Abstract
AIMS
The aims of the study were to assess the pharmacokinetics, pharmacodynamics, safety and tolerability of a novel, pegylated recombinant human consensus interferon-α variant (PEG-IFN-SA) in healthy volunteers. A pharmacokinetic and pharmacodynamic comparison of PEG-IFN-SA and peginterferon-α-2a in healthy subjects was evaluated.
METHODS
A randomized, dose-escalating, single administration dose phase I clinical study was conducted. Thirty healthy subjects received PEG-IFN-SA as a single dose of 0.5–2.0 μg kg−1 by subcutaneous (s.c.) injection in four parallel groups. Eight subjects received peginterferon-α-2a as a single dose of 180 μg s.c.
RESULTS
The incidence rates of adverse events for PEG-IFN-SA and peginterferon-α-2a were 29 of 30 and 7 of 8, respectively. The adverse events for PEG-IFN-SA were mild to moderate and similar to those of peginterferon-α-2a. Within 168 h after injection, the mean values of maximal concentration and area under the plasma concentration–time curve from time of dosing to 168 h [AUC(0–168h)] for 2′,5′-oligoadenylate, neopterin and β2-microglobulin for PEG-IFN-SA at 1.5 μg kg−1 s.c. were similar to or higher than those for peginterferon-α-2a at a dose of 180 μg s.c. After s.c. injection of PEG-IFN-SA at 1.5 μg kg−1, the mean geometric mean values of plasma half-life, time to maximal concentration, maximal concentration and AUC(0–168h) were 55.3 h, 26.9 h, 0.53 μg l−1 and 44.0 μg l−1 h, respectively.
CONCLUSIONS
The tolerance, pharmacokinetic and pharmacodynamic characteristics of PEG-IFN-SA support its administration by s.c. injection as a single dose of 1.5 μg kg−1 or at 2.0 μg kg−1 per week.
Keywords: PEG-IFN-SA, pharmacodynamics, pharmacokinetics, safety, tolerability
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Studies in animals have suggested that a novel, pegylated recombinant human consensus interferon-α variant (PEG-IFN-SA) provides enhanced drug exposure and has desirable pharmacokinetic properties and significantly sustained antiviral activity.
WHAT THIS STUDY ADDS
This first study in humans provides information on the tolerability, safety, pharmacokinetics and pharmacodynamics of PEG-IFN-SA, a potential new treatment for viral hepatitis and neoplastic disease.
The results of our study showed that the absorption and elimination of PEG-IFN-SA was faster than that of peginterferon-alfa-2a; however, the pharmacodynamic data on the novel formulation were comparable to or similar to those of peginterferon alfa-2a.
Introduction
Chronic hepatitis B and C are leading causes of hepatocellular carcinoma, cirrhosis and end-stage liver disease worldwide [1,2]. The prevalence of persons chronically infected with hepatitis B virus ranges from 10 to 20% in China [3]. Interferons (IFNs) have been used as a primary treatment of chronic hepatitis B and C [2,3]. Interferons are naturally occurring proteins produced by cells in response to antigenic stimulation with viral RNA, bacterial products or tumour proteins and are considered part of the innate immune system [4,5].
Interferon-α is a type I IFN with antiviral, antiproliferative and immunomodulatory functions. The consensus interferon could be used in the clinic, and it has been developed and is available as interferon alfacon-1 (Infergen®; Amgen, Inc., Thousand Oaks, CA, USA) and IFNα-n1 (Wellferon®; Glaxo-Wellcome Ltd., London, UK), which have been used successfully to treat various viral infections and tumours [5–7]. However, as with many unmodified protein drugs, some types of IFN have a rapid systemic clearance and relatively short lifespan in the systemic circulation, and they must be given frequently to maintain the desired therapeutic effects [5,7]. Protein conjugation with polyethylene glycol (PEG) is considered to be one method by which to overcome these disadvantages. The following PEG-modified interferons have been developed and are commercially available: PEG (a branched 40 kDa) IFN-α-2a (Pegasys, PEGASYS®; Hoffmann-La Roche, Inc., Basel, Switzerland) and PEG (a linear 12 kDa) IFN-α-2b (PEG-INTRON®; Schering Corp., Innishannon County Cork, Ireland). These PEG-modified interferons have a longer half-life and achieve more sustained therapeutic effects. Additionally, they allow a decrease of dosing frequency from three times to once per week, which reduces the inconvenience and pain for the patients [8,9]. In China, however, the drugs are expensive, and new treatments are required.
The recombinant human consensus interferon-α variant (IFN-SA), a novel consensus interferon-α with 171 amino acid residues, was developed by Chongqing Fagen Biomedical Inc., China. Compared with a naturally occurring IFN-α protein, IFN-SA has demonstrated enhanced (by ∼10-fold) antiviral activity in vitro. The PEG-modified IFN-SA (PEG-IFN-SA) investigated in the present study was produced by the covalent attachment of a single 20 kDa methoxy polyethylene glycol-propionaldehyde (mPEG-ALD) molecule to the N-terminal of IFN-SA, forming a molecule with an average molecular weight of ∼40 kDa, resulting in a longer half-life and sustained antiviral action. In vitro studies have shown that the antiviral mechanism of action of PEG-IFN-SA is identical to that of IFN-SA, which is stimulation of the expression of pro-inflammatory cytokines and co-stimulatory molecules, leading to the transition to an adaptive antiviral immunity [10]. Studies in animals have suggested that PEG-IFN-SA provides enhanced drug exposure and has desirable pharmacokinetic properties and significantly sustained antiviral activity [10].
The aims of this study were to evaluate the pharmacokinetics, pharmacodynamics, safety and tolerability of single doses of PEG-IFN-SA in healthy volunteers and to establish the optimal dosing regimen for subsequent studies. For comparison, a Pegasys control arm was included (using the dose indicated for chronic hepatitis B and C patients in clinical practice [2,11]), and pharmacokinetic and pharmacodynamic data were collected.
Methods
Subjects
Healthy, nonsmoking men or women aged 18–45 years with a body mass index of 18–25 kg m−2 were eligible for inclusion in the study. The health status was determined by a prestudy medical history, physical examination and clinical laboratory evaluations. Women who were nursing or pregnant and subjects who were infected with human immunodeficiency virus, hepatitis B or hepatitis C viruses were excluded from the study. The subjects were not allowed to consume alcohol, caffeine, tea or grapefruit-containing products for 7 days prior to screening and during admission and follow-up.
Design
An open, single-centre, randomized, positive control, dose-escalating study in healthy male and female subjects was conducted. The study was conducted at the Phase I Unit of West China Hospital (37# GuoXue Xiang, Chengdu, Sichuan, China) and sponsored by Chongqing Fagen Biomedical Inc. The study protocol was reviewed and approved by an independent ethics committee (Ethics approval number: WCHIEC 20100030, Ren min nan road 3, Chengdu, Sichuan 610041, China) and conducted under the Good Clinical Practices (GCP) of the China Food Drug Administration (CFDA); the study was in compliance with the Declaration of Helsinki. The study was registered at clinical trials.gov.cn (ChiCTR-TRC-11001393). After a full explanation of the study had been provided, written informed consent was obtained from each subject before the start of any protocol-specific procedures.
Thirty-eight subjects were randomized, according to a computer-generated simple random number list, to receive the one dose treatment (the test group or control group). In the test group, 30 subjects were randomized into four cohorts (four, 10, 10 and six; an equal number of women and men in each cohort). The subjects in the test group received, in sequential ascending order, PEG-IFN-SA 0.5, 1.0, 1.5 or 2.0 μg kg−1 by subcutaneous (s.c.) injection. Eight subjects (six and two) received Pegasys as a single dose of 180 μg (the usual therapeutic dose for chronic hepatitis B and C) by s.c. injection to act as the reference groups for the 1.5 and 2.0 μg kg−1 PEG-IFN-SA groups, respectively. All the doses were taken in the morning, in a fasted state. The subjects remained fasted for 2 h after administration of the medicine. During each period, the subjects were hospitalized during the first 48 h after the injections under strict medical supervision. Collection of the blood samples and monitoring for adverse reactions was continued for 168 h. Standard meals were provided during the study procedures. In all cases, testing of a higher dose was initiated only after it had been shown that the lower doses were adequately tolerated.
Eight subjects in the PEG-IFN-SA 1.5 μg kg−1 group and six subjects in the Pegasys group took part in the pharmacokinetic (PK) and pharmacodynamic (PD) cohorts. In these cohorts, blood serum samples were collected for pharmacokinetic and pharmacodynamic assessment [pharmacodynamic targets: 2′,5′-oligoadenylate (2,5-A), neopterin and β2-macroglobulin (β2M)] and their antiviral activity in vivo. In the PK and PD cohorts, the blood serum samples were collected over a 168 h period at the following time points: prior to dosing (0 h) and at 3, 6, 9, 12, 16, 24, 36, 48, 60, 72, 84, 96, 120, 144 and 168 h after dosing. For each sample, ∼4 ml of blood was collected into a vacuum tube containing no anticoagulant.
Except for the 0.5 μg kg−1 cohorts, the 20 volunteers (18 for the PEG-IFN-SA group and two for the Pegasys group) participated in the pharmacodynamic study. Additionally, the pharmacodynamic targets (2,5-A and neopterin) were evaluated. The blood serum samples for the pharmacodynamic assessments were collected over a 168 h period at the following time points: 0 (predose), 24, 48 or 72 and 168 h after the administration of each treatment.
Pharmacokinetic assessments
The concentrations of PEG-IFN-SA and Pegasys were detected by a commercially available enzyme-linked immunosorbent assay (ELISA) kit (PBL Biomedical Laboratories, Piscataway, NJ, USA; kit number, 41110) that had been validated for human serum. Briefly, a standard curve was prepared ranging from 12.5 to 1000 pg ml−1 for human IFN-α, and two standard curves were prepared ranging from 50 to 2500 pg ml−1 for PEG-IFN-SA and Pegasys. The values of absorbance at 450 nm (OD450) were determined according to the specifications of the test kit. The intra- and interassay variations were determined with blank human serum samples containing various concentrations of PEG-IFN-SA and Pegasys throughout the range of the assay. Three samples with different known concentrations were assayed 10 times in one test and assayed in 10 separate tests to assess the intra- and interassay precision. The recovery of PEG-IFN-SA and Pegasys with three different levels within the quantitative range of the assay from the human serum samples was evaluated. Some endogenous proteins, such as interferon β (IFN-β), recombinant hirudin 2 (HV2), ciliary neurotrophic factor (CNTF), human growth factor (HG), parathyroid hormone (PTH), human endostatin (Edno) and human interleukin 11 (IL-11), were used for the evaluation of the cross-reaction of these proteins with PEG-IFN-SA and Pegasys. The results showed that the linear ranges of PEG-IFN-SA and Pegasys were 50–2500 pg ml−1. The lower limits of quantification for PEG-IFN-SA and Pegasys were 50 pg ml−1. The intra- and interassay levels of PEG-IFN-SA were <7.9 and <9.9%, respectively. The intra- and interassay levels of Pegasys were <7.2 and <8.3%, respectively. The mean recovery for PEG-IFN-SA and Pegasys was 100 ± 2.1 and 105.0 ± 4.3%, respectively. The cross-reactivity rates were all below 0.5%.
Pharmacodynamic assessments
A commercially available radioimmunoassay kit (Eiken Chemical, Taito, Tokyo, Japan) that had been validated for human serum (the lower limit of quantification was 10 pmol dl−1) was used to detect the 2,5-A concentrations. The intra- and interassay precision levels of 2,5-A were <10.9 and <8.7%, respectively. The mean recovery for 2,5-A was 98.9 ± 1.8%.
The neopterin concentration was detected using a commercially available ELISA kit (IBL International GmbH, Flughafenstrasse, Hamburg, Germany; kit number, RE59321) that had been validated for human serum (the lower limit of quantification was 1.35 nmol l−1). The intra- and interassay precision levels of neopterin were <10.6%.
The β2M concentration was detected using a commercially available ELISA kit (R&D Systems, Minneapolis, MN, USA; kit number, DBM200) that had been validated for human serum (the lower limit of quantification was 0.4 μg ml−1). The intra- and interassay precision levels of β2M were <8.1 and <9.2%, respectively.
The ex vivo antiviral activity of PEG-IFN-SA and Pegasys in the serum of healthy volunteers was assessed using a cytopathic effect bioassay that reflected the ability of the protein to protect the human amnion WISH cells against cytotoxicity induced by infection with the vesicular stomatitis virus. The assay was performed in accordance with the Appendix to Volume III of the Chinese Pharmacopoeia (2005 Edition) [12]. Briefly, WISH cells (3.0 × 105 cells (100 μl)−1 per well in complete MEM medium containing 10% fetal bovine serum) were seeded in flat-bottomed 96-well plates and incubated at 37°C, in air supplemented with 5% CO2 for 5 h. Serial dilutions of serum samples or the IFN-α World Health Organization international standard were added to the wells, and the cells were challenged with vesicular stomatitis virus 24 h later. The viable cells were stained with 1% crystal violet in 15% ethanol and were quantified 24 h after the virus challenge. The cell viability was assessed by absorbance at 570 nm (OD570) with a reference wavelength of 630 nm using a microplate reader. The samples were tested in triplicate. The titre of the samples was based on the 50% cytopathic effect of the assay. A standard curve was generated for each plate, and it served to determine the IFN-α antiviral activity in each test serum sample. The antiviral activity, expressed as EC50 (the concentration to achieve 50% of the maximal effect), was calculated using a four-parameter curve fitting with Origin 7.5 software (Labconco, Kansas City, MO, USA).
Safety assessment
The following clinical safety assessments were included in the study: a physical examination; the vital signs (blood pressure, pulse rate, oral temperature and respiratory rate); 12-lead ECG cardiac monitoring (starting 30 min prior to the study drug administration and for 2 h postdose); and recording of the adverse events. Adverse experiences were monitored throughout the study. Clinical laboratory tests (full blood count, blood chemistry, free triiodothyronine, free thyroxine, thyrotrophin-stimulating hormone, coagulation tests and urinalysis) were performed on day 8 after dosing. The investigators evaluated all the clinical adverse events in terms of the intensity (mild, moderate or severe), duration, severity, outcome and relationship to the study drug.
Data and statistical analysis
The serum concentration–time data for PEG-IFN-SA, Pegasys, 2,5-A, neopterin and β2M were analysed by noncompartmental methods using WinNonlin 6.1 (version 6.0, 2012; Pharsight, Inc., Mountain View, CA, USA). The following PK and PD parameters were determined from the serum concentration–time data: the maximal concentration (Cmax); the time to the maximal concentration (Tmax); the half-life (t1/2); and the area under the concentration–time curve to the last measured concentration (AUC0–t). The concentration values below the lower limit of quantification were excluded from the PK and PD analysis. The Cmax and Tmax were recorded directly from the experimental observations for each treatment period. The apparent terminal elimination rate constant (λz) was estimated using least-squares regression analysis of the terminal log–linear portion of the plasma concentration–time profiles. The t1/2 was calculated as ln(2)/λz. The AUC0–168h was calculated by linear and log–linear trapezoidal summations using the mixed log–linear algorithm.
The geometric mean and coefficient of variation (CV) for each parameter were calculated. A two-sided 90% confidence interval was constructed for the geometric mean ratio (PEG-IFN-SA/Pegasys) of the PD parameter values using an independent two-sample t-test. The statistical analysis of the data was performed with the Statistical Package for Social Science program (SPSS) for Windows, version 16.0 (SPSS, Chicago, IL, USA). The tests of the statistical hypotheses were performed at the 5% significance level.
Results
Subject characteristics
A total of 38 subjects (19 men and 19 women) were enrolled in the single rising dose study. The demographic and baseline characteristics of the subjects are presented in Table 1. The hypothesis of homogeneity among the groups was accepted. All the individuals complied with the treatment and evaluations.
Table 1.
Baseline subject characteristics
| Characteristic | PEG-INF-SA | Pegasys | ||||
|---|---|---|---|---|---|---|
| 0.5 μg kg−1 | 1.0 μg kg−1 | 1.5 μg kg−1 | 2.0 μg kg−1 | 180 μg kg−1* | 180 μg kg−1† | |
| Number of subjects | 4 | 10 | 10 | 6 | 6 | 2 |
| Age (years) | 24.75 (21–28) | 24.00 (22–27) | 23.50 (21–28) | 23.00 (21–25) | 24.13 (22–26) | 24.00 (24–24) |
| Height (cm) | 168.00 (165–174) | 165.20 (155–173) | 163.63 (156–180) | 168.33 (158–181) | 167.75 (153–190) | 165.00 (162–181) |
| Weight (kg) | 59.00 (55–64) | 57.00 (46–70) | 59.25 (48–70) | 60.33 (50–78) | 59.88 (46–88) | 56.00 (55–57) |
| Body mass index (kg m−2) | 20.94 (19.49–23.50) | 20.64 (19.10–23.00) | 21.23 (19.30–24.50) | 21.14 (19.05–24.89) | 21.00 (19.60–24.30) | 20.58 (20.20–20.96) |
Values are given as the mean (range).
Control group for the PEG-INF-SA 1.5 μg kg−1 group.
Control group for the PEG-INF-SA 2.0 μg kg−1 group.
Very low serum concentrations of Pegasys were found in one subject (B3) after the injection during the trial period, and a pharmacodynamic effect was not found. The data from this patient were excluded from the pharmacokinetic and pharmacodynamic analyses and were taken into account only for the safety investigation.
Safety
The adverse events (AEs) were recorded during the entire study and are summarized in Table 2; they were mild to moderate and well controlled. The test (29 of 30) and control groups (7 of 8) had no statistically significant difference (P = 0.862) in the incidence of adverse events. The types, degree and incidence of adverse events between s.c. PEG-IFN-SA at a dose of 1.5 or 2.0 μg kg−1 and s.c. Pegasys at 180 μg were similar.
Table 2.
Incidence of all adverse events in at least one subgroup
| PEG-INF-SA | Pegasys | |||||
|---|---|---|---|---|---|---|
| 0.5 μg kg−1 | 1.0 μg kg−1 | 1.5 μg kg−1 | 2.0 μg kg−1 | 180 μg kg−1* | 180 μg kg−1† | |
| Number of subjects | 4 | 10 | 10 | 6 | 6 | 2 |
| Number of subjects reporting at least one adverse event | 4 | 10 | 9 | 6 | 5 | 2 |
| Adverse event | ||||||
| Dizziness‡ [n (%)] | 4 (100) | 3 (30) | 5 (50) | 3 (50) | 3 (50) | 2 (100) |
| Weakness‡ [n (%)] | 4 (100) | 1 (10) | 1 (10) | 6 (100) | 1 (17) | 1 (50) |
| Myalgia‡ [n (%)] | 0 | 0 | 1 (10) | 2 (33) | 1 (17) | 1 (50) |
| Headache [n (%)] | 1 (25) | 6 (60) | 7 (70) | 4 (67) | 2 (33) | 0 |
| Degree (mild/moderate) | 1/0 | 5/1 | 7/0 | 1/3 | 1/1 | |
| Fever [n (%)] | 2 (50) | 2 (20) | 7 (70) | 3 (50) | 2 (33) | 1 (50) |
| Degree (mild/moderate) | 2/0 | 1/1 | 5/2 | 2/1 | 1/1 | 1/0 |
| Leucopenia [n (%)] | 3 (75) | 7 (70) | 5 (50) | 5 (83) | 4 (67) | 2 (100) |
| Degree (mild/moderate) | 3/0 | 5/2 | 3/2 | 2/3 | 4/0 | 0/2 |
| Thyroid dysfunction‡ [n (%)] | 0 | 0 | 1 (10) | 0 | 0 | 0 |
| Somnolence‡ [n (%)] | 1 (25) | 1 (10) | 0 | 0 | 0 | 0 |
| Nasal obstruction‡ [n (%)] | 1 (25) | 0 | 0 | 0 | 0 | 0 |
| Cough‡ [n (%)] | 0 | 1 (10) | 0 | 0 | 0 | 0 |
| Diarrhoea‡ [n (%)] | 0 | 0 | 0 | 1 (17) | 0 | 0 |
| Injection site pain‡ [n (%)] | 0 | 0 | 0 | 2 (33) | 0 | 1 (50) |
| Poor appetite‡ [n (%)] | 0 | 0 | 0 | 0 | 0 | 1 (50) |
| Nausea‡ [n (%)] | 0 | 1 (10) | 0 | 0 | 0 | 0 |
Abbreviations are as follows: n, number of subjects.
Control group for the PEG-INF-SA 1.5 μg kg−1 group.
Control group for the PEG-INF-SA2.0 μg kg−1 group.
The degree was mild.
For the PEG-IFN-SA groups, the types of adverse reactions in each group were similar. With the dose escalation, more moderate adverse reactions were found in the 1.5 and 2.0 μg kg−1 groups, particularly for leucopenia and influenza-like symptoms. In the 2.0 μg kg−1 group, absolute neutrophil counts lower than 1.0 × 109 l−1 were found in one-half of the subjects. Considering the safety of the healthy volunteers, the follow-up test was stopped. No serious or severe adverse events occurred, and all the adverse events were transient and could be returned to normal or baseline after drug withdrawal.
Pharmacokinetics
The mean serum concentration–time data and PK parameters of PEG-IFN-SA (1.5 μg kg−1) and Pegasys (180 μg) are shown in Table 3 and Figure 1. Given that PK blood samples were only collected for 168 h after the treatment in this study, the elimination phase of Pegasys was not observed, and the calculation of Pegasys elimination half-life was unavailable.
Table 3.
Comparison of pharmacokinetic parameters following a single subcutaneous dose of PEG-IFN-SA (1.5 μg kg−1) and Pegasys (180 μg) in healthy volunteers
| Parameter | PEG-IFN-SA (n = 8) | Pegasys (n = 5) |
|---|---|---|
| Geometric mean (%CV) | Geometric mean (%CV) | |
| AUC(0–168h) (μg l−1 h) | 44.0 (47.2) | 1103.1 (75.8) |
| Cmax (μg l−1) | 0.53 (57.7) | 12.3 (61.9) |
| Tmax (h)* | 26.9 (16, 60) | 90.7 (34, 168) |
| t1/2 (h)* | 55.3 (22.3, 113.2) | – |
Abbreviations are as follows: AUC(0–168h), area under the plasma concentration–time curve from time of dosing to168 h; Cmax, maximal observed concentration; CV, coefficient of variation; Tmax, time to reach Cmax.
Values are given as the median (range).
Figure 1.
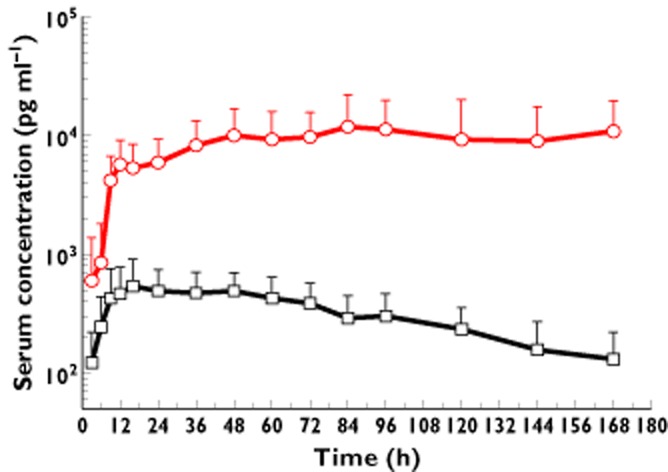
The mean serum concentration–time profiles following a single subcutaneous administration of PEG-IFN-SA and Pegasy in healthy volunteers at doses of 1.5 μg kg−1 and 180 μg, respectively. Each data point represents the mean value of the serum concentrations that were obtained from the PEG-IFN-SA (n = 8) and Pegasy (n = 5) groups, while the error bar shows the SD.  , PEG-IFN-SA;
, PEG-IFN-SA;  , Pegasys
, Pegasys
There were differences in the drug dosages, and the serum concentrations of PEG-IFN-SA were lower than those of Pegasys. The PEG-IFN-SA was readily absorbed after administration. Within 9–72 h after administration, the PEG-IFN-SA serum concentration was maintained at high levels (mean concentrations of 386.7–425.2 pg ml−1), and 168 h after administration the mean serum concentration could be detected at 131.0 pg ml−1. The serum concentration of Pegasys increased within 9–168 h after administration at a dose of 180 μg s.c., and the serum concentration remained at high levels. The mean t1/2 of PEG-IFN-SA (55.3 h) was shorter than that of Pegasys (50–130 h) [9,11]. The mean Tmax of PEG-IFN-SA was significantly earlier than that of Pegasys (26.9 vs. 90.7 h, respectively, P = 0.005). The PK parameters of both drugs showed that PEG-IFN-SA had relatively more rapid absorption and excretion than did Pegasys in the healthy subjects.
Pharmacodynamics
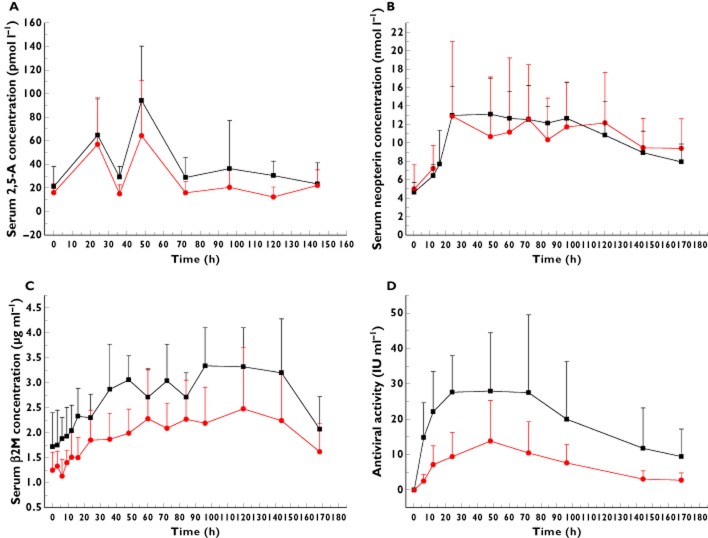
After administration of PEG-IFN-SA 1.5 μg kg−1 s.c. and Pegasys 180 μg s.c., the serum concentrations of 2,5-A, neopterin and β2M in both groups increased and presented similar trends (Figure 2A–C). The primary PD parameters of PEG-IFN-SA (1.5 μg kg−1) and Pegasys (180 μg) are shown in Table 4. The data on the PD parameters of PEG-IFN-SA and Pegasys showed that the mean AUC(0–168h) and Cmax for 2,5-A and neopterin in both groups were similar. Additionally, the data showed that the mean AUC(0–168h) of β2M with PEG-IFN-SA (1.5 μg kg−1 s.c.) was higher than that with Pegasys (180 μg s.c.). In the healthy volunteers, the ex vivo antiviral activity of PEG-IFN-SA was higher than that of Pegasys (Figure 2D).
Figure 2.
The mean serum concentration–time profiles of 2′,5′-oligoadenylate (2,5-A; A), neopterin (B) and β2-microglobulin (β2M; C) and the ex vivo antiviral activity (D) following a single subcutaneous administration of PEG-IFN-SA and Pegasys in healthy volunteers at doses of 1.5 μg kg−1 and 180 μg, respectively. Each data point represents the mean value of the serum concentrations that were obtained from the PEG-IFN-SA (n = 8) and Pegasy (n = 5) groups, while the error bar shows the SD.  , PEG-IFN-SA;
, PEG-IFN-SA;  , Pegasys
, Pegasys
Table 4.
Comparison of pharmacodynamic parameters following a single subcutaneous dose of PEG-IFN-SA (1.5 μg kg−1) and Pegasys (180 μg) in healthy volunteers
| Biomarker | Parameter | PEG-IFN-SA (n = 8) | Pegasys (n = 5) | GMR (T/C) | 90% Confidence interval for GMR | P Value |
|---|---|---|---|---|---|---|
| Geometric mean (%CV) | Geometric mean (%CV) | |||||
| 2′,5′-Oligoadenylate | AUC(0-168h) (pmol dl−1 h) | 5716.8 (36.5) | 3553.8 (36.7) | 1.61 | (0.97–1.70) | 0.053 |
| Cmax (pmol dl−1) | 99.5 (36.9) | 74.5 (50.7) | 1.34 | (0.85–1.53) | 0.309 | |
| Tmax (h)* | 40.4 (24–48) | 38.1 (24–48) | 1.06 | (0.79–1.21) | 0.756 | |
| Neopterin | AUC(0-168h) (nmol l−1 h) | 1797.0 (12.8) | 1645.4 (20.9) | 1.09 | (0.67–1.26) | 0.446 |
| Cmax (nmol l−1) | 16.4 (9.6) | 15.5 (24.5) | 1.06 | (0.87–1.13) | 0.147 | |
| Tmax (h)* | 56.2 (24–120) | 56.9 (24–120) | 0.98 | (0.94–1.03) | 0.988 | |
| β2-Macroglobulin | AUC(0–168h) (mg l−1 h) | 473.5 (13.8) | 329.8 (30.0) | 1.44 | (1.16–1.65) | 0.024 |
| Cmax (mg l−1) | 3.9 (14.0) | 2.8 (39.3) | 1.39 | (0.97–1.52) | 0.199 | |
| Tmax (h)* | 88.4 (36–144) | 91.9 (60–144) | 0.96 | (0.85–1.18) | 0.940 |
Abbreviations are as follows: AUC(0–168h), area under the plasma concentration–time curve from time of dosing to 168 h; C, Pegasys group; Cmax, maximal observed concentration; GMR, geometric mean ratio; T, PEG-IFN-SA group; Tmax, time of Cmax.
Values are given as the geometric mean (range).
The serum concentrations of 2,5-A and neopterin following a single dose of s.c. administration of PEG-IFN-SA (1–2 μg kg−1) in healthy volunteers had a similar increased trend in all the groups. The pharmacodynamic parameters of 2,5-A and neopterin are listed in Table 5 and presented no dose response relationship.
Table 5.
Pharmacodynamic parameters following a single subcutaneous dose of PEG-IFN-SA (1–2 μg kg−1) in healthy volunteers
| Biomarker | Parameter | PEG-IFN-SA | ||
|---|---|---|---|---|
| 1 μg kg−1 (n = 10) | 1.5 μg kg−1 (n = 2) | 2 μg kg−1 (n = 6) | ||
| 2′,5′-Oligoadenylate | AUC (0-168h) (pmol dl−1 h) | 7610 (37.0) | 11495.9 (3.8) | 31892.3 (36.7) |
| Cmax (pmol dl−1) | 64.7 (57.8) | 95.0 (5.4) | 273.4 (30.5) | |
| Tmax (h)* | 51.7 (24–72) | 48 (48–48) | 64 (24–168) | |
| Neopterin | AUC(0–168h) (nmol l−1 h) | 1993.0 (35.9) | 1128.5 (27.1) | 1697.6 (11.3) |
| Cmax (nmol l−1) | 15.86 (39.4) | 9.5 (30.3) | 13.7 (18.6) | |
| Tmax (h)* | 57.8 (24–72) | 48 (48–48) | 41.6 (24–72) | |
Abbreviations are as follows: AUC(0–168h), area under the plasma concentration–time curve from time of dosing to 168 h; Cmax, maximal observed concentration; Tmax, time of Cmax. Data are expressed as the geometric mean (%CV) or *geometric mean (range).
Discussion
This study provides the first safety, tolerability, PK and PD data for PEG-IFN-SA, in healthy volunteers. Single doses of up to 2 μg kg−1 were well tolerated. A previous study reported influenza-like symptoms, such as dizziness, weakness, headache and fever, as common adverse events of interferons [8,9]. The commonest laboratory abnormality resulting from interferon treatment was leucopenia [8,9,11]. The presentation of adverse events in healthy volunteers after PEG-IFN-SA s.c. found in our study was similar to those of previous reports [8,9,11].
In the comparison of the PK parameters of PEG-IFN-SA with those of general unpegylated interferon, the mean t1/2 of PEG-IFN-SA was 55.3 h, which was longer than that of the general unpegylated interferon-α (with a mean t1/2 of 3–10 h [5,13,14]), revealing long-acting activity. The PK parameters of PEG-IFN-SA in healthy volunteers were consistent with those found in monkeys and rats [10].
In our study, PEG-IFN-SA presented a relatively more rapid absorption and excretion than did Pegasys in healthy subjects. The PK characteristics might differ because of the different molecular weight, modification methods and modified loci of PEG modification. Pegasys is a 40 kDa molecular weight PEG, predominantly by amide linkage covalent bonding in lysine loci, specifically in the middle of the Lys 31,121,131,134 branch key modifiers, with priority for Lys121 [9]. However, PEG-IFN-SA is produced by the covalent attachment of a single 20 kDa methoxy polyethylene glycol-propionaldehyde (mPEG-ALD) molecule to the N-terminal of IFN-SA, forming a molecule with an average molecular weight of approximately 40 kDa. It has been reported that the hydrodynamic volume of branched PEG-proteins and variations in the position and number of PEG adducts results in variations in characteristics relevant to clinical and other application-related effects [15–18].
We found high individual variability in our study, which was similar to the results in other interferon PK studies [8,13,18]. Particularly for subject B3, whose serum concentrations ranged from 204.3 to 262.8 pg ml−1, the interferon PK levels were much lower than the mean concentration levels (4000–10 000 pg ml−1) in the Pegasys group. The same results were found in the PD targets of subject B3, and the data from this subject were excluded from the PK and PD analyses. No special events were found in the female subject during the trial. Many factors led to the individual differences of PK and PD in the interferon study. The major reason might be the individual variables. Interferon is a type of natural synthetic material in the human body, and there are individual differences in interferon levels and regulatory mechanisms [3,4,19].
The PK characteristics of dose escalation and multiple doses were not obtained in this study, which was the first study of this type to be conducted in humans, and they will be investigated in a study of patients with hepatitis C and hepatitis B.
The key effect protein of interferon is 2′,5′-oligoadenylate synthetase (2′,5′-OAS), which is an accurate indicator of the antiviral activity in the body [3,20]. In our study, the detection of 2,5-A could reflect the activity of 2′,5′-OAS. Neopterin is a GTP metabolic product and a type of immune activation regulator. The serum neopterin level is considered to be a specific biomarker of the body's immune activation by a viral infection or inflammation [21]. In addition, β2M plays an important role in the control of tumour growth and metastasis and is another biomarker reflecting the biological activities of interferon [22].
In this study, the PD end-point and watch points were referenced to past reports on Pegasys [22–25]. The geometric mean Tmax of 2,5-A, neopterin and β2M of PEG-IFN-SA at a dose of 1.5 μg kg−1 s.c. lagged behind the mean Tmax of the drug concentrations in the blood samples (∼26.9 h), at 40.4, 56.2, and 88.4 h, respectively. These changes in the pharmacodynamic targets were because of the interferon stimulation. The results in our study were consistent with those of previous studies [23–26]. The pharmacodynamic parameters of 2,5-A and neopterin following a single s.c. dose of PEG-IFN-SA (1–2 μg kg−1) in healthy volunteers had a similar increased trend but presented no dose–response relationship. This result may be influenced by the small sample size and the individual variables.
Although the serum concentration levels of PEG-IFN-SA were lower than those of Pegasys, the data in Table 4 show the similarity for PEG-IFN-SA and Pegasys of the AUC(0–168h) and Cmax for 2,5-A and neopterin. Additionally, the mean AUC(0–168h) of β2M for PEG-IFN-SA 1.5 μg kg−1 s.c. was higher than that for Pegasys 180 μg s.c. Thus, PEG-IFN-SA 1.5 μg kg−1 s.c. might present a clinical therapeutic effect similar to that of Pegasys 180 μg s.c. in chronic hepatitis B or C patients.
These outcomes were possibly a result of the particular structure of IFN-SA. Unlike general consensus interferon or natural interferon containing 166 amino acid residues, the novel recombinant human consensus interferon-α variant (IFN-SA) is a consensus interferon-α with 171 amino acid residues. The structural innovation of IFN-SA is in 121, 166 amino acid positions, Lys, Glu mutated into Arg, and Asp, and in the N-terminal five amino acid residues that were added for linking to PEG. These changes led to enhanced antiviral activity. The purity of PEG-IFN-SA and IFN-SA were determined to be 98.8 and 98.0%, respectively, by high-performance liquid chromatography, and the antiviral activity in vitro was 1.0 × 108 and 7.8 × 108 IU (mg protein)−1, respectively. However, for Pegasys, the antiviral activity in vitro was ∼1.0 × 106 to 3 × 106 IU (mg protein)−1 [11,27]. In our study, the higher ex vivo antiviral activity of PEG-IFN-SA in healthy volunteers might be a reason for the PK/PD results (Figure 2D).
Considering the small sample size, the clinical therapeutic effect of PEG-INF-SA should be confirmed by a future phase II–III study.
Conclusion
This first human trial of PEG-IFN-SA indicated that the medicine was well tolerated up to 2.0 μg kg−1 in healthy volunteers. Our research showed that absorption and excretion were more rapid for PEG-IFN-SA than for Pegasys, and they had similar or comparable pharmacodynamic responses. The tolerance, PK and PD characteristics of PEG-INF-SA support its administration by s.c. injection, at a single dose of 1.5 or 2.0 μg kg−1 once per week.
Acknowledgments
The study was sponsored by Chongqing Fagen Biomedical Inc. and supported by Ministry of Science and Technology of China funds 2011ZX09302-001. The authors wish to thank Professor Fan Kai for his comments on the study and thank all the volunteers and clinical staff involved in the conduct of the study in the GCP Center of West China Hospital.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: ZL, LMP, GZP, WY, XN, CYM and LH had support from Chongqing Fagen Biomedical Inc. for the submitted work; LH is a full-time employee of Chongqing Fagen Biomedical Inc.; ZL, GZP, WY and XN are full-time employees of GCP Center, West China Hospital of Sichuan University; LMP is a full-time employee of the First Affiliated Hospital of Chongqing Medical University; CYM is a full-time employee of Tianjin Institute of Pharmaceutical Research; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Kim BK, Revill PA, Ahn SH. HBV genotypes: relevance to natural history, pathogenesis and treatment of chronic hepatitis B. Antivir Ther. 2011;16:1169–1186. doi: 10.3851/IMP1982. [DOI] [PubMed] [Google Scholar]
- 3.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 4.Bonjardim CA, Ferreirab PCP, Kroon EG. Interferons. Signaling, antiviral and viral evasion. Immunol Lett. 2009;122:1–11. doi: 10.1016/j.imlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melian EB, Plosker GL. Interferon alfacon-1 a review of its pharmacology and therapeutic efficacy in the treatment of chronic hepatitis C. Drugs. 2001;61:1661–1691. doi: 10.2165/00003495-200161110-00009. [DOI] [PubMed] [Google Scholar]
- 6.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T, Tursz T. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. Groupe Français d'Immunothérapie. N Engl J Med. 1998;338:1272–1278. doi: 10.1056/NEJM199804303381805. [DOI] [PubMed] [Google Scholar]
- 7.Thitinan S, McConville JT. Interferon alpha delivery systems for the treatment of hepatitis C. Int J Pharm. 2009;369:121–135. doi: 10.1016/j.ijpharm.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 8.Glue P, Fang JW, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S. Pegylated interferon-alpha2b: pharmacokinetics, pharmacodynamics, safety, and preliminary efficacy data. Hepatitis C intervention therapy group. Clin Pharmacol Ther. 2000;68:556–567. doi: 10.1067/mcp.2000.110973. [DOI] [PubMed] [Google Scholar]
- 9.Reddy KR, Wright TL, Pockros PJ, Shiffman M, Everson G, Reindollar R, Fried MW, Purdum PP, Jensen D, Smith C, Lee WM, Boyer TD, Lin A, Pedder S, De Pamphilis J. Efficacy and safety of pegylated (40-kDa) interferon alpha-2a compared with interferon alpha-2a in noncirrhotic patients with chronic hepatitis C. Hepatology. 2001;33:433–438. doi: 10.1053/jhep.2001.21747. [DOI] [PubMed] [Google Scholar]
- 10.Cai Y, Zhang Z, Fan K, Zhang J, Shen W, Li M, Si D, Luo H, Zeng Y, Fu P, Liu C. Pharmacokinetics, tissue distribution, excretion, and antiviral activity of pegylated recombinant human consensus interferon-α variant inmonkeys, rats and guinea pigs. Regul Pept. 2012;173:74–81. doi: 10.1016/j.regpep.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Maribel RT, Jos'e FRO, Carlos FRB, Alberto FC, Elsa GL, Rosa SM, Acisclo MMC. Efficacy and safety of peg-IFN alfa-2a with ribavirin for the treatment of HCV/HIV coinfected patients who failed previous IFN based therapy. J Clin Virol. 2007;38:32–38. doi: 10.1016/j.jcv.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Pharmacopoeia Commission of the Ministry of Health of the People's Republic of China. Beijing, China: People's Medical Publishing House; 2005. Pharmacopoeia of the People's Republic of China. [Google Scholar]
- 13.Corssmit EPM, Heijligenberg R, Hack CE, Endert E, Sauerwein HP, Romijn JA. Effects of interferon-alpha (IFN-α) administration on leucocytes in healthy humans. Clin Exp Immunol. 1997;107:359–363. doi: 10.1111/j.1365-2249.1997.269-ce1161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruno R, Sacchi P, Cima S, Maiocchi L, Novati S, Filice G, Fagiuoli S. Comparison of peginterferon pharmacokinetic and pharmacodynamic profiles. J Viral Hepat. 2012;19(Suppl. 1):33–36. doi: 10.1111/j.1365-2893.2011.01519.x. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Du Y, Tian H, Gao XD, Yao WB. Quantitative determination of pegylated consensus interferon in rhesus monkey serum using a competitive enzyme-linked immunosorbent assay. Immuno Pharmacol Immunotoxicol. 2009;31:543–549. doi: 10.3109/08923970902814111. [DOI] [PubMed] [Google Scholar]
- 16.Fishburn CS. The pharmacology of PEGylation: balancing PD with PK to generate novel therapeutics. J Pharm Sci. 2008;97:4167–4183. doi: 10.1002/jps.21278. [DOI] [PubMed] [Google Scholar]
- 17.Fee CJ. Size comparison between proteins PEGylated with branched and linear poly(ethylene glycol) molecules. Biotechnol Bioeng. 2007;98:725–731. doi: 10.1002/bit.21482. [DOI] [PubMed] [Google Scholar]
- 18.Jonkman JHG, Nicholson KG, Farrow PR, Eckert M, Grasmeijer G, Oosterhuis B, De Noord OE, Guentert TW. Effects of α-interferon on theophylline pharmacokinetics and metabolism. Br J Clin Pharmacol. 1989;27:795–802. doi: 10.1111/j.1365-2125.1989.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonjardim CA. Interferons (IFNs) are key cytokines in both innate and adaptive antiviral immune responses – and viruses counteract IFN action. Microbes Infect. 2005;7:569–578. doi: 10.1016/j.micinf.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8:559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002;3:175–187. doi: 10.2174/1389200024605082. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. J Cell Physiol. 2003;195:346–355. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 23.Di Bisceglie AM, Fan X, Chambers T, Strinko J. Pharmacokinetics, pharmacodynamics, and hepatitis C viral kinetics during antiviral therapy: the null responder. J Med Virol. 2006;78:446–451. doi: 10.1002/jmv.20560. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Rakhit A, Ginsberg M, Rittweger K, Vuky J, Yu R, Fettner S, Hooftman L. Phase I trial of 40-kd branched pegylated interferon alfa-2a for patients with advanced renal cell carcinoma. J Clin Oncol. 2001;19:1312–1319. doi: 10.1200/JCO.2001.19.5.1312. [DOI] [PubMed] [Google Scholar]
- 25.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, Craxi A, Lin A, Hoffman J, Yu J. PEG IFN alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 26.Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A, Hoffman J, Brunda MJ. PEG IFN alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 27.Baron S, Tyring SK, Fleischmann WR, Jr, Coppenhaver DH, Niesel DW, Klimpel GR, Stanton GJ, Hughes TK. The interferons. Mechanisms of action and clinical applications. JAMA. 1991;266:1375–1383. doi: 10.1001/jama.266.10.1375. [DOI] [PubMed] [Google Scholar]