Abstract
The auditory region of pinnipeds has seldom been described. Here we describe and analyze the ontogenetic trajectory of the tympanic bulla of the southern elephant seal, Mirounga leonina (Phocidae, Mammalia). This species is extremely sexually dimorphic and highly polygynous (organized in harems). We examined 118 specimens, arranged in three age classes (CI, CII, and CIII), ranging from newborn to adults (males and females). To analyze the overall size and shape of the tympanic bulla we performed a geometric morphometric analysis including 87 skulls. Females reach definitive shape and size of the bulla at earlier ontogenetic stages than males, in agreement with their earlier involvement in reproductive activities. The internal anatomy of the tympanic region (e.g. form and extension of the paries) does not show remarkable differences between sexes or age classes. The greatest differences between age classes are related to bone thickness, resulting from the apposition of new annual layers. An examination of possible sex-related external differences among age classes shows significant shape differences between males and females in CIII. The morphology observed in neonates is conserved across all individuals from CI, which included specimens up to 1 year old. Clear morphological differences were observed between CI individuals, on one hand, and CII individuals plus CIII females on the other. During cranial development of both male and females, the glenoid cavity expands and compresses the bulla; this condition reaches its maximum expression in CIII males. CIII males showed the greatest morphological differences, with respect to both CI and CII individuals, and CIII females.
Keywords: dimorphism, ear region, ontogeny, southern elephant seal
Introduction
Studies of the morphology of the otic region of pinniped carnivorans offer the opportunity to investigate the correlates of an aquatic habitat and phylogenetic transformations, but this region of the anatomy has not been analyzed in depth for most species of this group. The auditory region of the Carnivora in general has been widely studied by many authors (e.g. Van Kampen, 1905; Pocock, 1921, 1929; Van der Klaauw, 1931; Thenius, 1949; Hough, 1952; Ginsburg, 1966; Beaumont, 1968; Hunt, 1974; Arnaudo et al. 2014). It is noteworthy that the analysis of auditory features, such as the presence or absence of the septum bullae (Hough, 1948; Ivanoff, 2000), has supported the classification of the members of Carnivora into three large groups: Arctoidea, Aeluroidea, and Cynoidea (Flower, 1869), currently recognized as different clades (Eisenberg, 1989; Wozencraft, 1989, 2005; Wyss & Flynn, 1993; Ivanoff, 2001).
Within the pinnipeds (Otariidae, Phocidae and Odobenidae) the auditory region has been briefly described for several Northern Hemisphere species (e.g. Thenius, 1949; King, 1964; Odend'hal & Poulter, 1966; Graham, 1967; Solntseva, 1972, 1973a,b, 1975; Hunt, 1974; Marsh, 2001; Berta et al. 2006) and a few Southern Hemisphere ones (e.g. Wyss, 1987, 1988), and other contributions on which the ears of pinnipeds are treated together with those of other groups of mammals (e.g. Repenning, 1972; Fleischer, 1978; Nummela, 1995, and references therein). In addition, studies using a physiological approach, i.e. hearing (Mohl, 1967, 1968) and audiometric (Kastak & Schusterman, 1999) analyses, have been performed on several Holarctic species such as Mirounga angustirostris (Phocidae, Carnivora). Species of this genus offer a rich subject of investigation given the chance to integrate the anatomical studies with biological information on the species.
The southern elephant seal (Mirounga leonina) (Phocidae) is an extremely sexually dimorphic and highly polygynous species. Adult males are much larger and almost three times heavier than females; they possess an enlarged proboscis and large canines (King, 1983; Laws, 1993, 1994). The main reproductive events of this species take place during the spring of the Southern Hemisphere, along a wide latitudinal range (Carrick et al. 1962; Condy, 1979; Bester, 1980; Bester & Lenglart, 1982; Baldi, 1992; Campagna & Lewis, 1992; Campagna et al. 1993; Lewis et al. 1998; Galimberti & Boitani, 1999) that extends from Valdés Peninsula (42°S, 64°W) in the Argentinean Patagonia to Isla 25 de Mayo/King George Island (62°S, 58°W) in Antarctica (Laws, 1994; McMahon et al. 2005; Carlini et al. 2006; Mennucci et al. 2012). Many aspects of the biology of this species, such as demography and distribution (Carrick et al. 1962; Condy, 1979; Bester, 1980; Bester & Lenglart, 1982; Baldi, 1992; Campagna et al. 1993; Lewis et al. 1998; Galimberti & Boitani, 1999; McMahon et al. 2005) and even behavior (McCann, 1981; Modig, 1996; Negrete et al. 2011) have been extensively studied. Furthermore, anatomical studies regarding its skull allometry and ontogeny have recently been published (Tarnawsky et al. 2013).
Materials and methods
The tympanic region was studied using the complete skulls of 100 specimens, as well as 18 isolated auditory regions (see Appendix 1), and some comparisons were made with specimens of M. angustirostris (n = 3), Hydrurga leptonyx (n = 52), Phoca sp. (n = 90), Leptonychotes weddellii (n = 74), Lobodon carcinophagus (n = 49), and Ommatophoca rossii (n = 8). We are focusing on the morphology of the tympanic bone (endotympanic and ectotympanic) either on its external and internal views, and on the change of shape and size along its postnatal ontogeny. In addition some aspects of the morphological and topographically related basicranial region were considered. The specimens are deposited in the following collections:
FMM: Fundación Mundo Marino (San Clemente del Tuyu, Argentina)
IAA: Instituto Antártico Argentino Departamento Biología Predadores Tope (Buenos Aires, Argentina)
LAMAMA, (CENPAT): Laboratorio de Mamíferos Marinos del Centro Nacional Patagónico, CONICET (Puerto Madryn, Argentina)
MACN: Museo de Ciencias Naturales Bernardino Rivadavia (Buenos Aires, Argentina)
MHNM: Museo de Historia Natural de Montevideo Uruguay (Montevideo, Uruguay)
MLP: Museo de La Plata (La Plata, Argentina)
MNHN: Muséum National d'Historie Naturelle, Collection de Anatomie Compareé (Paris, France)
MVZ: University of California Museum of Vertebrate Zoology (Berkeley, CA, USA)
NMB: Naturhistorisches Museum Basel (Basel, Switzerland)USNM: United States National Museum of Natural History, Smithsonian Institution (Washington DC, USA)
ZM-UZH: Zoologisches Museum der Universität Zurich, Vertebrate Collection (Zurich, Switzerland)
Among the specimens with whole skulls, seven M. leonina individuals of both sexes of known ages were selected to be analyzed using high resolution computed axial tomography (CAT), to observe the internal morphology on 3D reconstructions. CAT scans were performed at the Centro de Imágenes Médicas (CIMED, in La Plata, Buenos Aires) using a Scan Philips Brilliance 64 with 0.56-mm resolution between slices. For comparative analyses, an additional CAT scan of an M. angustirostris female was obtained with permission from the Digimorph.org website and Tim Rowe's Digital Libraries Grant from NSF. The 3D reconstructions were made using the software programs mimics 10.01, and imagej 1.49f.
Different methods have been proposed to establish age classes for this species, consequently, different categories exist, which are in many cases not completely equivalent. For instance, external and behavioral characters are used for living individuals (see Laws, 1994), whereas in the case of skeletal materials, age is established on the basis of suture lines (see Morejohn & Brigs, 1973), growth lines observed in tooth sections (see Laws, 1953; Carrick et al. 1962; Loza et al. 2011) or sequence of tooth eruption, condyle-basal length and degree of extraoccipital bone fusion (see Tarnawsky et al. 2013). To be able to count the number of growth lines in tooth sections, we removed organic matter using H2O2 (100 vol. 1/10), then we cut thin sections mechanically after the sections were decalcified with 20% formic acid, and finally stained the sections with graphite; lines were counted under a binocular microscope (Fig. 1).
Figure 1.

Transverse section of an upper canine of a Mirounga leonina male, showing the annual growth lines in dentin and cementum. Scale bar: 5 mm.
Prior to deciding which system for age-class separation would best fit the goals of this work and the characteristics of the morphological structures to be analyzed, we divided all the specimens in our sample into groups following the age-class criteria proposed by other authors (e.g. Tarnawsky et al. 2013). Thus, we were able to verify that the separation of postnatal specimens (from newborns to adults), and even full-term unborn individuals, into more categories than the ones used here, did not reflect the differences observed in a statistically significant manner. Therefore, in this work, and because only cranio-dental materials were available, age classes (and consequently, assignation of specimen to them) were determined using the following criteria:
Absolute age obtained by us (see Loza et al. 2011) from the count of growth lines in tooth sections (only for specimens deposited in the collections IAA, MACN, and MLP) (see Fig. 1).
Sequence of tooth eruption of the permanent dentition.
Condyle-basal length and degree of suture fusion (taken in part from Tarnawsky et al. 2013).
On the basis of these indicators, the following age classes were established:
Class I (CI). Specimens younger than 1 year old, with erupted incisors and postcanines, canines partially erupted in females and not erupted in males. Condyle-basal length < 234 mm; undoubtedly sexually immature (Ling & Bryden, 1981, and bibliography therein).
Class II (CII). Specimens 1–4 years old (females), and 1–7 years old (males); tooth series completely erupted, with condyle-basal length > 260 mm. As Ling & Bryden (1981, and bibliography therein) pointed out, most specimens (males and females) included in those time periods are not sexually mature or, if so, are not actively reproducing (Ling & Bryden, 1981, p. 302). For instance, sexual maturity in males of this species may be reached at ca. 4 years of age (Laws, 1956) but physically immature juveniles of this age are prevented from participating in breeding activities until they are 7–9 years old (Carrick et al. 1962), with very few and fortuitous exceptions (Negrete et al. 2012); a 1- to 3-year-old female was observed breeding on one occasion in Potter Peninsula (J. Negrete, personal observation).
Class III (CIII). Specimens both sexually and physically mature. Females: over 4 years old, condyle-basal length > 276 mm; males: over 7 years old, condyle-basal length > 400 mm.
The cranial regions studied here were measured in each specimen using digital calipers (accuracy 0.1 mm); angles were measured using a goniometer (accuracy 1°), and skulls were photographed in traditionally used views.
Nomenclature for foramina, ducts and canals follows the Nomina Anatomica Veterinaria (2012).
Measurements and morphometric landmarks
The auditory regions were measured with the goal of identifying variables that could be correlated with the previously defined age classes (Fig. 2). The following measurements were taken:
Figure 2.
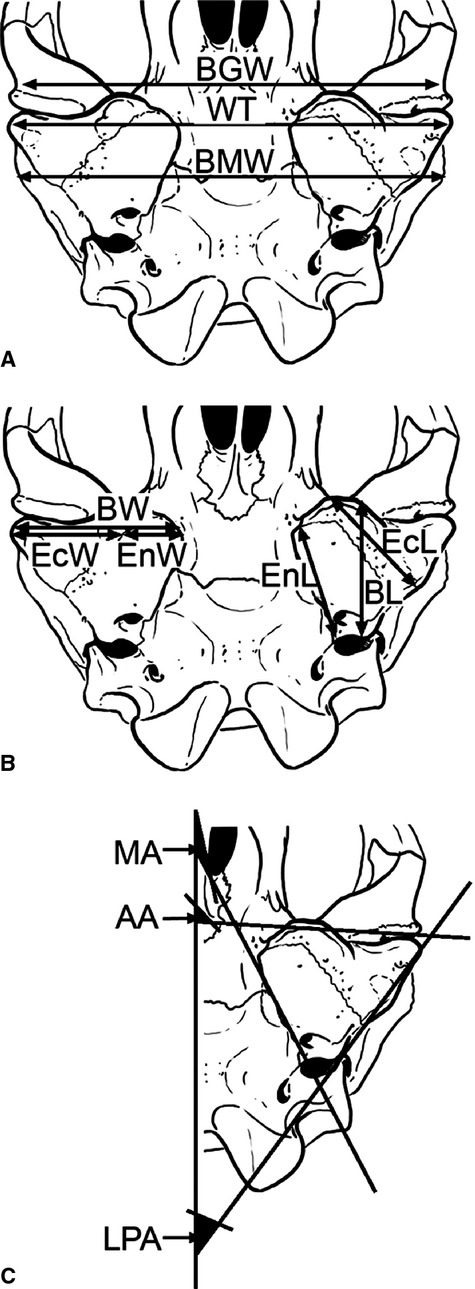
A and B: Ventral view of the skull of Mirounga leonina showing measurements and angles used in this work; C: detail of left bulla showing the angles). Anterior base angle (AA), biglenoid width (BGW), maximum length of the bulla (BL), bimastoid width (BMW), maximum width of bulla (BW), length of ectotympanic bone (EcL), ectotympanic width (EcW), maximum length of endotympanic bone (EnL), endotympanic width (EnW), latero-posterior base angle (LPA), medial base angle (MA), width between tubers (WT).
BGW (biglenoid width) – distance between lateral margins of articular surfaces of both glenoid fossae.
BL (maximum length of bulla) – antero-posterior distance between most anterior point of bulla (behind the glenoid fossa, not considering apophyses or processes that may or may not be present anteriorly to the posterior limit of this fossa), and most posterior point (which coincides with the triple suture between ectotympanic, mastoid, and basioccipital).
BMW (bimastoid width) – distance between most lateral extremes of mastoid apophyses.
BW (maximum width of bulla) – distance between medial margin of endotympanic and most exterior point of meatus acusticus externus.
CBL (condylo-basal length) – distance between the condylar plane and the distal tip of the palate.
EcL (length of ectotympanic bone) – distance between most anterior point of ectotympanic and posterior margin of the foramen stylomastoideum (FSM, see below).
EcW (ectotympanic width) – distance between endo-/ectotympanic suture and most lateral margin of ectotympanic tuber.
EnL (maximum length of endotympanic bone) – distance between most anterior point of endotympanic and FCP.
EnW (endotympanic width) – distance between medial margin of endotympanic and endo-/ectotympanic suture.
WT (width between tubers) – distance between lateral ectotympanic tubers.
Angles
AA (anterior base angle) – between anterior face of bulla and the sagittal plane (Fig. 2c).
LPA (posterior base angle) – between a line passing through most lateral point of external auditory meatus and jugular foramen (FY) and extended on posterior wall of the bulla, and the sagittal plane.
MA (medial base angle) – between medial face of bulla and the sagittal plane.
Specimen information
The geometric morphometric analysis included the 87 M. leonina skulls with available sex data on their labels obtained from seven collections (see Appendix 1). Digital images in palatal view were obtained using a Nikon Coolpix L120 digital camera mounted on a stand. Each photograph included a scale in order to account for size in the analyses, and all skulls were placed in the same position. Both sexes were nearly equally represented (42 females, 45 males).
Morphometric analysis
We used landmark-based geometric morphometric methods to analyze overall size and shape of the tympanic bulla in M. leonina (Rohlf & Marcus, 1993; Adams et al. 2004, 2013; Zelditch et al. 2004). These methods quantify the shape of anatomical objects from the coordinates of homologous locations, after the effects of non-shape variation (i.e. orientation, position, and scale) are held mathematically constant (Adams et al. 2013; Kelly et al. 2013). Two-dimensional coordinates of five homologous landmarks were digitized. Additionally, 24 equidistant semilandmarks (Gunz & Mitteroecker, 2013) were placed along the boundary between the endo- and ectotympanic bones to capture the shape and curvature of this structure (Fig. 3).
Figure 3.
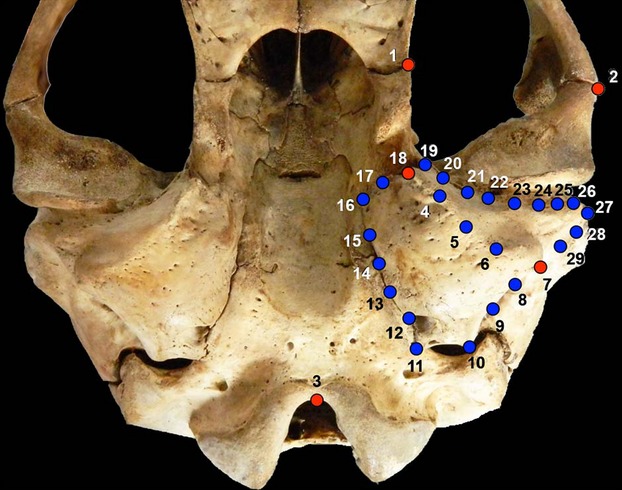
Ventral view of the skull of a CIII female of Mirounga leonina, showing the used morphometric points; red dots (1, 2, 3, 7, 18), landmarks; blue dots, semilandmarks.
The landmarks and semilandmarks were subjected to a generalized Procrustes analysis (GPA) (Rohlf & Slice, 1990). This procedure translates all specimens to the origin, scales them to unit centroid size, and optimally rotates them to minimize the total sums-of-squares deviations of the landmark coordinates from all specimens to the average configuration (Berns & Adams, 2013). During this procedure, semilandmarks are allowed to slide along their tangent directions (Bookstein, 1997; Bookstein et al. 1999) so as to minimize Procrustes distance between specimens (Pérez et al. 2006; Gunz & Mitteroecker, 2013). After superimposition, the aligned Procrustes shape coordinates are projected orthogonally into a linear tangent space yielding Kendall's tangent space coordinates (Dryden & Mardia, 1998; Rohlf, 1999; Claude, 2008; Berns & Adams, 2013), which are then treated as a set of shape variables to be used in the exploration of shape variation. Centroid size was also retained for further analyses.
The digitizing process was performed using tpsdig2 (Rohlf, 2009) and morphometric analyses were performed in r 3.0.2 (R Development Core Team, 2013) using routines in the package ‘geomorph’ (Adams & Otárola-Castillo, 2013).
Analysis of size and shape
The main focus of this work is the examination of possible sexual shape dimorphism and ontogenetic allometry of the tympanic bulla in M. leonina. Two sets of analyses were performed to assess patterns of sexual dimorphism. Initially, a principal components analysis (PCA) of the tangent space coordinates was performed to visualize patterns of shape variation in the shape space. Secondly, a Procrustes anova with permutation was used statistically to assess possible shape differences between males and females. Allometric patterns were visualized through a series of plots that describe the multivariate relationship between size and shape derived from landmark data. The abscissa of the plot is log (centroid size) and the ordinate represents shape, calculated as the common allometric component of the shape data, which is in turn an estimate of the average allometric trend within groups (Mitteroecker et al. 2004). In addition, a stylized graphic of the allometric trend was obtained for better visualization following Adams & Nistri (2010).
Results
External morphology of the tympanic region
Remarkably, the neonate specimens (IAA 01-14, LAMAMA ML024, LAMAMA ML026), defined as individuals ranging from newborn to 3 weeks old, did not show morphological differences with respect to CI individuals, and were therefore considered as part of the latter age class for these analyses (Figs 4 and 5).
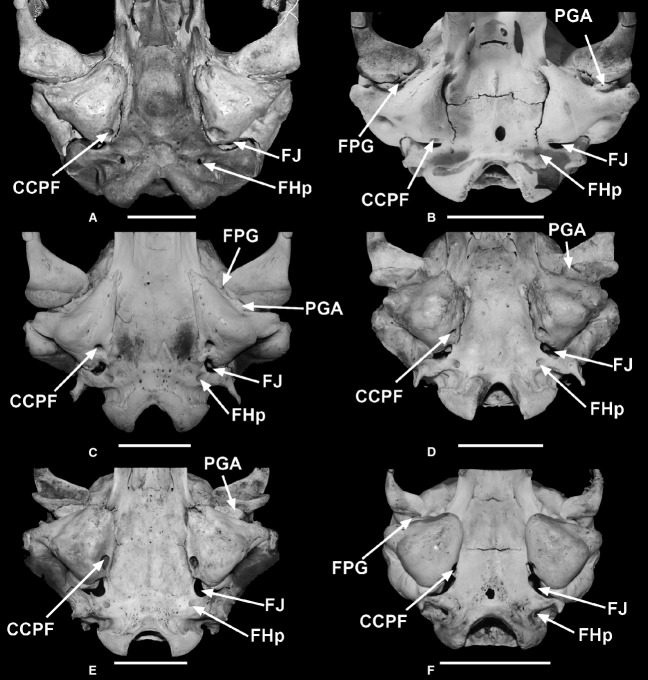
Figure 4.

Ventral views of the skull of Mirounga leonina, showing foramina and apophyses discussed in the text. (A) Ventral view, (B) latero-posterior view, (C) antero-ventral view. ‘A'F; ‘A’ foramen; AL, alisphenoid; BO, basioccipital; BS, basisphenoid; CCPF, posterior foramen of canalis caroticus; CG, glenoid fossa; Co, occipital condyle; EA, Eustachian apophysis; Ec/EnS, ectotympanic/endotympanic suture; EcT, ectotympanic bone; EcTT, ectotympanic tubercle; EnT, endotympanic bone; FCV, Fossa condylaris ventralis; FHp, foramen hypoglossis; FJ, foramen jugulare; FM, foramen magnum; FPG, foramen postglenoideum; FSM, foramen stylomastoideum; JA, jugular apophysis; JAa, jugular apophysis; anterior crista; MAE, meatus acusticus externus; PGA, postglenoid apophysis; PM, processus mastoideus; PPo, processus paraoccipitalis; PR, processus retroarticularis; PS, parasphenoid; SMA, stylomastoideum apophysis; STA, sulcus tubae auditivae.
Figure 5.

Isolated tympanic bulla of Mirounga leonina; generated by 3D reconstruction of the CAT of a CI specimen showing its internal and external anatomy. (A) Reconstructed ventral view, (B) reconstructed dorsal view, (C) reconstructed medial view, (D) reconstructed lateral view. CCAF, anterior foramen of canalis caroticus; CCLE, lateral extension of canalis caroticus; CCPF, posterior foramen of canalis caroticus; ET, Eustachian tube; MAE, meatus acusticus externus; STA, sulcus tubae auditivae. a, anterior; d, dorsal; l, lateral; m, medial; p; posterior.
The tympanic bulla of M. leonina presents the morphology characteristics of Phocidae (King, 1983; Wyss, 1988; Berta et al. 2006): it is primarily globose and triangular in outline, with a smooth surface and no markedly developed apophyses or processes (Figs 6 and 7). In contrast to the condition observed in Otariidae, the endotympanic bone is larger than the ectotympanic; the suture between these two bones is squamous and not always apparent, especially in the case of CIII individuals, in which it is completely obliterated and is represented only by a row of vascular foramina that indicate its approximate location. The mastoid region develops a rather evident pachyostosis (Berta et al. 2006), which grows in thickness concurrently with annual growth (and thus allows straightforward estimation of specimen age), hiding the sutures between the bulla, petrosal, mastoid, and squamosal. Only in some CI specimens is it possible with certainty to identify these boundaries in a CAT reconstruction (see Fig. 6).
Figure 6.
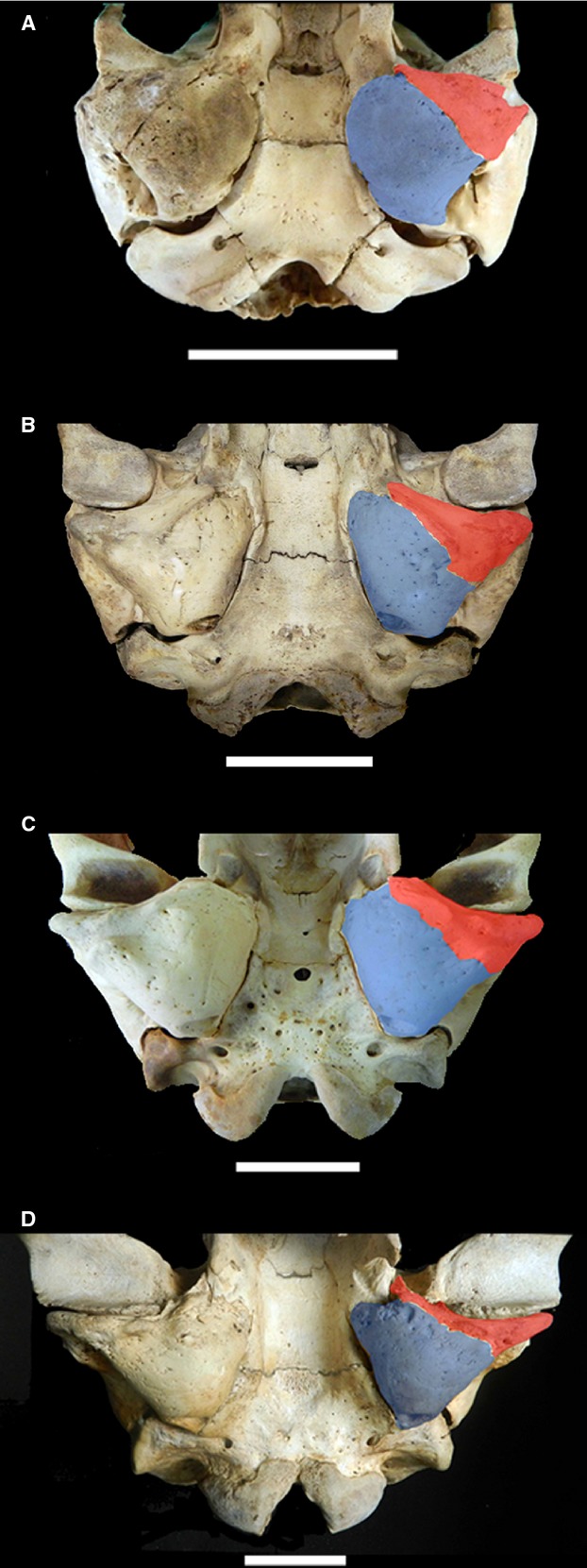
External morphology of tympanic bulla of Mirounga leonina showing differences in development of the ectotympanic (red) and endotympanic (blue) bones in different age classes: (A) Class I, (B) Class II, (C) Class III female, (D) Class III male. Scale bar: 5 cm.
Figure 7.
Ventral views of the skull of: (A) Mirounga angustirostris, (B) Ommatophoca rossii, (C) Hydrurga leptonyx, (D) Lobodon carcinophagus, (E) Leptonychotes weddellii, and (F) Phoca vitulina. CCPF, posterior foramen of canalis caroticus; FHp, foramen hypoglossis; FJ, foramen jugulare; FPG, foramen postglenoideum; PGA, postglenoid apophysis.
The following foramina, ducts, and passages (which are characteristic of most members of Arctoidea) were recognized (Figs 4 and 5):
Meatus acusticus externus (MAE) (Fig. 4B) is the largest opening, located laterally; it is smaller relative to skull length in males than in females; its size is similar to that of Lobodon, Phoca, and Leptonychotes, but smaller than in Ommatophoca, and larger than in Hydrurga; its contour is mostly circular in males and oval in females (Table1).
Sulcus tubae auditivae (STA), or (external foramen of the Eustachian tube) sensu Pocock 1916), is the anterior bony opening of the middle ear cavity continuous with the Eustachian tube (Fig. 4C). It is an obvious opening and generally oval in outline: relative to skull length, the foramen of males is the smallest of the family, whereas in females, its size is similar to those of Lobodon, Leptonychotes, and Phoca, and smaller that in Ommatophoca (Fig. 7). In other carnivorans, e.g. procyonids, hyaenids, and ursids, it is known as the ‘anterior opening of auditory tube’ (see Ivanoff, 2001) (Table1).
Anterior foramen of the canalis caroticus (CCAF), or foramen lacerum, is located posterior to the alisphenoid and represents the opening for a branch of the internal carotid artery (Fig. 5B,C). In this species, the CCAF does not open on the basicranial surface and is therefore not visible in the ventral view of the skull, as in most species except L. weddellii. In some groups (e.g. Primates) it is known as the ‘middle lacerate foramen’ (MacPhee, 1981; Wible, 1991).
Posterior foramen of the canalis caroticus (CCPF) is the posterior opening of the endotympanic traversed by the carotid (Figs 4A and 5A,C). Frequently, this foramen is not identified as such because in some other mammals it opens into a common vestibule with the foramen jugulare, and is therefore included as part of the latter (e.g. Wible, 1991, 2010). However, this foramen is always separate in both Phocidae and Otariidae, and quite conspicuous especially in the former. In M. leonina it has a circular outline and is the largest foramen (Fig. 7; Table1).
Foramen jugulare (FJ) is located between the bulla tympanica and the occipital bone; this opening is traversed by the glossopharyngeal (IX), vagus (X), and accessory (XI) nerves and the internal jugular vein: in this species the greater axis of this foramen is perpendicular to the sagittal plane. In relation to skull length, this foramen is always larger in females than in males of M. leonina, and it is the smallest in Hydrurga. It is mentioned in some texts as the ‘posterior lacerate foramen’ (e.g. canids, ursids, procyonids) (Figs 4A,B and 7; Table1).
Foramen stylomastoideum (FSM) is located postero-latero-dorsal to the bulla and posterior to the meatus acusticus externus, between the tympanic bulla and the mastoid process; it is traversed by the facial nerve (VII) and the stylomastoid vein (which goes through the inner ear) (Fig. 4B); it is always evident in both sexes and diverse age classes, but as in the case of the FJ, it is larger in females, and smallest in Phoca and Hydrurga.
Foramen post-glenoideum (FPG) is located immediately posterior to the postero-medial angle of the glenoid fossa, anterior to the tympanic bulla; it is the opening for the external jugular vein. This foramen tends to be small in the Phocidae, and in the case of M. leonina it is frequently absent in all age classes (Figs 4A and 7).
Foramen ‘A’ is located latero-posterior to the foramen stylomastoideum; it is not present in all specimens; when present, it may be well defined and separated from FSM, or joined to the latter, or appear as a blind depression (Fig. 4B).
Table 1.
Relative sizes of CCPF, FJ, Fhp, MAE, and FSM with respect to LBC, AMA, and AMB on six species of phocids.
| CBL/CCPF | CBL/FJ | CBL/FHp | CBL/MAE | CBL/FSM | CBL/STA | |
|---|---|---|---|---|---|---|
| Mirounga leonina (male) | 42–47 | 22–18 | 65–63 | 28–30 | 64–84 | 87 |
| Mirounga leonina (female) | 34–37 | 12 | 42 | 26 | 44–64 | 38–28 |
| Leptonychotes weddellii | 33 | 15 | 56 | 22 | 49 | 31 |
| Lobodon carcinophagus | 46 | 22 | 58 | 24 | 45 | 31 |
| Ommatophoca rossii | 31 | 23 | 41 | 14 | 59 | 24 |
| Hydrurga leptonyx | 43 | 30 | 78 | 43 | 389 | 59 |
| Phoca sp. | 42 | 13 | 71 | 25 | 102 | 40 |
| BMW/CCPF | BMW/FJ | BMW/FHp | BMW/MAE | BMW/FSM | BMW/STA | |
|---|---|---|---|---|---|---|
| Mirounga leonina (male) | 26–34 | 14–13 | 46–41 | 18–21 | 41–38 | 52 |
| Mirounga leonina (female) | 26 | 9 | 30–35 | 18–13 | 32–46 | 20–24 |
| Leptonychotes weddellii | 22 | 10 | 38 | 15 | 33 | 20 |
| Lobodon carcinophagus | 26 | 13 | 33 | 15 | 26 | 216 |
| Ommatophoca rossii | 23 | 17 | 30 | 10 | 44 | 18 |
| Hydrurga leptonyx | 24 | 17 | 43 | 24 | 218 | 35 |
| Phoca sp. | 22 | 7 | 39 | 13 | 54 | 21 |
| BW/CCFP | BW/FJ | BW/FHp | BW/MAE | BW/FSM | BW/STA | |
|---|---|---|---|---|---|---|
| Mirounga leonina (male) | 9–13 | 4 | 17 | 6–8 | 23–13 | 17 |
| Mirounga leonina (female) | 8–9 | 3 | 12 | 4–7 | 11–17 | 7–9 |
| Leptonychotes weddellii | 7 | 3 | 12 | 4 | 10 | 6 |
| Lobodon carcinophagus | 8 | 4 | 10 | 4 | 7 | 6 |
| Ommatophoca rossii | 8 | 6 | 11 | 4 | 16 | 7 |
| Hydrurga leptonyx | 7 | 5 | 13 | 7 | 66 | 10 |
| Phoca sp. | 7 | 2 | 13 | 4 | 18 | 2 |
Lastly, the foramina in the cranial base are:
Foramen hypoglossus (FHp): for the passage of the hypoglossal nerve (XII); due to its location close to the occipital condyles, it is also known as ‘condylar foramen’ (Figs 4A,B and 7). This foramen is relatively larger in females than in males (Table1).
Foramen ovale (FOv) is located on the alisphenoid, for the passage of the mandibular branch of the trigeminal nerve (V3).
The foramina and openings with the most consistent presence in this region are: meatus acusticus externus, foramen for the canalis caroticus posterior, jugulare, hypoglossis (reduced compared with the condition in Otariidae), stylomastoideum and postglenoideum (reduced or absent), and the ‘A’ foramen of uncertain homology.
The bulla tympanica presents a small anterior crista (JAa) (Fig. 4A) on the ectotympanic that extends along the anterior third of the latter, and a posterior crista (CP) that extends parallel to the ecto-/endotympanic suture. These two structures probably correspond to the ‘jugular apophysis’ of Otariidae; under this assumption, they may be described as two cristae (portions) of the ‘jugular apophysis’ (JA), one anterior (JAa) and the other posterior (JAp).
The tympanic bone also has a postglenoid apophysis (PGA), or tympanic process, smaller than that of Lobodon and Leptonychotes, that surrounds the FPG posteriorly and extends perpendicularly to the sagittal plane (Fig. 7). This apophysis should not be confused with the processus retroarticularis (PR) of the glenoid fossa; the latter surrounds the fossa whereas the PGA corresponds to the ectotympanic (Fig. 4A). Another apophysis is located adjacent to the anterior opening of the Eustachian tube (Fig. 4C). The ectotympanic bone develops a lateral tuberosity (EcTT) whose tip is more pointed in adult males than females.
Furthermore, both the mastoid region with its processus mastoideus (PM) and the paraoccipital region with its processus paraoccipitalis (PPo) (Fig. 4A) are well developed in CIII males and females and in some CII males.
External morphology and age classes
In external view, specimens of CI and CII do not possess well developed apophyses in the tympanic region; instead, they present markedly rugose areas. In CIII individuals the apophysis EA and the anterior process of JA are already visible (growth of the latter is already observable in CII). Remarkably, only JA shows a morphological trajectory that differs between males and females (see below and Fig. 6).
The EcTT was not observed in CI; this structure is well developed in CII and CIII, and its enlargement is accompanied by progressive elongation of the MAE.
In the course of ontogeny, the glenoid fossa reaches an apparently more posterior position so that it extends further onto the anterior wall of the tympanic bulla (Fig. 6). This seems to be brought about by the greater development of the retroarticular process from CII; this process becomes more vertical, thus providing stronger support for the mandibular condyle, mainly in CIII males (Fig. 6D).
External morphology in males and females
In CI specimens, external morphology of males and females is similar, even though males are slightly larger; sexual dimorphism begins to be evident from CII onwards.
Given that this is a clearly dimorphic species, by CIII morphological differences are evident, and the size difference is quite marked. In females the surface of the endotympanic bone is evidently globose, whereas it is flatter in males (Fig. 6C,D); and the ectotympanic of males bears evident rugosities that become more marked in older specimens.
The outline of the tympanic bulla maintains a rather constant shape in females of the three age classes; in contrast, its characteristic angular outline changes from CI through CIII, and in CIII, the apex of EcTT is angular in males and rounded in females.
Angularity (referring to change in the angle formed by the anterior base of the bulla and the sagittal plane of the skull) decreases in males, so that the AJ reaches a position almost perpendicular to the sagittal plane, whereas in females this angle remains at values greater than 90° (close to the values for juveniles).
Internal morphology of the bulla
From an anatomical and functional viewpoint, the middle ear can be divided into three well differentiated parts: the annexae mastoidae, the cavum tympani and the Eustachian tube, or Tuba pharyngotympanica (Thomassin et al. 2008) (Figs 1). The middle ear is formed by a pars petrosa, a pars tympanica, and a pars escamosa; these three parts are also involved in the conformation of the external and inner ear (Fig. 8A).
Figure 8.
Transversal section (TS) from CAT of the skull of Mirounga leonina at the auditory region level. (A) Posterior TS showing the paries labyrinthica, pars petrosa (pink), pars squamosa (green), and pars tympanica (brown). (B) More anterior TS showing the endotympanic/ectotympanic suture and the relationship of CCLE (lateral extension of canalis caroticus), as part of the paries tegmentalis or roof of the tympanic cavity. Skulls in lateral view show the precise line of each TS.
Following the work of Gray (1858), below we describe the six walls that delimit the tympanic bulla and its cavum tympani.
Paries tegmentalis (tectum) (Fig. 8B) – formed by the ventral wall of the petrosal, the tegmen tympani in which the recessus epitympanicus is excavated; the latter is a conspicuous hemispheric cavity that contains the large incus.
In addition, this roof is also formed medially by a thin bone layer that extends from the lateral margin of the canalis caroticus and covers the petrosal ventrally, and the ventro-lateral surface of the petrosal; this conformation is visible in the most cranial portion of the bulla (Figs 8 and 9). Both parts separate the cavum tympani from the base of the skull. The thin bone plate of the canalis caroticus (a well developed channel that pierces the endotympanic bone in an antero-dorsal to postero-ventral direction) is antero-posteriorly extended in the middle zone, forming the first third of the roof in the cranial medial half; due to the triangular outline of the bulla (which forms a wedge toward the rear), this bone plate forms all of the roof in the posterior or caudal half, with no participation of the petrosal (Fig. 9).
Paries jugularis (floor) (Figs 10B and 11A) –extends below the level of the lower wall of the MAE; its anterior region bears the recessus hypotympanicus (Fig. 10A) just below the recessus epitympanicus, the rest of its surface is slightly concave and smooth.
Paries labyrinthica (inner wall) (Fig. 11C) –includes the following structures: fenestra ovalis, fenestra rotunda, and promontorium. Gray (1858) also described a Fallopian aqueduct that we did not observe in M. leonina. Due to the enormous relative size of the petrosal, and to its oblique position (antero-medial and postero-lateral) with respect to the skull base, the paries labyrinthica is part of the dorsomedial and dorsolateral boundaries of the cavum tympani (at its anterior and posterior parts, respectively). This paries is completed medial by the lateral wall of the canalis caroticus (Fig. 12).
Paries mastoidea (posterior wall) (Fig. 11A,B) – contacts with the annexae mastoidae which are poorly developed in CI but are evident in CIII individuals, especially males; this may be due to the fact that the formation of the annexae mastoidae begins during fetal life, but takes place mostly after birth (e.g. Homo, see Tran Ba Huy & Teissier, 2011).
Paries carotica (anterior wall) (Fig. 11A,B) – circumscribes the posterior bony opening of the Eustachian tube, or tuba pharyngotympanica, in both adults and juveniles.
Paries membranaceus (external wall) (Fig. 11B,C) – defines the meatus acusticus externus (part of the external ear), which becomes proportionally narrower and longer during ontogeny. The meatus acusticus externus runs postero-ventrally from the cavum tympani to the exterior.
Figure 9.
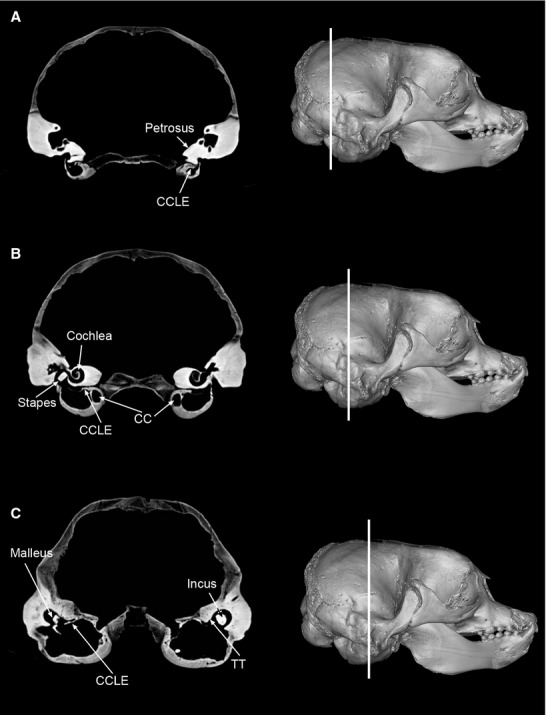
Three consecutive TS views from CAT of Mirounga leonina, in posterio-anteror sequence, from a CAT, showing variation in extension of the paries tegmentalis along the bulla and gradual reduction of the tympanic cavity. (A) Caudal TS, showing the petrosal excluded from the tympanic bullar roof, which is completely formed by the CCLE, and tympanic cavity restricted to a small space. (B) Middle TS, showing a bigger tympanic cavity, with a visible tegmen tympani (TT), and lateral extension of the canalis caroticus (CCLE). (C) Anterior TS, showing a large cavity, recessus epitympanicus that contains the malleus and incus (both large), and tegmen tympani (TT), associated to the lateral extension of the canalis caroticus (CCLE). Skulls in lateral view show the precise line of each TS.
Figure 10.

Two consecutive TS views from CAT of auditory region of Mirounga leonina showing the paries that limit the tympanic cavity. (A) View of posterior TS. (B) View of more anterior TS. Skulls in lateral view show the precise line of each TS.
Figure 11.

Three views from CAT of auditory region of Mirounga leonina showing the paries that limit the tympanic cavity. (A) View of a lateral, parasagittal section. (B) View of section through a ventral horizontal plane. (C) View of section through ventral horizontal plane comparing two specimens of different age classes CI (to the left) and CIII (to the right), showing the difference in relative development of the meatus acusticus externus and the Eustachian tube, as well as different internal morphology of the bulla. Skulls in dorsal and lateral view show the precise line of each section.
Figure 12.
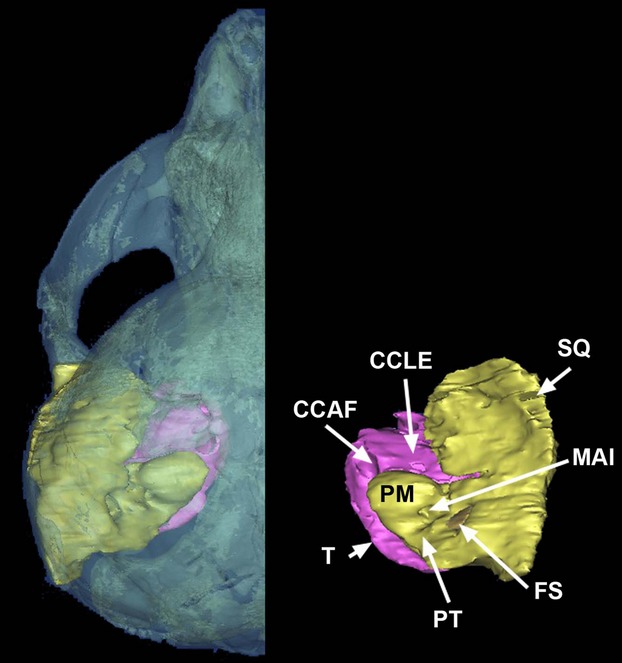
3D reconstruction of a skull of a dorsal view of Mirounga leonina with left auditory region, and detail of right auditory region, showing the tympanic bulla (pink) and the petrosal fused to the squamosal (yellow). CCAF, foramen of the canalis caroticus anterior; CCLE, lateral extension of the canalis caroticus; FS, fossa subarcuata; MAI, meatus acusticus internus; PM, promontorium; PT, petrosal; SQ, squamosal; T, tympanic.
Morphometrics
The first two principal components explained 73% of the total shape variation, and showed several distinct clusters of specimens (Fig. 13). In general, males and females were at opposite extremes of the plot, with some degree of overlapping, implying that shape differences between sexes exist. A statistical evaluation was performed using Procrustes anova with permutation, revealing significant shape differences both between sexes and between age stages, but the interaction term was marginally non-significant (Table2).
Figure 13.
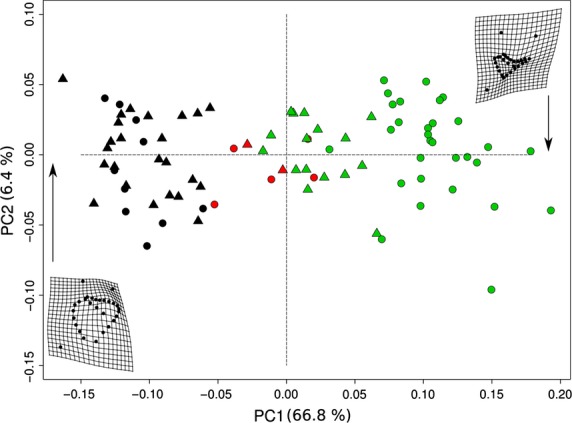
Principal components analysis of shape variables in tangent space showing shape variation. Deformation grids display the shape of specimens of Mirounga leonina at the ends of the range of variability along PC1. Black, CI. Red, CII. Green, CIII. Circles correspond to males and triangles to females.
Table 2.
Table listing results of statistical analyses on the Mirounga leonina specimens. For further details, see Materials and methods
| Factor | df | SS.obs | MS | F | P-value | R 2 |
|---|---|---|---|---|---|---|
| Sex | 1 | 0.208 | 0.208 | 38.530 | 0.0001 | 0.1746 |
| Stage | 2 | 0.495 | 0.247 | 45.672 | 0.0001 | 0.4140 |
| Sex : Stage | 2 | 0.052 | 0.026 | 4.878 | 0.0891 | 0.0442 |
df, degrees of freedom; F, F ratio; MS, mean squares; SS, sum of square. P-value in bold.
Significant allometry was detected by means of a multivariate regression of shape on size (centroid size) (P-value < 0.01). Males and females have approximately parallel trajectories, showing a common allometric pattern (Berge & Penin, 2004; Mitteroecker et al. 2004) (Fig. 14). However, when different age stages were taken into account, the patterns of allometric trajectories differed (Fig. 15). The allometric patterns as reported for stage CI were clearly different compared with those of stages CII and CIII (Fig. 15).
Figure 14.
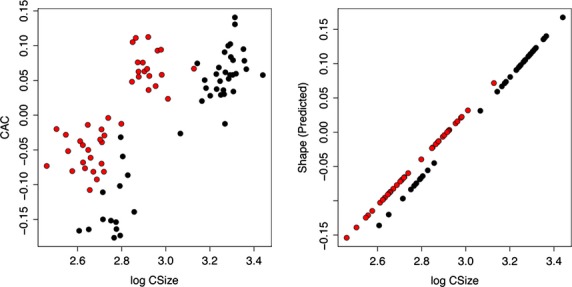
Allometric trends of males and females of Mirounga leonina showing the multivariate relationship between size and shape. (A) The common allometric component (CAC) vs. size (log centroid size). (B) Stylized trend following Adams & Nistri (2010). Black, CI. Red, CII. Green, CIII. Circles correspond to males and triangles to females.
Figure 15.
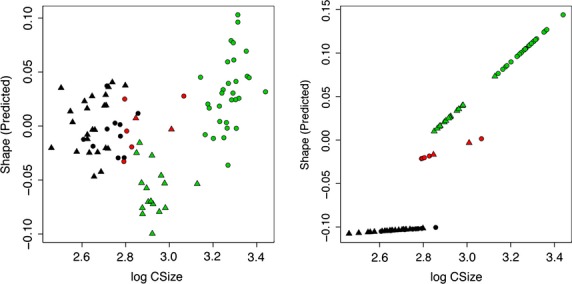
Allometric trends of different age stages of Mirounga leonina showing the multivariate relationship between size and shape. (A) The common allometric component (CAC) vs. size (log centroid size). (b) Stylized trend following Adams & Nistri (2010). Black, CI. Red, CII. Green, CIII. Circles correspond to males and triangles to females.
Discussion
The study of a large sample of M. leonina made possible the characterization of the ear anatomy of this specialized carnivoran and the exploration of ontogenetic and sex differences. Very few changes were detected after birth on the internal anatomy of the auditory region, between both sexes and age classes. However, significant differences between age classes were found in bone thickness, resulting from the apposition of new annual layers. Furthermore, there are possible sex-related external morphology differences among age classes that show significant shape differences in CIII. The external morphology observed in neonates is conserved across all individuals from CI (including specimens up to 1 year old); however, morphological differences were observed between them, on one hand, and CII individuals plus CIII females on the other, and the CIII males showed the greatest morphological change, with respect to all the individuals regardless of age class or sex. Most foramina of the tympanic-basicranial region of M. leonina are relatively smaller in adult males than in adult females in relation to skull length. In turn, the foramina of females are similar in relative size to those of Leptonychotes and Lobodon, but generally larger in relative size than in Hydrurga and generally smaller compared with Ommatophoca. The relative size of these foramina in Phoca is heterogeneous (Table1).
The MAE is clearly evident in all age classes and both sexes. Although its outline may vary in shape between CIII males and females, this may be related to sex differences in growth patterns.
The STA is a large foramen in all classes and both sexes, probably in relation to the need to compensate for differential pressures at great depths, as this foramen is continuous with the Eustachian tube.
The CCAF and CCPF in all specimens examined are large and associated to the carotid ramus that brings blood to the encephalon and inner ear; their size could be related to the physiology of diving and the need for large volumes of blood to be transported to avoid collapse of the circulatory system.
The FPG is absent or very reduced (probably due to the expansion of the post-glenoid process onto the tympanic bulla) and this has led to the assumption that the cephalic jugular drainage must follow a different path to ensure efficient venous return.
The large size of the foramina can be observed from early ages (CI); this could be associated to the short duration of nursing and parental dependence prior to their first independent feeding season. The foramina are also large in CII males and females and in CIII females. In contrast, in males the foramina are relatively smaller after sexual maturation (CIII males).
All preceding observations about the relative size of foramina were made with respect to skull length; the results are slightly different if the bimastoid width or tympanic width are used as reference. Nevertheless, in this case, again the foramina of M. leonina males (CIII) are relatively smaller than those of females (CIII), in accordance with the change in male morphology associated to sexual maturity in a conspicuously dimorphic species. Also, the relative sizes of foramina in M. leonina females are similar to those of Ommatophoca, Leptonychotes, Phoca, and Lobodon, but Hydrurga consistently has the smallest foramina (Fig. 7; Table1).
Finally, the homology of the FA is uncertain; it could either correspond to the vagal foramen or alternatively it could represent a deviation of the FSM, given that the FA is associated to the latter in all observed cases.
The large size of the apophyses and processes (e.g. PPO and PM together with the lambdoidal ridge of the skull) in CIII individuals is likely associated to the attachment of neck and mandibular muscles. The angle of JA changes as a consequence of the direction of growth of the ectotympanic, which becomes increasingly more perpendicular with respect to the sagittal axis (Fig. 6).
As stated above, the internal anatomy of the auditory region does not show great differences between sexes or age classes; however, bullar volume appears to decrease and some ducts increase in length and diameter (e.g. MAE, STA), surely as a consequence of the skull growth pattern of this species. The greatest differences between age classes are related to bone thickness resulting from the apposition of new layers year after year.
The results of the Procrustes anova show significant shape differences between sexes and age classes. Although the interaction term (sex : classes) is marginally non-statistically significant (P-value ∽0.08), an examination of possible sex differences within age classes shows that there are significant shape differences between males and females in CIII. This is partly consistent with Mitteroecker et al.'s (2004) findings, in which individuals were rather similar in early ontogeny and subsequently diverged in adult morphology.
The shape observed in neonates was the same as that of individuals in CI, a result that is quite striking because these two classes differ markedly in size and because newborn pups suckle milk, whereas CI individuals have already spent a season feeding independently from their mothers. No statistically significant differences in tympanic-basicranial structure were found in CI; all individuals were characterized by a markedly globose bulla without well developed apophyses or processes; the endo-/ectotympanic suture was visible in some specimens; bones presented a spongy or porous structure, and the bullar wall was thin because its thickness increases due to consecutive deposition of annual layers (Fig. 16). The specimens that were subjected to CAT showed agreement as to their age as determined from bullar growth lines and the absolute age determined a priori from tooth sections.
Figure 16.

Four views from CAT of auditory region of Mirounga leonina showing the bone thickness resulting from the apposition of new layers year after year. (A) CI with no lines of growing, (B) CII with one line, (C) CII with three lines, (D) CIII with at least five lines.
CII specimens showed relatively less development of the ectotympanic and more compact bone structure than CI specimens. CII specimens of both sexes did not differ greatly from CIII females regarding bullar morphology. Furthermore, CII individuals already presented most of the characteristics observed in CIII specimens. Thus, there are clear morphological differences between CI individuals on one hand, and CII individuals plus CIII females on the other. Some of the most noticeable differences are the smaller size of the ectotympanic relative to the endotympanic, the formation (and growth) of rugosities on the ectotympanic surface, clear development of a more triangular outline, and distal growth of the retroarticular process (PR) of the glenoid cavity (CG), which becomes more posterior and vertical. Likewise, as a result of the mesio-distal growth of the CG, the latter ends up resting on the anterior surface of the bulla, partially compressing it; this condition reaches its maximum expression in CIII males.
Females attain the definitive shape and size of the bulla at earlier stages of ontogeny compared with males, in agreement with their earlier involvement in reproductive activities. Thus, the bullar morphology of females is similar in all age classes; this could be related to the depths reached when diving in search of food (Boyd & Arnbom, 1991; Campagna & Lewis, 1992; Hindell & Bryden, 1992; McConnell et al. 1992, 2002; Campagna et al. 1993, 1995, 1998, 1999, 2000, 2007; Jonker & Bester, 1998; Hindell et al. 1999; Lewis et al. 2006; Field et al. 2007; Eder et al. 2010; McIntyre et al. 2010a,b). CIII males showed the greatest morphological differences, with respect to both CI and CII individuals and CIII females. CIII males dive to the greatest depths recorded so far for the species, more than 2000 m deep, whereas females and CII males have not been recorded below 1500 m depth, and CI individuals (males and females) make short shallow dives, reaching ca. 100 m depth (McIntyre et al. 2010a).
The morphological differences between the bullae of males and females could be related to the different behavior of the sexes during the breeding season, too. Whereas females must be able to recognize the call of their pup in the harem (at short range), males participate in agonistic interactions, for which the recognition of vocalizations emitted by other males at greater distances is essential. In addition, males engage in intraspecific combats, during which the posterolateral region of the skull is hit quite often.
Mirounga leonina did not show remarkable differences compared with specimens of its sister species from the Northern Hemisphere, M. angustirostris (USNM 260867 and USNM- A21890), and compared with a CAT from the Digimorph.org website (MVZ 184140), in either its internal or external morphology and proportions, despite the larger size of M. leonina. Moreover, if the values for the M. angustirostris female are included in the morphometric analysis and PCA, the specimen falls within the CI–CII range.
Acknowledgments
We thank the curators and staff responsible for the collections consulted: I. Olivares and D. Verzi (MLP); D. Flores, B. Tarnawsky and S. Lucero (MACN); E. González and S. Riverón (MHNM of Montevideo, Uruguay); C. Lefévre (MNHN of Paris, France); L. Costeur (NMB); M. Haffner (ZM-UZH); M.E. Márquez, N. Coria, A. Menucci, M. Juárez, M. Santos. Sr. Aldo Corbalán (IAA); E. Crespo and N. Garcia (CENPAT); J. Loureiro and scientific staff at FMM; C. Morgan for the English version; technicians at CIMED La Plata for their assistance during tomographies; to the Digimorph.org website, and Tim Rowe's Digital Libraries Grant from NSF, for allow us to use the CAT of M. angustirostris; Jorge González for scientific illustrations; M. Cordeiro de Castro and F. Galliari for critical reading of an early version of this paper; B. Tarnawsky and M. E. del Corro for their help in age determination; C. Desimone for her help in measuring the specimens. We especially thank the two reviewers and the Editor who improved our early version of the paper. This project was partially founded by UNLP N-593 and N-724 (to A.A.C.), and N-700 and N-732 (to L.H.S.). We also wish to express our deep respects to the memory of Dr. Alejandro R. Carlini, who dedicated years to scientific research in Antarctica, and was one of the fundamental pillars for the development of this and many other projects.
Appendix 1
Specimens of Mirounga leonina examined in this study
| Collection number | Sex | Age class | BL | |
|---|---|---|---|---|
| 1 | MACN 20608* | M | CIII | 89.88 |
| 2 | MACN 24.91* | M | CIII | 104.53 |
| 3 | MACN 13.26* | M | CIII | 107.76 |
| 4 | MACN 24.93* | M | CIII | 88.33 |
| 5 | MACN 24.49 | M | CIII | 92.48 |
| 6 | MACN 24.92* | M | CIII | 90.65 |
| 7 | MACN 22611* | F | CIII | 63.57 |
| 8 | MACN 26222* | M | CII | 63.18 |
| 9 | MACN 22614* | F | CIII | 70.45 |
| 10 | MACN 22612* | F | CIII | 73.48 |
| 11 | MACN 22613* | F | CIII | 69.32 |
| 12 | MACN 49.52* | F | CI | 60.31 |
| 13 | MACN 22615* | F | CI | 48.66 |
| 14 | MLP 947* | M | CIII | 109.63 |
| 15 | MLP 26.IV.00.13* | M | CII | 109.81 |
| 16 | MLP 1504* | M | CIII | 95.59 |
| 17 | MLP 14.IV.48.13 | M | CI | 57.15 |
| 18 | MLP 1966* | F | CI | 51.4 |
| 19 | MLP 1971* | F | CI | 49.49 |
| 20 | FMM 107 | M | CIII | 99.77 |
| 21 | FMM 109* | M | CIII | 80.01 |
| 22 | IAA AA-A* | F | CIII | 70.46 |
| 23 | IAA AA-B* | M | CI | 58.86 |
| 24 | IAA AA-C* | M | CI | 59.49 |
| 25 | IAA AA-7* | F | CI | 55.97 |
| 26 | IAA AA-6* | M | CI | 58.21 |
| 27 | IAA AA-8* | F | CI | 50.88 |
| 28 | IAA AA-2* | M | CI | 52.89 |
| 29 | IAA AA-11* | F | CI | 57.5 |
| 30 | IAA AA-10* | F | CI | 52.53 |
| 31 | IAA AA-9* | F | CI | 56.5 |
| 33 | IAA 02.14* | M | CI | 58.09 |
| 34 | IAA 02.19* | M | CI | 61.6 |
| 35 | IAA 02.25* | F | CI | 50.23 |
| 36 | IAA 02.18 | M | CI | 59.38 |
| 37 | IAA 02.30* | M | CI | 57.44 |
| 38 | IAA 02.28* | F | CI | 59.03 |
| 39 | IAA 02.22* | M | CI | 59.98 |
| 40 | IAA 02.29* | F | CI | 55.11 |
| 41 | IAA 02.20* | F | CI | 59.34 |
| 42 | IAA 02.26* | M | CI | 58.16 |
| 43 | IAA 02.23* | F | CI | 55.21 |
| 44 | IAA 02.24* | F | CI | 58.68 |
| 45 | IAA 02.17* | F | CI | 51.54 |
| 46 | IAA 02.21* | M | CI | 62.61 |
| 47 | IAA 02.12* | M | CIII | 78.92 |
| 48 | IAA 99.5* | M | CIII | 108.8 |
| 49 | IAA 00.8* | M | CIII | 112.43 |
| 50 | IAA 03.5* | F | CIII | 73.91 |
| 51 | IAA 03.4* | F | CIII | 73.55 |
| 52 | IAA 96.1* | F | CIII | 70.92 |
| 53 | IAA 00.9* | F | CIII | 70.34 |
| 54 | IAA 01.14* | F | CI | 44.64 |
| 55 | IAA 02.16* | M | CII | 63.85 |
| 56 | IAA 02.27 | M | CI | 53.52 |
| 57 | LAMAMA ML-25* | F | CII | 79.46 |
| 58 | LAMAMA CNP-105* | M | CIII | 108.33 |
| 59 | LAMAMA CNP-035* | M | CIII | 106.17 |
| 60 | LAMAMA ML-059* | M | CIII | 105.13 |
| 61 | LAMAMA CNP-102* | M | CIII | 97.91 |
| 62 | LAMAMA CNP-104* | M | CIII | 103.31 |
| 63 | LAMAMA CNP-111* | F | CIII | 73.21 |
| 64 | LAMAMA CNP-109 | M | CII | 64.56 |
| 65 | LAMAMA 103* | M | CIII | 100.12 |
| 66 | LAMAMA CNP-101* | M | CII | 65.06 |
| 67 | LAMAMA CNP-037* | F | CI | 61.98 |
| 68 | LAMAMA CNP-100 | F | CII | 64.54 |
| 69 | LAMAMA ML-32* | M | CIII | 107.43 |
| 70 | LAMAMA ML-34* | M | CIII | 96.92 |
| 71 | LAMAMA ML-28* | M | CIII | 100.55 |
| 72 | LAMAMA ML-35* | F | CIII | 75.94 |
| 73 | LAMAMA ML-36* | F | CI | 53.45 |
| 74 | LAMAMA ML-29 | M | CII | 71.04 |
| 75 | LAMAMA ML-30 | M | CII | 72.8 |
| 76 | LAMAMA ML-33 | F | CI | 42.85 |
| 77 | LAMAMA ML-23 | M | CI | 49.52 |
| 78 | LAMAMA ML-24* | F | CI | 47.43 |
| 79 | LAMAMA ML-26* | F | CI | 59.9 |
| 80 | LAMAMA ML-31* | F | CIII | 73.61 |
| 81 | LAMAMA ML-1* | F | CI | 53.77 |
| 82 | LAMAMA ML-4* | F | CI | 50.51 |
| 83 | LAMAMA ML-3* | M | CII | 71.39 |
| 84 | LAMAMA ML-9 | F | CIII | 74.82 |
| 85 | MHNM 5767* | M | CIII | 111.7 |
| 86 | MHNM 5766* | M | CIII | 105.89 |
| 87 | MHNM 1277* | M | CIII | 104.2 |
| 88 | MHNM 5768* | M | CIII | 99.5 |
| 89 | MHNM S/N* | F | CI | 54.32 |
| 90 | MNHN 1972-647* | M | CIII | 107.72 |
| 91 | MNHN 1971-113* | M | CIII | 103.34 |
| 92 | MNHN 2012-983* | M | CIII | 112.9 |
| 93 | MNHN 1972-652* | M | CII | 82.21 |
| 94 | MNHN 2012-986* | M | CIII | 120.17 |
| 95 | MNHN 1978-347* | F | CIII | 73.85 |
| 96 | MNHN 2012-985* | F | CIII | 70.18 |
| 97 | MNHN 1972-651* | F | CIII | 75.46 |
| 98 | MNHN 1939-449* | M | CIII | 109.33 |
| 99 | MNHN 1972-142* | F | CII | 66.09 |
| 100 | MNHN 1977-20* | M | CIII | 110.8 |
| 101 | IAA AA-14 a | M | CII | n/a |
| 102 | IAA AA-14 b | M | CII | n/a |
| 103 | IAA AA-14 c | M | CII | n/a |
| 104 | IAA AA-14 d | M | CII | n/a |
| 105 | IAA AA-14 e | indet | CI | n/a |
| 106 | IAA AA-14 f | indet | CI | n/a |
| 107 | IAA AA-14 g | indet | CI | n/a |
| 108 | IAA AA-14 h | indet | CI | n/a |
| 109 | IAA AA-14 i | indet | CI | n/a |
| 110 | IAA AA-14 j | indet | CI | n/a |
| 111 | MLP 777-C | indet | CI | n/a |
| 112 | MLP 781-C | indet | CI | n/a |
| 113 | MLP 775-C | indet | CI | n/a |
| 114 | MLP 782-C | M | CIII | n/a |
| 115 | MLP 779-C | indet | CI | n/a |
| 116 | MLP 783-C | F | CIII | n/a |
| 117 | MLP 784-C | indet | CI | n/a |
| 118 | MLP 785-C | indet | CI | n/a |
F, females; M, males; n/a, not available data because of isolated, or partially broken, otic regions.
For museum abbreviations, see Materials and methods.
Specimens used in the morphometric analyses.
References
- Adams DC, Nistri A. Ontogenetic convergence and evolution of foot morphology in European cave salamanders (Family: Plethodontidae) BMC Evol Biol. 2010;10:216. doi: 10.1186/1471-2148-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DC, Otárola-Castillo E. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol. 2013;4:393–399. [Google Scholar]
- Adams DC, Rohlf FJ, Slice DE. Geometric morphometrics: ten years of progress following the ‘revolution’. Ital J Zool. 2004;71:5–16. [Google Scholar]
- Adams DC, Rohlf FJ, Slice DE. A field comes of age: geometric morphometrics in the 21st century. Hystrix. 2013;24:8. [Google Scholar]
- Arnaudo ME, Soibelzon LH, Bona P, et al. First description of the auditory region of a Tremarctine (Ursidae, Mammalia) Bear: the case of Arctotherium angustidens. J Mamm Evol. 2014;21:321–330. [Google Scholar]
- Baldi R. Efecto del espacio disponible sobre el comportamiento social y el éxito reproductivo del elefante marino del sur, Mirounga leonina, en la Patagonia. Puerto Madryn, Argentina: Tesis de Grado, Facultad de Ciencias Naturales, Universidad Nacional de la Patagonia; 1992. [Google Scholar]
- Beaumont G. Note sur la region auditive de quelques Carnivores. Arch Sci. 1968;21:211–224. [Google Scholar]
- Berge C, Penin X. Heterochrony, and interspecific differences in the skull of African apes, using tridimensional procrustes analysis. Am J Phys Anthropol. 2004;124:124–138. doi: 10.1002/ajpa.10333. Ontogenetic allometry. [DOI] [PubMed] [Google Scholar]
- Berns CM, Adams DC. Becoming different but staying alike: patterns of sexual size and shape dimorphism in bills of hummingbirds. Evol Biol. 2013;40:246–260. [Google Scholar]
- Berta A, Sumich JM, Kovacs KM. Marine Mammals: Evolutionary Biology. 2nd edn. Burlington and California, USA: Elsevier; 2006. [Google Scholar]
- Bester MN. The southern elephant seal (Mirounga leonina) at Gough Island. S Afr J Zool. 1980;15:235–239. [Google Scholar]
- Bester MN, Lenglart PY. An analysis of the southern elephant seal Mirounga leonina breeding population at Kerguelen. S Afr J Antarct Res. 1982;12:11–16. [Google Scholar]
- Bookstein FL. Landmark methods for forms without landmarks: localizing group differences in outline shape. Med Image Anal. 1997;1:225–243. doi: 10.1016/s1361-8415(97)85012-8. [DOI] [PubMed] [Google Scholar]
- Bookstein F, Schafer K, Prossinger H, et al. Comparing frontal cranial profiles in archaic and modern homo by morphometric analysis. Anat Rec. 1999;257:217–224. doi: 10.1002/(SICI)1097-0185(19991215)257:6<217::AID-AR7>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Boyd IL, Arnbom T. Diving behavior in relation to water temperature in the southern elephant seal: foraging implications. Polar Biol. 1991;11:259–266. [Google Scholar]
- Campagna C, Lewis M. Growth and distribution of a southern elephant seal colony. Mar Mamm Sci. 1992;8:387–396. [Google Scholar]
- Campagna C, Lewis M, Baldi R. Breeding biology of southern elephant seals in Patagonia. Mar Mamm Sci. 1993;9:34–47. [Google Scholar]
- Campagna C, Le Boeuf BJ, Blackwell S, et al. Diving behaviour and foraging location of female southern elephant seals from Patagonia. J Zool. 1995;236:55–71. [Google Scholar]
- Campagna C, Quintana F, Le Boeuf BJ, et al. Diving behaviour and foraging ecology of male southern elephant seals from Patagonia. Aquat Mamm. 1998;4:1–11. [Google Scholar]
- Campagna C, Fedak MA, McConnell BJ. Post-breeding distribution and diving behavior of adult male southern elephant seals from Patagonia. J Mammal. 1999;4:1341–1352. [Google Scholar]
- Campagna C, Rivas AL, Marin MR. Temperature and depth profiles recorded during dives of elephant seals reflect distinct ocean environments. J Mar Syst. 2000;24:299–312. [Google Scholar]
- Campagna C, Piola AR, Maron MR, et al. Deep divers in shallow seas: southern elephant seals on the Patagonia shelf. Deep Sea Res Part 1. 2007;54:1792–1814. [Google Scholar]
- Carlini AR, Poljak S, Daneri GA, et al. The dynamics of male harem dominance in southern elephant seals (Mirounga leonina) at the South Shetland Islands. Polar Biol. 2006;29:796–805. [Google Scholar]
- Carrick R, Csordas SE, Ingham SE. Studies on the southern elephant seal, Mirounga leonina (L.). IV. Breeding and development. 1962;7:161–197. CSIRO Wildl Res. [Google Scholar]
- Claude J. Morphometrics with R. New York, USA: Springer; 2008. [Google Scholar]
- Condy PR. Annual cycle of the southern elephant seal Mirounga leonina (Linn.) at Marion Island. S Afr J Zool. 1979;14:95–102. [Google Scholar]
- Dryden IL, Mardia KV. Statistical Shape Analysis. Chichester: Wiley; 1998. [Google Scholar]
- Eder BE, Lewis MN, Campagna C, et al. Evidence of demersal foraging fron stable isotope analysis of juvenile elephant seals from Patagonia. Mar Mamm Sci. 2010;26:430–442. [Google Scholar]
- Eisenberg JF. An introduction to the Carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Ithaca, NY: Cornell University Press; 1989. pp. 1–9. [Google Scholar]
- Field IC, Bradshaw JA, van den Hoff J, et al. Age-related shifts in the diet composition of southern elephant seal expand overall foraging niche. Mar Biol. 2007;150:1441–1452. [Google Scholar]
- Fleischer G. Evolutionary principles of the mammalian middle ear. Adv Anat Embryol Cell Biol. 1978;55:1–70. doi: 10.1007/978-3-642-67143-2. [DOI] [PubMed] [Google Scholar]
- Flower WH. On the value of the characters of the Carnivora, and on the systematic position of Bassaris and other disputed forms. Proc Zool Soc Lond. 1869;37:4–37. [Google Scholar]
- Galimberti F, Boitani L. Demography and breeding biology of a small, localized population of southern elephant seals (Mirounga leonina. Mar Mamm Sci. 1999;15:159–178. [Google Scholar]
- Ginsburg L. Les amphicyons des Phosphorites du Quercy. Ann Paleontol. 1966;52:23–64. [Google Scholar]
- Graham SF. Seal ears. Science. 1967;155:489. doi: 10.1126/science.155.3761.489. [DOI] [PubMed] [Google Scholar]
- Gray H. Gray's Anatomy. Anatomy, Descriptive and Surgical. 15th revised edition. New York, USA: Gramercy Books, Random House Value Publishing; 1858. [Google Scholar]
- Gunz P, Mitteroecker P. Semilandmarks: a method for quantifying curves and surfaces. Hystrix. 2013;24:7. [Google Scholar]
- Hindell MA, Bryden MM. Physiological implications of continuous, prolonged, and deep dives of the southern elephant seal (Mirounga leonina. Can J Zool. 1992;70:370–379. [Google Scholar]
- Hindell MA, McConnell BJ, Fedak MA, et al. Enviromental and physiological determinants of successful foraging by naïve southern elephant seal pups during their first trip to sea. Can J Zool. 1999;77:1807–1821. [Google Scholar]
- Hough JR. The auditory region in some members of the Procyonidae, Canidae, and Ursidae. Its significance in the phylogeny of the Carnivora. Bull Am Mus Nat Hist. 1948;92:67–118. [Google Scholar]
- Hough JR. Auditory region in North American Felidae: significance in phylogeny. Geol Surv Prof Pap. 1952;243:95–115. [Google Scholar]
- Hunt RM., Jr The auditory bulla in Carnivora: an anatomical basis for reappraisal of carnivore evolution. J Morphol. 1974;143:21–76. doi: 10.1002/jmor.1051430103. [DOI] [PubMed] [Google Scholar]
- Ivanoff DV. Origin of the septum in the canid auditory bulla: evidence from morphogenesis. Acta Theriol. 2000;45:253–270. [Google Scholar]
- Ivanoff DV. Partitions in the carnivorian auditory bulla: their formation and significance for systematics. Mamm Rev. 2001;31:1–16. [Google Scholar]
- Jonker FC, Bester MN. Seasonal movements and foraging areas of adult southern female elephant seals, Mirounga leonine, from Marion Island. Antarct Sci. 1998;10:21–30. [Google Scholar]
- Kastak D, Schusterman RJ. In-air and underwater hearing sensitivity of a northern elephant seal (Mirounga angustirostris. Can J Zool. 1999;77:1751–1758. [Google Scholar]
- Kelly CD, Folinsbee KE, Adams DC, et al. Intraspecific sexual size and shape dimorphism in an Australian freshwater fish differs with respect to a biogeographic barrier and latitude. Evol Biol. 2013;40:408–419. [Google Scholar]
- King JE. Seals of the World. London: British Museum (Natural History); 1964. pp. 125–126. [Google Scholar]
- King JE. Seals of the World. 2nd edn. Ithaca, NY: Cornell University Press; 1983. [Google Scholar]
- Laws RM. A new method of determination in mammals with special reference to elephant seal (Mirounga leonina L.) Falkland Isl Depend Surv Sci Rep. 1953;2:1–11. [Google Scholar]
- Laws RM. The elephant seal (Mirounga leonina, Linn.), II: general, social and reproductive behavior. Falkland Isl Depend Surv Sci Rep. 1956;13:1–88. [Google Scholar]
- Laws RM. Identification of species. In: Laws RM, editor. Antarctic Seals. Research Methods and Techniques. Cambridge, UK: Cambridge University Press; 1993. pp. 1–28. [Google Scholar]
- Laws RM. History and present status of southern elephant seal populations. In: Le Boeuf BJL, Laws RM, editors. Elephant Seals: Population Ecology, Behavior, and Physiology. Berkeley: University of California Press; 1994. pp. 49–65. [Google Scholar]
- Lewis M, Campagna C, Quintana F, et al. Estado actual y distribución de la población del elefante marino del sur en la Península Valdés, Argentina. Mastozool Neotrop. 1998;5:29–40. [Google Scholar]
- Lewis RO, Connel T, Lewis M, et al. Sex-specific foraging strategies and resource portioning in the southern elephant seal (Mirounga leonina. Proc Biol Sci. 2006;273:2901–2907. doi: 10.1098/rspb.2006.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling JK, Bryden MM. Southern elephant seal Mirounga leonina Linnaeus, 1758. In: Ridgway SH, Harrison RJ, editors. Handbook of Marine Mammals, Vol. 2 Seals. London: Academic Press; 1981. pp. 297–327. [Google Scholar]
- Loza CM, Tarnawsky BA, Menucci JA. 2011. p. 117. ( Dimorfismo sexual cráneo-dentario en los primeros estadíos post-natales del desarrollo en Mirounga leonina (Phocidae, Carnivora). XXIV Jornadas Argentinas de Mastozoología, SAREM, La Plata, resúmenes.
- MacPhee R, et al. Auditory regions of primates and eutherian insectivores: morphology, ontogeny, and character analysis. Contrib Primatol. 1981;18:1–282. [Google Scholar]
- Marsh SE. 2001. Rice University, The Massachusetts Institute of Technology and The Woods Hole Oceanographic Institution Morphometric analyses of ears in two families of the pinnipeds. PhD Dissertation.
- McCann TS. Population structure and social organization of southern elephant, Mirounga leonina (L) Biol J Linn Soc. 1981;14:133–150. [Google Scholar]
- McConnell B, Chambers C, Fedak MA. Foraging ecology of southern elephant seals in relation to the bathymetry and productivity of the Southern Ocean. Antarct Sci. 1992;4:393–398. [Google Scholar]
- McConnell B, Fedak M, Burton H, et al. Movements and foraging areas of naïve, recently weaned southern elephant seal pups. J Anim Ecol. 2002;71:65–78. [Google Scholar]
- McIntyre T, Bruyn PJN, Ansorge IJ, et al. A lifetime at depth: vertical distribution of southern elephant seals in the water column. Polar Biol. 2010a;33:1037–1048. [Google Scholar]
- McIntyre T, Tosh CA, Plottz J, et al. Segregation in a sexually dimorphic mammal: a mixed-effects modeling analysis of diving behavior in southern elephant seals. Mar Ecol Prog Ser. 2010b;412:293–304. [Google Scholar]
- McMahon C, Bester MN, Burton HR, et al. Population status, trends and a re-examination of the hypotheses explaining the recent declines of the Southern elephant seal Mirounga leonina. Mamm Rev. 2005;35:82–100. [Google Scholar]
- Mennucci JA, Negrete J, Juáres MA. 2012. Seasonal variation in the number of breeding females of Southern Elephant Seal, at 25 de Mayo/King George Island. XXXII SCAR and Open Science Conference. Portland, Oregón, USA, 16–19 July.
- Mitteroecker P, Gunz P, Bernhard M, et al. Comparison of cranial ontogenetic trajectories among great apes and humans. J Hum Evol. 2004;46:679–697. doi: 10.1016/j.jhevol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Modig AO. Effects of body size and harem size on male reproductive behaviour in the southern elephant seal. Anim Behav. 1996;51:1295–1306. [Google Scholar]
- Mohl B. Frequency discrimination in the common seal and discussion of the concept of upper hearing limit. In: Albers V, editor. Underwater Acoustics. New York: Plenum Press; 1967. pp. 43–54. Vol. 2. [Google Scholar]
- Mohl B. Auditory sensivity of the common seal in air and water. J Aud Res. 1968;8:27–38. [Google Scholar]
- Morejohn GV, Briggs KT. Post-mortem studies of northern elephant seal pups. J Zool (Lond) 1973;171:67–77. [Google Scholar]
- Negrete J, Juáres MA, Ferrari HR, et al. Comportamiento agonístico en machos de elefante marino del sur (Mirounga leonina, Carnivora: Phocidae) en la Isla 25 de Mayo, Antártida. Acta Zool Lilloana. 2011;55:247–260. [Google Scholar]
- Negrete J, Carlini AR, Ferrari RH. 2012. pp. 1–7. ( Notes on the behaviour of precocious elephant seal (Mirounga Leonina) males in harems at the South Shetland Islands, Antarctica (Comunicación). Contribuciones Científicas del Instituto Antártico Argentino, Contribución No. 549.
- Nomina Anatomica Veterinaria. International Committee on Veterinary Gross Anatomical Nomenclature. 5th edn. Hannover (Germany), Columbia, MO (USA), Ghent (Belgium), Sapporo (Japan): Editorial Committee; 2012. (revised version). [Google Scholar]
- Nummela S, et al. Scaling of the mammalian middle ear. Hear Res. 1995;85:18–30. doi: 10.1016/0378-5955(95)00030-8. [DOI] [PubMed] [Google Scholar]
- Odend'hal S, Poulter TC. Pressure regulation in the middle ear cavity of the sea lions: a possible mechanism. Science. 1966;153:768–769. doi: 10.1126/science.153.3737.768. [DOI] [PubMed] [Google Scholar]
- Pérez SI, Bernal V, González PN. Differences between sliding semi-landmark methods in geometric morphometrics, with an application to human craniofacial and dental variation. J Anat. 2006;208:769–784. doi: 10.1111/j.1469-7580.2006.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock RI. The tympanic bulla in hyenas. Proc Zool Soc Lond. 1916;86:303–307. Vol. [Google Scholar]
- Pocock RI. The external characters and classification of the Procyonidae. Proc Zool Soc Lond. 1921;91:389–422. Vol. [Google Scholar]
- Pocock RI. The structure of the auditory bulla in the Procyonidae and the Ursidae, with a note on the bulla of Hyaena. Proc Zool Soc Lond. 1929;98:963–974. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Version 3.0.2. Vienna: R Foundation for Statistical Computing; 2013. http://cran.R-project.org. [Google Scholar]
- Repenning CA. Underwater hearing in seals: functional morphology. In: Harrison RJ, editor. Functional Anatomy of Marine Mammals. London: Academic Press; 1972. pp. 307–331. Vol. 1. [Google Scholar]
- Rohlf FJ. Shape statistics: procrustes superimpositions and tangent spaces. J Classif. 1999;16:197–223. [Google Scholar]
- Rohlf RJ. TPSDIG, version 2.12. Stony Brook: Department of Ecology and Evolution, State University of New York; 2009. http://life.bio.sunysb.edu/morph/ [Google Scholar]
- Rohlf FJ, Marcus LF. A revolution in morphometrics. Trends Ecol Evol. 1993;8:129–132. doi: 10.1016/0169-5347(93)90024-J. [DOI] [PubMed] [Google Scholar]
- Rohlf FJ, Slice D. Extensions of the procrustes method for the optimal superimposition of landmarks. Syst Zool. 1990;39:40–59. [Google Scholar]
- Solntseva GN. Comparative anatomical peculiarities of the middle ear structure in terrestrial, semi aquatic and aquatic mammals. Abstr 5th AllUnion Conf Mar Mam Res Makhachkala. 1972;2:216–220. Abstract (in Russian) [Google Scholar]
- Solntseva GN. Biomechanical features of the middle ear in terrestrial, semi-aquatic and aquatic mammals. 8th Un Acoust Conf Moscow. 1973a;128:29–32. Abstract. [Google Scholar]
- Solntseva GN. Dolk. Astrakhan: Sessii Kasp NIRKH porabotam za 1972; 1973b. Morphological and biomechanic features of the middle ear of the Caspian seal (Pusa caspica; pp. 74–76. [Google Scholar]
- Solntseva GN. Morphofunctional peculiarities of the auditory organ in terrestrial, semi-aquatic and aquatic mammals. Zool Zhurn. 1975;44:1529–1539. [Google Scholar]
- Tarnawsky BA, Cassini GH, Flores DA. Skull allometry and sexual dimorphism in the ontogeny of the southern elephant seal (Mirounga leonina. Can J Zool. 2013;92:19–31. [Google Scholar]
- Thenius E. Zur Revision der Insektivoren des steirische Tertiärs. Beiträge zur Kenntnis der Säugetierreste des steirischen Tertiärs II. Sitzungsb Österr Ak Wiss math-naturwissensch Klas Abt I. 1949;159:671–693. [Google Scholar]
- Thomassin JM, Dessi P, Danvin JB. EMC. 2008. ( Anatomía del oído medio (Elsevier Masson SAS, París) Oto-rhino-laryngologie, doi: 20-015-A-10.
- Tran Ba Huy P, Teissier N. 2011. EMC Embriología del oído medio (Elsevier Masson SAS, París) Oto-rhino-laryngologie, doi: 20-005-A-30.
- Van der Klaauw CJ, et al. The auditory bulla in some fossil mammals. Bull Am Mus Nat Hist. 1931;62:1–135. [Google Scholar]
- Van Kampen PN. Die Tympanalgegend des Saugetierschadels. Morph Jahrb. 1905;34:321–722. [Google Scholar]
- Wible JR. Origin of mammalia: the craniodental evidence reexamined. J Vertebr Paleontol. 1991;11:1–28. [Google Scholar]
- Wible JR. Petrosal anatomy of the nine-banded armadillo, Dasypus novemcinctus Linnaeus, 1758 (Mammalia, Xenarthra, Dasypodidae) Ann Carnegie Mus. 2010;79:1–28. [Google Scholar]
- Wozencraft WC. The phylogeny of the recent Carnivora. In: Gittleman JL, editor. Carnivore Behavior, Ecology, and Evolution. Baltimore: Cornell University Press; 1989. pp. 495–535. [Google Scholar]
- Wozencraft WC. Order Carnivora. In: Wilson DE, Reeder DM, editors. Mammal Species of the World. 3rd edn. Baltimore: The Johns Hopkins University Press; 2005. pp. 532–628. [Google Scholar]
- Wyss A. The walrus auditory region and the monophyly of pinnipeds. Am Mus Novit. 1987;2871:1–32. [Google Scholar]
- Wyss A. On ‘Retrogression’ in the evolution of the phocinae and phylogenetic affinities of the monk seals. Am Mus Novit. 1988;2924:1–40. [Google Scholar]
- Wyss AR, Flynn JJ. A phylogenetic analysis and definition of the Carnivora. In: Szalay FS, Novacek MJ, McKenna MC, editors. Mammal Phylogeny: Placentals. New York: Springer-Verlag; 1993. pp. 32–52. [Google Scholar]
- Zelditch M, Swiderski D, Sheets D. Geometric Morphometrics for Biologists. London, UK: Elsevier Academic Press; 2004. [Google Scholar]