Abstract
AIM
This study aimed to quantitate the efficacy of soy isoflavones in the treatment of menopausal hot flashes.
METHODS
Model based meta-analysis (MBMA) was used to quantitate the efficacy of soy isoflavones. We conducted a systemic literature search to build a time–effect model for placebo and soy isoflavones in treating menopausal hot flashes. Studies were identified, subjected to inclusion and exclusion criteria, and reviewed.
RESULTS
From 55 articles, 16 studies of soy isoflavones met the inclusion criteria, and contained 65 and 66 mean effect values in placebo and soy isoflavone groups, respectively, from about 1710 subjects. Interestingly, the developed model was found to describe adequately the time course of hot flashes reduction after administration of placebo and soy isoflavones. Using this model, we found that the maximal percentage change of hot flashes reduction by soy isoflavones was 25.2% after elimination of the placebo effect, accounting for 57% of the maximum effects of estradiol (Emax-estradiol = 44.9%). However, a time interval of 13.4 weeks was needed for soy isoflavones to achieve half of its maximal effects, much longer than estradiol, which only required 3.09 weeks. These results suggest that treatment intervals of 12 weeks are too short for soy isoflavones, which require at least 48 weeks to achieve 80% of their maximum effects.
CONCLUSIONS
Soy isoflavones show slight and slow effects in attenuating menopausal hot flashes compared with estradiol.
Keywords: estradiol, hot flashes, menopause, model based meta analysis, soy isoflavones
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Menopause hot flashes can be effectively treated with hormone replacement therapy (HRT). However, possible adverse effects of long term HRT have promoted the use of complementary therapies by menopausal women.
There has been debate on the benefits of soy isoflavones in the treatment of menopausal hot flashes.
WHAT THIS STUDY ADDS
The effects of soy isoflavones on menopausal hot flashes was quantitated adequately by the model based meta-analysis.
The developed model provided the robust evidence that soy isoflavones have slight and slow effects in attenuating menopausal hot flashes compared with estradiol.
Introduction
Menopause, the time in a woman's life when the ovaries lose their reproductive function, is often accompanied by a range of symptoms. However, only hot flashes and vaginal dryness are consistently associated with this stage of life [1]. Fortunately, hot flashes can be effectively treated with hormone replacement therapy (HRT) [2]. However, possible adverse effects [3] of long term HRT have promoted the use of complementary therapies by menopausal women. Recently, soy isoflavones, black cohosh and red clover have been reported to be widely used to relieve menopausal hot flashes [4], but their efficacies remain largely unknown. A meta-analysis published in JAMA in 2006 showed that no definitive evidence existed to support the efficacy of those plant medicines in treating menopausal hot flashes [5]. Nevertheless, this work presented some limitations. First, the efficacy data obtained at different time points were combined, neglecting the effect of time on treatment efficiency. Then, only a small number of studies were included, with most using small sample sizes. The efficacy data of hot flashes reduction usually display a large variability. Therefore, the conclusions need to be re-examined.
An approach recently described as model-based meta-analysis (MBMA) has been developed and used to estimate the comparative efficacy of different treatments [6]. MBMA can be used to distinguish inter-trial, inter-treatment arm and inter-individual variability. It also can test the impact on efficacy of different factors, including dose, duration, drug formulation and other variables. Therefore, compared with the conventional meta-analysis, MBMA is more powerful in identifying treatment effects.
Soy isoflavones are widely present in soy products, which are convenient to take, with little adverse effects. However, it is still unclear how much soy isoflavones should be taken and how long they need to be used. This study used the MBMA method to evaluate quantitatively the efficacy of soy isoflavones on hot flashes, comparing these compounds with estradiol, which has proven efficacy on hot flashes. This work provides useful information for a deeper understanding of the efficacy of soy isoflavones on menopausal hot flashes.
Method
Search strategy
A comprehensive literature search in the PubMed database was performed from January 1990 to January 2014, using the terms ‘hot flash’ or ‘hot flush’, and ‘soy’ or ‘genistein’ or ‘daidzin’ or ‘glycitein’. Additionally, our search was limited to clinical trials and articles published in English. Inclusion criteria were (1) placebo controlled clinical trial, (2) longitudinal data of frequency or decreased rates of hot flashes provided in tables or graphics and (3) if the study was a crossover design, only data from the first period were analyzed.
Data extraction
A Microsoft Excel database was created to catalogue the relevant features of various studies. The information collected from each selected clinical study included authors, year of publication, sample size, dosage, study duration, subject category and mean percentage of hot flashes reduction at each time point. The mean percentage change in hot flashes from baseline was used as the evaluation index for modelling, thus eliminating the potential baseline effects on the evaluation of treatment efficiency.
Digitizing software Engauge Digitizer (version 4.1, 2002 by Mark Mitchell) was used to extract the graphical data. If only frequency of hot flashes was reported in a study, the mean percentage change in hot flashes from baseline was estimated as follows:
| (1) |
where Et is the frequency of hot flashes at time t and Ebaseline represents the frequency of hot flashes at baseline.
All data were extracted independently by two researchers (LL and LX), and any disagreement between them was resolved by discussion. Data extraction errors between the two researchers should not exceed 2%. Mean values were considered final data.
Model development
We hypothesized that placebo and soy isoflavone effects would vary with time and reach a plateau. Therefore, the effect profiles for placebo and soy isoflavones were described with the sigmoid Emax model, with time considered as an independent variable. The basic model was described as follows:
| (2) |
where
| (3) |
| (4) |
In equation 2, Ei,j is the observed mean effect in ith study at time j, Eplacebo,i,j represents the predicted mean placebo effect in ith study at time j, Esoy,i,j is the predicted mean effect of soy isoflavones in ith study at time j, Noi,j is the sample size in ith study at time j, IAV represents the inter-treatment arm variability, assumed to be normally distributed with a mean of 0 and variance of ωarm2/Noi,j and εi,j is the residual error in ith study at time j, assumed to be normally distributed with a mean of 0 and variance of σ2/Noi,j. IAV and εi,j are weighted by the sample size.
In equations 3 and 4, Emax-placebo is the maximal effect of placebo, Emax-soy represents the maximal effect of soy isoflavones, ET50-placebo is the time to achieve 50% of Emax-placebo, ET50-soy represents the time to achieve 50% of Emax-soy, Tj is the time j, η1,i represents the inter-trial variability of Emax-placebo and η2,i is the inter- trial variability of Emax-soy. η1,I; η2,i are assumed to be normally distributed with a mean of 0 and variance of ω12 and ω22.
Once the basic model was established, the dose of soy isoflavones was considered a covariate to be added into Emax-soy. A difference in objective function value (OFV) of 6.63 (χ2, α = 0.01, d.f. = 1) was considered statistically significant in the covariate model building process.
Model validation
The accuracy of the model fit was evaluated by graphic assessment. Monte Carlo simulations were performed 1000 times to predict 90% confidence intervals of the effects of placebo and soy isoflavones.
The final model was also evaluated by the leave-one-out cross validation method. Briefly, values from one trial were sequentially dropped from the full data set, and the final model was applied. The parameter estimates obtained from each data set were compared to investigate stability of the final model.
Software
The model estimation and simulation were performed using nonmem 7 (Level 1.0, ICON Development Solutions, USA). Diagnostic graphics and visual predictive check were performed using the R software (version 3.0.1, The R Foundation of Statistical Computing).
Results
Characteristics of the selected studies
A total of 55 studies were assessed for inclusion in the analysis, and 39 were excluded based on study design that did not meet our inclusion criteria. The remaining 16 studies [7–22] containing 66 and 65 mean effect data of soy isoflavones and placebo, respectively, were included for model building (Figure 1, Table 1).
Figure 1.
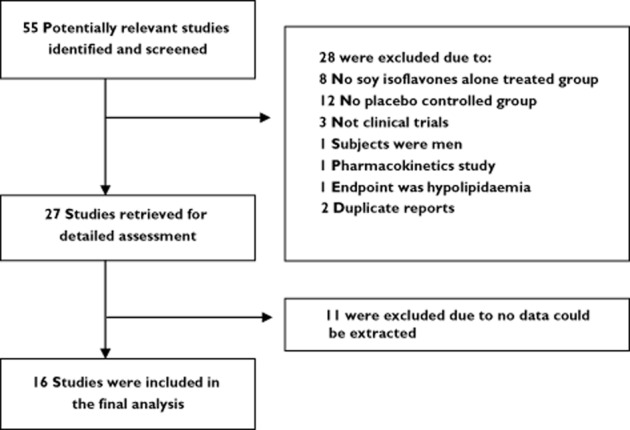
Flowchart for screening relevant articles
Table 1.
Summary of included placebo-controlled trials of soy isoflavones
| Source | Number of participants | Sample | Therapy | Study duration | Study design | Baseline of hot flashes/results |
|---|---|---|---|---|---|---|
| Albertazzi et al. [7] | 104 | Post-menopausal women; ≥7 hot flashes per day; average age, 52–53 years; Italy. | Isolated soy protein, 76 mg of isoflavones (aglycone units) | 12 weeks | Parallel | The baseline frequency: 11.4(10.7–12.7) per day for the soy group, 10.9(10.2–11.8) per day for the placebo group; Reduced frequency (45% vs. 30%, P < 0.01) with soy vs. placebo. |
| Quella et al. [8] | 177 | Women had breast cancer; ≥14 hot flashes per week; over 18 years of age; United States | Soy tablet, 150 mg daily (with 40–45% genistein, 40–45% daidzein, and 10–20% glycitein) | 4 weeks | Crossover | The baseline frequency: 7 ± 4.5 per day; No differences in frequency or severity score between groups, 36% of placebo vs. 24% of soy tablet. |
| Scambia et al. [9] | 39 | Post-menopausal women; average age, 53–54 years; Italy | Soy tablet, 50 mg daily of genistein and daidzein | 6 weeks | Parallel | The baseline frequency: 33 ± 5.1 per week for the soy group, 27 ± 5.1 per week for the placebo group; Reduced frequency (40% vs. 25%, P < 0.05) with soy tablet vs. placebo. |
| Upmalis et al. [10] | 177 | Healthy post-menopausal women; ≥5 hot flashes per day; aged 50 years or older; American | Soy tablet, 50 mg daily of genistein and daidzein | 12 weeks | Parallel | The baseline frequency: 8.8 ± 6.2 per day for the soy group, 9.4 ± 6.0 per day for the placebo group; No difference in frequency between groups. |
| Knight et al. [11] | 24 | Post-menopausal women; ≥3 hot flashes per day; aged 40–45 years; Australia. | Soy powder, 134.4 mg daily of genistein, daidzein and glycitein compounds. | 12 weeks | Parallel | The baseline frequency: 50.2 ± 13.6 per week for the soy group, 56.2 ± 26.5 per week for the placebo group; Reduced frequency (43% vs. 20%, P = 0.32) with soy vs. placebo |
| Faure et al. [12] | 75 | Post-menopausal women; ≥7 hot flashes per day; average age, 53–54 years; France | Soy capsule, 70 mg daily of genistein and daidzein | 16 weeks | Parallel | The baseline frequency: 10.1 ± 6.4 per day for the soy group, 9.4 ± 3.4 per day for the placebo group; Reduced frequency with soy vs. placebo (61%vs. 21%, P = 0.01). |
| Van Patten et al. [13] | 157 | Women had breast cancer; ≥10 hot flashes per week; average age, 55–56 years; England. | Soy beverage, 90 mg of isoflavones | 12 weeks | Parallel | The baseline frequency: 7.1 ± 4.3 per day for the soy group, 7.4 ± 6.4 per day for the placebo group; No differences in frequency between groups. Reduced frequency (25.4% vs. 33.8%) with soy vs. placebo. |
| Penotti et al. [14] | 62 | Post-menopausal women; ≥7 hot flashes per day; aged 45–60 years; Italy | Soy tablet, 72 mg daily isoflavone | 6 mo | Parallel | The baseline frequency: 9.5 ± 3.4 per day for the soy group, 8.8 ± 1.4 per day for the placebo group; 40% reduction in frequency in both groups; No between-group differences. |
| Crisafulli et al. [15] | 90 | Post-menopausal women; aged 47–57 years; Italy. | Genistein, 54 mg daily; | 1y | Parallel | The baseline frequency: 4.6 ± 3.2 per day for the genistein group, 4.7 ± 3.2 per day for the placebo group; Reduced frequency for genistein vs. placebo (24% mean difference, P < 0.01). |
| Campagnoli et al. [16] | 36 | Healthy post-menopausal women; ≥5 hot flashes per day; aged 45–58 years; Italy | 200 mg standardized soy extract, (with15% daidzein, 15% genistein, 20% saponin) | 12 weeks | Crossover | The baseline frequency: 38 per week; No differences in frequency between groups. Reduced frequency (26.5% vs. 24.3%) with Soy vs. placebo. |
| Levis et al. [17] | 99 | Post-menopausal women; aged 45–60 years; Canada | Muffins with 42 mg of isoflavones | 16 weeks | Parallel | The baseline frequency: 4.1 ± 2.4 per day for the isoflavones group, 4.7 ± 3.0 per day for the placebo group; Reduced frequency (17.4% vs. 19.7%) with soy vs. placebo. |
| Nahas et al. [18] | 80 | Healthy post-menopausal women; ≥5 hot flashes per day; aged 45 years or older; Brazil | Soy capsule, 100 mg daily of isoflavone | 10 mo | Parallel | The baseline frequency: 9.6 ± 3.9 per day for the soy group, 10.1 ± 4.9 per day for the placebo group; Reduced frequency (3.1 ± 2.3 and 5.9 ± 4.3 per day, P < 0.001) with soy tablet vs. placebo. |
| D'Anna et al. [19] | 236 | Post-menopausal women; average age, 54–55 years; Italy | Genistein tablet, 54 mg daily of total isoflavone | 24 mo | Parallel | The baseline frequency: 4.4 ± 3.4 per day for the genistein group, 4.2 ± 3.7 per day for the placebo group; In the genistein group, there was a significant decrease in the mean number (−56.4%) of the hot flushes. |
| Ferrari [20] | 180 | Post-menopausal women or 6 weeks after bilateral oophorectomy; ≥5 hot flashes per day; aged 40–65 years; Italy | Soybean extract, 80 mg daily isoflavones (with 60 mg genistein and genistin, 16 mg daidzein and daizin, 3 mg glycitein and glycitin). | 12 weeks | Parallel | The baseline frequency: 8.0 ± 3.3 per day for the isoflavones group, 7.5 ± 2.8 per day for the placebo group; Reduced frequency (41.2% vs. 29.3%, P = 0.023) with soy vs. placebo at 12 weeks. |
| Evans et al. [21] | 84 | Healthy post-menopausal women; ≥40 hot flashes per week; aged 40–65 years; Canada | Capsules, 30 mg genistein | 12 weeks | Parallel | The baseline frequency: 9.4 ± 3.8 per day for the genistein group, 9.9 ± 3.9 per day for the placebo group; Reduced frequency (51.2% vs. 29.8%, P = 0.046) with genistein vs. placebo at 12 weeks. |
| Ye et al. [22] | 90 | Post-menopausal women; aged 45–60 years; China | Soy germ isoflavone extract powder, low dose group, 84 mg daily isoflavones; high dose group, 126 mg daily isoflavones (with 52% daidzin, 15% genistin, and 33% glycitin aglcone equivalents) | 24 weeks | Parallel | The baseline frequency: 20 ± 11.0 per week for the genistein group, 21 ± 12.5 per week for the placebo group; Reduced frequency (44.3% vs. 48.5% vs. 27.8, P < 0.01) with isoflavone low dose group vs. isoflavone high dose group vs. placebo. |
The included studies were published between 1998 and 2012 in some professional journals, such as Menopause and Maturitas. All the studies taken together comprised 1710 subjects, ranging from 24 to 236 individuals per report (median 90). Study duration ranged between 4 weeks and 2 years, with a median of 12 weeks (Table 2).
Table 2.
Parameter estimation of soy isoflavones and placebo
| Parameters | Value | RSE (%) | 95% CI |
|---|---|---|---|
| Emax-placebo, % | 35.8 | 11.3 | 27.9, 43.7 |
| Emax-soy, % | 25.2 | 26.9 | 11.9, 38.5 |
| ET50-placebo, week | 2.99 | 31.6 | 1.1, 4.8 |
| ET50-soy, week | 13.4 | 30.1 | 5.5, 21.3 |
| η(Emax-placebo), % | 13.1 | 48.9 | 0.5, 25.7 |
| η(Emax-soy), % | 24.4 | 43.0 | 3.8, 45.0 |
| Correlation coefficient [η(Emax-placebo) – η(Emax-soy)] | 0.424 | – | – |
| IAV, % | 21.0 | 23.5 | 11.3, 30.7 |
| ε, % | 26.5 | 121.6 | 0, 89.7 |
Model establishment
The parameter estimates in the basic model are shown in Table 2. The typical value of Emax-soy was estimated at 25.2%, and the time to achieve 50% of Emax-soy was 13.4 weeks. Meanwhile, the typical value obtained for Emax-placebo was 35.8% and 2.99 weeks were needed to achieve 50% of Emax-placebo. Inter-trial variability of the Emax-soy showed moderate correlation with that of the Emax-placebo, with a correlation coefficient of 0.424.
The predicted Emax-soy value was not significantly correlated with the dose of soy isoflavones (Figure 2D). After the dose factor of soy isoflavones was introduced into the Emax-soy parameters, the OFV was not significantly decreased. Therefore, the basic model established above was considered final.
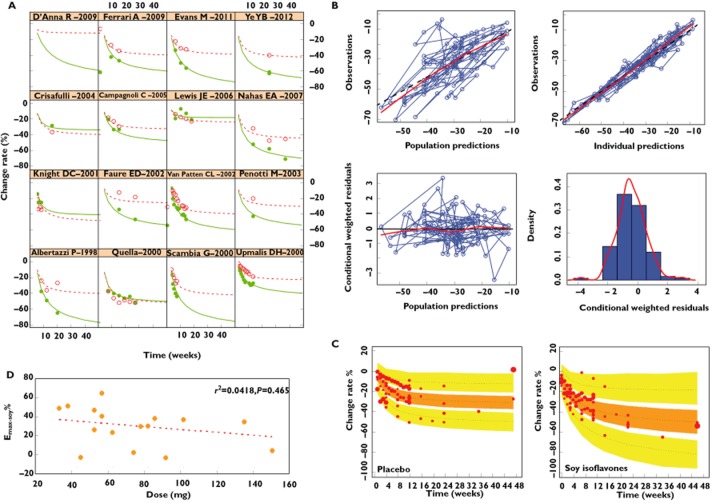
Figure 2.
Model evaluation. (A) Time course of mean % change of hot flashes from baseline for each individual study. (B) Model diagnosis graph. (C) Prediction corrected visual predictive check of the model. The dashed lines are the 5th, 50th and 95th percentiles of observed data. The shaded areas are the corresponding confidence intervals of the simulated data. The solid points represent observed data, and the symbol size is proportional to the number of subjects in each study. (D) The relationship between the dose of soy isoflavones and the predicted Emax-soy value.  , observed value-soy isoflavones;
, observed value-soy isoflavones;  , observed value-placebo;
, observed value-placebo;  , predicted value-soy isoflavones;
, predicted value-soy isoflavones;  , predicted value-placebo
, predicted value-placebo
Model evaluation
Figure 2A depicts the application of the final model to each individual trial, showing that the data observed in trials were consistent with the predicted values. The goodness-of-fit plots for the final model are presented in Figure 2B. Generally, there was good accordance between observed (OBS) and population model-predicted (PRED) effects, and between OBS and individual model-predicted (IPRED) effects. The WRES magnitude was small and randomly distributed around a straight line through 0, and located within ± 4 from the centre.
Monte Carlo simulations (1000 times) showed that the 90% CI of the 5%, median and 95% predicted percentiles covered the corresponding observed data, which indicated that the model had an adequate prediction capability (Figure 2C).
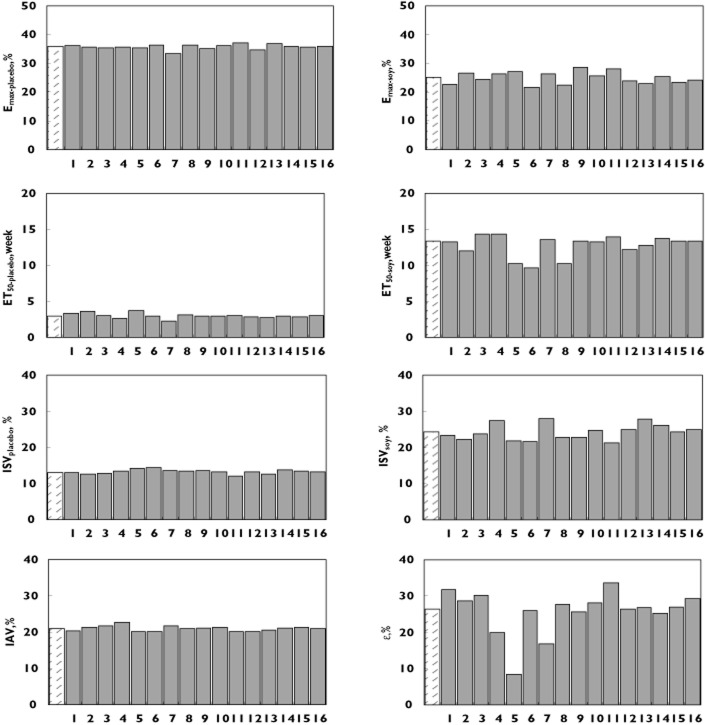
In order to test the impact of each individual study on the model parameters, leave-one-out cross validation was conducted. The results (Figure 3) showed that expect for ε, the distribution of most parameters was stable and only slightly affected by the individual trial.
Figure 3.
Bar charts of the typical parameter values obtained from the final model in a leave-one-out method. The abscissa represents the study number dropped from the full data and the ordinate, the typical value of parameters. The left shadow bar represents parameters obtained from full data. Study 1: Faure et al. [12], 2: Penotti et al. [14], 3: Scambia et al. [9], 4: Upmalis et al. [10], 5: Quella et al. [8], 6: Crisafulli et al. [15], 7: Albertazzi et al. [7], 8: Knight et al. [11], 9: Van Patten et al. [13], 10: Campagnoli et al. [16], 11: Levis et al. [17], 12: Nahas et al. [18], 13: D'Anna et al. [19], 14: Ferrari [20], 15: Evans et al. [21], 16: Ye et al. [22]
Efficacy comparison with estradiol
The efficacy of estradiol on menopausal hot flashes was also evaluated by MBMA (Supply materials) [15,23–50]. The results showed that the typical value of Emax-estradiol was estimated at 44.9%, which is about 1.8 times that of Emax-soy, and the time to achieve 50% of Emax-estradiol was only 3.09 weeks, much shorter than that of soy isoflavones (Table 3). It should be noted that the Emax-placebo in estradiol trials was 55.6%, overtly higher than the value obtained from trials with soy isoflavones.
Table 3.
Parameter estimation of estradiol and placebo
| Parameters | Value | RSE (%) | 95% CI |
|---|---|---|---|
| Emax-placebo, % | 55.6 | 5.4 | 49.7, 61.5 |
| Emax-estradiol, % | 44.9 | 7.0 | 38.7, 51.1 |
| ET50-placebo, weeks | 1.96 | 11.4 | 1.5, 2.4 |
| ET50-estradiol, weeks | 3.09 | 19.1 | 1.9, 4.2 |
| η(Emax-placebo), % | 12.4 | 26.6 | 5.9, 18.9 |
| η(Emax-estradiol), % | 12.3 | 39.1 | 2.9, 21.7 |
| Correlation coefficient [η(Emax-placebo) – η(Emax-estradiol)] | 0.453 | – | – |
| IAV, % | 0, Fixed | – | – |
| ε, % | 46.2 | 14.4 | 33.2,. 59.2 |
Due to the large difference in ET50 between soy isoflavones and estradiol, estradiol reached 80% of its Emax after 12 weeks treatment, while soy isoflavones only reached 47% of Emax. It was only after at least 48 weeks that treatment with soy isoflavones resulted in 80% of Emax (Figure 4).
Figure 4.
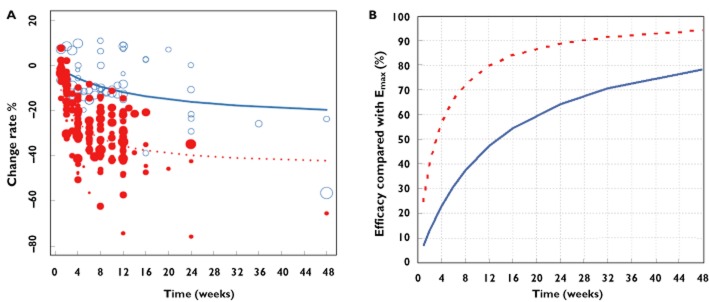
Typical predicted effect (lines) and observed data (points) of soy isoflavones and estradiol after elimination of the placebo effect (A). The corresponding efficacy ratio compared with the Emax value is presented in Figure 4B.  , estradiol;
, estradiol;  , soy isoflavones
, soy isoflavones
Discussion
Soy isoflavones are phytoestrogens with potential hormonal activity due to their structural similarity with 17-β-estradiol. The increasing availability of soy isoflavones in food and through the use of supplements has prompted extensive research on biological benefits to menopausal women in chronic disease prevention and health maintenance. It has been reported that approximately 70–80% of USA women of menopausal and peri-menopausal age experience hot flashes, in comparison with approximately 10–20% of Asian women [51]. Interestingly, the average blood concentration of the soy isoflavone genistein in Asian women is approximately 25 ng ml−1, while only 2 ng ml−1 is found in USA women [51]. The sharp contrast between frequency of hot flash symptoms and soy genistein concentrations has led researchers to assess soy isoflavones for their potential to prevent hot flashes.
Unfortunately, according to the published meta-analyses, there is still an ongoing debate on the efficiency of soy isoflavones in treating menopausal hot flashes. In the Forest plot [1], it seemed that the treatment efficiency in patients administered soy isoflavones was superior to those treated with placebo. However, no statistical difference (P > 0.05) was observed due to a wide confidence interval, which can be explained by the following: 1) the evaluation of treatment efficiency was relatively subjective as the daily frequency of hot flashes was reported by the patients themselves. The accuracy was therefore not guaranteed with a large variation. The relative standard deviation (RSD) in most studies was in the range of 50 ∼100%, 2) most clinical studies were of small sample size, precisely enrolling less than 60 subjects in each treatment group and 3) the time points for final observation varied by trial. Despite these fluctuations, the results obtained were analyzed in a combined manner in the meta-analysis, which neglected the effects of time on treatment efficiency. All these contributed to errors, which may affect the evaluation of treatment efficiency.
Unfortunately, these errors could not be distinguished using conventional meta-analysis. However, MBMA overcomes the disadvantages of conventional meta-analysis through detailed description of efficacy data using a mathematical model, as well as separation of inter-trial variation, inter-treatment arm variations and residual errors. Therefore, accurate evaluation of the efficiency of soy isoflavones was carried out using MBMA.
To eliminate the potential effects of baseline frequency on the evaluation of treatment efficiency, the evaluation index was defined as the descending ratio of frequency of hot flashes after different treatments compared with the baseline frequency. Significant placebo effects were observed with a maximal value of 35.8% in the soy isoflavones trial, and much higher in the estradiol trial (maximal value of 55.6%). This might be attributed to the fact that most subjects were aware that the effects of soy isoflavones were definitely not superior to those of estradiol, and the lower expectation in the effects of soy isoflavones resulted in the lower placebo effects observed in the soy isoflavone trials. After eliminating the placebo effect, the maximum effects of soy isoflavones was 25.2%, accounting for 57% of the maximum effects of estradiol (Emax-estradiol = 44.9%). However, soy isoflavones need 13.4 weeks to achieve half of the peak activity, much longer than estradiol, which only requires 3.09 weeks. Therefore, after 12 weeks treatment, estradiol achieved 80% of its maximum effects, while the effects of soy isoflavones only reached 47% of maximum effects. Of note, 12 weeks is the recommended treatment interval in the FDA guide for estrogen and estrogen/progestin drug products for treatment of vasomotor symptoms [52]. This time frame is very reasonable for estradiol, but too short for soy isoflavones. After 48 weeks of treatment, the effects of soy isoflavones were just close to 80% of ceiling effects.
In the 16 reports included in this MBMA, the dose of isoflavones ranged from 30 to 200 mg day−1. Meanwhile, the active components of various isoflavone preparations were different, most of which were isoflavone extracts of soybean containing genistein, diadzein, and glycitein. Genistein monotherapy was performed in three studies [15,19,21]. In our study, we attempted to adjust the parameter Emax-soy by dosage of soy isoflavones. However, no significant correlation was observed between the inter-trial variations of dosage and Emax-soy. Finally, dose was not introduced in this model. In this study, we could not evaluate the efficacy of a single active component in treating hot flashes, as isoflavone components varied by study. Therefore, the large inter-trial variances observed among these studies may be partly induced by the dose and type of soy isoflavones.
In conclusion, this study quantified the efficacy of soy isoflavones in treating menopausal hot flashes for the first time. Important parameters, such as the maximum effect and time associated with an effect equal to 50% of Emax, were obtained by the MBMA. These results demonstrate that soy isoflavones have slight and slow effects in attenuating the menopausal hot flashes, and provide a valuable reference for a rational use of soy isoflavones.
Acknowledgments
This study was financially supported by Shanghai 085 Project of Higher Education Connotation Construction (085ZY1202), Shanghai Municipal Education Commission (SYZ11056, ZYSNXD-CC-LCP, 2013JW19-A1-20140364), and the National Natural Science Funds (81303279).
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1
Goodness-of-fit plots for the model. A) Population predicted effect data vs. observed effect data; B) individual predicted effect data vs. observed effect data; C) conditional weighted residuals vs. population predicted effect data; D) Density distribution of conditional weighted residuals. The black and red lines in A and B represent identity and regression lines, respectively, whereas in C the black line is the position where conditional weighted residual equals 0 and the red lines are the nonparametric regression lines. The red line in D is density distribution line
Figure S2
Prediction corrected visual predictive check of the final model. The dash lines are the 5th, 50th and 95th percentiles of observed data. The shaded areas are the corresponding confidence intervals of the simulated data. The solid points represent observed data, and the symbol size is proportional to the number of subjects in each study
Table S1
Summary of included placebo-controlled trials of estradiol
References
- 1.Nelson HD. Menopause. Lancet. 2008;371:760–770. doi: 10.1016/S0140-6736(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 2.Ohta H. [Hormone replacement Up-to-date. Practical tips for hormone replacement therapy in women] Clin Calcium. 2007;17:1407–1413. [PubMed] [Google Scholar]
- 3.Hardie C, Bain C, Walters M. Hormone replacement therapy: the risks and benefits of treatment. J R Coll Physicians Edinb. 2009;39:324–326. doi: 10.4997/JRCPE.2009.408. [DOI] [PubMed] [Google Scholar]
- 4.Carroll DG. Nonhormonal therapies for hot flashes in menopause. Am Fam Physician. 2006;73:457–464. [PubMed] [Google Scholar]
- 5.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 6.Mould DR. Model-based meta-analysis: an important tool for making quantitative decisions during drug development. Clin Pharmacol Ther. 2012;92:283–286. doi: 10.1038/clpt.2012.122. [DOI] [PubMed] [Google Scholar]
- 7.Albertazzi P, Pansini F, Bonaccorsi G, Zanotti L, Forini E, De Aloysio D. The effect of dietary soy supplementation on hot flushes. Obstet Gynecol. 1998;91:6–11. doi: 10.1016/s0029-7844(97)00597-8. [DOI] [PubMed] [Google Scholar]
- 8.Quella SK, Loprinzi CL, Barton DL, Knost JA, Sloan JA, LaVasseur BI, Swan D, Krupp KR, Miller KD, Novotny PJ. Evaluation of soy phytoestrogens for the treatment of hot flashes in breast cancer survivors: a North Central Cancer Treatment Group Trial. J Clin Oncol. 2000;18:1068–1074. doi: 10.1200/JCO.2000.18.5.1068. [DOI] [PubMed] [Google Scholar]
- 9.Scambia G, Mango D, Signorile PG, Anselmi Angeli RA, Palena C, Gallo D, Bombardelli E, Morazzoni P, Riva A, Mancuso S. Clinical effects of a standardized soy extract in postmenopausal women: a pilot study. Menopause. 2000;7:105–111. doi: 10.1097/00042192-200007020-00006. [DOI] [PubMed] [Google Scholar]
- 10.Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2000;7:236–242. doi: 10.1097/00042192-200007040-00005. [DOI] [PubMed] [Google Scholar]
- 11.Knight DC, Howes JB, Eden JA, Howes LG. Effects on menopausal symptoms and acceptability of isoflavone-containing soy powder dietary supplementation. Climacteric. 2001;4:13–18. [PubMed] [Google Scholar]
- 12.Faure ED, Chantre P, Mares P. Effects of a standardized soy extract on hot flushes: a multicenter, double-blind, randomized, placebo-controlled study. Menopause. 2002;9:329–334. doi: 10.1097/00042192-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Van Patten CL, Olivotto IA, Chambers GK, Gelmon KA, Hislop TG, Templeton E, Wattie A, Prior JC. Effect of soy phytoestrogens on hot flashes in postmenopausal women with breast cancer: a randomized, controlled clinical trial. J Clin Oncol. 2002;20:1449–1455. doi: 10.1200/JCO.2002.20.6.1449. [DOI] [PubMed] [Google Scholar]
- 14.Penotti M, Fabio E, Modena AB, Rinaldi M, Omodei U, Vigano P. Effect of soy-derived isoflavones on hot flushes, endometrial thickness, and the pulsatility index of the uterine and cerebral arteries. Fertil Steril. 2003;79:1112–1117. doi: 10.1016/s0015-0282(03)00158-4. [DOI] [PubMed] [Google Scholar]
- 15.Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, Adamo EB, Marini R, D'Anna R, Corrado F, Bartolone S, Frisina N, Squadrito F. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause. 2004;11:400–404. doi: 10.1097/01.gme.0000109314.11228.e5. [DOI] [PubMed] [Google Scholar]
- 16.Campagnoli C, Abba C, Ambroggio S, Peris C, Perona M, Sanseverino P. Polyunsaturated fatty acids (PUFAs) might reduce hot flushes: an indication from two controlled trials on soy isoflavones alone and with a PUFA supplement. Maturitas. 2005;51:127–134. doi: 10.1016/j.maturitas.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Levis S, Strickman-Stein N, Ganjei-Azar P, Xu P, Doerge DR, Krischer J. Soy isoflavones in the prevention of menopausal bone loss and menopausal symptoms: a randomized, double-blind trial. Arch Intern Med. 2011;171:1363–1369. doi: 10.1001/archinternmed.2011.330. [DOI] [PubMed] [Google Scholar]
- 18.Nahas EA, Nahas-Neto J, Orsatti FL, Carvalho EP, Oliveira ML, Dias R. Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized, double-blind, and placebo-controlled study. Maturitas. 2007;58:249–258. doi: 10.1016/j.maturitas.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.D'Anna R, Cannata ML, Marini H, Atteritano M, Cancellieri F, Corrado F, Triolo O, Rizzo P, Russo S, Gaudio A, Frisina N, Bitto A, Polito F, Minutoli L, Altavilla D, Adamo EB, Squadrito F. Effects of the phytoestrogen genistein on hot flushes, endometrium, and vaginal epithelium in postmenopausal women: a 2-year randomized, double-blind, placebo-controlled study. Menopause. 2009;16:301–306. doi: 10.1097/gme.0b013e318186d7e2. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari A. Soy extract phytoestrogens with high dose of isoflavones for menopausal symptoms. J Obstet Gynaecol Res. 2009;35:1083–1090. doi: 10.1111/j.1447-0756.2009.01058.x. [DOI] [PubMed] [Google Scholar]
- 21.Evans M, Elliott JG, Sharma P, Berman R, Guthrie N. The effect of synthetic genistein on menopause symptom management in healthy postmenopausal women: a multi-center, randomized, placebo-controlled study. Maturitas. 2011;68:189–196. doi: 10.1016/j.maturitas.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Ye YB, Wang ZL, Zhuo SY, Lu W, Liao HF, Verbruggen M, Fang S, Mai HY, Chen YM, Su YX. Soy germ isoflavones improve menopausal symptoms but have no effect on blood lipids in early postmenopausal Chinese women: a randomized placebo-controlled trial. Menopause. 2012;19:791–798. doi: 10.1097/gme.0b013e31823dbeda. [DOI] [PubMed] [Google Scholar]
- 23.Archer DF. Percutaneous 17beta-estradiol gel for the treatment of vasomotor symptoms in postmenopausal women. Menopause. 2003;10:516–521. doi: 10.1097/01.GME.0000070526.74726.8A. [DOI] [PubMed] [Google Scholar]
- 24.Archer DF, Pickar JH, MacAllister DC, Warren MP. Transdermal estradiol gel for the treatment of symptomatic postmenopausal women. Menopause. 2012;19:622–629. doi: 10.1097/gme.0b013e31823b8867. [DOI] [PubMed] [Google Scholar]
- 25.Bacchi-Modena A, Bolis P, Campagnoli C, De Cicco F, Meschia M, Pansini F, Pisati R, Huls G. Efficacy and tolerability of Estraderm MX, a new estradiol matrix patch. Maturitas. 1997;27:285–292. doi: 10.1016/s0378-5122(97)00039-x. [DOI] [PubMed] [Google Scholar]
- 26.Bachmann GA, Schaefers M, Uddin A, Utian WH. Lowest effective transdermal 17beta-estradiol dose for relief of hot flushes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2007;110:771–779. doi: 10.1097/01.AOG.0000284450.51264.31. [DOI] [PubMed] [Google Scholar]
- 27.Buster JE, Koltun WD, Pascual ML, Day WW, Peterson C. Low-dose estradiol spray to treat vasomotor symptoms: a randomized controlled trial. Obstet Gynecol. 2008;111:1343–1351. doi: 10.1097/AOG.0b013e318175d162. [DOI] [PubMed] [Google Scholar]
- 28.de Vrijer B, Snijders MP, Troostwijk AL, The S, Iding RJ, Friese S, Smit DA, Schierbeek JM, Brandts H, van Kempen PJ, van Buuren I, Monza G. Efficacy and tolerability of a new estradiol delivering matrix patch (Estraderm MX) in postmenopausal women. Maturitas. 2000;34:47–55. doi: 10.1016/s0378-5122(99)00085-7. [DOI] [PubMed] [Google Scholar]
- 29.Endrikat J, Graeser T, Mellinger U, Ertan K, Holz C. A multicenter, prospective, randomized, double-blind, placebo-controlled study to investigate the efficacy of a continuous-combined hormone therapy preparation containing 1mg estradiol valerate/2mg dienogest on hot flushes in postmenopausal women. Maturitas. 2007;58:201–207. doi: 10.1016/j.maturitas.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 30.Gass MS, Rebar RW, Cuffie-Jackson C, Cedars MI, Lobo RA, Shoupe D, Judd HL, Buyalos RP, Clisham PR. A short study in the treatment of hot flashes with buccal administration of 17-beta estradiol. Maturitas. 2004;49:140–147. doi: 10.1016/j.maturitas.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Good WR, John VA, Ramirez M, Higgins JE. Double-masked, multicenter study of an estradiol matrix transdermal delivery system (Alora) versus placebo in postmenopausal women experiencing menopausal symptoms. Alora Study Group. Clin Ther. 1996;18:1093–1105. doi: 10.1016/s0149-2918(96)80064-6. [DOI] [PubMed] [Google Scholar]
- 32.Haas S, Walsh B, Evans S, Krache M, Ravnikar V, Schiff I. The effect of transdermal estradiol on hormone and metabolic dynamics over a six-week period. Obstet Gynecol. 1988;71:671–676. [PubMed] [Google Scholar]
- 33.Haines C, Yu SL, Hiemeyer F, Schaefers M. Micro-dose transdermal estradiol for relief of hot flushes in postmenopausal Asian women: a randomized controlled trial. Climacteric. 2009;12:419–426. doi: 10.1080/13697130902748967. [DOI] [PubMed] [Google Scholar]
- 34.Honjo H, Taketani Y. Low-dose estradiol for climacteric symptoms in Japanese women: a randomized, controlled trial. Climacteric. 2009;12:319–328. doi: 10.1080/13697130802657888. [DOI] [PubMed] [Google Scholar]
- 35.Lee BS, Kang BM, Yoon BK, Choi H, Park HM, Kim JG. Efficacy and tolerability of estradiol 1 mg and drospirenone 2 mg in postmenopausal Korean women: a double-blind, randomized, placebo-controlled, multicenter study. Maturitas. 2007;57:361–369. doi: 10.1016/j.maturitas.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Lin SQ, Sun LZ, Lin JF, Yang X, Zhang LJ, Qiao J, Wang ZH, Xu YX, Xiong ZA, Zhou YZ, Wang ML, Zhu J, Chen SR, Su H, Yang CS, Wang SH, Zhang YZ, Dong XJ. Estradiol 1 mg and drospirenone 2 mg as hormone replacement therapy in postmenopausal Chinese women. Climacteric. 2011;14:472–481. doi: 10.3109/13697137.2011.553971. [DOI] [PubMed] [Google Scholar]
- 37.Notelovitz M, Cassel D, Hille D, Furst KW, Dain MP, VandePol C, Skarinsky D. Efficacy of continuous sequential transdermal estradiol and norethindrone acetate in relieving vasomotor symptoms associated with menopause. Am J Obstet Gynecol. 2000;182:7–12. doi: 10.1016/s0002-9378(00)70483-2. [DOI] [PubMed] [Google Scholar]
- 38.Notelovitz M, Lenihan JP, McDermott M, Kerber IJ, Nanavati N, Arce J. Initial 17beta-estradiol dose for treating vasomotor symptoms. Obstet Gynecol. 2000;95:726–731. doi: 10.1016/s0029-7844(99)00643-2. [DOI] [PubMed] [Google Scholar]
- 39.Notelovitz M, Mattox JH. Suppression of vasomotor and vulvovaginal symptoms with continuous oral 17beta-estradiol. Menopause. 2000;7:310–317. doi: 10.1097/00042192-200007050-00005. [DOI] [PubMed] [Google Scholar]
- 40.Panay N, Ylikorkala O, Archer DF, Gut R, Lang E. Ultra-low-dose estradiol and norethisterone acetate: effective menopausal symptom relief. Climacteric. 2007;10:120–131. doi: 10.1080/13697130701298107. [DOI] [PubMed] [Google Scholar]
- 41.Poller L, Thomson JM, Coope J. A double-blind cross-over study of piperazine oestrone sulphate and placebo with coagulation studies. Br J Obstet Gynaecol. 1980;87:718–725. doi: 10.1111/j.1471-0528.1980.tb04606.x. [DOI] [PubMed] [Google Scholar]
- 42.Rovati LC, Setnikar I, Genazzani AR. Dose-response efficacy of a new estradiol transdermal matrix patch for 7-day application: a randomized, double-blind, placebo-controlled study. Italian Menopause Research Group. Gynecol Endocrinol. 2000;14:282–291. doi: 10.3109/09513590009167695. [DOI] [PubMed] [Google Scholar]
- 43.Schurmann R, Holler T, Benda N. Estradiol and drospirenone for climacteric symptoms in postmenopausal women: a double-blind, randomized, placebo-controlled study of the safety and efficacy of three dose regimens. Climacteric. 2004;7:189–196. doi: 10.1080/13697130410001713698. [DOI] [PubMed] [Google Scholar]
- 44.Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once-a-week 17beta-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause. 2002;9:195–207. doi: 10.1097/00042192-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 45.Simon JA. Estradiol in micellar nanoparticles: the efficacy and safety of a novel transdermal drug-delivery technology in the management of moderate to severe vasomotor symptoms. Menopause. 2006;13:222–231. doi: 10.1097/01.gme.0000174096.56652.4f. [DOI] [PubMed] [Google Scholar]
- 46.Simon JA, Bouchard C, Waldbaum A, Utian W, Zborowski J, Snabes MC. Low dose of transdermal estradiol gel for treatment of symptomatic postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2007;109:588–596. doi: 10.1097/01.AOG.0000254160.62588.41. [DOI] [PubMed] [Google Scholar]
- 47.Stevenson JC, Durand G, Kahler E, Pertynski T. Oral ultra-low dose continuous combined hormone replacement therapy with 0.5 mg 17beta-oestradiol and 2.5 mg dydrogesterone for the treatment of vasomotor symptoms: results from a double-blind, controlled study. Maturitas. 2010;67:227–232. doi: 10.1016/j.maturitas.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 48.Studd J, Pornel B, Marton I, Bringer J, Varin C, Tsouderos Y, Christiansen C. Efficacy and acceptability of intranasal 17 beta-oestradiol for menopausal symptoms: randomised dose-response study. Aerodiol Study Group. Lancet. 1999;353:1574–1578. doi: 10.1016/s0140-6736(98)06196-0. [DOI] [PubMed] [Google Scholar]
- 49.von Holst T, Salbach B. Efficacy and tolerability of a new 7-day transdermal estradiol patch versus placebo in hysterectomized women with postmenopausal complaints. Maturitas. 2000;34:143–153. doi: 10.1016/s0378-5122(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 50.von Holst T, Salbach B. Efficacy of a new 7-day transdermal sequential estradiol/levonorgestrel patch in women. Maturitas. 2002;41:231–242. doi: 10.1016/s0378-5122(01)00297-3. [DOI] [PubMed] [Google Scholar]
- 51.Kurzer MS. Soy consumption for reduction of menopausal symptoms. Inflammopharmacology. 2008;16:227–229. doi: 10.1007/s10787-008-8021-z. [DOI] [PubMed] [Google Scholar]
- 52.2003. Guidance for industry: estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms -recommendations for clinical evaluation. FDA.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Goodness-of-fit plots for the model. A) Population predicted effect data vs. observed effect data; B) individual predicted effect data vs. observed effect data; C) conditional weighted residuals vs. population predicted effect data; D) Density distribution of conditional weighted residuals. The black and red lines in A and B represent identity and regression lines, respectively, whereas in C the black line is the position where conditional weighted residual equals 0 and the red lines are the nonparametric regression lines. The red line in D is density distribution line
Figure S2
Prediction corrected visual predictive check of the final model. The dash lines are the 5th, 50th and 95th percentiles of observed data. The shaded areas are the corresponding confidence intervals of the simulated data. The solid points represent observed data, and the symbol size is proportional to the number of subjects in each study
Table S1
Summary of included placebo-controlled trials of estradiol