5694/j.1326-5377.2005.tb06700.x. [DOI] [PubMed] [Google Scholar]
- Cottrell J, Street J, Chong A, Roder D. Comparing cancer profiles and survival of aboriginal and non-aboriginal patients in South Australia: where are the opportunities for improving Aboriginal health? Asian Pac J Cancer Prev. 2007;8:495–501. [PubMed] [Google Scholar]
- Cunningham J, Rumbold AR, Zhang X, Condon JR. Incidence, aetiology, and outcomes of cancer in Indigenous peoples in Australia. Lancet Oncology. 2008;9:585–595. doi: 10.1016/S1470-2045(08)70150-5. [DOI] [PubMed] [Google Scholar]
- Curado MP, Edwards B, Shin HR, et al., editors. IARC Scientific Publications No 160. Lyon: IARC; 2007. Cancer Incidence in Five Continents Vol. IX. [Google Scholar]
- Dachs GU, Currie MJ, McKenzie F, et al. Cancer disparities in indigenous Polynesian populations: Maori, Native Hawaiians, and Pacific people. Lancet Oncol. 2008;9:473–484. doi: 10.1016/S1470-2045(08)70127-X. [DOI] [PubMed] [Google Scholar]
- de Vathaire F, Le Vu B, Vathaire CC. Thyroid cancer in French Polynesia between 1985 and 1995: influence of atmospheric nuclear bomb tests performed at Mururoa and Fangataufa between 1966 and 1974. Cancer Causes Control. 2000;11:59–63. doi: 10.1023/a:1008961503506. [DOI] [PubMed] [Google Scholar]
- DiSchino M, Merouze F, Huerre M, et al. Cutaneous malignant melanomas in New Caledonia. Study of the Cancer Registry (1977–1987) Med Trop. 1989;49:271–276. (in French). [PubMed] [Google Scholar]
- Dowse GK. Incidence of NIDDM and the natural history of IGT in Pacfic and Indian Ocean populations. Diabetes Res Clin Pract. 1996;34:S45–S50. doi: 10.1016/s0168-8227(96)90007-8. [DOI] [PubMed] [Google Scholar]
- Evans M, Sinclair RC, Fusimalohi C, Liava’a V. Diet, health and the nutrition transition: some impacts of economic and socio-economic factors on food consumption patterns in the Kingdom of Tonga. Pac Health Dialog. 2002;9:309–315. [PubMed] [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin DM. IARC CancerBase No. 5, version 2.0. Lyon: IARC Press; 2004. GLOBOCAN 2002: Cancer Incidence, Mortality and Prevalence Worldwide. [Google Scholar]
- Finau SA, Tukuitonga CF. Cancer in Niue: analysis of a cancer register 1962–1985. Pac Health Dialog. 2001;8:94–98. [PubMed] [Google Scholar]
- Foliaki S, Fakakovikaetau T, Waqatakirewa L, Pearce N. Health research in the Pacific. Pac Health Dialogue. 2004a;11:199–203. [PubMed] [Google Scholar]
- Foliaki S, Jeffreys M, Wright C, Blakey K, Pearce N. Cancer in Pacific people in New Zealand: a descriptive study. Pacific Health Dialogue. 2004b;11:94–100. [PubMed] [Google Scholar]
- Foster HM, Webb SJ. Skin cancer in the North Solomons. Aust NZ J Surg. 1988;58:397–401. doi: 10.1111/j.1445-2197.1988.tb01087.x. [DOI] [PubMed] [Google Scholar]
- Garland SM, Brotherton JM, Skinner SR, et al. Human papillomavirus and cervical cancer in Australasia and Oceania: risk-factors, epidemiology and prevention. Vaccine. 2008;26(Suppl 12):M80–M88. doi: 10.1016/j.vaccine.2008.05.041. [DOI] [PubMed] [Google Scholar]
- Gleize L, Laudon F, Sun LY, et al. Cancer registry of French Polynesia: results for the 1990–1995 period among native and immigrant population. Eur J Epidemiol. 2000;16:661–667. doi: 10.1023/a:1007641009097. [DOI] [PubMed] [Google Scholar]
- Goldberg M, Goldberg P, Leclerc A, et al. A 10-year incidence survey of respiratory cancer and a case-control study within a cohort of nickel mining and refining workers in New Caledonia. Cancer Causes Control. 1994;5:15–25. doi: 10.1007/BF01830722. [DOI] [PubMed] [Google Scholar]
- Haddock RL. Oral cancer incidence disparity among ethnic groups on Guam. Pac Health Dialog. 2005;12:153–154. [PubMed] [Google Scholar]
- Haddock RL, Naval CL. Cancer on Guam, especially among Micronesians. Pac Health Dialog. 2002;9:222–224. [PubMed] [Google Scholar]
- Haddock RL, Talon RJ, Whippy HJ. Ethnic disparities in cancer mortality among residents of Guam. Asian Pac J Cancer Prev. 2006;7:411–414. [PubMed] [Google Scholar]
- Haddock RL, Whippy HJ, Talon RJ, Montano MV. Ethnic disparities in cancer incidence among residents of Guam. Asian Pac J Cancer Prev. 2009;10:571–562. [PMC free article] [PubMed] [Google Scholar]
- Halder A, Morewya J, Watters DA. Rising incidence of breast cancer in Papua New Guinea. ANZ J Surg. 2001;71:590–593. doi: 10.1046/j.1445-2197.2001.02205.x. [DOI] [PubMed] [Google Scholar]
- Haynes R, Pearce J, Barnett R. Cancer survival in New Zealand: ethnic, social and geographical inequalities. Soc Sci Med. 2008;67:928–937. doi: 10.1016/j.socscimed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Henderson BE, Kolonel LN, Dworsky R, et al. Cancer incidence in the islands of the Pacific. Natl Cancer Inst Monogr. 1985;69:73–81. [PubMed] [Google Scholar]
- Hiawalyer G. Smoking prevalence among young people in Papua New Guinea. Pac Health Dialog. 2002;9:209–213. [PubMed] [Google Scholar]
- Hodge AM, Dowse GK, Toelupe P, et al. Dramatic increase in the prevalence of obesity in western Samoa over the 13 year period 1978–1991. Int J Obes Relat Metab Disord. 1996;18:419–428. [PubMed] [Google Scholar]
- Hodge AM, Dowse GK, Toelupe P, Collins VR, Zimmet PZ. The association of modernization with dyslipidaemia and changes in lipid levels in the Polynesian population of Western Samoa. Int J Epidemiol. 1997;26:297–306. doi: 10.1093/ije/26.2.297. [DOI] [PubMed] [Google Scholar]
- Hubbell FA, Luce PH, Afeaki WP, et al. Legacy of the Pacific Islander cancer control network. Cancer. 2006;107:2091–2098. doi: 10.1002/cncr.22154. [DOI] [PubMed] [Google Scholar]
- Hughes CK, Tsark JU, Kenui CK, Alexander GA. Cancer research studies in Native Hawaiians and Pacific Islanders. Ann Epidemiol. 2000;10:S49–S60. doi: 10.1016/s1047-2797(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Jamrozik K. Regional variation of oral cancer in Papua New Guinea. PNG Med J. 1985;28:9–13. [PubMed] [Google Scholar]
- Kaholokula JK, Braun KL, Kana’iaupuni S, Grandinetti A, Chang HK. Ethnic-by-gender differences in cigarette smoking among Asian and Pacific Islanders. Nicotine Tob Res. 2006;8:275–286. doi: 10.1080/14622200500484600. [DOI] [PubMed] [Google Scholar]
- Kuska B. Breast cancer increases on Papua New Guinea. J Natl Cancer Inst. 1999;91:994–996. doi: 10.1093/jnci/91.12.994. [DOI] [PubMed] [Google Scholar]
- Laurvick CL, Semmens JB, Holman CD, Leung YC. Ovarian cancer in Western Australia (1982–98): incidence, mortality and survival. Aust N Z J Public Health. 2003;27:588–595. doi: 10.1111/j.1467-842x.2003.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Hankin JH, Bach F, et al. An ecological study of diet and lung cancer in the South Pacific. Int J Cancer. 1995;63:18–23. doi: 10.1002/ijc.2910630105. [DOI] [PubMed] [Google Scholar]
- Le Marchand L, Wilkens LR, Kolonel LN. Ethnic differences in the lung cancer risk associated with smoking. Cancer Epidemiol Biomarkers Prev. 1992;1:103–107. [PubMed] [Google Scholar]
- Leon Guerrero RT, Workman RL. Physical activity and nutritional status of adolescents on Guam. Pac Health Dialog. 2002;9:177–185. [PubMed] [Google Scholar]
- Le Vu B, de Vathaire F, de Vathaire CC, et al. Cancer incidence in French Polynesia 1985–95. Trop Med Int Health. 2000;5:722–731. doi: 10.1046/j.1365-3156.2000.00624.x. [DOI] [PubMed] [Google Scholar]
- Lindeberg S, Eliasson M, Lindahl B, Ahren B. Low serum insulin in traditional Pacific Islanders--the Kitava Study. Metabolism. 1999;48:1216–1219. doi: 10.1016/s0026-0495(99)90258-5. [DOI] [PubMed] [Google Scholar]
- Lovelace CE, Aalbersberg WG. Aflatoxin levels in foodstuffs in Fiji and Tonga islands. Plant Foods Hum Nutr. 1989;39:393–399. doi: 10.1007/BF01092077. [DOI] [PubMed] [Google Scholar]
- Lumukana R, King T. Smoking and chewing habits of oral cancer patients in the Solomon Islands. Pac Health Dialog. 2003;10:41–44. [PubMed] [Google Scholar]
- Martin WM, Sengupta SK, Murthy DP, Barua DL. The spectrum of cancer in Papua New Guinea. An analysis based on the Cancer Registry 1979–1988. Cancer. 1992;70:2942–2950. doi: 10.1002/1097-0142(19921215)70:12<2942::aid-cncr2820701235>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- McCredie M, Cox B, Stewart JH. Smoking-related Cancers in Maori and non-Maori in New Zealand, 1974–1993: Fewer Bladder Cancers among Maori. Asian Pac J Cancer Prev. 2000;1:221–225. [PubMed] [Google Scholar]
- McMullin JM, Taumoepeau L, Talakai M, Kivalu F, Hubbell FA. Tongan perceptions of cancer. Cancer Detect Prev. 2008;32(Suppl 1):S29–S36. doi: 10.1016/j.cdp.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menvielle G, Luce D, Fevotte J, et al. Occupational exposures and lung cancer in New Caledonia. Occup Environ Med. 2003;60:584–589. doi: 10.1136/oem.60.8.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SI, Luce-Aoelua PH, Wilkens LR. Cancer among indigenous populations. The experience of American Samoans. Cancer. 1996;78(7 Suppl):1553–1557. [PubMed] [Google Scholar]
- Morewaya J, Koriyama C, Akiba S, et al. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncol Rep. 2004;12:1093–1098. [PubMed] [Google Scholar]
- Murthy DP, Ray U, Morewaya J, SenGupta SK. A study of the correlation of prostatic pathology and serum prostate-specific antigen (PSA) levels: a perspective from Papua New Guinea. P N G Med J. 1998;41:59–64. [PubMed] [Google Scholar]
- Natsuhara K, Inaoka T, Umezaki M, et al. Cardiovascular risk factors of migrants in Port Moresby from the highlands and island villages, Papua New Guinea. Am J Hum Biol. 2000;12:655–664. doi: 10.1002/1520-6300(200009/10)12:5<655::AID-AJHB11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Palafox NA, Gunawardsane K, Demei Y. Pacific island partnership: the Pacific Cancer Initiative. J Cancer Education. 2006;21(suppl):S87–S90. doi: 10.1207/s15430154jce2101s_15. [DOI] [PubMed] [Google Scholar]
- Paksoy N, Montaville B, McCarthy SW. Cancer occurrence in Vanuatu in the South Pacific, 1980–86. Asia Pac J Public Health. 1989;3:231–236. doi: 10.1177/101053958900300310. [DOI] [PubMed] [Google Scholar]
- Paksoy N, Bouchardy C, Parkin DM. Cancer occurrence in Western Samoa. Int J Epidemiol. 1991;20:634–641. doi: 10.1093/ije/20.3.634. [DOI] [PubMed] [Google Scholar]
- Pinhey TK, Heathcote GM, Craig UK. Health status of diabetic persons in an Asian-Pacific population: evidence from Guam. Ethn Dis. 1997;7:65–71. [PubMed] [Google Scholar]
- Sawata S, Hidaka H, Yasuda H, et al. Prevalence of cardiovascular diseases in the Kingdom of Tonga. Jpn Heart J. 1988;29:11–18. doi: 10.1536/ihj.29.11. [DOI] [PubMed] [Google Scholar]
- Roder D. Comparative cancer incidence, mortality and survival in Indigenous and non-Indigenous residents of South Australia and the Northern Territory. Cancer Forum. 2005;29:7–9. [Google Scholar]
- Roder D, Currow D. Cancer in Aboriginal and Torres Strait Islander People of Australia. Asian Pac J Cancer Prev. 2009;10:729–734. [PubMed] [Google Scholar]
- Ruidas L, Adaoag A, Williams VT, Sesepasara ML. Cancer in American Samoa. Pac Health Dialog. 2004;11:17–22. [PubMed] [Google Scholar]
- Shaw C, Blakely T, Sarfati D, Fawcett J, Peace J. Trends in colorectal cancer mortality by ethnicity and socio-economic position in New Zealand, 1981–99: one country, many stories. Aust N Z J Public Health. 2006;30:64–70. doi: 10.1111/j.1467-842x.2006.tb00088.x. [DOI] [PubMed] [Google Scholar]
- South Australian Cancer Registry. Incidence and mortality 1996. Adelaide: Lutheran Press; 1997. Epidemiology of cancer in South Australia. Incidence, mortality and survival 1977–1996. [Google Scholar]
- Sporle A, Koea J. Maori responsiveness in health and medical research: key issues for researchers (part 1) N Z Med J. 2004;117:U997. [PubMed] [Google Scholar]
- Steiner GG. The correlation between cancer incidence and kava consumption. Hawaii Med J. 2000;59:420–422. [PubMed] [Google Scholar]
- Supramaniam R, Grindley H, Pulver LJ. Cancer mortality in Aboriginal people in New South Wales, Australia, 1994–2002. Aust N Z J Public Health. 2006;30:453–456. doi: 10.1111/j.1467-842x.2006.tb00463.x. [DOI] [PubMed] [Google Scholar]
- Talonu NT. Observations on betel-nut use, habituation, addiction and carcinogenesis in Papua New Guinea. PNG Med J. 1989;32:195–197. [PubMed] [Google Scholar]
- Taylor R, Parker M, Ansford A, Davison A. Cancer in Solomon Islands, 1970–82. PNG Med J. 1983;26:102–110. [PubMed] [Google Scholar]
- Thomas SJ, Harris R, Ness AR, et al. Betel quid not containing tobacco and oral leukoplakia: a report on a cross-sectional study in Papua New Guinea and a meta-analysis of current evidence. Int J Cancer. 2008;123:1871–1876. doi: 10.1002/ijc.23739. [DOI] [PubMed] [Google Scholar]
- Thompson AG. Regional differences in incidence of gastric and colonic cancer in the Maori of New Zealand. Postgrad Med J. 2002;78:419–421. doi: 10.1136/pmj.78.921.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson B, Shaw I. A comparison of risk and protective factors for colorectal cancer in the diet of New Zealand Maori and non-Maori. Asian Pac J Cancer Prev. 2002;3:319–324. [PubMed] [Google Scholar]
- Tomisaka K, Lako J, Maruyama C, et al. Dietary patterns and risk factors for type 2 diabetes mellitus in Fijian, Japanese and Vietnamese populations. Asia Pac J Clin Nutr. 2002;11:8–12. doi: 10.1046/j.1440-6047.2002.00257.x. [DOI] [PubMed] [Google Scholar]
- Valery PC, Coory M, Stirling J, Green AC. Cancer diagnosis, treatment, and survival in Indigenous and non- Indigenous Australians: a matched cohort study. Lancet. 2006;367:1842–1848. doi: 10.1016/S0140-6736(06)68806-5. [DOI] [PubMed] [Google Scholar]
- Wahi S, Gatzka CD, Sherrard B, et al. Risk factors for coronary heart disease in a population with a high prevalence of obesity and diabetes: a case-control study of the Polynesian population of Western Samoa. J Cardiovasc Risk. 1997;4:173–178. [PubMed] [Google Scholar]
- Wakai K, Matsuo K. The JICA training course, Community-based Cancer Prevention for the Asian and Pan-Pacific Countries, fiscal year 2006 (epidemiological approach) Asian Pac J Cancer Prev. 2007;8:150–154. [PubMed] [Google Scholar]
- Wilson N, Ruff TA, Rana BJ, Leydon J, Locarnini S. The effectiveness of the infant hepatitis B immunisation program in Fiji, Kiribati, Tonga and Vanuatu. Vaccine. 2000;18:3059–3066. doi: 10.1016/s0264-410x(00)00080-3. [DOI] [PubMed] [Google Scholar]