Abstract
AIMS
To compare the pharmacokinetics of metformin between diabetic Indigenous (Aboriginal and Torres Strait Islander) and non-Indigenous patients.
METHODS
An observational, cross-sectional study was conducted on type 2 diabetic Indigenous and non-Indigenous patients treated with metformin. Blood samples were collected to determine metformin, lactate, creatinine and vitamin B12 concentrations and glycosylated haemoglobin levels. A population model was used to determine the pharmacokinetic parameters.
RESULTS
The Indigenous patients (median age 55 years) were younger than the non-Indigenous patients (65 years), with a difference of 10 years (95% confidence interval 6–14 years, P < 0.001). The median glycosylated haemoglobin was higher in the Indigenous patients (8.5%) than in the non-Indigenous patients (7.2%), with a difference of 1.4% (0.8–2.2%, P < 0.001). Indigenous patients had a higher creatinine clearance (4.3 l h−1) than the non-Indigenous patients (4.0 l h−1), with a median difference of 0.3 l h−1 (0.07–1.17 l h−1; P < 0.05). The ratio of the apparent clearance of metformin to the creatinine clearance in Indigenous patients (13.1, 10.2–15.2; median, interquartile range) was comparable to that in non-Indigenous patients (12.6, 9.9–14.9). Median lactate concentrations were also similar [1.55 (1.20–1.88) vs. 1.60 (1.35–2.10) mmol l−1] for Indigenous and non-Indigenous patients, respectively. The median vitamin B12 was 306 pmol l−1 (range 105–920 pmol l−1) for the Indigenous patients.
CONCLUSIONS
There were no significant differences in the pharmacokinetics of metformin or plasma concentrations of lactate between Indigenous and non-Indigenous patients with type 2 diabetes mellitus. Further studies are required in Indigenous patients with creatinine clearance <30 ml min−1.
Keywords: Aboriginal and Torres Strait Islander patients, kidney disease, metformin, pharmacokinetics, type 2 diabetes mellitus
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Metformin is widely used in the treatment of type 2 diabetes mellitus.
Metformin is also used in patients with one or more contraindications, such as renal disease.
The incidence of type 2 diabetes mellitus and renal disease in the Australian Indigenous population is amongst the highest in the world.
WHAT THIS STUDY ADDS
The pharmacokinetics of metformin was not clinically different between the Indigenous and non-Indigenous patients.
Dose simulations of metformin in patients with kidney disease from previous studies can also be applied to the Indigenous population.
Introduction
Metformin is the drug of first choice in the treatment of type 2 diabetes mellitus (T2DM), a common disease in Indigenous Australians (Australian Aborigines and Torres Strait Islanders). The Torres Strait Islands are part of Australia lying between Mainland Australia and New Guinea. The Islanders are Melanesians, who are similar to peoples in New Guinea but distinct from Australian Aborigines. Indigenous Australians experience T2DM at an earlier age, with an incidence up to four times higher than non-Indigenous Australians and mortality rates from diabetes approximately 12 times greater than other Australians [1,2]. Additionally, there is a very high prevalence of nephropathy in Indigenous patients, particularly in patients who have T2DM [2,3]. This strong association between T2DM and risk of nephropathy suggests that improving the control of diabetes may prevent or delay the onset of kidney disease [3]. However, not all cases of nephropathy in Indigenous patients are associated with diabetes [4,5]. Some factors that may contribute to the high rate of T2DM and nephropathy among Indigenous patients include genetic susceptibility, a sedenatary lifestyle, reduced access to healthcare and high rates of infections, especially streptococcal bacterial infections [4,6,7]. Owing to the lower health status of Indigenous Australians, improving Indigenous health remains one of the major research and health priority areas in Australia [8].
Metformin is largely excreted unchanged in urine. Accumulation of metformin in renal impairment is purported to be a cause of lactic acidosis. Hence, there is uncertainty about the appropriate dosage of metformin in patients with renal impairment. Lactic acidosis is a syndrome which has considerable mortality, particularly in patients with dehydration and poor tissue perfusion [9]. The problem for prescribers is that variable lower limits of creatinine clearance have been placed on the use of metformin. These range from 60 ml min−1 (Product Label; [10]), with the suggestion that the dose be reviewed if the estimated glomerular filtration rate is <45 ml min−1 and ceased if the estimated glomerular filtration rate is <30 ml min−1 (National Institute for Health and Clinical Excellence [11]). In Australia, a creatinine clearance (CLCR) limit of 30 ml min−1 (Australian Medicines Handbook; [12]) has been suggested, while a recent recommendation is that metformin can be used, in selected patients, at lower levels (<30 ml min−1; [13]).
We recently developed a population pharmacokinetic model to investigate the pharmacokinetics of metformin that accommodates patients with a range of renal function (CLCR 15–120 ml min−1; [14]). The model was constructed with data collected from healthy English and Malaysian subjects and also included non-Indigenous Australian patients with T2DM [14]. We have now extended our studies to include Indigenous patients residing in New South Wales and Far North Queensland. These locations contain the largest estimated resident Indigenous populations in Australia, with 29% of the total Indigenous population residing in New South Wales and 28% in Queensland [15].
The objective of our study was to investigate and compare the clearance of metformin in Indigenous and non-Indigenous patients with T2DM. Additionally, subgroup analyses were conducted to investigate pharmacokinetic differences between Indigenous groups. We have suggested suitable doses of metformin over a range of renal function in non-Indigenous patients [14]. Significantly lower clearances in Indigenous patients could lead to excessive accumulation and increased risk of lactic acidosis. The effect of impaired renal function on metformin pharmacokinetics in Indigenous patients was of particular interest. Plasma concentrations of lactate and vitamin B12 were also measured in Indigenous patients. High plasma concentrations of plasma lactate (>5 mmol l−1) are considered to be a risk factor for lactic acidosis [16], while low concentrations of vitamin B12 (<120 pmol l−1) are suggested to be an adverse effect of metformin [17].
Methods
Patients
Indigenous patients with T2DM (n = 50) were recruited from Diabetes Outpatients' Clinics (La Perouse Aboriginal Centre and Cairns Base Hospital) and the hospital inpatient ward (Cairns Base Hospital). Subjects were included in the study if they provided informed consent, were being treated with any metformin formulation and if they had Aboriginal and/or Torres Strait Islander ancestry (at least one parent of Aboriginal and/or Torres Strait Islander ancestry). The Indigenous patients were also asked about the ethnicity of their parents and grandparents. Subgroup pharmacokinetic analysis was conducted to investigate whether there are differences in the pharmacokinetics of metformin between Indigenous groups. This involved separating data from Indigenous patients into Aboriginal (32 patients with at least one Aboriginal parent) or Torres Strait Islander groups (10 patients with at least one Torres Strait Islander parent). The remaining eight patients had both Aboriginal and Torres Strait Islander ancestry and were excluded from this particular analysis.
Non-Indigenous patients were recruited from hospital inpatients at St Vincent's Hospital, Sydney, NSW, Australia and from outpatients from the Diabetes and Renal Clinics of the hospital.
The study was approved by the Human Research Ethics Committee at St Vincent's Hospital and University of New South Wales (08209/SVH08/035), South Eastern Sydney and Illawarra Area Health Service (11/G/039), NSW Aboriginal Health and Medical Research Council (785/11) and Cairns Base Hospital (11/QCH/33-727). This study was registered in the Australian and New Zealand Clinical Trials Registry (ANZCTR; ID: ACTRN12611000935932).
Demographics, history and dosage of metformin
The demographics, medical history and concomitant medications of the patients were recorded. The dose of metformin, dose formulation (immediate release or extended release), times of the last two doses and dosing regimen were recorded. One to six blood samples were collected from each patient to determine metformin plasma concentrations. Sparse blood samples were collected as close as possible to the estimated time of peak (2–4 h postdose, immediate release; 6–8 h postdose, extended release) and trough concentrations (10–12 h postdose, immediate release; 20–24 h postdose extended release). On the first visit, blood samples were also collected to determine glycosylated haemoglobin (HbA1C), creatinine, vitamin B12 and lactate concentrations. The CLCR was calculated using the Cockcroft–Gault equation [15] based upon lean bodyweight.
Analytical methods
Blood samples for metformin determination were collected as previously described [14]. For samples collected at Cairns Base Hospital, plasma was stored at −4°C before transport to the central laboratory (Department of Clinical Pharmacology and Toxicology, St Vincent's Hospital). Plasma metformin was quantified using a validated, reverse-phase high-performance liquid chromatography assay with ultraviolet detection (236 nm; [14]). A Zorbax Cyano column (150 mm × 4.6 mm, 5 μm particles; temperature set to 40°C) was used, and the mobile phase consisted of methanol : acetonitrile : phosphate buffer (20:60:20, v : v : v; pH adjusted to 7.0). The peak height ratios of six calibration standards (range 0.05–10 mg l−1) were used to establish the standard curves. Two quality-control samples (1.0 and 3 mg l−1) were included in each run. The standard curves were linear (r2 ≥ 0.997), and the between-day accuracy (percentage difference from nominal value) of the quality controls was <10%, with a precision (measured as the coefficient of variation) below 15%.
Pharmacokinetic and statistical analyses
A population pharmacokinetic model was constructed using nonlinear mixed-effects modelling (NONMEM® VII, ICON Development Solutions, Ellicott City, MD, USA) and used to investigate the pharmacokinetic parameters of metformin. The detailed analysis was described in our previous publication [14], and the details are shown in the Supplementary section of this manuscript (Supplementary Tables S1 and S2). In brief, the time course of metformin was fitted by a two-compartment model with first-order absorption for the immediate-release formulation and zero-order absorption for the extended-release formulation. The mean pharmacokinetic parameters from this model were used as initial estimates in the present pharmacokinetic analysis. Data from 50 Indigenous Australians were added to this model to determine their individual pharmacokinetics. The individual estimates of apparent clearance of metformin (CL/F) and apparent volume of the central compartment (V1/F) in the Indigenous and non-Indigenous patients were then compared.
Statistical comparisons were performed using SPSS (version 20.0; SPSS, Chicago, IL, USA). Results were presented as medians and interquartile ranges because some data were not normally distributed. The Mann–Whitney U-test and the Hodges–Lehman estimate were used to compare patient demographics and pharmacokinetic parameters between the Indigenous and non-Indigenous patients. A P value of <0.05 was considered statistically significant. The number of Indigenous patients (n = 50) was chosen as a number which could reasonably be investigated in non-Indigenous clinics. Subsequent power analysis indicated that significant contrasts between the most important pharmacokinetic parameter (the ratio of CL/F to CLCR) in Indigenous and non-Indigenous patients would be detected (P = 0.02) if the coefficient of variation in the Indigenous patients (4%) was approximately the same as in non-Indigenous patients (Supplementary Methods).
Results
Demographics
The characteristics of the subjects are summarized in Table 1. The Indigenous patients were younger (median 55 years) than the non-Indigenous Australian patients (69 years), with a median difference (95% confidence interval) of 10 years (6–14 years, P < 0.001; Table 1). Most Indigenous patients (n = 35/50) reported a family history of T2DM. The median CLCR in the Indigenous patients [4.3 (3.3–5.7) l h−1; median (interquartile range, IQR)] was higher than that for the non-Indigenous patients [4.0 (2.8–4.9) l h−1; P < 0.05), with a median difference (95% confidence interval) of 0.3 l h−1 (0.07–1.17 l h−1); Table 1).
Table 1.
Characteristics of Indigenous and non-Indigenous patients during treatment with metformin
| Characteristic | Indigenous | Non-Indigenous |
|---|---|---|
| n | 50 | 120 |
| Age (years) | 55 (48–62) | 65 (59–74)** |
| Bodyweight (kg) | 85 (74–95) | 86 (75–102) |
| Body mass index (kg m−2) | 29 (27–35) | 30 (26–34) |
| Creatinine clearance (l h−1) | 4.3 (3.4–5.6) | 4.0 (2.8–4.9)* |
| Metformin dosage (mg day−1)† | 1500 (500−3000) | 2000 (250–3000) |
| Haemoglobin A1C (%) | 8.5 (7.1–10.4) | 7.2 (6.5–7.8)** |
| Lactate (mmol l−1) | 1.55 (1.20–1.88) | 1.60 (1.35–2.10) |
| Vitamin B12 (pmol l−1)† | 306 (105–920) | – |
The data are shown as the median and interquartile range.
P < 0.05,
P < 0.001.
Data are shown as the median and range.
The Indigenous patients had higher HbA1C [8.5% (7.1–10.4%); median (IQR)] compared with the non-Indigenous patients [7.2% (6.5–7.8%)], with a median difference of 1.3% (95% confidence interval 0.8–2.2%; P < 0.001). The duration of diabetes was not significantly different between the Indigenous and non-Indigenous patients (9 vs. 7 years, respectively). There was no significant difference in the lactate concentrations between the Indigenous patients and non-Indigenous patients (1.6 vs. 1.7 mmol l−1; Table 1). The median plasma concentration of vitamin B12 in the Indigenous patients was 310 pmol l−1 (replete concentration >180 pmol l−1). Vitamin B12 was not measured in the non-Indigenous patients.
Pharmacokinetics
There was good agreement between the observed and predicted plasma concentrations in all individual subjects (Supplementary Figures S1 and S2) and also in the corresponding plot for the Indigenous and non-Indigenous patients (Figure 1). However, there are several instances where the observed plasma concentrations were more than 60% lower than predicted. These plasma concentrations were <1 mg l−1, and the data came from patients in the Indigenous group (n = 2) and patients in the non-Indigenous group (n = 6; Figure 1).
Figure 1.
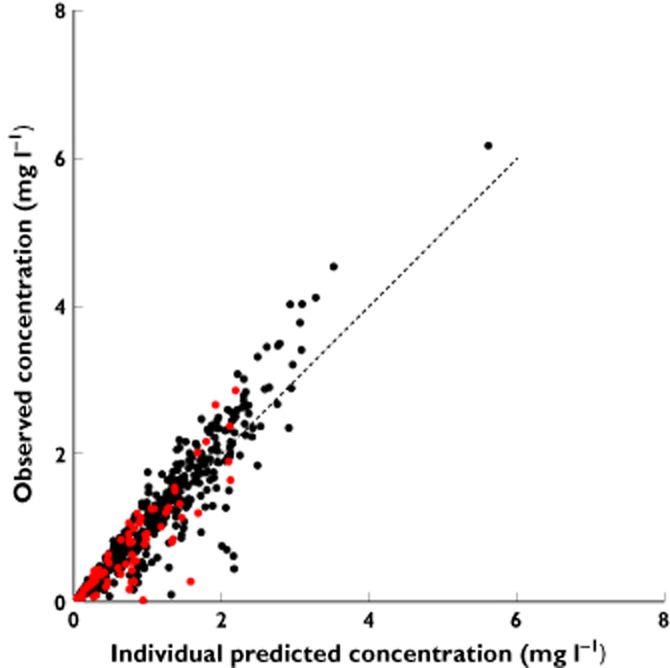
The observed concentrations of metformin vs. the individual predicted metformin concentration of the final population model of metformin with Indigenous patients.  , non-Indigenous patient concentrations;
, non-Indigenous patient concentrations;  , Indigenous patient concentrations. Dashed line is the line of identity
, Indigenous patient concentrations. Dashed line is the line of identity
The median CL/F was statistically higher in Indigenous patients than in the non-Indigenous patients (P < 0.05). The median difference between the two groups, however, was small (8 l h−1; 95% confidence interval 2.5–18.9 l h−1) and there was marked overlap between them (Figure 2). Normalizing CL/F to CLCR (renal function) reduced this variation, but a considerable spread remained (Figure 2, Table 1). The difference between the mean values of the ratio of CL/F to CLCR was small and not statistically significant (medians 13.1 and 12.6 l h−1, for Indigenous and non-Indigenous patients respectively). The V1/F was comparable between the two groups (Figure 2, Table 1). There was no difference in the pharmacokinetic parameters of metformin between the Aboriginal and Torres Strait Islander groups (Figure 3).
Figure 2.
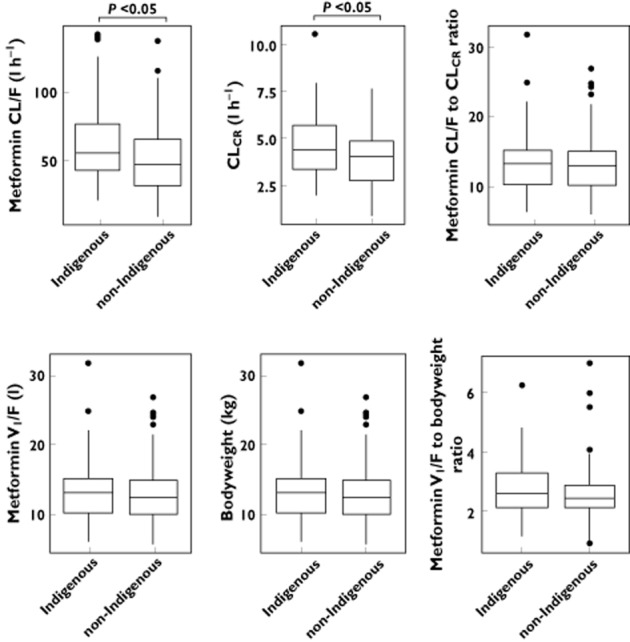
The comparative pharmacokinetics of metformin in Indigenous and non-Indigenous patients. The distribution of the apparent clearance of metformin (CL/F), creatinine clearance (CLCR), CL/F to CLCR ratio, central volume of distribution (V1/F), bodyweight and V1/F to bodyweight ratio are shown
Figure 3.
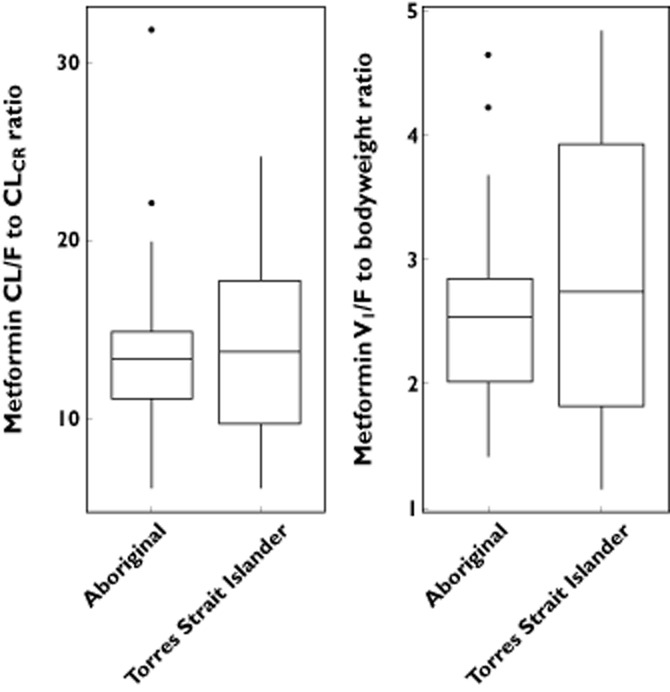
Comparative ratios of metformin apparent clearance (CL/F) to creatinine clearance (CLCR) and the central volume of distribution (V1/F) to bodyweight in 32 Aboriginal and 10 Torres Strait Islander patients
Discussion
Metformin is started at a low dosage (typically, 0.5 g daily) and increased gradually depending on the patient's renal function and gastrointestinal tolerance of the drug [14,18]. At least 80% of metformin is excreted unchanged in urine, and renal function is an important factor controlling the steady-state dosage of metformin. As outlined in our previous communication [14], suitable steady-state doses of metformin were 0.5, 1, 2 and 3 g daily for CLCR of 15, 30, 60 and 120 ml min−1, respectively. The lack of any substantial effect of ethnicity in Australian patients indicates that these doses can also be prescribed for Indigenous patients (Table 2, Supplementary data and Results).
Table 2.
Pharmacokinetic parameters of metformin in Indigenous and non-Indigenous patients
| Parameter | Indigenous | Non-Indigenous |
|---|---|---|
| n | 50 | 120 |
| CL/F (l h−1) | 54 (42–77) | 46 (31–64)* |
| Ratio of CL/F to CLCR | 13.1 (10.2–14.9) | 12.6 (9.9–14.9) |
| V1/F (l) | 221 (177–278) | 206 (167–248) |
| Ratio of V1/F to bodyweight | 2.5 (2.2–3.2) | 2.4 (2.1–2.9) |
Values are presented as the median and interquartile range. Abbreviations are as follows: CL/F, apparent clearance of metformin; CLCR, creatinine clearance; V1/F, apparent volume of the central compartment.
P < 0.05.
Several reasons may be put forward to explain the wide variation in the most clinically significant pharmacokinetic parameter of metformin, namely the ratio of the apparent metformin clearance (CL/F) to creatinine clearance (CLCR); these include poor adherence, interpatient and intrapatient variations in F (which ranges from about 25 to 75% [19]) and interpatient variation in the ratio of the renal clearance of metformin to CLCR which is independent of F [18]. Although clear relationships between the clinical response and plasma concentrations of metformin have not been demonstrated, the steady-state dose of metformin should be reduced in patients with low creatinine clearances although, in principle, it should be individualized at any level of renal function.
Another approach to investigate the reasons for interpatient differences in the pharmacokinetics of metformin is to group patients by genotype rather than by ethnicity. This can be achieved by examining the effects of single nucleotide polymorphisms (SNPs) of metformin transporters on metformin pharmacokinetics. The SNPs in metformin transporters (organic cation transporters, OCT1–OCT3; multidrug and toxin extrusion 1, MATE1; and plasma membrane monoamine transporter, PMAT) were investigated previously in non-Indigenous subjects [14]. Although variant SNPs were not associated with a significant effect on metformin pharmacokinetics, this may have been due to the low numbers of patients carrying a homozygous variant SNP. Only four patients (out of 120 non-Indigenous Australians) possessed one or more low-activity variants of the OCT1 gene (Arg61Cys, Gly401Ser, M420del or Gly465Arg), which, in the literature, was reported to be associated with either a decrease in metformin CL/F [20] or an increase in metformin trough concentration [21]. At present, the minor allele frequency of these transporters is unknown in the Indigenous Australian population, and permission was not sought in the present study to genotype these patients. Had significant contrasts between the pharmacokinetics of metformin in Indigenous and non-Indigenous been detected, then approval for genetic testing would have been requested from NSW Aboriginal Health and Medical Research Council and Cairns Base Hospital.
It had been anticipated that a large proportion of the Indigenous patients would have stage 3 kidney disease (CLCR < 30 ml min−1) because of the known higher incidence of T2DM and kidney disease in this population. On the contrary, no Indigenous patient had a CLCR below 30 ml min−1, whereas 13 of the 120 non-Indigenous Australian patients had CLCR below 30 ml min−1 [14]. This finding may be due to different prescribing practices in the various clinics and hospitals. Many Indigenous patients come from remote locations, with limited health services. It is possible that cautious prescribing was undertaken in this cohort due to the low attendance rates at these health services [2,22].
Our work supports the use of metformin down to 30 ml min−1 for both Indigenous and non-Indigenous Australians [9,11] provided that the dosage is reduced according to renal function. Our data further indicate that non-Indigenous Australian patients tolerate metformin at CLCR below 30 ml min−1. This is also likely to apply to the Indigenous population. However, as there were no Indigenous patients with CLCR below 30 ml min−1, further studies are required to ensure that the plasma concentrations of metformin and lactate are not significantly elevated in this group.
Five of the 50 Indigenous patients had a vitamin B12 concentration below the replete concentration (180 pmol l−1), two of which were considered deficient (<120 pmol l−1). No other concentrations from these patients were available to determine whether these were aberrant results. Metformin CL/F did not correlate with vitamin B12 concentrations (data not shown), thus, the pharmacokinetics is not an explanation for this finding.
This is the first study to examine the pharmacokinetics of metformin in Indigenous Australians. Given that T2DM and kidney disease are highly prevalent in the Indigenous population, the adherence to the guideline of a cut-off down to 30 ml min−1 should allow more Indigenous patients to benefit from metformin therapy [23]. In particular, the product information recommending that metformin should not be used when CLCR is below 60 ml min−1 appears to be overly cautious for both Indigenous and non-Indigenous Australian patients with T2DM.
Acknowledgments
We would like to acknowledge the support of the local Aboriginal Communities, La Perouse Local Aboriginal Lands Council and Wuchopperen Aboriginal Health Service. We also gratefully acknowledge the support and assistance of the Renal and Diabetes Clinic in Cairns Base Hospital (Dr Murty Mantha, Professor Ashim Sinha and Ms Stella Green), Dr Louise Maple-Brown, Dr Simon Chalkley and the La Perouse Aboriginal Health Link Advisory Group. We would like to thank Dr Toong Lee and Dr Peter Timmins for providing healthy subject data sets. Funding for this study was provided by the NH&MRC Programme Grant 568612, Australian Research Council Grant LP 0990670.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: JKD and SSK had support from the Australian Research Council (ARC) Linkage Grant LP 0990670 for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1
The diagnostic plots of the final population model of metformin with Indigenous patients. Plots of observed vs. population predicted (A) and individual predicted (B) concentrations of metformin were plotted with the line of identity and the conditional weighted residuals vs. population predicted (C) and time after dose (D) were plotted with a smooth curve
Figure S2
The prediction-corrected visual predictive check (VPC) of the final model. The 5th, 50th and 95th percentiles of the observed concentrations (red lines) were plotted with the 5th, 50th and 95th percentiles of the predictions (black lines). The 95% confidence interval (shaded grey area) is shown for the prediction intervals
Table S1
The study design of the datasets used for the population model
Table S2
The forward inclusion and backward elimination of covariates for inclusion in the model. The data are expressed as means and RSEs
References
- 1.Burke V, Zhao Y, Lee AH, Hunter E, Spargo RM, Gracey M, Smith RM, Beilin LJ, Puddey IB. Predictors of type 2 diabetes and diabetes-related hospitalisation in an Australian Aboriginal cohort. Diabetes Res Clin Pract. 2007;78:360–368. doi: 10.1016/j.diabres.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 2.McDermott RA, Li M, Campbell SK. Incidence of type 2 diabetes in two Indigenous Australian populations: a 6-year follow-up study. Med J Aust. 2010;192:562–565. doi: 10.5694/j.1326-5377.2010.tb03636.x. [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Hoy WE. Diabetes and lifetime risk of ESRD in high-risk remote-dwelling Australian Aboriginal people: a 20-year cohort study. Am J Kidney Dis. 2013;62:845–846. doi: 10.1053/j.ajkd.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, Kile E, Walker KA, Wang Z. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998;54:1296–1304. doi: 10.1046/j.1523-1755.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 5.McDonald SP, Russ GR. Burden of end-stage renal disease among indigenous peoples in Australia and New Zealand. Kidney Int Suppl. 2003;63:S123–127. doi: 10.1046/j.1523-1755.63.s83.26.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoy WE, White AV, Tipiloura B, Singh GR, Sharma S, Bloomfield H, Swanson CE, Dowling A, McCredie DA. The influence of birthweight, past poststreptococcal glomerulonephritis and current body mass index on levels of albuminuria in young adults: the multideterminant model of renal disease in a remote Australian Aboriginal population with high rates of renal disease and renal failure. Nephrol Dial Transplant. 2014 doi: 10.1093/ndt/gfu241. doi: 10.1093/ndt/gfu241. [DOI] [PubMed] [Google Scholar]
- 7.McDonald SP, Russ GR. Current incidence, treatment patterns and outcome of end-stage renal disease among indigenous groups in Australia and New Zealand. Nephrology (Carlton) 2003;8:42–48. doi: 10.1046/j.1440-1797.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher FR, Shannon C, Dunbar TE. The National Health and Medical Research Council Road Map: a strategic framework for improving Aboriginal and Torres Strait Islander health through research. Med J Aust. 2008;188:525–526. doi: 10.5694/j.1326-5377.2008.tb01767.x. [DOI] [PubMed] [Google Scholar]
- 9.Davoren P. Safe prescribing of metformin in diabetes. Aust Prescr. 2014;37:2–5. [Google Scholar]
- 10.Diabex Tablets. Carole Park, Australia: Alphapharm Pty Ltd; 2013. [Google Scholar]
- 11.Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update) London: Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- 12.Rossi S. Australian Medicines Handbook 2013. Adelaide: Australian Medicines Handbook Pty Ltd; 2013. [Google Scholar]
- 13.Duong JK, Roberts DM, Furlong TJ, Kumar SS, Greenfield JR, Kirkpatrick CM, Graham GG, Williams KM, Day RO. Metformin therapy in patients with chronic kidney disease. Diabetes Obes Metab. 2012;14:963–965. doi: 10.1111/j.1463-1326.2012.01617.x. [DOI] [PubMed] [Google Scholar]
- 14.Duong JK, Kumar SS, Kirkpatrick CM, Greenup LC, Arora M, Lee TC, Timmins P, Graham GG, Furlong TJ, Greenfield JR, Williams KM, Day RO. Population pharmacokinetics of metformin in healthy subjects and patients with type 2 diabetes mellitus: simulation of doses according to renal function. Clin Pharmacokinet. 2013;52:373–384. doi: 10.1007/s40262-013-0046-9. [DOI] [PubMed] [Google Scholar]
- 15.Population Distribution. Aboriginal and Torres Strait Islander Australians. Canberra: edStatistics ABo; 2006. [Google Scholar]
- 16.Lalau JD, Lemaire-Hurtel AS, Lacroix C. Establishment of a database of metformin plasma concentrations and erythrocyte levels in normal and emergency situations. Clin Drug Invest. 2011;31:435–438. doi: 10.2165/11588310-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 17.Reinstatler L, Qi YP, Williamson RS, Garn JV, Oakley GP., Jr Association of biochemical B12 deficiency with metformin therapy and vitamin B12 supplements: the National Health and Nutrition Examination Survey, 1999–2006. Diabetes Care. 2012;35:327–333. doi: 10.2337/dc11-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, Furlong TJ, Greenfield JR, Greenup LC, Kirkpatrick CM, Ray JE, Timmins P, Williams KM. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Tucker GT, Casey C, Phillips PJ, Connor H, Ward JD, Woods HF. Metformin kinetics in healthy subjects and in patients with diabetes mellitus. Br J Clin Pharmacol. 1981;12:235–246. doi: 10.1111/j.1365-2125.1981.tb01206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu Y, Brown C, Castro RA, Shi RJ, Lin ET, Owen RP, Sheardown SA, Yue L, Burchard EG, Brett CM, Giacomini KM. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Clin Pharmacol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen MM, Brasch-Andersen C, Green H, Nielsen F, Damkier P, Beck-Nielsen H, Brosen K. The pharmacogenetics of metformin and its impact on plasma metformin steady-state levels and glycosylated hemoglobin A1c. Pharmacogenet Genomics. 2011;21:837–850. doi: 10.1097/FPC.0b013e32834c0010. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Hoy WE, Si D. Incidence of type 2 diabetes in Aboriginal Australians: an 11-year prospective cohort study. BMC Public Health. 2010;10:487. doi: 10.1186/1471-2458-10-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas M, Weekes AJ, Thomas MC. The management of diabetes in indigenous Australians from primary care. BMC Public Health. 2007;7:303. doi: 10.1186/1471-2458-7-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
The diagnostic plots of the final population model of metformin with Indigenous patients. Plots of observed vs. population predicted (A) and individual predicted (B) concentrations of metformin were plotted with the line of identity and the conditional weighted residuals vs. population predicted (C) and time after dose (D) were plotted with a smooth curve
Figure S2
The prediction-corrected visual predictive check (VPC) of the final model. The 5th, 50th and 95th percentiles of the observed concentrations (red lines) were plotted with the 5th, 50th and 95th percentiles of the predictions (black lines). The 95% confidence interval (shaded grey area) is shown for the prediction intervals
Table S1
The study design of the datasets used for the population model
Table S2
The forward inclusion and backward elimination of covariates for inclusion in the model. The data are expressed as means and RSEs


