Abstract
AIM
The incidence of adverse drug events (ADEs) in surgical and non-surgical patients may differ. This individual patient data meta-analysis (IPDMA) identifies patient characteristics and types of medication most associated with patients experiencing ADEs and suggests target areas for reducing harm and implementing focused interventions.
METHODS
Authors of eligible studies on preventable ADEs (pADEs) were approached for collaboration. For assessment of differences among (non-)surgical patients and identification of associated factors descriptive statistics, Pearson chi-square, Poisson and logistic regression analyses were performed. For identification of high risk drugs (HRDs), a model was developed based on frequency, severity and preventability of medication related to ADEs.
RESULTS
Included were 5367 patients from four studies. Patients aged ≥ 77 years experienced more ADEs and pADEs compared with patients aged ≤ 52 years (odds ratios (OR) 2.12 (95% CI 1.70, 2.65) and 2.55 (95% CI 1.70, 3.84), respectively, both P < 0.05). Polypharmacy on admission also increased the risk of ADEs (OR 1.21 (95% CI 1.03, 1.44), P < 0.05) and pADEs (OR 1.85 (95% CI 1.34, 2.56), P < 0.05). pADEs were associated with more severe harm than non-preventable ADEs (54% vs. 32%, P < 0.05). The top five HRDs were antibiotics, sedatives, anticoagulants, diuretics and antihypertensives. Events associated with HRDs included diarrhoea or constipation, abnormal liver function test and central nervous system events. Most pADEs resulted from prescribing errors (90%).
CONCLUSION
Elderly patients with polypharmacy on admission and receiving antibiotics, sedatives, anticoagulants, diuretics or antihypertensives were more prone to experiencing ADEs. Efficiency in prevention of ADEs may be improved by targeted vigilance systems for alertness of physicians and pharmacists.
Keywords: adverse drug events, epidemiology, medication safety, non-surgical patient, surgical patient
WHAT IS ALREADY KNOWN ABOUT THE SUBJECT
Adverse drug events cause serious morbidity and mortality in hospitalized patients.
The admission pathway of surgical and non-surgical patients differs.
Drug use is associated with an increased risk of post-operative complications.
WHAT THIS STUDY ADDS
Individual patient data analysis of patient characteristics and types of medication associated with (preventable) adverse drug events (ADEs) during admission with a substantial increase of statistical power.
Difference in occurrence of ADEs in surgical and non-surgical patients.
Suggestions for focused interventions for preventing ADEs in surgical and non-surgical patients.
Introduction
Adverse drug events (ADEs) constitute a considerable cause of morbidity and mortality in hospitalized patients [1]. Most studies on the occurrence and preventability of ADEs were performed in cohorts of non-surgical patients such as paediatric, medical and intensive care patients [2]. A study on risk factors associated with drug-related admissions to the hospital focused on the drug groups, based on frequency of events [3]. Another review on medication errors or ADEs in hospitalized patients concluded a wide variability of the occurrence of medication errors and adverse events or reactions. Important risk factors for errors included the insufficient pharmacological knowledge of health care professionals. Polypharmacy, female gender, drugs with a narrow therapeutic range, renal elimination of drugs, age over 65 years and use of anticoagulants or diuretics are important risk factors for adverse events [4]. Differences in the admission process of surgical and non-surgical patients may affect the risk for ADEs during hospitalization. For instance, during the surgical process many patient handovers associated with the intervention take place [5]. Handovers between physicians in hospitals are routinely mediated through a verbal or written ‘sign-out’. Important information is often not transmitted at sign-out [6]. These failures in communication can lead to uncertainty in patient care decisions resulting in patient harm [7]. A paper by Kennedy et al. demonstrated that regular drug use for co-morbidity was associated with increased risk of post-operative complications related to the co-morbidity at hand. Moreover, if the length of a paucity in medication use in preparation for the surgery increased, then the complication rate increased as well. Hence, the increased risk certainly reflects the severity of co-morbidity as a confounder. These authors further suggested that the patients' needs for drugs to withstand the stresses of the post-operative period of an operation might also contribute to an increased risk of complications [8]. On the other hand, non-surgical patients may be older and often use more kinds of medication during their admission. All these aspects can affect the occurrence of ADEs in both groups. It would be interesting to know if the admission to a surgical or to a non-surgical ward differentially associates with the occurrence of in-hospital (p)ADEs.
Different means for improving patient safety have been advocated through the years. The recent development in patient safety improvement is to provide individual care systems. A system approach is based on patient characteristics as well. Our study group is developing a medication safety programme using a combination of a system approach and an individual care approach tailored by patient characteristics [9].
A meta-analysis of individual patient data was used to provide more detailed information on factors associated with ADEs during admission of patients to hospital. Another major advantage of an individual patient data meta-analysis (IPDMA) was a substantial increase in statistical power. It allowed subgroup analyses and enabled correction for potential effect modifiers or confounders.
This IPDMA aimed to identify patient characteristics and types of medication associated with (preventable) ADEs during admission, focusing on surgical and non-surgical patients. If these factors can be identified, interventions can be developed to prevent patients from having ADEs during admission or to detect ADEs as early as possible.
Methods
Search and study selection
To identify studies that registered ADEs in adult hospitalized patients a literature search was conducted on PubMed and Embase (from 2000 to April 2011). The combined search term consisted of the following keywords in the title or abstract regarding ADEs: ‘adverse drug events’, ‘ADE’, ‘medication related problems’, ‘adverse drug reaction reporting system’ or ‘drug therapy/adverse effects’. In order to find studies that included surgical patients as well as non-surgical patients, to specify surgical patients, ‘surgical’, ‘surgery’, ‘operation’, ‘pre-operative’, ‘peri-operative’ or ‘post-operative’ were added. Then the terms ‘hospitalized’ or ‘hospitalised’, ‘hospitalization’ or ‘hospitalisation’, ‘hospital’ or ‘inpatients’ were included in order to retrieve studies on hospitalized patients, i.e. studies that included ADEs during admission. Lastly the keywords ‘frequency’, ‘incidence’ or ‘epidemiology’ were added to include epidemiological studies. No language restrictions were used.
To exclude children and incidents registered in the emergency department, study titles containing the terms ‘child’, ‘children’, ‘paediatrics’ or ‘emergency’ were excluded. A manual cross-reference search of eligible papers was performed to identify other relevant articles. Two studies on ADEs from research groups at our hospital, one in surgical patients and one in medical patients using the same methodology, also met the inclusion criteria [10,11].
After completion of the study and study manuscript we updated the search in August 2014 to make sure that in the meantime no vital studies had been published while the current study was running.
Data collection process
The corresponding authors of the studies meeting the inclusion criteria of the present IPDMA were approached by e-mail, including the research protocol, to collaborate on this project. When collaboration was confirmed, available variables in the datasets were compared. Variables were considered for harmonization if included in at least two studies. After this step, a definite list of the IPDMA variables was created. With respect to privacy, the transferred databases and cumulative database did not contain identifiable personal data, only unique study numbers. All data were handled and stored anonymously in the IPDMA database.
Data items
The data items were defined before article selection. Item definitions had to be comparable in two or more studies. Moreover, data items could only be included and merged if the definitions were similar. The included items and their definitions were relevant items and used widely in patient and medication safety studies. The selection of patient characteristics in the final analysis consisted of age, gender, clinical service (surgical or non-surgical), urgency of admission (acute or planned) and polypharmacy. Age was categorized in four age categories: ≤52 years, 53–64 years, 65–76 years and ≥77 years. Age was first categorized in under and over 65 years old and each category subsequently separated in two subcategories based on their median ages (52 and 77 years, respectively). Information on urgency of admission was available in three studies. In the fourth dataset the urgency was assessed based on the reason for admission. Polypharmacy was dichotomized to include all studies in the analysis and defined as more than five drugs used on admission. One study (de Boer et al.) only supplied the dichotomous variable. In the literature, this cut-off point is commonly used [12,13]. A study by Linjakumpu et al. concluded that using five or more drugs was associated with poor physical and psychic health [13]. The selected ADE variables were trigger used for ADE detection, causality, severity, preventability, type of medication accountable for the ADE, type of event and type of medication error. Triggers used for identification of ADEs were classified as laboratory values, clinical symptoms or both. Assessment of the probability for a causal relationship between an adverse event and a drug was classified as certain, probable/likely and possible. For assessment of the severity of ADEs, the Common Terminology Criteria for Adverse Events (CTCAE) classification was used [14]. The CTCAE identifies five categories: mild, moderate, severe, life-threatening and death. For the purpose of this IPDMA, these five categories were recoded into two categories, mild and moderate were recoded as mild and severe, life-threatening and death were recoded as severe. Medication accountable for ADEs was categorized based on major medication groups or, in the case of high number of ADEs, on subgroups. ADEs caused by medication errors were deemed preventable (pADEs). To all pADEs a stage of medication error was attributed. The categorization consisted of five error stages: prescribing (including ordering and monitoring), transcribing, dispensing, administering and across stage. ADEs not caused by medication errors were considered non-preventable ADEs.
For quality assessment, the Methodological Index for Non-randomized Studies (MINORS)-checklist was used, developed by Slim et al. This checklist was developed to assess the methodological quality in comparative and non-comparative studies. The checklist consists out of 12 items, eight for non-comparative studies and four additional items for comparative studies, including the risk of bias. The items were scored on a three point scale, ranging from 0–2. The maximum score was 16 for non-comparative studies and 24 for comparative studies, with higher scores indicating better quality [15].
Summary measures and synthesis of results
After pooling the datasets, occurrences of ADEs and pADEs per 100 admissions (and their 95% confidence intervals) were calculated with a Poisson regression analysis. Furthermore the risk factors for ADEs in surgical and non-surgical patients were identified. The associations of patient characteristics with ADE occurrence were expressed as odds ratios (and their 95% confidence intervals) following uni- and multivariable binary logistic regression analyses with candidate factors for the multivariable analyses selected from the univariable analyses with P > = 0.10 as the removal criterion. Study participants were listwise deleted in regression analyses, in the case of missing data on any variable of the predictor sets. If, due to this set-up, adding a variable to a regression model excluded a whole site, then the previous regression model without the added variable was assessed with and without data from the excluded site(s) to assess the potential bias resulting from the complete case analyses.
In a second step of the analysis we assessed whether the heterogeneity among studies in the pooled dataset had an impact on the identification of factors significantly associated with (p)ADEs by adding ‘Study’ to the final multivariable models and observing if the associations remained significant. Steps in model building were fully documented.
For the assessment of medications accountable for ADEs, i.e. high-risk drugs (HRDs), a weighing model was applied. This model was based on the fraction of all ADEs related to the type of medication (fADE), the medication-related proportion of severe ADEs (pS), the relative weight of severe (wS) compared with mild ADEs, and the medication-related proportions of preventable severe (prevS) and mild (prevM) ADEs: fADE*(pS*wS*prevS + (1 − pS)*prevM). All parameters except wS stem from the included data. The relative weight of severe vs. mild ADEs (wS) was arbitrarily set at 5 (severe ADEs being five times as worse as mild ADEs). The medications were ranked according to their weight based on this formula and the top five were considered HRDs. Because the relative weight of 5 was set arbitrarily, it was varied within a range from 2 to 10 in an additional scenario analysis in order to assess the robustness of this ranking.
Analysis of the triggers for detecting the ADEs and of type of medication errors in the different wards were performed with Pearson's Chi-square. The level of significance was set at P < 0.05. Statistical analyses were performed using SPSS, version 19.0.
Results
Study selection and characteristics
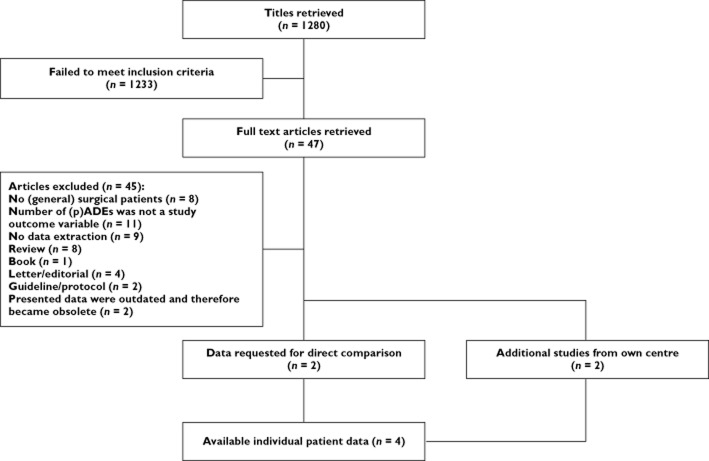
The literature search in 2011 yielded 1280 titles. After screening title and abstract, 47 papers were eligible and their full text was retrieved. Only two studies fulfilled the inclusion criteria and provided information on ADEs and their preventability in surgical as well as non-surgical patients. Their data were combined with two eligible studies performed in our centre [10,11,16,17] (Figure l). All four included studies were prospective observational multicentre studies. The methodological quality of the studies, based on the MINORS criteria, was good, and scored in the upper quartile of the quality score range (13–15 points, possible maximum score was 16 points). The characteristics of the included studies are shown in Table 1.
Figure 1.
Flow chart article selection
Table 1.
Study and patient characteristics per included cohort
| de Boer et al. [10] | Klopotowska et al. [11] | Morimoto et al. [17] | Berga Culleré et al. [16] | ||
|---|---|---|---|---|---|
| Trial design characteristics | |||||
| Publication year | 2013 | 2013 | 2010 | 2009 | |
| Cohort year | 2009 | 2007 | 2004 | 2007 | |
| Quality score (maximum 16) | 13 | 14 | 14 | 15 | |
| Number of hospitals | 3 | 3 | 5 | 5 | |
| Number of patients | 567 | 250 | 3 000 | 1550 | |
| Surgical | 567 | – | 1 469 | 775 | |
| Non-surgical | – | 250 | 1531 | 775 | |
| Patient days | |||||
| Surgical | 5367 | – | 30 457 | 5876 | |
| Non-surgical | – | 2151 | 25 751 | 7252 | |
| Method of ADE detection | Chart review based on selected triggers; assessment by an expert panel | Chart review enhanced by a IHI trigger tool; assessment by an expert panel | Chart review, direct observation and voluntary incident reports; assessment by physician reviewers | Chart review based on selected warning signs and daily observation; assessment by the research team | |
| Patient characteristics | |||||
| Age (%) | ≤52 years | 130 (23) | 0 | 572 (19) | 336 (22) |
| 53–64 years | 172 (30) | 0 | 549 (18) | 259 (17) | |
| 65–76 years | 173 (31) | 126 (50) | 995 (33) | 397 (26) | |
| ≥77 years | 92 (16) | 124 (50) | 884 (29) | 558 (36) | |
| Gender (%) | Male | 278 (49) | 117 (47) | 1 668 (56) | 894 (58) |
| Female | 289 (51) | 133 (53) | 1 332 (44) | 656 (42) | |
| Medication on admission (%) n = 4271 | ≤5 | 392 (69) | 82 (33) | 2 288 (76) | – |
| >6 | 170 (30) | 168 (67) | 712 (24) | – | |
| Urgency of admission (%) n = 5754 | Planned | 567 (100) | 37 (15) | 1 561 (52) | 465 (30) |
| Acute | - | 213 (85) | 1 439 (48) | 1013 (65) | |
| Patients with ADE (%) | 130 (23) | 36 (14) | 656 (22) | 159 (10) | |
| Patients with pADE (%) | 23 (4) | 21 (8) | 116 (4) | 81 (5) | |
The Japan Adverse Drug Events (JADE) study by Morimoto and colleagues investigated the incidence and preventability of ADEs and medication errors in Japan [17]. This study provided data of 1469 surgical patients and 1531 non-surgical patients. Another 459 patients admitted to the ICU ward were excluded for this analysis.
The Ward-oriented pharmacy In Newly admitted Geriatric Seniors (WINGS) study by Klopotowska and colleagues investigated the incidence and preventability of ADEs in hospitalized seniors (>65 years) [11]. Chart review enhanced by a modified Institute for Healthcare Improvement (IHI) trigger-tool was used to identify ADEs [18]. An expert team (physician and pharmacist) conducted the ADE assessment. For the purpose of the IPDMA only ADEs detected by the modified IHI ADE trigger-tool were included in the analyses. ADEs not related to triggers (i.e. detected by chart review only) were excluded.
The updated literature search in 2014 yielded 45 titles and abstracts. Just one additional study, with data on (p)ADEs in Saudi Arabia, was identified and we decided to reflect upon its outcomes in the discussion section below [19].
Summary measures and synthesis of results
Data from a total of 5367 admitted patients were available for the present IPDMA, 2811 surgical and 2556 non-surgical patients. The overall number of ADEs was 1304 of which 265 (20%) were preventable. Per 100 admissions, 24.3 (95% CI 22.8, 25.9) ADEs and 4.9 (95% CI 4.3, 5.6) pADEs were counted. ADEs occurred less frequently in surgical patients compared with non-surgical patients without reaching statistical significance (P value = 0.061), with 22.9 (95% CI 20.9, 25.1) ADEs per 100 admissions vs. 25.9 (95% CI 23.6, 28.3). The occurrence of pADEs was significantly lower in surgical patients, with 4.2 (95% CI 3.5, 5.1) pADEs per 100 admissions vs. 5.7 (95% CI 4.8, 6.8) in non-surgical patients (P value = 0.024).
Patient factors associated with ADEs
All patients were evaluated to define factors associated with one or more ADEs using a univariable and multivariable analysis. In the univariable analysis, the variables age and polypharmacy on admission significantly contributed to the occurrence of ADEs and pADEs. In line with the higher occurrence of pADEs in non-surgical patients, a non-surgical service was identified as a factor associated with pADEs as well (Table 2). In a multivariable logistic regression analysis, age was the only identified factor associated with ADEs, while age and polypharmacy on admission were both factors associated with pADEs. Equal results were found in a subgroup analysis in patients over 65 years (Table 2).
Table 2.
Factors associated with (preventable) ADEs
| All patients | Patients > 65 years | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Factors | OR (95% CI) ADE | P value | OR (95% CI) pADE | P value | OR (95% CI) ADE | P value | OR (95% CI) pADE | P value | |
| Age (years) | ≤ 52 years | 1 | 1 | – | – | ||||
| 53–64 years | 1.62 (1.26, 2.08) | 0.000 | 0.84 (0.49, 1.45) | 0.540 | – | – | |||
| 65–76 years | 1.75 (1.40, 2.19) | 0.000 | 1.45 (0.94, 2.24) | 0.093 | 1 | 1 | |||
| ≥ 77 years | 2.12 (1.70, 2.65) | 0.000 | 2.55 (1.69, 3.84) | 0.000 | 1.21 (1.02, 1.43) | 0.027 | 1.76 (1.30, 2.38) | 0.000 | |
| Gender | Male | 1 | 1 | 1 | 1 | ||||
| Female | 1.09(0.95, 1.25) | 0.242 | 1.07 (0.82, 1.38) | 0.616 | 0.99 (0.83, 1.17) | 0.875 | 1.02 (0.76, 1.38) | 0.874 | |
| Clinical service | Surgical | 1 | 1 | 1 | 1 | ||||
| Non-surgical | 1.04 (0.91, 1.20) | 0.534 | 1.30 (1.01, 1.69) | 0.045 | 0.92 (0.77, 1.08) | 0.306 | 1.05 (0.78, 1.41) | 0.751 | |
| Urgency of admission (n = 5295) | Planned | 1 | 1 | 1 | 1 | ||||
| Acute | 0.94 (0.82, 1.08) | 0.367 | 1.22 (0.94, 1.58) | 0.137 | 0.97 (0.82, 1.14) | 0.687 | 1.16 (0.86, 1.56) | 0.330 | |
| Polypharmacy * (n = 3812) | No | 1 | 1 | 1 | 1 | ||||
| Yes | 1.21 (1.03, 1.44) | 0.024 | 1.85 (1.34, 2.56) | 0.000 | 1.10 (0.90, 1.35) | 0.335 | 1.58 (1.10, 2.27) | 0.013 | |
The variable ‘Study’ did not affect the identification of factors associated with (p)ADEs in the hierarchical regression model for the whole group, as well as the senior group.
Detection and nature of ADEs
All studies used triggers such as clinical symptoms, laboratory values or a combination of both to detect ADEs. The greater part of the ADEs was detected by clinical symptoms, 937 of 1304 ADEs (72%). The role of laboratory values in detecting ADEs and pADEs was significantly higher in the severe ADE severity category (P < 0.001) (Table 3).
Table 3.
Triggers identifying ADEs
| Severity* | Preventability | |||||
|---|---|---|---|---|---|---|
| Mild (%) | Severe (%) | P value | No (%) | Yes (%) | P value | |
| Clinical | 596 (83) | 212 (54) | 767 (74) | 170 (64) | ||
| Laboratory | 116 (16) | 159 (40) | 0.000 | 253 (24) | 82 (31) | 0.001 |
| Both | 3 (0) | 21 (5) | 19 (2) | 13 (5) | ||
The probability of a causal relationship between adverse event and an administered drug was deemed certain in 12%, probable/likely in 42% and possible in 46% of the ADEs. Causality was significantly more often certain in pADEs compared with non-preventable ADEs (30% vs. 9%, P < 0.05) [10,11,17].
Next, the focus was on the type of medication accountable for ADEs [10,11,16,17]. For the additional analysis, data from only three studies were used [10,11,17], since one study had only analyzed the severity on pADEs [16]. Applying the weighing model as described in the methods section, resulted in a top five of HRDs: antibiotics, sedatives, anticoagulants, diuretics and antihypertensives (Table 4).
Table 4.
Medication accountable for ADEs
| Type of medication | Frequency (% of all ADEs n = 1304) | Severe*† | Mild*† | Ranking HRDs*‡ | ||
|---|---|---|---|---|---|---|
| All (% of all severe ADEs) | Preventable | All (% of all mild ADEs) | Preventable | |||
| Sedatives | 89 (7) | 81 (21) | 24 | 7 (1) | 1 | 1 (0.109) |
| Antibiotics | 375 (29) | 89 (23) | 12 | 252 (35) | 9 | 2 (0.062) |
| Antithrombotics– anticoagulants | 41 (3) | 22 (6) | 9 | 13 (2) | 5 | 3 (0.045) |
| Diuretics | 35 (3) | 18 (5) | 7 | 9 (1) | 2 | 4 (0.033) |
| Antihypertensives | 56 (4) | 24 (6) | 6 | 26 (4) | 5 | 5 (0.032) |
| Electrolytes or fluids | 28 (2) | 2 (1) | 2 | 24 (3) | 23 | 6 (0.030) |
| Antidiabetics | 25 (2) | 11 (3) | 6 | 8 (1) | 2 | 7 (0.029) |
| Analgesics – opioids | 143 (11) | 21 (5) | 4 | 101(14) | 6 | 8 (0.024) |
| Analgesics – NSAIDs | 90 (7) | 40 (10) | 4 | 35 (5) | 6 | 9 (0.023) |
| Other drugs | 163 (13) | 34 (9) | 3 | 73 (10) | 9 | 10 (0.022) |
| Antipsychotics | 26 (2) | 17 (4) | 3 | 6 (1) | 1 | 11 (0.014) |
| Cardiovascular drugs | 25 (2) | 8 (2) | 2 | 8 (1) | 0 | 12 (0.009) |
| Gastrointestinal drugs | 123 (9) | 13 (3) | 0 | 103 (14) | 4 | 13 (0.004) |
| Antithrombotics–antiplatelets | 19 (1) | 5 (1) | 0 | 13 (2) | 2 | 14 (0.002) |
| Nutritional agents and vitamins | 13 (1) | 1 (0) | 0 | 8 (1) | 2 | 15 (0.002) |
| Anaesthetics | 21 (2) | 2 (1) | 0 | 11 (2) | 0 | 16 (0) |
| Antiepileptics | 13 (1) | 4 (1) | 0 | 6 (1) | 0 | 17 (0) |
| Combination of medication | 12 (1) | 0 | 0 | 10 (1) | 0 | 18 (0) |
| Antifungals | 4 (0) | 0 | 0 | 2 (0) | 0 | 19 (0) |
ADEs, adverse drug events; HRDs, high risk drugs; NSAIDs, non-steroidal anti-inflammatory drugs.
n = 1107, severity was not assessable in three cases.
Weighing algorithm based on the fraction of all ADEs related to the type of medication (fADE), the medication-related proportion of severe ADEs (pS), the relative weight of severe (wS) compared with mild ADEs, and the medication-related proportions of preventable severe (prevS) and mild (prevM) ADEs: fADE*(pS*wS*prevS + (1 − pS)*prevM). wS was set at 5 in this calculation.
The type of events associated with HRDs in the various medication groups was evaluated [10,11,17]. An overview of all event types, the preventability and severity of the events can be found in Table 5 [10,11,16,17]. The events were ordered based on their association with HRDs. The event types associated with HRDs were often associated with abnormal laboratory values, such as abnormal liver function tests (19%) or impaired haemostasis (4%). Other event types were diarrhoea or constipation (35%), central nervous system event (18%) and skin and/or allergic reaction (11%).
Table 5.
ADE classification
| Type of ADE | Frequency (% of all ADEs n = 1304)˜ | Preventable (% of all pADEs n = 265) | Severe (% of all severe ADEs)*† | Associated with high risk drugs (%) |
|---|---|---|---|---|
| Diarrhoea or constipation | 345 (26) | 16 (6) | 24 (7) | 188 (35) |
| Abnormal liver function tests | 161 (12) | 5 (2) | 79 (52) | 101 (19) |
| Central nervous system event | 184 (14) | 48 (18) | 133 (83) | 96 (18) |
| Skin and/or allergic reaction | 156 (12) | 42 (16) | 7 (5) | 62 (11) |
| Impaired haemostasis | 23 (2) | 13 (5) | 13 (65) | 19 (4) |
| Renal function disorder | 38 (3) | 16 (6) | 20 (69) | 18 (3) |
| Cardiovascular event | 58 (4) | 28 (11) | 16 (43) | 13 (2) |
| Other | 85 (7) | 28 (11) | 20 (47) | 12 (2) |
| Haemorrhage | 65 (5) | 19 (7) | 50 (79) | 11 (2) |
| Electrolyte imbalance | 32 (2) | 22 (8) | 3 (23) | 8 (1) |
| Respiratory insufficiency | 12 (1) | 5 (2) | 9 (90) | 7 (1) |
| Nausea and/or vomiting | 131 (10) | 13 (5) | 10 (9) | 6 (1) |
| Thromboembolic event | 3 (0) | 3 (1) | 0 | 0 |
| Hypoglycaemia | 11 (1) | 7 (3) | 8 (80) | 0 |
A non-significantly higher proportion of pADEs in non-surgical patients was identified, 146 of 661 non-surgical ADEs (22%) vs. 119 of 643 surgical ADEs (19%, P = 0.108). A total of 13 ADEs directly contributed to the death of a patient, seven of which were judged as preventable. Significantly more pADEs were classified severe compared with the non-preventable ADEs (55% vs. 32%, P < 0.001; in data from three cohorts [10,11,17]). Furthermore, more severe ADEs were seen in non-surgical patients compared with the surgical patients (43% vs. 28%, P < 0.001).
For the 265 pADEs associated with medication errors, the medication error stage could be determined in 264 of the 265 pADEs. The majority of medication errors were found in the prescribing stage (90%). A slightly higher number of prescribing errors occurred in surgical patients (94%), compared with non-surgical patients (87%), but just failed to reach significance (P = 0.055). Importantly, in the severe pADE severity category 96% of the errors were associated with prescribing errors.
Discussion
The overall occurrence of ADEs and more specific pADEs constitutes a serious problem in hospitalized patients. The patients and/or drugs factors associated with the occurrence of a (p)ADE as provided by the present IPDMA can be used to target tailored interventions aimed at reducing ADEs. Non-surgical, elderly patients with polypharmacy on admission and/or receiving HRDs (antibiotics, sedatives, anticoagulants, diuretics and antihypertensives) require increased alertness.
This IPDMA led us to conclude that non-surgical patients are the ones who have a higher risk of pADEs compared with surgical patients. An explanation for this conclusion may be the age difference in both groups. Non-surgical patients in the included studies were older than surgical patients. This age difference remained when excluding the WINGS study that was conducted exclusively in elderly patients. Other grounds for this contrast might be the urgency of admission and length of hospital stay. Surgical admissions were more frequently planned admissions, whereas internal medicine has more acute admissions. The length of stay could increase the risk for developing pADEs as the longer the hospital stay, the more time patients are exposed to possible errors and their adverse effects. However, in the IPDMA, hospital stay of surgical admissions was comparable with non-surgical admissions.
Previous studies determined patient factors associated with ADEs in ambulatory care, nursing home residents and adult hospitalized patients. They concluded that age, gender, number of drugs, comorbidity and medical (non-surgical) service are important factors [20–23]. These studies were performed 20 years ago and perhaps are now outdated. Moreover, most of these studies were case-control studies that have a high risk of bias in comparison with prospective studies. One prospective cohort study was found. However, it relied on patient interviews for identifying ADEs and was entirely lacking objective measurements, which leads to highly biased results [21]. This IPDMA is comprised exclusively of prospective patient data. Therefore, this large international dataset has an explicit additional value in determining the genuine factors associated with ADEs.
Next to identification of patient groups with an increased risk of developing (p)ADEs, medication types seem another important focus in identifying ADE risks. The 5 Million Lives Campaign by the Institute of Healthcare Improvement focused on specific high ADE risk medication groups. In that campaign 12 interventions aimed to reduce morbidity and mortality due to medication errors were proposed. One of these interventions was ‘Prevent Harm from High-Alert Medications … starting with a focus on anticoagulants, sedatives, narcotics and insulin’ [24]. The campaign focused on prevention of all harm caused by medication, not solely harm perceived as preventable. The necessity of increased alertness when prescribing anticoagulants and sedatives was confirmed by the present IPDMA. According to this IPDMA, antibiotics, diuretics and antihypertensives should be considered as high risk drugs as well. To label certain categories of drugs as high risk drugs, here not only frequency of ADEs was taken into account but also the severity and the preventability of them. A robust model was used for ranking types of drugs, attributing a higher weight to those types causing severe ADEs. It may nevertheless still be worthwhile to put more effort into determining quantitative severity weights for different ADEs among groups of professionals and patients in future research.
Most errors in the medication order process occurred at the prescribing stage. The stage of prescribing is the most well documented stage of the medication order process. Also errors at this stage are the root of most errors. Nurses or anyone else will not likely intercept a wrong prescription. Kale et al. estimated that in a hospital where 6 million doses a year were administrated, more than 4200 preventable ADEs attributable to medication administration errors occur annually. The costs could range anywhere between $25 and $33 million in a 700-bed teaching hospital annually [25]. Another study concluded that the most important factor resulting in errors was the number of items on a prescription [26].
The important value of this IPDMA compared with single observational studies is the availability of ADE data from very diverse and large patient populations. Therefore, we were able to identify factors that can contribute to an ADE based on patient characteristics and medication type. The originating countries and baseline characteristics of the included studies were heterogeneous, meaning that identified factors associated with ADEs likely apply to various patient populations. If tailored intervention strategies to prevent ADEs are based on these factors they likely apply to any setting. The Agency for Healthcare Research and Quality (AHRQ) reported that ‘Adverse drug events cannot be predicted by patient characteristics or drug type’ [27]. With this IPDMA, however, due to the large population it was deemed possible to predict ADEs based on patient characteristics or drug type. On the other hand the diversity of studies unfortunately is a limitation as well. The population size varied. About half (56%) of the study population in this IPDMA consisted of patients from the JADE study [17]. Moreover, due to the minor differences in study design and recorded variables, only a small number of corresponding variables could be included in this IPDMA. In doing so, some more specific patient factors that presumably influence the risk for an ADE might have been left out in this analysis. For example the health status of the admitted patient, based on comorbidities and expressed as ASA classification, the body mass index or social class could not be retrieved from all studies and were therefore not included. These potential confounders, if known, could have influenced the observed association patterns. Hence, further differentiation in targeting areas for future interventions like elderly people with particular morbidity or disease statuses is well conceivable. For now, the IPDMA focused on information that is known for most patients on admission and thereby readily available for involved caregivers.
The study by Berga Culleré et al. focused on pADEs and while variables regarding these events were fairly complete, data on severity of non-preventable ADEs were absent [16]. The urgency of admission was manually determined based on the reason for admission, if sufficient information was available. The study by Berga Culleré et al. was excluded from analysis whenever the missing variables were required for that specific analysis, such as analysis of severity for non-preventable as well as preventable ADEs. Fortunately data from the Berga Culleré et al. study could be used in analyses for appointing patient characteristics that could be an indicator for the occurrence of (p)ADEs. To prevent bias due to their missing values, an additional patient risk factor analysis was performed, while excluding the Berga Culleré et al. study. It did not appear to be a confounding factor, since factors associated with (p)ADEs in this analysis (age and polypharmacy on admission) remained unchanged.
Some limitations of the data analysis must be noted as well. It was originally intended to include the variable ‘length of hospital stay’ in the multivariable analysis of factors associated with (p)ADEs. However, it was necessary to exclude this variable as a prospective risk factor for two main reasons: causality and unfitness for use as a predictor. First, it is difficult to discriminate between long hospital stays resulting in more time and opportunities for ADEs to take place on the one hand and prolonged hospitals stay resulting from experiencing an ADE on the other hand. Secondly, the length of hospital stay cannot be used as a predictive factor, since this factor develops during hospital stay and is not yet available on admission.
Another limitation might be that the factors potentially associated with (p)ADEs were all selected from available and accessible data from the included studies. Potential confounders like morbidity and disease status were not assessed, but could have influenced the observed association patterns, if known. Hence, further differentiation in targeting areas for future interventions like elderly people with particular morbidity or disease statuses is well conceivable.
Furthermore, two datasets from different studies at our centre were used. These studies were included because the methodology was similar, albeit not fully equal. In the WINGS study, the experts conducted a full chart review using the trigger-tool only as an aid and not for patient pre-selection [11]. In the Surepill study, a trigger tool was used to pre-select patients [10]. Only patients with identified triggers in their charts were further assessed by an expert team for ADEs [28]. For the purpose of this IPDMA, only ADEs that were identified by the trigger tool in the WINGS study were included, to optimize the comparability with the Surepill study. When using trigger tools to identify ADEs, ADEs not related to triggers can be missed [29].
The updated literature search yielded one prospective cohort study on the occurrence and nature of (p)ADEs in Saudi Arabia [19]. The study included 496 medical (non-surgical), 306 surgical and 175 ICU patients. The overall incidence of ADEs was 8.5 (95% CI 6.8, 10.4) and preventable ADEs 2.6 (95% CI 1.6, 3.7) per 100 admissions. The stage of errors was most frequent at the prescribing stage of the medication use process. The most frequent preventable ADEs according to drug classes were antibiotics, anticoagulants and antihypertensives. Significant factors associated with an increased odds ratio for ADEs were age, ICU, number of medications at admission and comorbidity. Surgical wards had a significantly lower odds ratio for ADEs.
In conclusion this IPDMA provided patient, drug and event characteristics that are associated with the occurrence of (p)ADEs. In particular elderly patients with polypharmacy on admission and use of high risk drugs are prone to experience ADEs. Efficiency in the prevention of ADEs can be improved by targeting vigilance systems for alertness of physicians and pharmacists.
Acknowledgments
We would like to thank the researchers Dr Codina Jané, Dr Tuset Creus and Dr De Andres of the SCFC-study, Professor Morimoto and Dr Sakuma of the JADE study and Professor Lie-A-Huen and Dr Klopotowska of the WINGS study for their collaboration in this project.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Kohn LT, Corrigan JM, Donaldson MS. To Err Is Human: Building A Safer Health System. New York: National Academies Press; 1999. [PubMed] [Google Scholar]
- 2.Cano FG, Rozenfeld S. Adverse drug events in hospitals: a systematic review. Cad. saúde pública / Ministério da Saúde, Fundação Oswaldo Cruz, Esc. Nac. Saúde Pública. 2009;25(Suppl. 3):S360–372. doi: 10.1590/s0102-311x2009001500003. [DOI] [PubMed] [Google Scholar]
- 3.Howard RL, Avery J, Slavenburg S, Royal S, Pipe G, Lucassen P, Pirmohamed M. Which drugs cause preventable admissions to hospital? A systematic review. Br J Clin Pharmacol. 2007;63:136–147. doi: 10.1111/j.1365-2125.2006.02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krähenbühl-Melcher A, Schlienger R, Lampert M, Haschke M, Drewe J, Krähenbühl S. Drug-related problems in hospitals: a review of the recent literature. Drug Saf. 2007;30:379–407. doi: 10.2165/00002018-200730050-00003. [DOI] [PubMed] [Google Scholar]
- 5.De Vries EN, Prins HA, Crolla RMPH, den Outer AJ, van Andel G, van Helden SH, Schlack WS, van Putten MA, Gouma DJ, Dijkgraaf MG, Smorenburg SM, Boermeester MA. Effect of a comprehensive surgical safety system on patient outcomes. N Engl J Med. 2010;363:1928–1937. doi: 10.1056/NEJMsa0911535. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz SM, Waggoner-Fountain L, Bass EJ, Sledd RM. Adequacy of information transferred at resident sign-out (in-hospital handover of care): a prospective survey. Qual Saf Health Care. 2008;17:6–10. doi: 10.1136/qshc.2006.019273. [DOI] [PubMed] [Google Scholar]
- 7.Arora V, Johnson J, Lovinger D, Humphrey HJ, Meltzer DO. Communication failures in patient sign-out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14:401–407. doi: 10.1136/qshc.2005.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kennedy JM, van Rij AM, Spears GF, Pettigrew RA, Tucker IG. Polypharmacy in a general surgical unit and consequences of drug withdrawal. Br J Clin Pharmacol. 2000;49:353–362. doi: 10.1046/1365-2125.2000.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramrattan MA, Boeker EB, Ram K, Burgers DMT, de Boer M, Lie-A-Huen L, Mulder WM, Boermeester MA. Evidence based development of bedside clinical drug rules for surgical patients. Int J Clin Pharm. 2014;36:581–588. doi: 10.1007/s11096-014-9941-x. [DOI] [PubMed] [Google Scholar]
- 10.De Boer M, Boeker EB, Ramrattan MA, Kiewiet JJS, Dijkgraaf MGW, Boermeester MA, Lie-A-Huen L. Adverse drug events in surgical patients: an observational multicentre study. Int J Clin Pharm. 2013;35:744–752. doi: 10.1007/s11096-013-9797-5. [DOI] [PubMed] [Google Scholar]
- 11.Klopotowska JE, Wierenga PC, Smorenburg SM, Stuijt CCM, Arisz L, Kuks PFM, Lie-A-Huen L. Recognition of adverse drug events in older hospitalized medical patients. Eur J Clin Pharmacol. 2012;69:75–85. doi: 10.1007/s00228-012-1316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veehof LJG, Steward RE, Haaijer-Ruskamp FM, Meyboom-de Jong B. The development of polypharmacy. A longitudinal study. Fam Pract. 2000;17:261–267. doi: 10.1093/fampra/17.3.261. [DOI] [PubMed] [Google Scholar]
- 13.Linjakumpu T, Hartikainen S, Klaukka T, Veijola J, Kivelä S-L, Isoaho R. Use of medications and polypharmacy are increasing among the elderly. J Clin Epidemiol. 2002;55:809–817. doi: 10.1016/s0895-4356(02)00411-0. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common Terminology Criteria for Adverse Events v.3.0and v.4.0 (CTCAE). [Internet]. Available at http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (last accessed 18 December 2012)
- 15.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 16.Berga Culleré C, Gorgas Torner MQ, Altimiras Ruiz J, Tuset Creus M, Besalduch Martin MT, Capdevila Sunyer M, Torres Gubert M, Casajoana Cortinas MT, Baró Sabaté E, Fernández Solà JR, Moron i Besolí A, Òdena Estradé E, Serrais Benavente J, Vitales Farrero MT, Codina Jané C. Detecting adverse drug events during hospital stay. Farm Hosp. 2009;33:312–323. [PubMed] [Google Scholar]
- 17.Morimoto T, Sakuma M, Matsui K, Kuramoto N, Toshiro J, Murakami J, Fukui T, Saito M, Hiraide A, Bates DW. Incidence of adverse drug events and medication errors in Japan: the JADE study. J Gen Intern Med. 2011;26:148–153. doi: 10.1007/s11606-010-1518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griffin F, Resar R. 2009. IHI global trigger tool for measuring adverse events (second edition). IHI Innov. Ser. White Pap.. Available at http://www.IHI.org (last accessed 18 December 2012)
- 19.Aljadhey H, Mahmoud M, Mayet A, Alshaikh M, Ahmed Y, Murray MD, Bates DW. Incidence of adverse drug events in an academic hospital: a prospective cohort study. Int J Qual Health Care. 2013;25:648–655. doi: 10.1093/intqhc/mzt075. [DOI] [PubMed] [Google Scholar]
- 20.Bates DW, Miller EB, Cullen DJ, Burdick L, Williams L, Laird N, Petersen LA, Small SD, Sweitzer BJ, Vander Vliet M, Leape LL. Patient risk factors for adverse drug events in hospitalized patients. ADE Prevention Study Group. Arch Intern Med. 1999;159:2553–2560. doi: 10.1001/archinte.159.21.2553. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi TK, Weingart SN, Borus J, Seger AC. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 22.Bates DW, Leape LL, Petrycki S. Incidence and preventability of adverse drug events in hospitalized adults. J Gen Intern Med. 1993;8:289–294. doi: 10.1007/BF02600138. [DOI] [PubMed] [Google Scholar]
- 23.Field TS, Gurwitz JH, Avorn J, McCormick D, Jain S, Eckler M, Benser M, Bates DW. Risk factors for adverse drug events among nursing home residents. Arch Intern Med. 2001;161:1629–1634. doi: 10.1001/archinte.161.13.1629. [DOI] [PubMed] [Google Scholar]
- 24.Federico F. Preventing harm from high-alert medications. Jt Comm J Qual Patient Saf. 2007;33:537–542. doi: 10.1016/s1553-7250(07)33057-2. [DOI] [PubMed] [Google Scholar]
- 25.Kale A, Keohane C, Maviglia S, Gandhi TK, Poon EG. Adverse drug events caused by serious medication administration errors. BMJ Qual Saf. 2012;21:933–938. doi: 10.1136/bmjqs-2012-000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seden K, Kirkham JJ, Kennedy T, Lloyd M, James S, McManus A, Ritchings A, Simpson J, Thornton D, Gill A, Coleman C, Thorpe B, Khoo SH. Cross-sectional study of prescribing errors in patients admitted to nine hospitals across North West England. BMJ Open. 2013;3:1–14. doi: 10.1136/bmjopen-2012-002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rockville M. Agency for Healthcare Research and Quality. 2001. Reducing and preventing adverse drug events to decrease hospital costs: research in action. Issue 1. Available at http://www.ahrq.gov/research/findings/factsheets/errors-safety/aderia/index.html (last accessed 18 December 2012) [DOI] [PubMed]
- 28.De Boer M, Kiewiet JJS, Boeker EB, Ramrattan MA, Dijkgraaf MGW, Lie-A-Huen L, Boermeester MA. A targeted method for standardized assessment of adverse drug events in surgical patients. J Eval Clin Pract. 2013;19:1073–1082. doi: 10.1111/jep.12033. [DOI] [PubMed] [Google Scholar]
- 29.Franklin BD, Birch S, Schachter M, Barber N. Testing a trigger tool as a method of detecting harm from medication errors in a UK hospital: a pilot study. Int J Pharm Pract. 2010;18:305–311. doi: 10.1111/j.2042-7174.2010.00058.x. [DOI] [PubMed] [Google Scholar]