Abstract
Monotremes have undergone remarkable changes to their digestive and metabolic control system; however, the monotreme pancreas remains poorly characterized. Previous work in echidna demonstrated the presence of pancreatic islets, but no information is available for platypus and the fine structure has not been described for either monotreme. Based on our recent finding that monotremes lack the ghrelin gene, which is expressed in mouse and human pancreatic islets, we investigated the structure of monotreme islets in more detail. Generally, as in birds, the islets of monotremes were smaller but greater in number compared with mouse. β-cells were the most abundant endocrine cell population in platypus islets and were located peripherally, while α-cells were observed both in the interior and periphery of the islets. δ-cells and pancreatic polypeptide (PP)-cells were mainly found in the islet periphery. Distinct PP-rich (PP-lobe) and PP-poor areas (non-PP-lobe) are present in therian mammals, and we identified these areas in echidna but not platypus pancreas. Interestingly, in some of the echidna islets, α- and β-cells tended to form two poles within the islets, which to our knowledge is the first time this has been observed in any species. Overall, monotreme pancreata share the feature of consisting of distinct PP-poor and PP-rich islets with other mammals. A higher number of islets and α- or β-cell only islets are shared between monotremes and birds. The islets of monotremes were larger than those of birds but smaller compared with therian mammals. This may indicate a trend of having fewer larger islets comprising several endocrine cell types during mammalian evolution.
Keywords: echidna, islets of Langerhans, monotremes, pancreas, platypus
Introduction
The egg-laying monotremes diverged prior to marsupial and eutherian lineages, and hence are of great importance for our understanding of mammalian evolution. Monotremes have undergone striking physiological, anatomical and genetic changes in regards to their digestive system. Previous work reported an extraordinarily small stomach, the lack of glands (the only glands present are the Brunner's glands) and a neutral pH (Krause, 1971; Griffiths, 1978). Subsequent work showed wholesale loss or inactivation of genes required for the formation of gastric juice and gastric function such as gastrin and pepsin (Ordonez et al. 2008). Our previous studies have shown the loss of another key metabolic gene in the monotreme genome, ghrl, that encodes ghrelin, a hormone known to be released from stomach and pancreatic ε-cells in eutherian mammals (Wierup et al. 2002; He et al. 2013).
The pancreas is an essential component of both the digestive and endocrine systems. It is divided into two components, the exocrine pancreas that produces digestive enzymes and bicarbonate that are released into the small intestine, and the endocrine pancreatic islets of Langerhans, which in humans and mice consist of five main endocrine cell types. These five major hormone-releasing cell types are α-cells (glucagon), β-cells (insulin), δ-cells (somatostatin), PP-cells (pancreatic polypeptide) and ε-cells (ghrelin) (Wierup et al. 2002). Islets play important roles in metabolic control, in particular in regulating blood glucose homeostasis, as well as influencing digestion through effects of endocrine hormones on exocrine pancreas secretions (Youngs, 1972; Henderson et al. 1981). The hormone-secreting islets of Langerhans evolved hundreds of millions of years ago in early vertebrates, and appear as multiple discrete entities within the exocrine pancreas in vertebrates (Bonner-Weir & Weir, 1979). Despite this conservation of islets, their architecture varies among species. For instance, the thoroughly studied rodent islets have a well-defined core-mantle structure, with β-cells clustered in the central core surrounded by non-β-cells in the periphery (Elayat et al. 1995), whilst human islets have a more scattered distribution of α- and β-cells (Cabrera et al. 2006; Bosco et al. 2010).
Very little is known about the anatomical structure and function of the platypus pancreas. Previous work in echidna pancreas found distinctive endocrine and exocrine parts and two different categories of islets: the β-islets composed of mostly β-cells with α-, δ- and PP-cells fewer in number; and PP-islets, containing predominantly PP-cells with few or no other endocrine cell types present (Yamada et al. 1990). Several studies revealed the relation between PP levels and human diseases. For instance, plasma PP levels were elevated in diabetic patients (Floyd et al. 1976) as well as in patients with pancreatic endocrine tumors (Larsson et al. 1976). A recent study also showed a diet-induced β-cell function improvement in patients with Type 2 diabetes was associated with decreased PP release (Kahleova et al. 2012).
The aim of this study was to characterize the structure and distribution of pancreatic islets and endocrine cells in monotreme pancreas in more detail. This revealed overall therian-like islets in monotremes, with some notable similarities with birds and also monotreme-specific characteristics.
Materials and methods
Sample collection
Platypus and echidna specimens were captured by netting (Animal ethics permits AEEC R.CG.07.03 and AEC S-49-200 to F.G.) at the Upper Barnard River (New South Wales, Australia) during breeding season. Specimens were killed with an intraperitoneal injection of pentobarbitone sodium (Nembutal) or pentobarbital (Lethabarb) at a dose of 0.1 mg g−1. Tissue samples including pancreatic tissue were snap-frozen or fixed in formalin and processed as described below.
Antibodies
Sequence comparison between platypus and human glucagon, insulin and somatostatin has been done to compare the conservation of antibody epitopes across species (Table1; Fig. S2). Only a partial amino acid sequence of platypus PP is available in the genome database (http://asia.ensembl.org/Ornithorhynchus_anatinus/Info/Index). All the primary antibodies utilized in the present study are listed in Table1.
Table 1.
Monoclonal (mAb) and polyclonal (pAb) antibodies used in the present study
| Antibodies | Host | Epitope | Source | Optimal dilutuon | Cat. no. |
|---|---|---|---|---|---|
| Anti-glucagon mAb | Rabbit | N-terminal | Abcam, Cambridge, UK | 1 : 1000 | ab92517 |
| (HSQGTFTSDYSKYLDSR) | |||||
| Anti-insulin pAb | Guinea pig | Unknown | Dako, Carpitneria, CA | 1 : 50 | A0564 |
| Anti-somatostatin-14 pAb | Rabbit | C-terminal | Abcam, Cambridge, UK | 1 : 100 | ab64053 |
| (AGCKNFFWKTFTSC) | |||||
| Anti-PP pAb | Goat | C-terminal | Sigma-Aldrich | 1 : 25 | SAB2500747 |
| (TRPRYGKRHKEDT) |
PP, pancreatic polypeptide.
Immunohistochemistry
Pancreas tissue was fixed in formalin and processed in butanol before embedding in low-melting paraffin. All pancreas samples were sectioned at 5 μm, and 10 consecutive sections were obtained with the first and last section stained with hematoxylin and eosin (H&E) following standard methods.
Before immunofluorescence, sections were deparaffinized and dehydrated with ethanol. The antigen-retrieval process was performed by 20 min treatment at 37 °C with proteinase K (1 : 1000; Roche; cat. no. 03115887001) for glucagon and insulin staining, or incubation in sodium citrate (0.01 m, pH 6.0) buffer for 20 min for somatostatin and PP staining. Ten percent normal horse serum in antibody diluent [NaCl, NaH2PO4, Na2HPO4·2H2O, 10% NaN3 in distilled water (pH 7.1)] was used as blocking agent. Primary antibodies (Table1) were diluted in 10% normal horse serum. Antigens were visualized using appropriate secondary antibodies, Alexa 488-conjugated goat anti-guinea pig (1 : 100; Invitrogen; cat. no. A11073), Alexa 568-conjugated goat anti-rabbit (1 : 100; Invitrogen; cat. no. A11011) and Alexa 488-conjugated donkey anti-goat (1 : 100; Invitrogen; cat. no. A11055). Primary antibodies were incubated on tissue sections overnight at 4 °C, followed by 4 × 5 min 1 × phosphate-buffered saline (PBS) washes. Secondary antibodies were incubated at room temperature for 2 h with 4 × 5 min 1 × PBS washes before mounting with ProLong® Gold antifade reagent with DAPI (Life Technologies).
Image acquisition and quantification
For imaging, a Zeiss (Jena, Germany) AxioImager Z1 microscope was used equipped with a × 10 ocular, and × 20 and × 63 objective lenses. Fluorescent tags were visualized using three filters for DAPI (blue), Alexa 568 (green) and Alexa 488 (red). Images were taken with an Axiocam charge-coupled device camera, and image analysis was performed using Zeiss Axiovision software. Morphometric analysis was performed on multiple consecutive tissue sections to ensure complete representation of both large and small islets in the data.
Statistical analysis
Data are expressed as mean values with standard deviation (SD). Statistical analyses were performed using paired Student's t-test. Differences were considered to be significant at P < 0.05.
Results
Histology of monotreme pancreatic tissue
Anatomically the pancreas of the platypus appeared diffuse and was difficult to discriminate from surrounding connective and adipose tissue, while in the echidna the pancreas was a more discrete organ readily distinguished from surrounding tissue (observed by F. Grützner and A. Casey during dissection). The overall pancreatic histology and cytology of platypus (Fig.1C,D) and echidna (Fig.1E,F) was similar to mouse (Fig.1A,B) and other mammals with well-structured exocrine acini, ducts, blood vessels and endocrine islets of Langerhans. H&E staining of monotreme pancreas showed numerous lobules of acinar glands consisting of basophilic cells. The lumen of the acinus is the origin of the secretory duct and contains centroacinar cells, which are pale staining and smaller in size than the acinar cells (Fig.1B,D,F).
Figure 1.
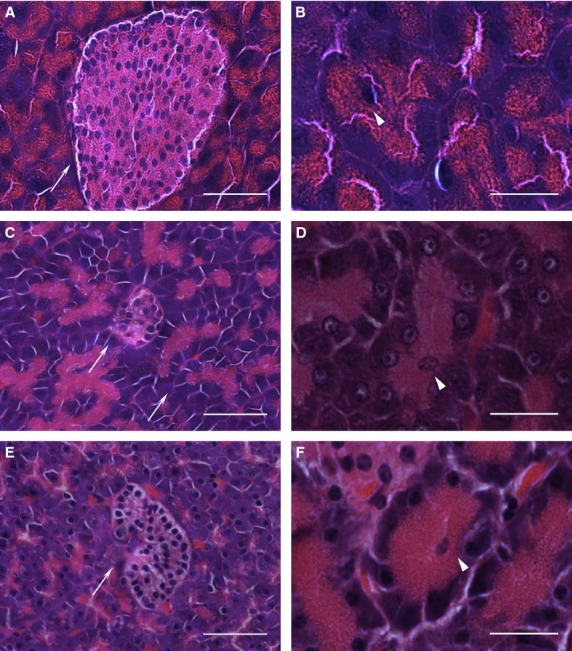
Histology of pancreatic tissue from mouse, platypus and echidna. H&E-stained sections of mouse pancreas (A, B), platypus pancreas (C, D) and echidna pancreas (E, F). Arrows point to the islets of Langerhans in (A), (C) and (E) (scale bar: 50 μm). Arrowheads show the centroacinar cells of the exocrine acinus in (B), (D) and (F) (scale bar: 20 μm).
The eosinophilic endocrine islets of Langerhans were scattered throughout the exocrine tissue. The size of islets varied considerably, from 10 or fewer cells to over 100 cells per islet. Generally, platypus (mean diameter 46 μm) and echidna islets (mean diameter 41 μm) were similar in size, but were generally smaller than mouse (mean diameter 88 μm) (Fig.2) or human islets (mean diameter 140 μm) (Hellman & Hellerström, 1969; Takei et al. 1994; Kim et al. 2009). Also, there appeared to be a lot of endocrine cells scattered either singly or in small groups of two or three through the exocrine region in the pancreas of both platypus and echidna. The shape of most islets in both species was round or oval, but some showed a more irregular shape (Fig. S3a). No distinct pattern of distribution was identified in terms of accumulation of islets in certain areas. Most islets were surrounded by exocrine tissue, but some were located within connective tissue next to blood vessels, ducts or between fat cells. The outlines of small islets were not always clearly delineated, whereas large islets tended to have more clear boundaries (Fig. S3b,c).
Figure 2.
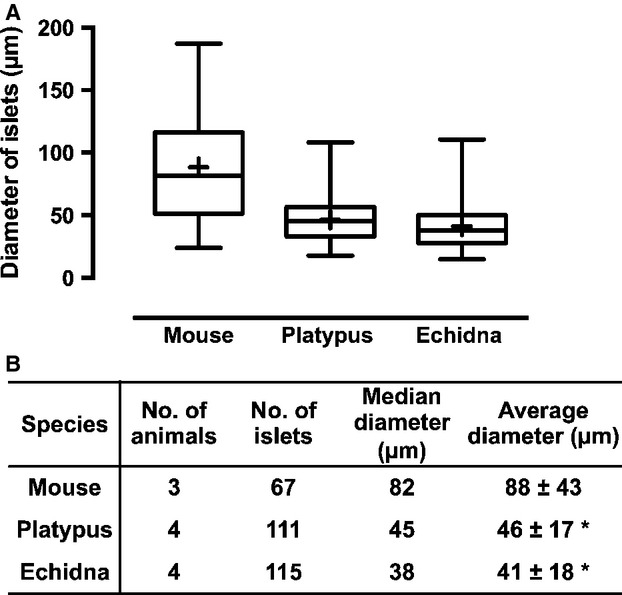
Size of the islets of Langerhans in mouse, platypus and echidna. Islet size was described as an effective diameter of a circle that depicts an area corresponding to a measured islet area. Means are shown as ‘+’ in the box and whiskers graph (A). The values shown in (B) are mean ± SD per animal. The tissue measured is H&E-stained. *P < 0.05 compared with mouse.
Immunolocalization of endocrine hormones
The mouse islets of Langerhans had a well-defined core-mantle structure, with β-cells accounting for 72.8% (Table2) of all endocrine cells, clustering in the central core surrounded by non-β-cells, mainly α-cells (12.4%), but also δ- (10.8%) and PP-cells (4.0%) in the periphery (Figs3A–C and 4A–C; Table2).
Table 2.
Cellular compositions of the islets of Langerhans in mouse, platypus, echidna, possum and chicken
| Species | No. of animals | No. of islets | α-Cells | β-Cells | δ-Cells | PP-cells | Reference | |
|---|---|---|---|---|---|---|---|---|
| Mouse | 3 | 53 | Periphery | Core | Periphery | Periphery | ||
| 12.4% | 72.8% | 10.8% | 4.0% | |||||
| Platypus | 3 | 135 | Core/periphery | Periphery | Periphery | Core/periphery | ||
| 21.6% | 64.4% | 10.6% | 3.4% | |||||
| Echidna | PP-lobe | 2 | 74 | Periphery | Periphery | Periphery | Core | |
| 1.1% | 24.8% | 19.0% | 55.1% | |||||
| Non-PP-lobe | 2 | 90 | Core | Core | Periphery | Periphery | ||
| 36.3% | 37.7% | 14.6% | 11.4% | |||||
| Possum | Core/periphery highest % (up to 70%) | Core/periphery | Core (few)/periphery | Periphery/scattered | (White & Harrop, 1975; Reddy et al. 1986) | |||
| Chicken | A-islets | Core | Few | Periphery | Periphery | (Epple & Farner, 1967; Watanabe et al. 1975; do Prado et al. 1989; Edwin & Leigh, 1993; Nascimento et al. 2007) | ||
| 22% | 11% | |||||||
| B-islets | Few | Core | ||||||
| Mixed islets | Periphery | Core | ||||||
The location of each cell type and the number of cells from each type that make up an islet is shown (%).PP, pancreatic polypeptide.
Figure 3.
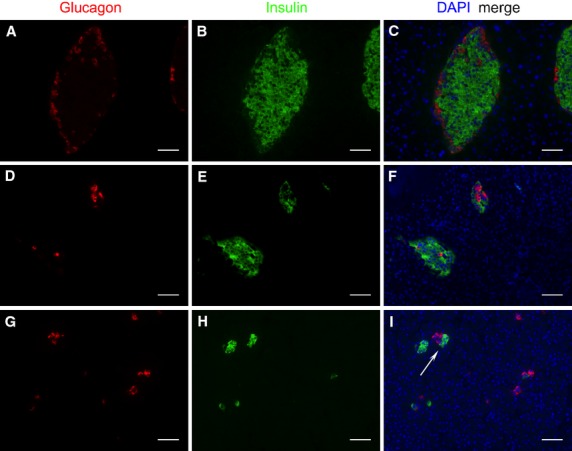
Immunohistochemical localization of glucagon (red), insulin (green) and nuclei (blue) in the endocrine pancreas of mouse (A–C), platypus (D–F) and echidna (G–I). Scanned with a Zeiss AxioImager 2.1 microscope. Scale bar: 50 μm.
Figure 4.
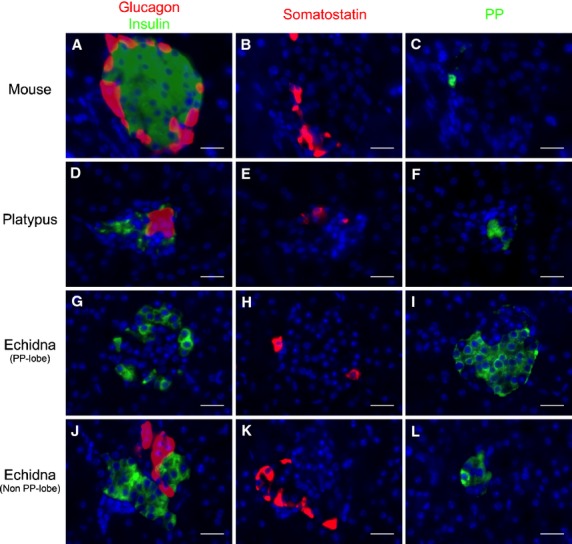
Immunohistochemical localization of glucagon, insulin, somatostatin and pancreatic polypeptide (PP) in the endocrine pancreas of mouse (A–C), platypus (D–F) and echidna (G–L). (A–C) Consecutive mouse pancreatic sections incubated with the anti-glucagon (A, red), anti-insulin (A, green), anti-somatostatin (B) and anti-PP (C) antibodies. (D–F) Consecutive platypus pancreatic sections incubated with the anti-glucagon (D, red), anti-insulin (D, green), anti-somatostatin (E) and anti-PP (F) antibodies. (G–L) Consecutive echidna pancreatic sections incubated with the anti-glucagon (G, J, red), anti-insulin (G, J, green), anti-somatostatin (H, K) and anti-PP (I, L) antibodies. In all three species, glucagon, insulin, somatostatin and PP immunoreactivities were present on different cell populations. Scale bar: 20 μm.
Glucagon- and insulin-immunoreactive cells were identified in platypus pancreas (Figs3D–F and 4D). In most islets, β-cells were the predominant cell type (64.4%) and were distributed peripherally, whereas α-cells were located both in the center and periphery of the islets (Table2). Somatostatin- and PP-immunoreactive cells were fewer in number, and were observed in the islet periphery. In some islets, PP-cells were also found in the core (Fig.4F).
The pancreatic tissue of echidna could be grouped into two categories based on the predominant cell type in the endocrine islets: the PP-lobe and the non-PP-lobe. In the PP-lobe, PP-cells predominated (55.1%) and were located in the core (Fig.4I). Insulin- (Fig.4G) and somatostatin- (Fig.4B) positive cells were also observed but mainly in the periphery. α-cells were rare in the PP-lobe. In contrast, in the non-PP-lobe, while some islets consisted predominantly of α-cells, others were predominantly made up of β-cells (Fig.3G–I). Interestingly, 17.6% of the islets (Fig.3I; Fig. S1) had a unique architecture where α- and β-cells clustered at opposite ends of the one islet. The overall proportion between α- and β-cells across the whole non-PP-lobe was about 1 : 1 (36.3 : 37.7%; Table2). δ-cells and PP-cells were identified in fewer numbers than α- and β-cells in the periphery, accounting for 14.6% and 11.4% of total islet cells, respectively.
In the exocrine region of both platypus and echidna pancreas, immunoreactive cells of all four types were observed to be scattered either singly or in small groups of two or three cells throughout.
Discussion
The structure and histology of exocrine pancreas is overall conserved between mammals and birds (Motta et al. 1997; Mobini, 2013). We demonstrated that conservation of the general anatomy of the exocrine pancreas is also maintained in monotremes, in spite of the large-scale physiological, anatomical and genomic changes in their digestive system. In contrast, endocrine pancreas cytoarchitecture can be quite different between species. This may reflect evolutionary adaptations to different diets or other environmental conditions (Cabrera et al. 2006). Consistent with their phylogenetic position, the histology of monotreme endocrine pancreas showed greater resemblance to that of birds and marsupials than to eutherian mammals (reviewed by Steiner et al. 2010; Heller, 2010).
The overall anatomy of the platypus pancreas appeared more diffuse compared with the distinct pancreas of echidna (Grützner & Casey, personal communication). Such differences are also observed in therian mammals: rodents and lagomorphs also feature a diffuse pancreas (Dimitrov et al. 2013), while they are more defined in carnivores (Griffith, 1989). How this might relate to dietary or feeding behavior is currently unclear.
In terms of islet anatomy, we found that generally the monotreme islets are smaller than those of other mammalian species (Fig.2), but larger than those of birds (Kim et al. 2009; Steiner et al. 2010). Overall this indicates a trend towards fewer islets with enlarged size during mammalian evolution.
Immunolocalization of glucagon, insulin, somatostatin and PP in the pancreas identified most main cell types within the islets. The arrangement of mouse pancreatic islets described here is consistent with numerous other studies of rodent islets, with a well-defined core-mantle structure of central β-cells surrounded by non-β-cells fewer in number in the periphery (Heller, 2010; Steiner et al. 2010). Unlike this characteristic distribution of rodent islet cells, platypus pancreas had an arrangement where β-cells were mainly located in the periphery, an arrangement previously observed in horse (Helmstaedter et al. 1976; Furuoka et al. 1989). Also the ratio between β- and α-cells was lower than that of mouse (∽ 3 : 1 in platypus compared with ∽ 6 : 1 in mouse; Table2). Immunohistochemical study of islet vascularization of monotreme islets was unsuccessful due to failure of several mammalian antibodies directed against vascular endothelial markers to cross-react with the monotreme antigens. For this reason we could not ascertain the role played by vascularization in monotreme islets and whether the endocrine cells were located adjacent to blood vessels as is seen in human islets (Cabrera et al. 2006).
The existence of a PP-cell-rich region is well established in the pancreas of several therian mammals (Orci et al. 1978; Edwin, 1979, 1987; Reddy et al. 1986; Wang et al. 2013). Interestingly, we did not see such an arrangement of PP-cells into a PP-rich lobe in platypus. This may be a result of the diffuse structure of the platypus pancreas, which made dissection and identification of distinct head, body or tail regions difficult. In contrast, a greater proportion of PP-cells has been reported in the head region of echidna pancreas (Edwin, 1987), and our work confirmed the existence of distinct PP- and non-PP-lobes in echidna.
In the non-PP-lobe of echidna pancreas, islets were either exclusively α-cells or β-cells or a mixture of both α- and β-cells (Fig.3I). This arrangement of α- and β-cells is different from those of mouse and human, where all islets always contain both α- and β-cells, except for those located in the PP-rich head regions (Steiner et al. 2010; Wang et al. 2013). Islets that consist of exclusively α-cells or β-cells are also seen in several marsupial species and birds (Hazelwood, 1973; White & Harrop, 1975; Reddy et al. 1986). In birds, pancreatic islets are divided into three different categories: (i) islets consisting of α-cells in the core and several δ-cells in periphery and a few β-cells; (ii) islets containing mainly β-cells, surrounded by several δ-cells and a few α-cells; and (iii) mixed islets consisting of α-, β- and δ-cells (Hazelwood, 1973; Watanabe et al. 1975; do Prado et al. 1989; Lucini et al. 1995). We observed all these three type of islets in echidna. However, we discovered that some of the mixed islets in echidna had a distinctive polarity where α- and β-cells were juxtaopposed to form two distinct hemispheres (Fig.3I; Fig. S1). This has not been reported in other species and maybe a fourth, possibly monotreme-specific category of islets. It is currently unclear what these different islet types may mean for the functioning of the islets in monotremes.
Evidence from a study by Griffiths (1965) suggests that the pancreatic islets function in a similar way to those of carnivore eutherians as, while there is a rapid response to high levels of orally administered glucose, echidnas appear to have a high tolerance to glucose. Their fasting blood glucose is, however, lower than some eutherians (Griffiths, 1965). A modest drop in blood glucose was measured in response to exogenous insulin administration. Curiously, a single experiment using crude preparations of echidna pancreas when injected into rabbits showed induction of a ‘striking and prolonged’ hyperglycemia, suggesting the presence of a relatively high glucagon concentration in the islets (Griffiths, 1965). However, our data suggest there are more β-cells than α-cells in the monotreme pancreas (Table2), which would imply that preliminary observation by Griffith is somewhat counterintuitive. Ultimately it would be interesting (but challenging in these wild animals) to measure the pancreatic insulin and glucagon levels under different conditions.
Several studies have suggested that in humans, heterologous contact between α- and β-cells favors insulin release stimulated by glucagon (Huypens et al. 2000; Wojtusciszyn et al. 2008). On the other hand, Bosco et al. (1989) showed that homologous interaction between β-cells improves insulin secretion in mouse. Whether the arrangement of different cell types in the monotreme islets has a functional significance relating to insulin secretion remains unknown. Moreover, studies of human and mouse pancreas suggested that there is considerable plasticity in the structure of islets influenced by physiological and pathophysiological conditions, such as pregnancy, fat mass or diabetes (Cabrera et al. 2006; Steiner et al. 2010). Hence, the differences in general pancreas anatomy as well as the arrangement of islet cells between platypus and echidna may be related to their different metabolic requirements and dietary habits. Platypuses are highly active aquatic animals that feed mainly on invertebrates (Burrell, 1927), whereas echidna are known to have a low metabolic rate and a diet of mainly ants and termites (Augee et al. 2006).
In summary, the pancreata of monotremes contained both endocrine and exocrine structures, and share a similar overall structure with other mammals. However, the endocrine islets of monotremes were smaller compared with therian mammals and larger than those of birds, indicating a trend towards the reduction of numbers whilst enlargement of islet size during mammalian evolution. The cytoarchitecture of the islets in platypus was more similar to those of eutherian specie, whilst echidna showed more resemblance to birds and marsupials. We confirmed the existence of PP-lobe and non-PP-lobe in echidna. In the echidna non-PP-lobe, which is similar to that in birds, there are three different islet types: islets containing exclusively α-cells or β-cells or a mixture of both α- and β-cells. Interestingly, the arrangement of α- and β-cells in some of the mixed islets, where α- and β-cells formed two poles of one islet, is unique and to our knowledge has not been observed in other species. Overall, this detailed histological analysis shows that the monotreme pancreas is largely mammal-like with some similarity to birds. Importantly the conservation of the islet structure contrasts the dramatic changes observed in other parts of the gastrointestinal system in monotremes.
Acknowledgments
The authors would like to acknowledge Dr Tasman Daish and Aaron Casey (University of Adelaide) for obtaining platypus and echidna tissues, and Dr Heshan Peiris (Flinders University) for the assistance with the immunohistochemistry experiments. Chuan He is supported by the China Scholarship Council (CSC) and the University of Adelaide. Frank Grützner is an ARC Research Fellow.
Supporting Information
Fig. S1. Immunohistochemical localization of glucagon (red), insulin (green) and nuclei (blue) in the six consecutive echidna endocrine pancreas sections (a–f, 5 μm apart), showing the unique cytoarchitecture of α- and β-cells clustering to two opposite hemispheres of the given islet. Scale bar: 20 μm.
Fig. S2. Sequence comparison between human and platypus pancreatic endocrine hormones. The epitope of each antibody has been underlined where known. All sequences were acquired from Ensembl (http://www.ensembl.org/index.html). hPPY, human pancreatic polypeptide prohormone.
Fig. S3. Histology of pancreatic tissue from echidna. H&E-stained sections of echidna pancreas showing islets with irregular shape (a), big islets with clear boundary (b), and small islets without a clear boundary (c). The islets of Langerhans are arrowed. Scale bar: 50 μm.
References
- Augee ML, Gooden B, Musser A. Echidna: Extraordinary Egg-Laying Mammal. Melbourne: CSIRO Publishing; 2006. p. 113. [Google Scholar]
- Bonner-Weir S, Weir GC. The organization of the endocrine pancreas: a hypothetical unifying view of the phylogenetic differences. Gen Comp Endocrinol. 1979;38:28–37. doi: 10.1016/0016-6480(79)90084-4. [DOI] [PubMed] [Google Scholar]
- Bosco D, Orci L, Meda P. Homologous but not heterologous contact increases the insulin secretion of individual pancreatic B-cells. Exp Cell Res. 1989;184:72–80. doi: 10.1016/0014-4827(89)90365-0. [DOI] [PubMed] [Google Scholar]
- Bosco D, Armanet M, Morel P. Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes. 2010;59:1202–1210. doi: 10.2337/db09-1177. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrell HJ. The Platypus: Its Discovery, Zoological Position, Form and Characteristics, Habits, Life History, etc. Sydney: Angus & Robertson; 1927. [Google Scholar]
- Cabrera O, Berman DM, Kenyon NS. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrov R, Russenov A, Stamatova-Yovcheva K. Ultrasonographic characteristics of rabbit's pancreas. İstanbul Üniversitesi Veteriner Fakültesi Dergisi. 2013;39:139–147. , et al. ( [Google Scholar]
- Edwin N. Quantitative estimation of islet tissue in echidnas. Singapore Med J. 1979;20:297–298. [PubMed] [Google Scholar]
- Edwin N. Quantitative estimation of pancreatic polypeptide (PP) cells of islet tissue of pancreas in Australian mammals. J Zool. 1987;213:665–671. [Google Scholar]
- Edwin N, Leigh CM. The endocrine pancreas in the Australian wedge-tailed eagle (Aquila audax) – an immunocytochemical study. Eur J Histochem. 1993;37:219–224. [PubMed] [Google Scholar]
- Elayat AA, el-Naggar MM, Tahir M. An immunocytochemical and morphometric study of the rat pancreatic islets. J Anat. 1995;186(Pt 3):629–637. [PMC free article] [PubMed] [Google Scholar]
- Epple A, Farner DS. The pancreatic islets of the white-crowned sparrow, Zonotrichia leucophrys gambelii, during its annual cycle and under experimental conditions. Z Zellforsch Mikrosk Anat. 1967;79:185–197. doi: 10.1007/BF00369284. [DOI] [PubMed] [Google Scholar]
- Floyd JC, Jr, Fajans SS, Pek S. A newly recognized pancreatic polypeptide; plasma levels in health and disease. Recent Prog Horm Res. 1976;33:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. , et al. ( [DOI] [PubMed] [Google Scholar]
- Furuoka H, Ito H, Hamada M. Immunocytochemical component of endocrine cells in pancreatic islets of horses. Jpn J Vet Sci. 1989;51:35–43. doi: 10.1292/jvms1939.51.35. , et al. ( [DOI] [PubMed] [Google Scholar]
- Griffiths M. Tachyglossidae. In: Richardson BJ, Walton DW, editors. Fauna of Australia. Canberra: Australian Government Publishing Service; 1989. p. 20. [Google Scholar]
- Griffiths M. Digestion, growth and nitrogen balance in an egg-laying mammal, Tachyglossus Aculeatus (Shaw) Comp Biochem Physiol. 1965;14:357–375. doi: 10.1016/0010-406x(65)90210-0. [DOI] [PubMed] [Google Scholar]
- Griffiths M. The Biology of Monotremes. New York: Academic Press; 1978. Food and feeding habits: digestive organs and digestion; pp. 77–81. [Google Scholar]
- Hazelwood RL. The avian endocrine pancreas. Am Zool. 1973;13:699–709. [Google Scholar]
- He C, Tsend-Ayush E, Myers MA. Changes in the ghrelin hormone pathway maybe part of an unusual gastric system in monotremes. Gen Comp Endocrinol. 2013;191:74–82. doi: 10.1016/j.ygcen.2013.06.003. , et al. ( [DOI] [PubMed] [Google Scholar]
- Heller RS. The comparative anatomy of islets. Adv Exp Med Biol. 2010;654:21–37. doi: 10.1007/978-90-481-3271-3_2. [DOI] [PubMed] [Google Scholar]
- Hellman B, Hellerström C. Histology and histophysiology of the islets of Langerhans in man. In: Pfeiffer EF, editor. Handbook of Diabetes Mellitus. Munich: Lehmanns; 1969. pp. 90–118. [Google Scholar]
- Helmstaedter V, Feurle GE, Forssmann WG. Insulin-, glucagon-, and somatostatin-immunoreactive endocrine cells in the equine pancreas. Cell Tissue Res. 1976;172:447–454. doi: 10.1007/BF00220331. [DOI] [PubMed] [Google Scholar]
- Henderson JR, Daniel PM, Fraser PA. The pancreas as a single organ: the influence of the endocrine upon the exocrine part of the gland. Gut. 1981;22:158–167. doi: 10.1136/gut.22.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huypens P, Ling Z, Pipeleers D. Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia. 2000;43:1012–1019. doi: 10.1007/s001250051484. , et al. ( [DOI] [PubMed] [Google Scholar]
- Kahleova H, Mari A, Nofrate V. Improvement in beta-cell function after diet-induced weight loss is associated with decrease in pancreatic polypeptide in subjects with type 2 diabetes. J Diabetes Complications. 2012;26:442–449. doi: 10.1016/j.jdiacomp.2012.05.003. , et al. ( [DOI] [PubMed] [Google Scholar]
- Kim A, Miller K, Jo J. Islet architecture: a comparative study. Islets. 2009;1:129–136. doi: 10.4161/isl.1.2.9480. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause WJ. Brunner's glands of the duckbilled platypus (Ornithorhynchus anatinus. Am J Anat. 1971;132:147–165. doi: 10.1002/aja.1001320203. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Schwartz T, Lundqvist G. Occurrence of human pancreatic polypeptide in pancreatic endocrine tumors. Possible implication in the watery diarrhea syndrome. Am J Pathol. 1976;85:675–684. , et al. ( [PMC free article] [PubMed] [Google Scholar]
- Lucini C, Castaldo L, Lai O. An immunohistochemical study of the endocrine pancreas of ducks. Eur J Histochem. 1995;40:45–52. [PubMed] [Google Scholar]
- Mobini B. Histochemical and histological studies on the pancreas in mature pigeon (Columba Livia. Eur J Exp Biol. 2013;3:148–152. [Google Scholar]
- Motta PM, Macchiarelli G, Nottola SA. Histology of the exocrine pancreas. Microsc Res Tech. 1997;37:384–398. doi: 10.1002/(SICI)1097-0029(19970601)37:5/6<384::AID-JEMT3>3.0.CO;2-E. , et al. ( [DOI] [PubMed] [Google Scholar]
- Nascimento AA, Sales A, Cardoso TR. Immunocytochemical study of the distribution of endocrine cells in the pancreas of the Brazilian sparrow species Zonotrichia Capensis Subtorquata. Braz J Biol. 2007;67:735–740. doi: 10.1590/s1519-69842007000400021. , et al. ( [DOI] [PubMed] [Google Scholar]
- Orci L, Malaisse-Lagae F, Baetens D. Pancreatic-polypeptide-rich regions in human pancreas. Lancet. 1978;2:1200–1201. doi: 10.1016/s0140-6736(78)92181-5. , et al. ( [DOI] [PubMed] [Google Scholar]
- Ordonez GR, Hillier LW, Warren WC. Loss of genes implicated in gastric function during platypus evolution. Genome Biol. 2008;9:R81. doi: 10.1186/gb-2008-9-5-r81. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Prado ML, Campos MN, Ricciardi Cruz AR. Distribution of A, B, and mixed pancreatic islets in two bird species (Anas platyrhincus Gallus gallus, Linne, 1758) – a morphometric study. Gegenbaurs Morphol Jahrb. 1989;135:379–384. [PubMed] [Google Scholar]
- Reddy S, Bibby NJ, Fisher SL. Immunolocalization of insulin, glucagon, pancreatic polypeptide, and somatostatin in the pancreatic islets of the possum, Trichosurus vulpecula. Gen Comp Endocrinol. 1986;64:157–162. doi: 10.1016/0016-6480(86)90042-0. , et al. ( [DOI] [PubMed] [Google Scholar]
- Steiner DJ, Kim A, Miller K. Pancreatic islet plasticity: interspecies comparison of islet architecture and composition. Islets. 2010;2:135–145. doi: 10.4161/isl.2.3.11815. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei S, Teruya M, Grunewald A. Isolation and function of human and pig islets. Pancreas. 1994;9:150–156. doi: 10.1097/00006676-199403000-00002. , et al. ( [DOI] [PubMed] [Google Scholar]
- Wang X, Zielinski MC, Misawa R. Quantitative analysis of pancreatic polypeptide cell distribution in the human pancreas. PLoS One. 2013;8:e55501. doi: 10.1371/journal.pone.0055501. , et al. ( [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Ki Paik Y, Yasuda M. Fine structure of the pancreatic islets in domestic fowl with special reference to the cell type and secretion. Arch Histol Jpn. 1975;38:259–274. doi: 10.1679/aohc1950.38.259. [DOI] [PubMed] [Google Scholar]
- White AW, Harrop CJF. The islets of Langerhans of the pancreas of macropodid marsupials: a comparison with eutherian species. Aust J Zool. 1975;23:309–319. [Google Scholar]
- Wierup N, Svensson H, Mulder H. The ghrelin cell: a novel developmentally regulated islet cell in the human pancreas. Regul Pept. 2002;107:63–69. doi: 10.1016/s0167-0115(02)00067-8. , et al. ( [DOI] [PubMed] [Google Scholar]
- Wojtusciszyn A, Armanet M, Morel P. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843–1852. doi: 10.1007/s00125-008-1103-z. , et al. ( [DOI] [PubMed] [Google Scholar]
- Yamada J, Krause WJ, Edwin N. A survey of endocrine cells in the pancreas of the echidna (Tachyglossus aculeatus) with special reference to pancreatic motilin cells. J Anat. 1990;171:223–231. , et al. ( [PMC free article] [PubMed] [Google Scholar]
- Youngs G. Hormonal control of pancreatic endocrine and exocrine secretion. Gut. 1972;13:154–161. doi: 10.1136/gut.13.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Immunohistochemical localization of glucagon (red), insulin (green) and nuclei (blue) in the six consecutive echidna endocrine pancreas sections (a–f, 5 μm apart), showing the unique cytoarchitecture of α- and β-cells clustering to two opposite hemispheres of the given islet. Scale bar: 20 μm.
Fig. S2. Sequence comparison between human and platypus pancreatic endocrine hormones. The epitope of each antibody has been underlined where known. All sequences were acquired from Ensembl (http://www.ensembl.org/index.html). hPPY, human pancreatic polypeptide prohormone.
Fig. S3. Histology of pancreatic tissue from echidna. H&E-stained sections of echidna pancreas showing islets with irregular shape (a), big islets with clear boundary (b), and small islets without a clear boundary (c). The islets of Langerhans are arrowed. Scale bar: 50 μm.


