Abstract
Little attention has been paid to adrenal sustentacular cells, and several major histology textbooks do not even describe them. This study presents a detailed morphological description of sustentacular cells using immuno-light microscopy and an antibody against brain-type fatty acid-binding protein. The immunopositive sustentacular cells and processes formed lattices with holes of various sizes and compactnesses or openness. In addition, weakly immunostained sheet-like structures with ill-defined contours were often associated with the processes and lattices. In the carotid body, which has traditionally been classified under the name of paraganglia in common with the adrenal medulla, immunostained sustentacular cell processes formed lattices in association with the weakly immunostained sheet-like structures, but the lattices with sheets were more compact and rigid than the adrenal medulla, and appeared like individually distinct compartments. In the ganglion, the immunostained satellite cell processes with the sheets tightly enclosed individual neurons. As a result, the immunostained sheet-like structures were regarded as en-face views of thinly flattened sustentacular cytoplasmic envelopes partially covering the chromaffin cells in the adrenal medulla, and widely in the carotid body in a way rather similar to the satellite cells in the ganglion. In brief, the terminal enclosing portions of adrenal sustentacular cell processes, in cut-views, were too thin/flat to be recognized as distinct lines in immuno-light microscopy because of its resolution limit. They are recognized in en-face views as entities of a substantially spacious extension in immuno-light microscopy.
Keywords: adrenal medulla, brain type-fatty acid-binding protein, immunohistochemistry, light microscopy, sustentacular cell, transmission electron microscopy
Introduction
The adrenal medulla as well as the autonomic ganglia are considered to be derived from the neural crest in development, and adrenal chromaffin cells and ganglionic neurons are homologous in ontogeny (Hamilton et al. 1972). Cells of similar light and electron microscopic features to the adrenal chromaffin cells in terms of the chromaffin reaction and catecholamine-containing electron-dense granules (chromaffin granules) are known to comprise extra-adrenal tissues such as the carotid body and para-aortic body (Zuckerkandl's organ). In addition, such cells are also known to exist, under the name of small intensely fluorescence cells, within autonomic ganglia themselves. All of the cells have been historically collectively termed ‘paraganglion’ cells (Kohn, 1903; Bock, 1982). Whether the chromaffin reaction is positive for all the paraganglion cells, especially those in the carotid body and within the ganglia, is uncertain.
It is well known that ganglionic neurons and their axons, similar to central neurons, are associated with satellite cells and Schwann cells, respectively, both of which are considered to be glial in nature (Belzer et al. 2010; Pannese, 2010). It is thus natural to expect that the adrenal chromaffin cells and other paraganglion cells are also associated with such cells of glial nature, and the existence of such associated cells, termed the sustentacular cells, has actually been well verified in electron microscopy (Bock, 1982). Little attention, however, is paid to the adrenal sustentacular cells as exemplified by the fact that their explanation is omitted in most, if not all, textbooks of histology, especially those written in English (Weiss, 1983; Fawcett 1994; Williams, 1995).
Based on the electron microscopic finding, thin cytoplasmic profiles of the associated cells of glial nature are often directly apposed to paraganglion cells as well as neurons/axons, The associated cells are generally known to envelope the paraganglionic and neuronal elements. The width of the thin cytoplasmic profiles is largely below the light microscopic resolution. On the other hand, Schwann cells and astrocytes are traditionally well known to have radiated multiple processes in light microscopy with the silver impregnation staining (Fawcett, 1994). Considering the view that such thread-formed processes in the light microscopic shape could not envelope the neuronal elements widely, but only circulate in forms of headbands, the processes must be highly flattened to envelope them. The same is the case for the sustentacular cells in relation to adrenal chromaffin and paraganglion cells, and it is important to recognize in light microscopy such flattened structures whose cross-views correspond to the thin cytoplasmic profiles in electron microscopy.
With this consideration, together with recent increasing attention paid to the functional significance of glial cells in the neural function (Fields & Stevens-Graham, 2002; Miller, 2005), the present study attempted to reveal the detailed entire morphology of the sustentacular cells in the adrenal gland of adult and newborn mice in comparison with those cells in the adult carotid body and sympathetic ganglion by immunohistochemistry at the light microscopic level using the antibody for brain-type-fatty acid-binding protein (B-FABP), which has already been shown to be a specific marker for the sustentacular cells as well as the other associated cells of glial nature (Yun et al. 2004; Owada et al. 2006). Special attention was paid to the morphological feature of the sustentacular cellular envelopes themselves.
Materials and methods
Male ICR mice of postnatal 1 day and postnatal 8 weeks, five animals at each stage, were perfused under pentobarbital anesthesia with phosphate-buffered saline (PBS), and followed by 4% paraformaldehyde/0.1 m phosphate buffer. The adrenal glands as well as the carotid bifurcations containing the carotid bodies and the superior cervical ganglia were removed, then postfixed with the same fixative for 2 h. Specimens were dipped into 30% sucrose–0.1 m phosphate buffer, then 30% sucrose/0.1 m phosphate buffer for cryoprotection. Cryosections of 20 μm thickness were made and permeabilized with 0.1% Triton X-100/PBS for 20 min at room temperature, incubated with 0.3% H2O2/methanol for 10 min, then 5% normal goat serum/PBS for 30 min. Sections were incubated with anti-mouse B-FABP rabbit IgG (2 μg mL−1) overnight at room temperature. The specificity of the antibodies has already been described elsewhere (Owada et al. 2006). The sections were subsequently incubated for 1 h at room temperature with biotinylated anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA) diluted at 1 : 200 for diaminobenzidine reaction by VECTASTAIN Elite ABC kit (Vector Laboratories). For immuno-electron microscopy, some of the immuno-reacted sections were further fixed with 2.5% glutaraldehyde in 0.1 m phosphate buffer, and subsequently postfixed with 1% OsO4 in 0.1 m phosphate buffer for 2 h. After en-bloc staining with 0.5% uranyl acetate, the sections were dehydrated with graded concentrations of alcohol and flatly embedded in Epon. Ultrathin sections were made and observed using electron microscopy. In addition, some of the specimens taken from the animals were processed for conventional epoxy-embedded transmission electron microscopy by fixation with 2.5% glutaraldehyde and 1% OsO4.
For stereo-viewing of immuno-light microscopic photographs, a given area of the immunostained sections on glass-slides was photographed with a 20 × objective lens first at 0 ° and then approximate 5 ° tiltings (by manual handling) of a glass-slide against the light microscopic observatory stage. This process was applied to more than five different domains of a tissue section and more than 10 different tissue sections, and more than 50 pairs of twice-tilted photographs were obtained. Then, the pairs of photographs were processed by anaglyph in Adobe Photoshop, and resulting photographs were observed with red/blue-colored glasses.
All subsequent procedures were conducted in accordance with Guidelines for the Care and Use of Laboratory Animals at Khon Kaen University. The study was reviewed and approved by the ethics board with the ethics number AEKKU 3/2014.
Results
Immuno-light microscopy
In adult mice, in accord with the previous report by us (Yun et al. 2004), the sustentacular cells were distinctly immunoreactive for B-FABP. Their perikarya were oval or polygonal in shape, and they had bipolar or multi-polar processes. The processes were often branched, but tended to be slender and relatively long in general. In some regions of the medulla, the cell perikarya were crowded, and their processes often formed lattices with holes being variable in shape and size. In some other regions, they were sparsely distributed and lattices of the immunopositive processes tended to be looser with holes being less compact, or to be open. These two types of regions occurred randomly throughout the medulla with a proportion of about 50 : 50. In addition to the distinctly immunostained and branched cell processes in thread forms, weak immunoreactivity for B-FABP was often seen in forms of irregular-shaped and ill-delineated sheets of variable sizes. The weakly immunostained sheet-like structures were associated with some of the distinctly immunopositive processes forming lattices, whereas some of the other immunopositive processes were free of the sheet-like structures (Figs1 and 3). Such features of the weakly immunostained sheet-like structures were more clearly appreciated in stereo-viewing immuno-light microscopy with the help of red/blue stereo glasses (Fig. 3a), and an imaginative architecture of the sustentacular cells based on the present findings is shown in line-drawings (Fig. 3b).
Figure 1.
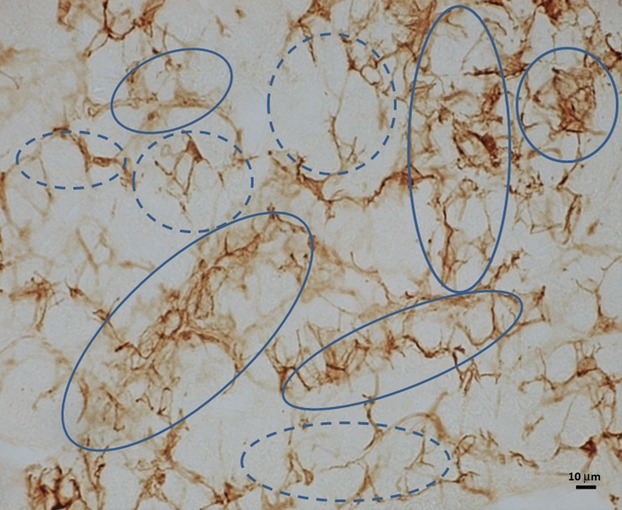
Light micrograph of adrenal medulla of adult mice immunostained for B-FABP. Note intensely immunostained lattices of sustentacular cell processes in association with weakly immunostained sheets (solid circles) and those without association to weakly immunostained sheets (broken lined circles).
Figure 3, 4.
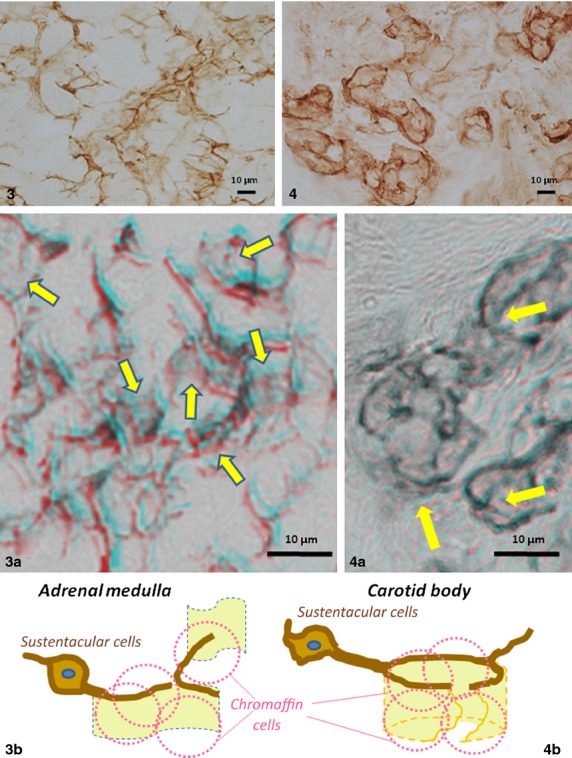
Comparison of light micrographs in morphology of B-FABP-immunostained processes and their lattices in association with weakly immunostained sheets between the adrenal medulla (Fig. 3) and carotid body (Fig. 4) of adult mice. Note open lattices and widely extended sheets of the adrenal sustantacular cells vs. rather close, though incomplete, lattices with sheets in forms of buckets with broken walls of the carotid body sustentacular cells. These differences in architecture of the sheets are shown in 3D light microscopy using red/blue glasses as indicated by arrows in Fig. 3a,b vs. Fig. 4a; and schematically shown in line drawings (Figs 3b and 4b) in which chromaffin cells are shaped in broken pink lines enveloped by sheets.
In newborn specimens, intensely B-FABP-immunopositive cell perikarya and their processes have already been recognized throughout the medulla. In some regions of the medulla, the immunopositive cells exhibited a distribution pattern of perikarya and process-lattices similar to the adult ones. In most other regions, however, the cell perikarya tended to be larger than the adult specimens, and their processes tended to be thinner and shorter and did not extend along their trajectory in such a straight manner (Fig.2). These two types of regions occurred randomly with the proportions of about 2 : 5. This feature resulted in the dominant appearance of the processes terminating more freely or in the immunopositive lattices being looser or more open than those in adults. The weakly immunostained sheet-like structures tended to occur in association with some immunostained lattices.
Figure 2.
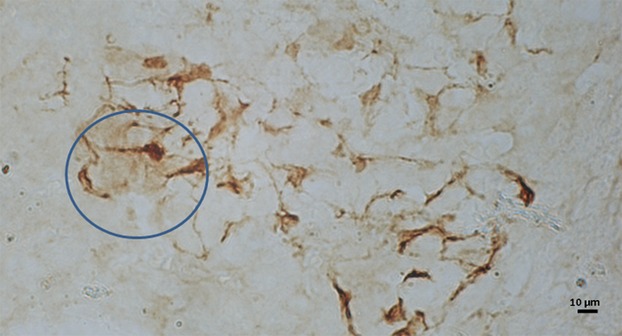
Light micrograph of adrenal medulla of newborn mice immunostained for B-FABP. Intensely immunopositive processes of sustentacular cells are shorter and less branched than the adult, and they generally tend to terminate freely without forming lattices, although lattices of the adult pattern in association with weakly immunostained sheets may be seen in some areas (solid circles).
In the carotid body of adult mice, on the other hand, although sustentacular cells and processes were also immunopositive for B-FABP, their immunopositive cell processes often formed more compact/close lattices with adjacent immunopositive processes. The individual lattices were almost always associated tightly with weakly immunostained sheet-like structures, resulting in the appearance of numerous, though incomplete, compartments with rather rigid contours, which contained several chromaffin cells. These features in sections appeared like bottomless buckets that were walled with the weakly immunostained sheets and fringed with distinctly immunopositive processes on one edge (Figs3 and 4). In the superior cervical ganglion, satellite cell processes were immunostained distinctly and they enclosed, together with associated sheet-like structures, individual neuronal somata almost completely in the form of almost complete buckets (Fig.5). Such features of the weakly immunostained sheet-like structures together with sustentacular and satellite cell processes were more clearly appreciated in stereo-viewing immuno-light microscopy with the help of red/blue stereo glasses (Figs 4a and 5a). Imaginative architectures of the sustentacular and satellite cells based on the present findings are shown in line-drawings (Figs 4b and 5b).
Figure 5.
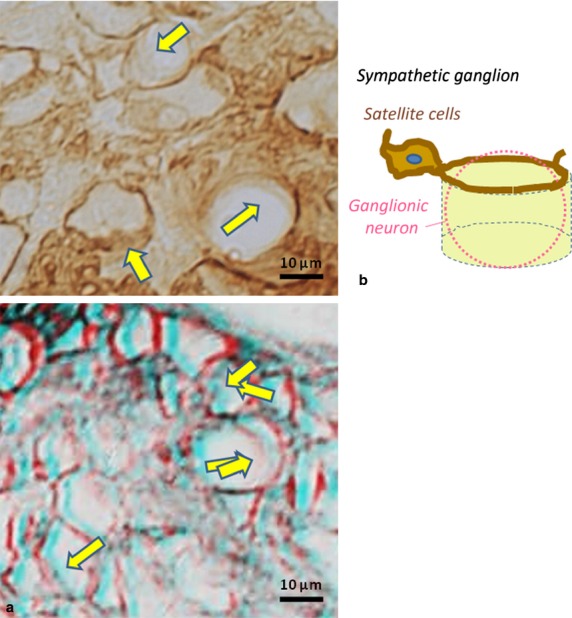
Light micrographs of B-FABP-immunostained satellite cell processes in superior cervical ganglion of adult mice in comparison with sustentacular cell processes of the adrenal medulla (Fig.3) and carotid body (Fig.4). Note almost complete buckets or compartmentations walled by weakly immunostained sheets of satellite cell processes. These features are shown in stereo (a) and line drawing (b) of the ganglion. Please use ‘red and blue’ glasses for 3-D viewing.
Immuno-electron microscopy
In immuno-electron microscopy, the immunopositive sustentacular cells and their processes were identified as electron-dense cell-profiles with the nucleus immunonegative in the adrenal medulla of adult mice. The cell processes were thin, less than 100 nm at the minimum and directly apposed to immunonegative chromaffin cells without the interstitial spaces intervening. The chromaffin cell surfaces were often directly exposed to the interstitial space widely without covering by the immunopositive sustentacular cell processes (Fig.6). These features were in accord with the previous conventional electron microscopic reports (see Bock, 1982).
Figure 6.
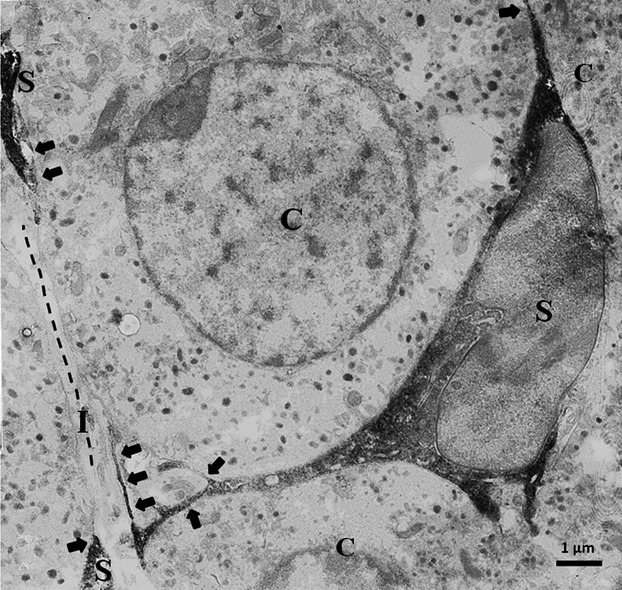
Immuno-electron micrograph of adrenal medulla. Intensely immunopositive processes of sustentacular cells (S) are directly apposed to adrenal chromaffin cells (C), and some of them are highly thin (arrows). Broken lines indicate direct exposure of chromaffin cells to interstitial space (I) without association of thin sustentacular cell processes.
Discussion
The present immunohistochemical study revealed for the first time, by employing the specific immunohistochemical marker, B-FABP, the morphological features of the adrenal sustentacular cells of adult mice in light microscopy. The cell processes took various arborization patterns such as bipolar and multipolar extensions from the perikarya. In addition to the free-ending form, they, together with adjacent processes, frequently formed lattices with various sizes, shapes and degrees of openness. Furthermore, the weakly immunostained sheet-like structures were noted for the first time, which were, as discussed in the next session below, identified as terminal forms of the cell processes to envelope the chromaffin cells. In newborn specimens, more open lattices or frequent free-ends of the sustentacular cell processes were often revealed, although the adult features noted above were also found to some extent. These features suggest the newborn sustentacular cells to be still under differentiation, which is compatible with the previous finding that the enveloped chromaffin cells are under a proliferative stage in newborn rodents (Mascorro & Yates, 1989; Powers et al. 2004).
Because of its soluble nature, B-FABP-immunoreactivity is expected to occur throughout the entire cytoplasm of the sustentacular cells, even in their highly thin cellular processes apposing to the chromaffin cell surfaces as seen in previous electron microscopic studies by others (Kondo, 1971; Bock, 1982). Because the thin cytoplasmic profiles are largely at degrees of thinness below the light microscopic resolution, they are not expected to be recognized as distinct lines in light microscopy even after being filled with the immunopositive materials. If, however, such thin cytoplasmic processes with the immunoreactive materials are actually extended flatly in substantial sizes, it is expected that they could be recognized when viewed en-face in light microscopy. In this regard, it should be noted that the weakly immunopositive sheet-like structures were seen in association with the distinctly immunopositive processes and lattices in the present light microscopy of B-FABP-immunostained adrenal medulla. It is thus reasonable to infer that the sheet-like structures represent en-face views of highly flattened and truly terminal cytoplasmic portions of thread-formed sustentacular cell processes filled with B-FABP-immunoreactive materials. In other words, it is inferred that such thin sheet-like structures become visible, only when viewed en-face, in immuno-light microscopy by weak but selective immunostaining because of B-FABP distributed in the highly flattened cytoplasm. This interpretation is supported by the present stereo-viewing of immuno-light micrographs, and further supported by the present immuno-light microscopic findings on the carotid body and sympathetic ganglion in comparison with the adrenal medulla: the weakly immunostained sheet-like structures were also noticed in these organs and they appeared like immunostained bottomless buckets whose fringes and walls are represented by the thread-like cell processes and the sheet-like structures, respectively. The buckets, containing multiple chromaffin (‘chief’) cells in the carotid body and individual neurons in the ganglion, were somehow loose in the carotid body, while they were almost complete or rigid in the ganglion. In contrast, the sheet-like structures did not take the form of buckets, but of more opened expansion in the adrenal medulla. These features are in accord with the well-known ultrastructural features of the sustentacular process profiles associated with a considerable surface area of the carotid body chief cell and are also in accord with that of the satellite cell processes associated with almost all surfaces of neurons (Kondo et al. 1982; Belzer et al. 2010; Pannese, 2010). In contrast, the surfaces of adrenal chromaffin cells are largely associated with the basal laminae, but rarely with the thin cytoplasmic profiles of the sustentacular cells (Kondo, 1971; Bock, 1982). The latter feature probably makes it difficult to pay attention to the adrenal sustentacular cells, resulting in the omission of the cells from descriptions in several major textbooks as noted in the Introduction.
What is the functional significance of the marked differences in the architecture and arrangement of the sheet-like structures of the sustentacular cells and satellite cells? The differences include the fact that the sustentacular cells enclose the adrenal cells only partially, while they enclose the main cells of the carotid body to a large extent (Kondo et al. 1982), and the ganglionic neurons almost entirely (Belzer et al. 2010; Pannese, 2010). These differences in the sustentacular and satellite cells among the different paraganglia and ganglia may represent differences in the functional implications of catecholamines in individual paraganglion/chromaffin cells and ganglionic neurons: endocrine functions in adrenal chromaffin cells, neural chemoreceptive/afferent synaptic function in carotid body chromaffin (‘chief’) cells; and remotely efferent synaptic functions in ganglionic neurons.
Based on the present findings, and also in view of the increasing evidence for the importance of glia–neuron cross-talk functions (Fields & Stevens-Graham, 2002; Miller, 2005), further experiments are expected to be performed for further understanding of the functional significance of the sustentacular cells, especially their sheet-like structures, in relation to the hormone secretion of chromaffin cells in the medulla. The functional significance of B-FABP in the sustentacular cells as well as ganglionic satellite cells and central astrocytes also remain to be elucidated.
Acknowledgments
This work was supported by Faculty of Medicine, Khon Kaen University through WH (No. I57201). The authors thank Miss Y. Polsan and Mr D. Hipkaeo for their technical support. The authors would also like to acknowledge Prof. James A. Will for editing the manuscript via Publication Clinic KKU, Thailand.
Authorship
S.P.: acquisition of data, data analysis/interpretation, drafting of the manuscript; S.C., Y.K., W.M., S.I. and T.S.: acquisition of data; W.H.: concept/design, data analysis/interpretation, drafting of the manuscript; Y.O. and H.K.: concept/design, critical revision of the manuscript and approval of the article.
References
- Belzer V, Shraer N, Hanani M. Phenotypic changes in satellite glial cells in cultured trigeminal ganglia. Neuron Glia Biol. 2010;6:237–243. doi: 10.1017/S1740925X1100007X. [DOI] [PubMed] [Google Scholar]
- Bock P. The Paraganglia (Handbuch der Mikroskopischen Anatomie des Menschen) Berlin: Springer; 1982. [Google Scholar]
- Fawcett DW. Fawcett, a Textbook of Histology. New York: WB Saunders; 1994. [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron–glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton WJ, Mossman HW, Boyd JD. Human Embryology: Prenatal Development of Form and Function. 4th edn. New York: Macmillan; 1972. [Google Scholar]
- Kohn A. Die paraganglion. Archiv fur Mikroskopische Anatomie. 1903;62:263–265. [Google Scholar]
- Kondo H. An electron microscopic study on innervation of the carotid body of guinea pig. J Ultrastruct Res. 1971;37:544–562. doi: 10.1016/s0022-5320(71)80024-2. [DOI] [PubMed] [Google Scholar]
- Kondo H, Iwanaga T, Nakajima T. Immunocytochemical study on the localization of neuron-specific enolase and S-100 protein in the carotid body of rats. Cell Tissue Res. 1982;227:291–295. doi: 10.1007/BF00210887. [DOI] [PubMed] [Google Scholar]
- Mascorro JA, Yates RD. Mitotic cell division in the extraadrenal chromaffin system of various species. J Electron Microsc Tech. 1989;12:323–330. doi: 10.1002/jemt.1060120405. [DOI] [PubMed] [Google Scholar]
- Miller G. The dark side of glia (News Focus) Science. 2005;308:778–781. doi: 10.1126/science.308.5723.778. [DOI] [PubMed] [Google Scholar]
- Owada Y, Abdelwahab SA, Kitanaka N, et al. Altered emotional behavioral responses in mice lacking brain-type fatty acid-binding protein gene. Eur J Neurosci. 2006;24:175–187. doi: 10.1111/j.1460-9568.2006.04855.x. [DOI] [PubMed] [Google Scholar]
- Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. 2010;6:3–10. doi: 10.1017/S1740925X10000037. [DOI] [PubMed] [Google Scholar]
- Powers JF, Brachold JM, Schelling K, et al. Potentiation of mitogenesis in adult rat chromaffin cell cultures by immunosuppressive agent FK506. Neurosci Let. 2004;356:5–8. doi: 10.1016/j.neulet.2003.10.083. [DOI] [PubMed] [Google Scholar]
- Weiss L. Cell and Tissue Biology, A Textbook of Histology. 6th edn. Baltimore, MD: Urban & Schwarzenberg; 1983. [Google Scholar]
- Williams PL. Gray's Anatomy. 38th edn. New York: Churchill Livingstone; 1995. [Google Scholar]
- Yun X, Nourani MR, Abdelwahab SA, et al. Differential localization of brain-type and epidermal-type fatty acid binding proteins in the adrenal gland of mice. Tohoku J Exp Med. 2004;203:77–86. doi: 10.1620/tjem.203.77. [DOI] [PubMed] [Google Scholar]


