Abstract
AIM
The aim of this review was to investigate the quality of the current literature on the transfer of anticonvulsants to breast milk to provide an overview of which anticonvulsants are in need of further research.
METHODS
We reviewed the quality of the available lactation studies for 19 anticonvulsants against the guidelines of the Food and Drug Administration (FDA) and the International Lactation Consultant Association (ILCA).
RESULTS
Except for one study on lamotrigine and one case report on gabapentin, no study on anticonvulsants had both the absolute infant dose (AID) and milk to plasma ratio (M : P) correctly assessed. Only one study on carbamazepine, phenytoin and vigabatrin was found that correctly assessed the AID. The main cause for this low number is the lack of essential details in published studies, since 25 of 62 studies were case reports, letters or abstracts. Other major shortcomings were the lack of information on sampling methods, the number of samples in a particular dose interval as well as the low number of study participants.
CONCLUSION
The quality of the current literature on the transfer of anticonvulsants to breast milk is low, except for lamotrigine, which makes it hard to draw conclusions about the safety of the use of anticonvulsants during the lactation period. Therefore, further research is needed.
Keywords: anti-epileptic drugs, anticonvulsants, breast milk, human milk, lactation
Introduction
Breastfeeding a child is the natural way to provide infants with essential nutrients. Human milk stimulates gastrointestinal function and improves host defences in the newborn [1–3]. Because of its overall benefits and low costs for the developing world, the World Health Organization recommends breastfeeding exclusively during the first 6 months and as supplement up to 2 years of age. In the United States, 75% of mothers have ever breastfed their infant in the early post-partum period and 36% are exclusively breastfeeding at 3 months [4]. One of the concerns in the lactation period is the safety of drugs being used by mothers, because transfer of the drug to the infant can occur through breastfeeding and may lead to adverse effects. This accounts especially for women with epilepsy, who often need long term anti-epileptic drugs to prevent seizures. In the United States nearly 1.1 million women with epilepsy are of the childbearing age [5]. Drowsiness, sedation, hepatitis, apnoea, methaemoglobinaemia and withdrawal symptoms are some of the reported adverse effects of anticonvulsant drug exposure in breast milk [6–11]. The long half-life of anticonvulsants in infants is another risk factor for developing adverse effects, since the drugs are not rapidly excreted [12]. Therefore, it is of great value to make a risk assessment of the possible adverse drug reactions but to not unnecessarily withhold infants of the great benefits of breast milk. A major element of this risk assessment is the estimated dose an infant receives through breastfeeding, as can be found in guidelines of the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) [13,14]. The other three major elements of risk assessment are infant plasma concentrations, observed adverse effects of the infant and follow-up of breast fed infants. This review focuses on the first element, the estimated infant dose and is not easily answered since studies about the pharmacokinetic and pharmacodynamic behaviour of anticonvulsant drugs in human milk vary in study design. Furthermore, the used methodology is in general not congruent with the International Lactation Consultant Association (ILCA) and FDA guidelines. Both guidelines provide a clear overview on the parameters which have to be determined to make a statement on the transfer of drugs into the breast milk and the infant. The absolute infant dose (AID) is considered as an informative and practical endpoint for the estimated dose an infant receives. The AID is defined as the dose an infant receives by ingestion of maternal milk after one single maternal dose or during a dose interval at steady-state. The milk to plasma ratio (M : P) is an estimate of the distribution of the drug between maternal plasma and milk. Recommendations to determine the AID and/or the M : P ratio are stated in these guidelines. We performed a literature review for lactation studies that measured the amount of anticonvulsants in breast milk and checked whether they met the ILCA and FDA guidelines on lactation studies to provide an overview of which anticonvulsants are in need of further research.
Methods
Anticonvulsants of interest
The following anticonvulsants are registered in the Netherlands: carbamazepine, clobazam, clonazepam, eslicarbazepine, ethosuximide, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, primidone, retigabine, rufinamide, stiripentol, topiramate, valproic acid and vigabatrin.
Inclusion of studies
The LactMed database was used to include studies. LactMed is part of the National Library of Medicine's (NLM) Toxicology data network and provides information about drug concentrations in breast milk and infant blood (http://lactmed.nlm.nih.gov). This database contains also information on possible adverse effects in breast fed infants. All data are peer reviewed and continually updated. LactMed is considered as one of the most accurate and extensive sources on information about drugs in breastfeeding mothers [15].
Drug records on LactMed contain a ‘drug concentrations’ segment in which findings of studies that reported amounts of drugs in breast milk are numerated. All studies in this ‘drug concentration’ segment were included in this review.
A Pubmed search was carried out to reveal any missing articles. The following query was used: (‘Milk, Human'[Mesh] OR ‘Breast Milk'[title/abstract] OR ‘Human Milk'[title/abstract]) AND (‘carbamazepine'[All Fields] OR ‘clobazam'[All Fields] OR ‘clonazepam'[All Fields] OR ‘eslicarbazepine'[All Fields] OR ‘ethosuximide'[All Fields] OR ‘gabapentin'[All Fields] OR ‘lacosamide'[All Fields] OR ‘lamotrigine'[All Fields] OR ‘levetiracetam'[All Fields] OR ‘oxcarbazepine'[All Fields] OR ‘phenobarbital'[All Fields] OR ‘phenytoin'[All Fields] OR ‘primidone'[All Fields] OR ‘retigabine'[All Fields] OR ‘rufinamide'[All Fields] OR ‘stiripentol'[All Fields] OR ‘topiramate'[All Fields] OR ‘valproic acid'[All Fields] OR ‘vigabatrin’).
Searches in LactMed and Pubmed were performed during December 2013. All studies published until then were eligible for inclusion. Studies that did not measure breast milk concentrations or any anticonvulsant of interest were excluded. Books were also excluded since they are not original research. Studies written in other languages than English, Dutch or German were excluded as well.
Criteria for quality review
The quality review of all studies was based on the ILCA and FDA protocol [13,14]. These protocols with guidelines on lactation studies, topics on study design, the amount of drugs in breast milk and determination of kinetics were merged to create a checklist (Table 1) and each study was checked using this checklist if it met the criteria. To characterize further the type of studies included, we stated if studies were case reports, letters, abstracts or full articles.
Table 1.
Quality checklist based upon the FDA and ILCA protocol
| Subject | Criterion |
|---|---|
| Publication status | No criterion: Registered type of study: full article, case report / letter or abstract |
| Study design | |
| Median number of patients (min−max) | No criterion: Registered number of included patients (and of which milk samples were obtained) |
| Longitudinal design | Obtained milk samples at multiple days post-partum in same patient |
| Amount of drug in breast milk | |
| Dose | Daily dose of patient is given |
| Timing of dose | Time between drug intake and milk sampling is given |
| Milk samples | Obtained pre- and post-feed samples OR total milk collection of one feeding and took aliquot for analysis |
| Determination of kinetics | |
| Milk concentrations | Obtained at least five milk samples in one dose interval |
| Plasma concentrations | Obtained at least five plasma samples in one dose interval |
| Active metabolites | Measured active metabolites, if any |
| Infant plasma | Obtained one plasma sample from nursed infant |
| Endpoints | |
| Milk : plasma ratio (M : P) | Correctly assessed if M : P is calculated from AUC in milk and plasma based on ≥ 5 milk and plasma levels |
| Absolute infant dose (AID) | Correctly assessed if entire volumes of milk in 24 h were collected OR by collecting ≥ five milk samples in dose interval |
Results
A total number of 62 articles was included for review (Figure 1). Using the LactMed database, we found 61 studies. One more article was added to this with the use of the PubMed query described in the methods section. The average number of investigated anticonvulsants per study was 1.20. Sixteen of the 62 studies were case reports, case series or letters and nine studies were abstracts. No studies were included for eslicarbazepine, retigabine, rufinamide, stiripentol, clobazam and lacosamide. Only one study was included for pregabalin and vigabatrin.
Figure 1.
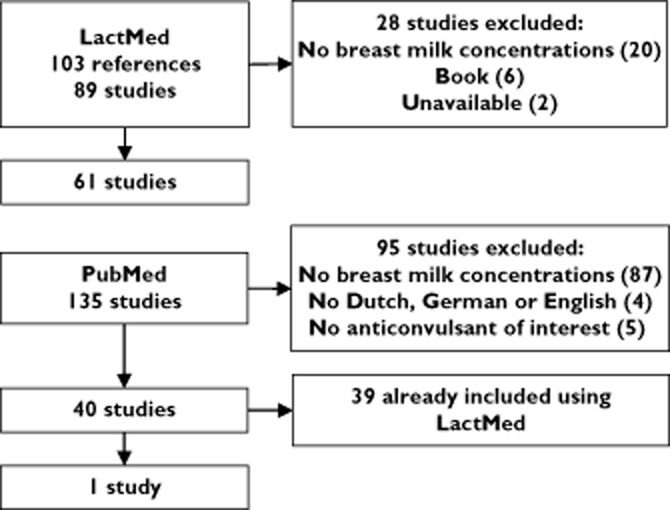
Results of study inclusion using LactMed and Pubmed
The results of our review are shown in Table 2. Regarding the endpoints, the AID was correctly assessed in one study for carbamazepine (three patients), phenytoin (one patient), gabapentin (one patient) and vigabatrine (two patients) and in two studies for lamotrigine (total of 29 patients). The milk to plasma ratio (M : P) was correctly determined in one study for lamotrigine (six patients) and gabapentin (one patient).
Table 2.
Results of quality review. All numbers represent the number of articles that matches corresponding criterion in Table 1
| Carbamazepine [8,19,40–49] | Ethosuximide [41,45,50–54] | Phenobarbital [20,41,55–58] | Phenytoin [20,41,45,59–64] | Lamotrigine [10,16,18,65–72] | Valproic acid [45,73–79] | Pregabalin [80] | Clonazepam [9,63] | Gabapentin [17,81,82] | Levetiracetam [83–86] | Oxcarbazepine [87–89] | Primidon [41,42,45,58,90] | Topiramate [91–93] | Vigabatrine [21] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies included | 12 | 7 | 6 | 9 | 11 | 8 | 1 | 2 | 3 | 4 | 3 | 5 | 3 | 1 |
| Publication status | ||||||||||||||
| Abstract | 2 | 2 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 2 | 1 | 0 | 1 | 0 |
| Case report/letter | 5 | 1 | 1 | 2 | 3 | 3 | 0 | 2 | 1 | 0 | 1 | 2 | 0 | 0 |
| Full article | 5 | 4 | 5 | 7 | 4 | 5 | 0 | 0 | 1 | 2 | 1 | 3 | 2 | 1 |
| Study design | ||||||||||||||
| Median number of patients (min−max) | 3 (1–56) | 2 (1–5) | 8 (1–13) | 3 (1–9) | 6 (1–34) | 4 (1–13) | 2 (2–2) | 1 (1–1) | 3 (1–5) | 12.5 (7–14) | 1 (1–3) | 7 (1–12) | 1 (1–3) | 2 (2–2) |
| Longitudinal design | 7 | 3 | 3 | 4 | 3 | 5 | 0 | 2 | 0 | 2 | 2 | 3 | 1 | 0 |
| Amount of drug in breast milk | ||||||||||||||
| Daily dose | 10 | 5 | 2 | 7 | 7 | 8 | 0 | 1 | 2 | 3 | 2 | 3 | 3 | 1 |
| Time of drug intake until sampling | 1 | 2 | 4 | 6 | 5 | 4 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 1 |
| Representative milk samples | 2 | 1 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 |
| Determination of kinetics | ||||||||||||||
| Milk concentrations | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Plasma concentrations | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Active metabolites | 5 | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | 2 | N/A | N/A | N/A |
| Infant plasma | 7 | 3 | 3 | 5 | 9 | 5 | 1 | 2 | 3 | 3 | 3 | 2 | 1 | 0 |
| Endpoints | ||||||||||||||
| M : P correctly assessed | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| AID correctly assessed | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 |
Values in bold correspond to endpoints.
AID, Absolute infant dose; M : P, Milk : plasma ratio; N/A, Not applicable.
Discussion
Our review showed that no anticonvulsant had both the AID and M : P correctly assessed according to the guidelines of the FDA and ILCA, except for one study on lamotrigine [16] and a case report on gabapentin [17]. Regarding the AID lamotrigine [18], carbamazepine [19], phenytoin [20] and vigabatrin [21] had only one study that assessed the AID correctly. Despite our results, these data are used in risk assessments worldwide every day. One of the reasons for this low number is that the ILCA and FDA protocols were not yet available as guidance, since most studies were published between 1970 and 2000. Another contributing factor for this low number is the publication status, since many studies have never been fully published. Therefore, most quality criteria could not be determined due to the brief character of the reports. This also accounts for the large number of case reports and letters that reported maternal milk concentrations.
As described in our results section, the number of participants in the studies that had the AID or M : P correctly assessed was low. Although the FDA and ILCA protocols do not mention a minimum number of study participants it is hard to state if the results of such small studies are representative for the whole group of lactating mothers. Even more some of these studies are case reports which are susceptible to selection bias due to their retrospective character.
The description of milk sampling was lacking for most studies. It was therefore not possible to take the drug distribution into account and the measured concentration could either be in foremilk, hindmilk or both. Because of the higher lipid content of hindmilk, lipophilic drugs will be more present at the end of feeding [22]. Studies that analyzed fore and hindmilk separately, showed that drug concentrations of some antidepressants were two to four times as high in post-feed compared with pre-feed samples [23,24].
Most studies only obtained one milk sample in a dose interval. The time milk samples were obtained with respect to dose intake was not reported in most studies. Studies investigating kinetics in milk samples showed that drug concentrations in milk vary during the dose interval, with the highest drug concentrations in the first half of the dose interval [25–28]. If only one sample is taken and/or the time of sampling to dose intake is not stated, the actual and calculated absolute infant dose may differ. A large number of studies did not report the ingested dose. Without this dose, interpreting the AID or reported breast milk concentration is impossible since both depend on the maternal dose.
The absolute exposure of anticonvulsants in the suckling infant can be revealed by measuring infant serum concentrations and is, beside the infant dose, another major element in the risk assessment according to the EMA [29]. Although this review only included studies in which breast milk concentrations were measured, studies with infant serum concentrations but without measuring breast milk concentrations were also found [30–38]. Most of these studies were either abstracts or case reports and were not correlated to the ingested infant dose, making it difficult to interpret reported infant serum concentrations. Therefore, measuring infant serum concentrations should be accompanied by measuring breast milk concentrations.
Longitudinal data to minimize intra-individual measurements are taken into account for most anticonvulsants. This is important to correct for the variation of analysis and variable milk composition during the post-partum period. Lipid content and pH vary in the first few weeks which can influence the extent of drug transfer to breast milk [39].
Conclusion
The clinical importance of qualitative studies that assess the AID or M : P is to determine if observed adverse effects in breast fed infants can be the result of nursing and if these effects are dose related. For this reason the European Medicine Agency (EMA) [29] and the FDA [14] state that the infant dose is one of the major elements for a risk assessment. We showed that most studies with anticonvulsants did not provide this infant dose. Another element in the risk assessment is to provide information on how to minimize exposure of the breast fed infant to the drug. With data on the kinetics of a drug in breast milk, timing of minimum and maximum drug concentrations become known, so mothers can be instructed to feed at specific times post-dose. We conclude that the quality of the current literature about this topic is rather poor. Better studies are needed to determine the extent of drug transfer to breast milk or M : P ratio for anticonvulsants.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work. IW had financial relationships, outside the submitted work, with organizations that might have an interest in the submitted work in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
Contributors
AW and PH designed the study. DM carried out the literature review with AW and PH. DM, AW, IW, BW and PH prepared the manuscript.
References
- 1.Kramer MS, Kakuma R. Optimal duration of exclusive breastfeeding. Cochrane Database Syst Rev. 2012;(8) doi: 10.1002/14651858.CD003517.pub2. CD003517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung M, Raman G, Trikalinos T, Lau J, Ip S. Interventions in primary care to promote breastfeeding: an evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:565–582. doi: 10.7326/0003-4819-149-8-200810210-00009. [DOI] [PubMed] [Google Scholar]
- 3.Breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–841. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 4.2012. Centers for Disease Control and Prevention. Breastfeeding report card – United States.
- 5.Yerby MS. Quality of life, epilepsy advances, and the evolving role of anticonvulsants in women with epilepsy. Neurology. 2000;55(5 Suppl. 1):S21–31. discussion S54–8. [PubMed] [Google Scholar]
- 6.Knott C, Reynolds F, Clayden G. Infantile spasms on weaning from breast milk containing anticonvulsants. Lancet. 1987;2:272–273. doi: 10.1016/s0140-6736(87)90853-1. [DOI] [PubMed] [Google Scholar]
- 7.Frey B, Braegger CP, Ghelfi D. Neonatal cholestatic hepatitis from carbamazepine exposure during pregnancy and breast feeding. Ann Pharmacother. 2002;36:644–647. doi: 10.1345/aph.1A326. [DOI] [PubMed] [Google Scholar]
- 8.Merlob P, Mor N, Litwin A. Transient hepatic dysfunction in an infant of an epileptic mother treated with carbamazepine during pregnancy and breastfeeding. Ann Pharmacother. 1992;26:1563–1565. doi: 10.1177/106002809202601215. [DOI] [PubMed] [Google Scholar]
- 9.Fisher JB, Edgren BE, Mammel MC, Coleman JM. Neonatal apnea associated with maternal clonazepam therapy: a case report. Obstet Gynecol. 1985;66(3 Suppl):34S–35. [PubMed] [Google Scholar]
- 10.Nordmo E, Aronsen L, Wasland K, Småbrekke L, Vorren S. Severe apnea in an infant exposed to lamotrigine in breast milk. Ann Pharmacother. 2009;43:1893–1897. doi: 10.1345/aph.1M254. [DOI] [PubMed] [Google Scholar]
- 11.Finch E, Lorber J. Methaemoglobinaemia in the newborn probably due to phenytoin excreted in human milk. J Obstet Gynaecol Br Emp. 1954;61:833–834. [PubMed] [Google Scholar]
- 12.Chen L, Liu F, Yoshida S, Kaneko S. Is breast-feeding of infants advisable for epileptic mothers taking antiepileptic drugs? Psychiatry Clin Neurosci. 2010;64:460–468. doi: 10.1111/j.1440-1819.2010.02126.x. [DOI] [PubMed] [Google Scholar]
- 13.Begg EJ, Duffull SB, Hackett LP, Ilett KF. Studying drugs in human milk: time to unify the approach. J Hum Lact. 2002;18:323–332. doi: 10.1177/089033402237904. [DOI] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. 2005. Clinical lactation studies – study design, data analysis and recommendations for labeling.
- 15.Akus M, Bartick M. Lactation safety recommendations and reliability compared in 10 medication resources. Ann Pharmacother. 2007;41:1352–1360. doi: 10.1345/aph.1K052. [DOI] [PubMed] [Google Scholar]
- 16.Page-Sharp M, Kristensen JH, Hackett LP, Beran RG, Rampono J, Hale TW, Kohan R, Ilett KF. Transfer of lamotrigine into breast milk. Ann Pharmacother. 2006;40:1470–1471. doi: 10.1345/aph.1G667. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen JH, Ilett KF, Hackett LP, Kohan R. Gabapentin and breastfeeding: a case report. J Hum Lact. 2006;22:426–428. doi: 10.1177/0890334406293421. [DOI] [PubMed] [Google Scholar]
- 18.Newport DJ, Pennell PB, Calamaras MR, Ritchie JC, Newman M, Knight B, Viguera AC, Liporace J, Stowe ZN. Lamotrigine in breast milk and nursing infants: determination of exposure. Pediatrics. 2008;122:e223–231. doi: 10.1542/peds.2007-3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pynnönen S, Kanto J, Sillanpää M, Erkkola R. Carbamazepine: placental transport, tissue concentrations in foetus and newborn, and level in milk. Acta Pharmacol Toxicol (Copenh) 1977;41:244–253. doi: 10.1111/j.1600-0773.1977.tb02145.x. [DOI] [PubMed] [Google Scholar]
- 20.Horning M, Stillwell W, Nowlin J, Lertratanangkoon K, Stillwell R, Hill R. Identification and quantification of drugs and drug metabolites in human breast milk using GC-MS-COM methods. Mod Probl Paediatr. 1975;15:73–79. [Google Scholar]
- 21.Tran A, O'Mahoney T, Rey E, Mai J, Mumford JP, Olive G. Vigabatrin: placental transfer in vivo and excretion into breast milk of the enantiomers. Br J Clin Pharmacol. 1998;45:409–411. doi: 10.1046/j.1365-2125.1998.t01-1-00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall B. Changing composition of human milk and early development of an appetite control. Lancet. 1975;305:779–781. doi: 10.1016/s0140-6736(75)92440-x. [DOI] [PubMed] [Google Scholar]
- 23.Ilett KF, Lebedevs TH, Wojnar-Horton RE, Yapp P, Roberts MJ, Dusci LJ, Hackett LP. The excretion of dothiepin and its primary metabolites in breast milk. Br J Clin Pharmacol. 1992;33:635–639. doi: 10.1111/j.1365-2125.1992.tb04093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kemp J, Ilett KF, Booth J, Hackett LP. Excretion of doxepin and N-desmethyldoxepin in human milk. Br J Clin Pharmacol. 1985;20:497–499. doi: 10.1111/j.1365-2125.1985.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rampono J, Hackett LP, Kristensen JH, Kohan R, Page-Sharp M, Ilett KF. Transfer of escitalopram and its metabolite demethylescitalopram into breastmilk. Br J Clin Pharmacol. 2006;62:316–322. doi: 10.1111/j.1365-2125.2006.02659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kristensen JH, Ilett KF, Hackett LP, Yapp P, Paech M, Begg EJ. Distribution and excretion of fluoxetine and norfluoxetine in human milk. Br J Clin Pharmacol. 1999;48:521–527. doi: 10.1046/j.1365-2125.1999.00040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verbeeck RK, Ross SG, McKenna EA. Excretion of trazodone in breast milk. Br J Clin Pharmacol. 1986;22:367–370. doi: 10.1111/j.1365-2125.1986.tb02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kristensen JH, Ilett KF, Rampono J, Kohan R, Hackett LP. Transfer of the antidepressant mirtazapine into breast milk. Br J Clin Pharmacol. 2007;63:322–327. doi: 10.1111/j.1365-2125.2006.02773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Medicine Agency. 2009. Guideline on risk assessment of medicinal products on human reproduction and lactation: from data to labelling.
- 30.Piontek CM, Baab S, Peindl KS, Wisner KL. Serum valproate levels in 6 breastfeeding mother-infant pairs. J Clin Psychiatry. 2000;61:170–172. doi: 10.4088/jcp.v61n0304. [DOI] [PubMed] [Google Scholar]
- 31.Stahl MM, Neiderud J, Vinge E. Thrombocytopenic purpura and anemia in a breast-fed infant whose mother was treated with valproic acid. J Pediatr. 1997;130:1001–1003. doi: 10.1016/s0022-3476(97)70292-0. [DOI] [PubMed] [Google Scholar]
- 32.Clark CT, Klein AM, Perel JM, Helsel J, Wisner KL. Lamotrigine dosing for pregnant patients with bipolar disorder. Am J Psychiatry. 2013;170:1240–1247. doi: 10.1176/appi.ajp.2013.13010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Precourt A, Morin C. Use of lamotrigine during breastfeeding: descriptive analysis of our population and report of five cases of premature neonates. Breastfeed Med. 2011;6(Suppl) S–18. [Google Scholar]
- 34.Cibert M, Gouraud A, Vial T, Tod M. A physiologically-based pharmacokinetic model to predict neonate exposure to drugs during breast-feeding: application to lamotrigine. Fundam Clin Pharmacol. 2010;24(Suppl):1–106. [Google Scholar]
- 35.Kacirova I, Grundmann M, Brozmanova H. Serum levels of lamotrigine in breastfeeding mothers, maternal milk and nursed infants. Basic Clin Pharmacol Toxicol. 2011;109(Suppl. 4):1–176. [Google Scholar]
- 36.Pote M, Kulkarni R, Agarwal M. Phenobarbital toxic levels in a nursing neonate. Indian Pediatr. 2004;41:963–964. [PubMed] [Google Scholar]
- 37.Birnbaum CS, Cohen LS, Bailey JW, Grush LR, Robertson LM, Stowe ZN. Serum concentrations of antidepressants and benzodiazepines in nursing infants: A case series. Pediatrics. 1999;104:e11. doi: 10.1542/peds.104.1.e11. [DOI] [PubMed] [Google Scholar]
- 38.Wisner KL, Perel JM. Serum levels of valproate and carbamazepine in breastfeeding mother-infant pairs. J Clin Psychopharmacol. 1998;18:167–169. doi: 10.1097/00004714-199804000-00009. [DOI] [PubMed] [Google Scholar]
- 39.Hibberd CM, Brooke OG, Carter ND, Haug M, Harzer G. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch Dis Child. 1982;57:658–662. doi: 10.1136/adc.57.9.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pynnonen S, Sillanpaa M. Carbamazepine and mother's milk. Lancet. 1975;2:563. doi: 10.1016/s0140-6736(75)90950-2. [DOI] [PubMed] [Google Scholar]
- 41.Kaneko S, Sato T, Suzuki K. The levels of anticonvulsants in breast milk. Br J Clin Pharmacol. 1979;7:624–627. doi: 10.1111/j.1365-2125.1979.tb04654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niebyl JR, Blake D, Freeman JM, Luff RD. Carbamazepine levels in pregnancy and lactation. Obstet Gynecol. 1979;53:139–140. [PubMed] [Google Scholar]
- 43.Kuhnz W, Jäger-Roman E. Carbamazepine and carbamazepine-10, 11-epoxide during pregnancy and postnatal period in epileptic mother and their nursed infants: pharmacokinetics and clinical effects. Pediatr Pharmacol. 1983;3:199–208. [PubMed] [Google Scholar]
- 44.Froescher W, Eichelbaum M, Niesen M, Dietrich K, Rausch P. Carbamazepine levels in breast milk. Ther Drug Monit. 1984;6:266–271. doi: 10.1097/00007691-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Meyer F, Quednow B, Potrafki A, Walther H. [The perinatal pharmacokinetics of anticonvulsant drugs] Zentralbl Gynakol. 1988;110:1195–1205. [PubMed] [Google Scholar]
- 46.Brent NB, Wisner KL. Fluoxetine and carbamazepine concentrations in a nursing mother/infant pair. Clin Pediatr (Phila) 1998;37:41–44. doi: 10.1177/000992289803700107. [DOI] [PubMed] [Google Scholar]
- 47.Lopes BR, Barreiro JC, Baraldi PT, Cass QB. Quantification of carbamazepine and its active metabolite by direct injection of human milk serum using liquid chromatography tandem ion trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2012;889–890:17–23. doi: 10.1016/j.jchromb.2012.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Shimoyama R, Ohkubo T, Sugawara K. Monitoring of carbamazepine and carbamazepine 10,11-epoxide in breast milk and plasma by high-performance liquid chromatography. Ann Clin Biochem. 2000;37(Pt 2):210–215. doi: 10.1258/0004563001899023. [DOI] [PubMed] [Google Scholar]
- 49.Kacirova I, Grundmann M, Brozmanova H. Therapeutic monitoring of carbamazepine concentrations in breastfeeding mothers, maternal milk and nursed infants. Ther Drug Monit. 2011;33:503. [Google Scholar]
- 50.Koup J, Rose J, Cohen M. Ethosuximide pharmacokinetics in a pregnant patient and her newborn. Epilepsia. 1977;19:535–539. doi: 10.1111/j.1528-1157.1978.tb05033.x. [DOI] [PubMed] [Google Scholar]
- 51.Rane A, Tunell R. Ethosuximide in human milk and in plasma of a mother and her nursed infant. Br J Clin Pharmacol. 1981;12:855–858. doi: 10.1111/j.1365-2125.1981.tb01320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kuhnz W, Koch S, Jakob S, Hartmann A, Helge H, Nau H. Ethosuximide in epileptic women during pregnancy and lactation period. Placental transfer, serum concentrations in nursed infants and clinical status. Br J Clin Pharmacol. 1984;18:671–677. doi: 10.1111/j.1365-2125.1984.tb02528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomson T, Villén T. Ethosuximide enantiomers in pregnancy and lactation. Ther Drug Monit. 1994;16:621–623. doi: 10.1097/00007691-199412000-00016. [DOI] [PubMed] [Google Scholar]
- 54.Soderman P, Rane A. Ethosuximide and nursing. Acta Pharm. 1986;59(Suppl) Abstract 513. [Google Scholar]
- 55.Westerink D, Glerum JH. [Separation and microdetermination of phenobarbital and phenytoin in human milk] Pharm Weekbl. 1965;100:577–583. [PubMed] [Google Scholar]
- 56.Gomita Y, Furuno K, Araki Y, Yamatogi Y, Ohtahara S. Phenobarbital in sera of epileptic mothers and their infants. Am J Ther. 1995;2:968–971. doi: 10.1097/00045391-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 57.Shimoyama R, Ohkubo T, Sugawara K. Characteristics of interaction between barbiturate derivatives and various sorbents on liquid chromatography and determination of phenobarbital in Japanese human breast milk. J Liq Chromatogr Relat Technol. 2000;23:587–599. [Google Scholar]
- 58.Kuhnz W, Koch S, Helge H, Nau H. Primidone and phenobarbital during lactation period in epileptic women: total and free drug serum levels in the nursed infants and their effects on neonatal behavior. Dev Pharmacol Ther. 1988;11:147–154. doi: 10.1159/000457682. [DOI] [PubMed] [Google Scholar]
- 59.Mirkin B. Diphenylhydantoin: Placental transport, fetallocalization, neonatal metabolism, and possible teratogenic effects. J Pediatr. 1971;78:329–337. doi: 10.1016/s0022-3476(71)80025-2. [DOI] [PubMed] [Google Scholar]
- 60.Rane A, Garle M, Borga O, Sjoqvist F. Plasma disappearance of transplacentally transferred diphenyl-hydantoin in the newborn studied by mass fragmentography. Clin Pharmacol Ther. 1974;15:39–45. doi: 10.1002/cpt197415139. [DOI] [PubMed] [Google Scholar]
- 61.Shimoyama R, Ohkubo T. Monitoring of phenytoin in human breast milk, maternal plasma and cord blood plasma by solid-phase extraction and liquid chromatography. J Pharm Biomed Anal. 1998;17:863–869. doi: 10.1016/s0731-7085(97)00277-x. [DOI] [PubMed] [Google Scholar]
- 62.Sugawara K, Shimoyama R, Ohkubo T. Determinations of psychotropic drugs and antiepileptic drugs by high-performance liquid chromatography and its monitoring in human breast milk. Hirosaki Med J. 1999;51(Suppl):81–86. [Google Scholar]
- 63.Soderman P, Matheson I. Clonazepam in breast milk. Eur J Pediatr. 1988;147:212–213. doi: 10.1007/BF00442230. [DOI] [PubMed] [Google Scholar]
- 64.Steen B, Rane A, Lönnerholm G, Falk O, Elwin CE, Sjöqvist F. Phenytoin excretion in human breast milk and plasma levels in nursed infants. Ther Drug Monit. 1982;4:331–334. doi: 10.1097/00007691-198212000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Ohman I, Vitols S, Tomson T. Lamotrigine in pregnancy: pharmacokinetics during delivery, in the neonate, and during lactation. Epilepsia. 2000;41:709–713. doi: 10.1111/j.1528-1157.2000.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 66.Rambeck B, Kurlemann G, Stodieck SR, May TW, Jürgens U. Concentrations of lamotrigine in a mother on lamotrigine treatment and her newborn child. Eur J Clin Pharmacol. 1997;51:481–484. doi: 10.1007/s002280050234. [DOI] [PubMed] [Google Scholar]
- 67.Tomson T, Ohman I, Vitols S. Lamotrigine in pregnancy and lactation: a case report. Epilepsia. 1997;38:1039–1041. doi: 10.1111/j.1528-1157.1997.tb01489.x. [DOI] [PubMed] [Google Scholar]
- 68.Berry D. The disposition of lamotrigine throughout pregnancy. Ther Drug Monit. 1999;21:450. [Google Scholar]
- 69.Ohman I, Tomson T, Vitols S. Lamotrigine (LTG) pharmacokinetics during delivery and lactation. Ther Drug Monit. 1999;21:478. [Google Scholar]
- 70.Fotopoulou C, Kretz R, Bauer S, Schefold JC, Schmitz B, Dudenhausen JW, Henrich W. Prospectively assessed changes in lamotrigine-concentration in women with epilepsy during pregnancy, lactation and the neonatal period. Epilepsy Res. 2009;85:60–64. doi: 10.1016/j.eplepsyres.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 71.Kacirova I, Grundmann M, Brozmanova H. Drug interaction between lamotrigine and valproic acid used at delivery and during lactation – a case report. Basic Clin Pharmacol Toxicol. 2011;109(Suppl):56–164. [Google Scholar]
- 72.Kacirova I, Grundmann M, Koristkova B, Brozmanova H. Therapeutic monitoring of lamotrigine during delivery, in the neonatal period, and during lactation. Ther Drug Monit. 2007;29:477. [Google Scholar]
- 73.Alexander F. Sodium valproate in pregnancy. Arch Dis Child. 1979;54:240–245. doi: 10.1136/adc.54.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dickinson R, Harland RC, Lynn RK, Smith WB, Gerber N. Transmission of valproic acid (Depakene) across the placenta: Half-life of the drug in mother and baby. J Pediatr. 1979;94:832–835. doi: 10.1016/s0022-3476(79)80172-9. [DOI] [PubMed] [Google Scholar]
- 75.Von Unruh G, Froescher W, Hoffmann F, Niesen M. Valproic acid in breast milk: how much is really there? Ther Drug Monit. 1984;6:272–276. doi: 10.1097/00007691-198409000-00003. [DOI] [PubMed] [Google Scholar]
- 76.Nau H, Koch S, Häuser I, Helge H. Valproic acid and its metabolites: placental transfer, neonatal pharmacokinetics, transfer via mother's milk and clinical status in neonates of epileptic mothers. J Pharmacol Exp Ther. 1981;219:768–777. [PubMed] [Google Scholar]
- 77.Nau H, Helge H, Luck W. Valproic acid in the perinatal period: decreased maternal serum protein binding results in fetal accumulation and neonatal displacement of the drug and some metabolites. J Pediatr. 1984;104:627–634. doi: 10.1016/s0022-3476(84)80567-3. [DOI] [PubMed] [Google Scholar]
- 78.Philbert A, Pedersen B, Dam M. Concentration of valproate during pregnancy, in the newborn and in breast milk. Acta Neurol Scand. 1985;72:460–463. doi: 10.1111/j.1600-0404.1985.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 79.Tsuru N, Maeda T, Tsuruoka M. Three cases of delivery under sodium valproate. Placental transfer, milk transfer and probable teratogenicity of sodium valproate. Jpn J Psychiatry Neurol. 1988;42:89–96. doi: 10.1111/j.1440-1819.1988.tb01960.x. [DOI] [PubMed] [Google Scholar]
- 80.Ohman I, de Flon P, Tomson T. Pregabalin kinetics in the neonatal period, and during lactation. Epilepsia. 2011;52:23–263. [Google Scholar]
- 81.Ohman I, Vitols S, Tomson T. Pharmacokinetics of gabapentin during delivery, in the neonatal period, and lactation: does a fetal accumulation occur during pregnancy? Epilepsia. 2005;46:1621–1624. doi: 10.1111/j.1528-1167.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- 82.Ohman I, Tomson T. Gabapentin kinetics during delivery, in the neonatal period, and during lactation. Epilepsia. 2009;50(Suppl):108. [Google Scholar]
- 83.Kramer G, Hosli I, Glanzmann R. Levetiracetam accumulation in human breast milk. Epilepsia. 2002;43(Suppl):105. [Google Scholar]
- 84.Greenhill L, Betts T, Yarrow H, Patsalos P. Breast milk levels of levetiracetam after delivery. Epilepsia. 2004;45(Suppl):230. [Google Scholar]
- 85.Johannessen SI, Helde G, Brodtkorb E. Levetiracetam concentrations in serum and in breast milk at birth and during lactation. Epilepsia. 2005;46:775–777. doi: 10.1111/j.1528-1167.2005.54804.x. [DOI] [PubMed] [Google Scholar]
- 86.Tomson T, Palm R, Källén K, Ben-Menachem E, Söderfeldt B, Danielsson B, Johansson R, Luef G, Ohman I. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia. 2007;48:1111–1116. doi: 10.1111/j.1528-1167.2007.01032.x. [DOI] [PubMed] [Google Scholar]
- 87.Bulau P, Paar W, von Unruh G. Pharmacokinetics of oxcarbazepine and 10-hydroxy-carbazepine in the newborn child of an oxcarbazepine-treated mother. Eur J Clin Pharmacol. 1988;34:311–313. doi: 10.1007/BF00540963. [DOI] [PubMed] [Google Scholar]
- 88.Lutz U, Wiatr G, Gaertner H, Bartels M. Oxcarbazepine treatment during breast-feeding: a case report. J Clin Psychopharmacol. 2007;27:730–732. doi: 10.1097/JCP.0b013e31815a5819. [DOI] [PubMed] [Google Scholar]
- 89.Ohman I, Tomson T. Pharmacokinetics of oxcarbazine in neonatal period and during lactation. Epilepsia. 2009;50(Suppl. 4):2–262. [Google Scholar]
- 90.Nau H, Rating D, Häuser I, Jäger E. Placental transfer and pharmacokinetics of primidone and its metabolites phenobarbital, PEMA and hydroxyphenobarbital in neonates and infants of epileptic mothers. Eur J Clin Pharmacol. 1980;18:31–42. doi: 10.1007/BF00561476. [DOI] [PubMed] [Google Scholar]
- 91.Ohman I, Vitols S, Luef G, Söderfeldt B, Tomson T. Topiramate kinetics during delivery, lactation, and in the neonate: preliminary observations. Epilepsia. 2002;43:1157–1160. doi: 10.1046/j.1528-1157.2002.12502.x. [DOI] [PubMed] [Google Scholar]
- 92.Westergren T, Hjelmeland K, Kristoffersen B, Johannessen SI, Kalikstad B. Topiramate-induced diarrhoea in a 2-month-old breastfed child. Drug Saf. 2009;32:875–993. doi: 10.1016/j.ebcr.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Froscher W, Jurges U. [Topiramate used during breast feeding] Aktuelle Neurol. 2006;33:215–217. [Google Scholar]


