Abstract
AIM
Drugs used for postoperative nausea and vomiting prophylaxis are believed to provoke torsadogenic changes in cardiac repolarization. The aim of this study was to assess the effect of small doses of droperidol on the parameters of cardiac repolarization, including the QTc interval and transmural dispersion of repolarization.
METHODS
A total of 75 patients were randomly allocated to receive 0.625 or 1.25 mg droperidol or 8 mg ondansetron. The QTc interval was calculated using Bazett's formula and the Framingham correction. The transmural dispersion of repolarization was determined as Tpeak–Tend time.
RESULTS
Transient QT prolongation, corrected with both formulae, followed 1.25 mg of droperidol 10 min after administration. No change in the QTc value was observed in the other groups. When corrected with Bazett's formula, QTc was prolonged above 480 ms in two patients receiving 1.25 mg droperidol (at the 10th and 20th minute of the study) and in one receiving ondansetron. No patients developed a QTcB prolongation over 500 ms. No increase above 480 ms was observed relative to the Framingham correction method. There were no significant differences in the Tpeak–Tend time either between or within the groups.
CONCLUSION
In men without cardiovascular disorders small doses (1.25 mg) of droperidol prophylaxis induced transient QTc prolongation without changes in transmural dispersion of repolarization. The apparently low risk of the drug applies only in low risk male patients with a low pro-QTc score.
Keywords: corrected QT interval, droperidol, ondansetron, postoperative nausea and vomiting, torsadogenic action, transmural dispersion of repolarization
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Small doses of droperidol are highly effective in the prevention of postoperative nausea and vomiting.
As droperidol provokes corrected QT interval prolongation and was reported to provoke serious cardiac arrhythmias, the FDA issued a ‘black box’ warning on droperidol.
Sparse data are known about the influence of droperidol on the transmural dispersion of repolarization, although only a concurrent assessment of any changes in this parameter and in the QTc could accurately determine its torsadogenic action.
WHAT THIS STUDY ADDS
Droperidol 1.25 mg induces transient QTc prolongation, but the lack of simultaneous changes in the transmural dispersion of repolarization makes the risk of its torsadogenic action low.
Ondansetron does not present any torsadogenic action in male patients without cardiovascular diseases.
The apparently low risk of the drugs applies only on low risk male patients with low pro-QTc score.
Introduction
Droperidol has been widely used for the prevention and treatment of postoperative nausea and vomiting (PONV) due to its high effectiveness and low cost [1]. In 2001, the FDA issued a ‘black box’ warning on droperidol because of concerns related to serious cardiac arrhythmias following its potential QT interval prolongation. The warning was based on 273 cases, out of which 127 resulted in serious adverse outcomes. However, in only two of these cases was droperidol administered in the appropriately low doses recommended by PONV management guidelines [2]. This ‘black box’ warning provoked a substantial decrease in droperidol use as the first line treatment for PONV, despite the fact that more than 90% of the responding members of the Society for Ambulatory Anesthesia did not believe that such a warning was warranted [3]. This paradoxical problem remains a source of contention [4–6].
Although many papers have described the influence of droperidol on cardiac repolarization, all of them have focused exclusively on QT and corrected QT (QTc) intervals. Most authors showed that droperidol provoked a dose-dependent QTc prolongation. Although QTc interval prolongation is a risk factor for ventricular arrhythmia development [7], it is not the sole determinant of a drug's potential to cause torsade de pointes polymorphic ventricular tachycardia (TdP) [8,9]. Another electrocardiographical marker of torsadogenic drug action is the increased transmural dispersion of repolarization (TDR), which indicates the time elapsed between the peak of the T wave and the end of the T wave (Tp–e) on the surface ECG (Figure 1). TDR represents differences in the repolarization of the myocardial ‘layers,’ such as the epicardium, M-cells and endocardium. It is believed that the induction of QT lengthening must be accompanied by a parallel increase in TDR to promote torsadogenesis [8].
Figure 1.

QT interval measurement using the tangent method (the end of the T wave is the intercept between the isoelectric line with the tangent drawn through the maximum down slope of the T wave). X represents Tpeak − Tend time (modified from http://en.ecgpedia.org/wiki)
At present, sparse data are known about the influence of droperidol on the TDR, although only a concurrent assessment of any changes in this parameter and in the QTc interval following drug administration could accurately determine its torsadogenic action. Therefore, the aim of this study was to verify whether the small doses of intravenous droperidol (0.625 mg and 1.25 mg) routinely used in PONV management provoked a parallel prolongation of the QT and QTc intervals and an increase of the TDR, thereby promoting ventricular arrhythmias. To blind the study properly, a control group of patients receiving ondansetron was included in the analysis. Ondansetron is widely used for PONV prophylaxis and treatment in adults and children and has also been reported to prolong the QTc interval [10].
Methods
After obtaining approval from the Bioethical Committee of the Medical University of Gdańsk and written informed consent from the study participants, 75 males were recruited to take part in the study (Trial Registry Number NCT01819857). All patients were already due to undergo elective orthopaedic surgery and were between 18 and 60 years of age, with an American Society of Anesthesiologists (ASA) grade I or II and a pre-operative QT and QTc (corrected with Bazett's formula) less than 440 ms. Patients with abnormal conductions and arrhythmias (including sinus bradycardia/tachycardia and sinus arrhythmia), those treated with drugs known to prolong QT duration, those suffering from coronary heart disease or heart failure and those with congenital or acquired heart defects and myocarditis in anamnesis were excluded. Patients were also excluded if they had any history of hypersensitivity to droperidol or ondansetron or pre-operative electrolytic imbalances (serum magnesium <0.7 mmol l−1, serum calcium <2.2 mmol l−1, serum potassium <3.5 mmol l−1) or if they had been treated with anti-arrhythmics, psychotropic drugs, macrolides or fluoroquinolones.
A randomization scheme was generated using the generator of Wichmann & Hill, as modified by McLeod (available at http://www.randomization.com). Patients were randomly allocated to receive either 0.625 mg or 1.25 mg droperidol (Xomolix® 2.5 mg ml−1 ProStrakan, Galashiels, UK), group D 0.625 or D 1.25, respectively or 8 mg ondansetron (Zofran®, Glaxo Wellcome, Brentford, UK) intravenously, group O. Both medicines were diluted to 10 ml using a 0.9% NaCl solution.
The risk of PONV was assessed using Apfel scores [11] the day before anaesthesia. The risk of TdP was calculated as the pro-QTc score proposed by Haguaa et al. [12]. All patients were premedicated with midazolam (7.5 mg orally 1 h before anaesthesia). After the patients' arrival in the operating theatre, routine monitoring, consisting of ECG, pulse oximetry and non-invasive blood pressure measurement, was begun. Then, 12-lead ECG electrodes were placed, and continuous acquisition commenced. Patients were positioned in the supine position throughout the study. A slow intravenous infusion of 0.9% NaCl was given. Immediately after the monitoring began, droperidol (0.625 mg or 1.25 mg) or ondansetron (8 mg) was administered from a previously prepared syringe, as marked with the randomization number. Both the patients and the staff members who administered the droperidol or ondansetron and performed the measurements were blinded to the substance administered. The staff member who prepared the solution and knew its composition was permanently and directly available by phone but did not participate during any stage of the study. The staff member who read the ECG charts was blinded to the administered drug. No surgical procedures, including patient positioning or urinary catheterization, were performed during the study period (until the moment of the last ECG chart registration). The electrocardiographic curve was recorded using a continuous 12-lead registration with a Siemens Megakart device. The obtained ECG charts were scanned, magnified and analyzed with AutoCad 2009 (Autodesk, San Rafael, USA) using appropriate scaling.
The effects of the tested drugs on repolarization were analyzed according to the E14 instructions developed by the International Conference on the Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [13].
The analysis included an assessment of the ECG parameters and of the values of arterial blood pressure before antiemetic administration, as well as 5, 10, 15 and 20 min following drug administration. The QT and RR intervals were measured on lead II of the ECG curve. Correction of the QT interval was calculated using Bazett's correction (QTcB = QT RR−1/2) and the Framingham formula (QTcF = QT + [1 − RR] × 0.154). Analysis of the categorical data was also performed, comparing the number of patients with maximal QTc prolongations (throughout the study) over 450, 480 and 500 ms in both study groups. The TDR was determined as the time between the peak and the end of the T wave and was measured on the V5 lead of the ECG. Both in QT interval measurement and in Tp−e time determination, the end of T wave was detected with the tangent method (Figure 1). The mean value of three successive cardiac cycles was included for statistical analysis. The results of the obtained ECG parameters were expressed in milliseconds. The measurement was performed without knowledge of the treatment the patient had received. The results were decoded after all electrocardiographic calculations had been carried out.
Statistical analysis
A minimal sample size of 23 persons per group was calculated assuming an α level = 0.05 and a β level = 0.90 for the detection of a mean difference of 15 ms of QTc interval between groups based on population data presented by Benhorin and colleagues: a mean (SD) QTc value of 414 (17) ms [14]. To allow for dropouts, 25 individuals were analyzed per group.
Data are expressed as the mean values (95% CI) or n (proportion). Statistical analysis was performed using STATISTICA 10.0 PL software (Polish version) (StatSoft, Tulsa, OK, USA). A normal data distribution was verified using the Shapiro–Wilk's test, and the homogeneity of variance was verified using Levene's test. Comparisons were performed using two-sided anova tests for repeated measurements with the subsequent analysis of significant differences using a post hoc method (Tukey HSD test) if appropriate or chi-squared test (with the Yates correction if necessary). P < 0.05 was adopted as indicative of a significant difference.
Results
Demographic data are presented in Table 1. There were no significant differences in the mean age, body mass or height among the three groups. Similarly, no differences in the ASA pre-operative status, Apfel scores as well as in serum magnesium, calcium and potassium concentrations were detected. No one individual had pro-QTc score greater than 0.
Table 1.
Patient details
| Parameter | Group | ||
|---|---|---|---|
| D 0.625 | D 1.25 | O | |
| n = 25 | n = 25 | n = 25 | |
| Age (years) | 35 (30–40) | 36 (31–42) | 34 (29–39) |
| Body mass (kg) | 84 (79–90) | 87 (81–93) | 87 (80–94) |
| Height (cm) | 179 (176–181) | 181 (179–184) | 180 (178–183) |
| ASA status; I:II | 24:1 | 23:2 | 23:2 |
| Apfel score; 1:2 | 12:13 | 16:9 | 14:11 |
| Arterial hypertension; yes:no | 1:24 | 2:23 | 2:23 |
| Pro-QTc score = 0 | 25 | 25 | 25 |
| Serum magnesium (mmol l−1) | 0.82 (0.78–0.86) | 0.81 (0.76–0.86) | 0.85 (0.80–0.90) |
| Serum calcium (mmol l−1) | 2.26 (2.20–2.32) | 2.28 (2.20–2.36) | 2.33 (2.26–2.40) |
| Serum potassium (mmol l−1) | 4.1 (3.9–4.3) | 4.0 (3.8–4.2) | 4.1 (3.9–4.3) |
ASA, American Society of Anesthesiologists.
Figure 2 presents the changes in the uncorrected QT time in the study groups. Sedative administration caused no prolonged ventricular repolarization in either group. Figures 3 and 4 present changes in the QT interval, corrected according to Bazett's formula and the Framingham correction, respectively. A significant prolongation of the QTcB and QTcF intervals, followed by a subsequent decrease, was observed in the D 1.25 group at the 10th and 15th minute, respectively. No significant changes in QTcB or QTcF were detected in the other groups. We did not observe any significant intergroup differences, irrespective of the method of correction applied.
Figure 2.

Mean and 95% confidence intervals of uncorrected QT intervals during different time points. +5, +10, +15, and +20 refer to the time in minutes from the study's beginning.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
Figure 3.

Mean and 95% confidence intervals of the QT intervals corrected using Bazett's formula (QTcB) across different time points. *P = 0.033 compared with initial values in the same group and P = 0.019 compared with subsequent measurements.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
Figure 4.
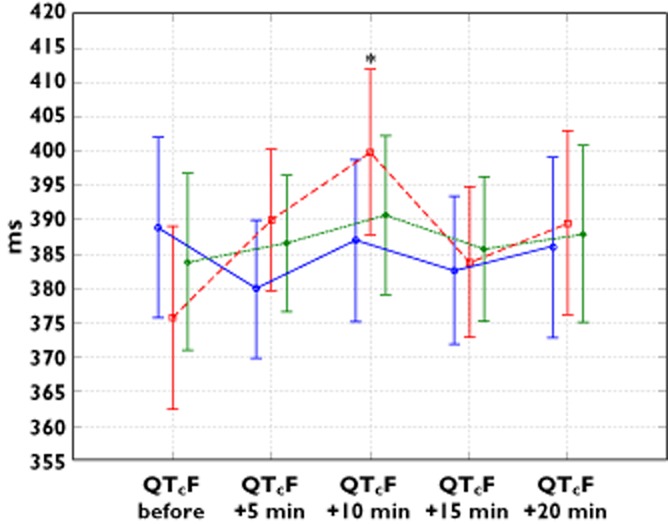
Mean and 95% confidence intervals of QT interval corrected using Framingham formula (QTcF) across different time points. *P = 0.0009 compared with initial values in the same group and P = 0.036 compared with subsequent measurements.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
The number of patients with QTcB prolongation over 450 and 480 ms and comparisons of the study groups are presented in Table 2. No patients developed a QTcB prolongation over 500 ms. QTcF increased above 450 ms in just one individual in the group D 0.625 at the 20th minute of the study. No increase above 480 ms was observed relative to the latter QTc correction method.
Table 2.
The number of patients with QTcB prolongation over 450 and 480 ms and comparisons of study groups
| 5th min | 10th min | 15th min | 20th min | P value | |
|---|---|---|---|---|---|
| QTcB >450 ms | |||||
| Group D 0.625 | 1 (4%) | 1 (4%) | 0 | 2 (8%) | 0.19 |
| Group D 1.,25 | 3 (12%) | 2 (8%) | 2 (8%) | 1 (4%) | |
| Group O | 0 | 0 | 0 | 1 (4%) | |
| QTcB >480 ms | |||||
| Group D 0.625 | 0 | 0 | 0 | 0 | 0.77 |
| Group D 1.25 | 0 | 1 (4%) | 0 | 1 (4%) | |
| Group O | 0 | 1 (4%) | 0 | 0 | |
Tpeak–Tend time did not change significantly in the groups, as shown in Figure 5. Changes in the heart rate and mean arterial blood pressure (MAP) are shown in Figures 6 and 7, respectively. Mild but significant reductions in MAP were observed in the three study groups when the initial and subsequent values were compared. In patients receiving droperidol, these reductions were detected earlier compared with those receiving ondansetron. No intergroup differences in MAP were found. Mild heart rate decreases were observed in both groups receiving droperidol, without any significant change in the ondansetron group.
Figure 5.

Changes in the Tpeak – Tend time during the study.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
Figure 6.
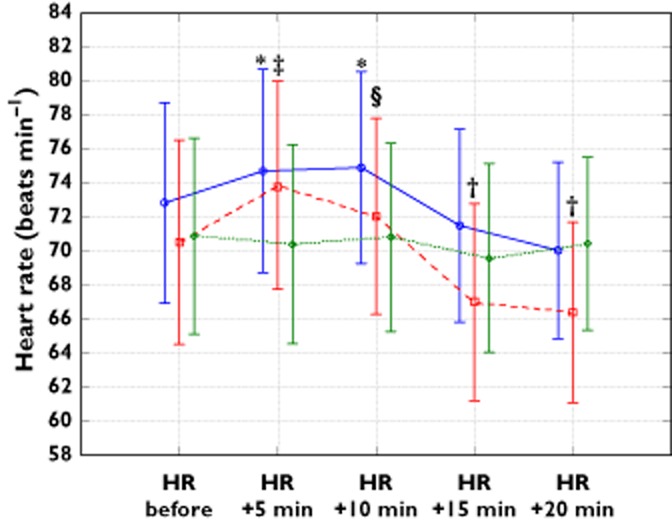
Mean and 95% confidence limits of the heart rate (HR) during the study. *P < 0.01 compared with the value at the 20th min in the same group; †P < 0.05 compared with initial value in the same group; ‡P < 0.00005 compared with values at 15th and 20th minute of the study; §P < 0.005 compared with values at 15th and 20th min of the study.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
Figure 7.

Mean and 95% confidence limits of the mean arterial pressure (MAP) during the study. *P < 0.05 compared with initial value in the same group; †P = 0.02, ‡P = 0.0005, §P = 0.002 compared with initial value in the same group.  , group D 0.625;
, group D 0.625;  , group D 1.25;
, group D 1.25;  , group O
, group O
Discussion
The major finding of this study was that small doses of droperidol, given intravenously, provoked slight and transient corrected QT interval prolongation without influencing the TDR. Ondansetron, when given as a control drug, does not alter any of the parameters of cardiac repolarization.
Independent of the correction method, the QTc interval increased significantly at the 10th minute after droperidol administration. This prolongation vanished 5 min later. We may suspect that such a time-related effect is due to droperidol's half-life of distribution, which is 10 min after its intravenous injection [15]. At this moment, the tissue concentration of this drug, including among cardiomyocytes, is the highest. Our analysis not only included changes in the actual values of QT and corrected QTc intervals but also any increases in these parameters above the values considered to be critical for TdP development. It is believed that, even though values greater than 500 ms for QTc represent a threshold of particular concern, some measure of attention should be paid even to shorter values (>450 ms) [13]. QTcB prolongation above 450 ms was observed in a small number of individuals in the group D, 1.25, whereas the Framingham formula indicated only one patient. No one individual had a QTc prolonged >500 ms. Our observations regarding the QT/QTc changes after droperidol and ondansetron administration are only partially in accordance with those published by other authors. Lischke and co-workers observed significant QTcB prolongation following intravenous droperidol, but they used high doses of the drug (0.1 to 0.25 mg kg−1) [16]. Smaller doses were used by Charbit et al. in two published studies. In the first of these studies, the patients received 0.75 mg of droperidol or 4 mg of ondansetron (control group). In both groups, a significant lengthening of QTcB was noted [17]. In our study, we observed no such effects of ondansetron, even after using a double dose. In the next study, patients received 1 mg of droperidol or 4 mg of ondansetron or droperidol, together with ondansetron or placebo. Significant QTc prolongation followed the administration both of droperidol and ondansetron, and this effect was significantly longer in the droperidol group [18]. On the other hand, White and colleagues did not observe any significant QTcB changes after the administration of 0.125 or 0.25 mg of droperidol compared with saline [19]. A slight prolongation of QTcB was observed 3–6 min following droperidol administration, earlier than was found in our results. Unfortunately, droperidol was administered during general anaesthesia with desflurane, which is also known to prolong cardiac repolarization [10].
In our study, neither inter- nor intragroup changes in TDR were detected. This finding allowed us to conclude that the torsadogenic potential of the studied drug is low. There are sparse data on the impact of medicines used in anaesthesia on the TDR. Published reports have described such an impact for propofol and sevoflurane in children [20,21]. Among anti-emetics, promethazine has been tested and was found to prolong the QTc interval without influencing the TDR [22]. The only currently existing study on droperidol and ondansetron was performed on children by Mehta and collaborators. These authors observed significant changes in QTcB (determined as clinically irrelevant) without parallel changes in TDR. The authors concluded that neither drug provoked any risk of TdP, as they had no influence on TDR [23].
Our statements about the low torsadogenic potential of droperidol may be supported by other clinical observations published last year. That study included 20 122 persons receiving a total of 35 536 doses of 0.625 mg of droperidol intravenously. Eight hundred and fifty-eight of those individuals had ECG charts that could be analyzed and no one developed polymorphic ventricular tachycardia or death as a direct result of droperidol administration [24]. On the other hand, TdP has clearly been reported associated with droperidol in cases published in the literature. It may be due to fact that some people are more prone to the drug-induced QTc lengthening and are at higher risk for TdP than others [25]. It is possible, that drug-induced TdP may be more probably observed in those with latent forms of congenital long QT syndrome (LQTS) medicated with QT-prolonging substances [26]. It is estimated, that LQTS mutation carriers are present in 1 of 1000 to 3000 individuals and are asymptomatic until the moment of QT-prolonging drug exposure [27].
In our study, we used ondansetron 8 mg as a control drug. This antiemetic is believed to be safe and is widely recommended and used for PONV prophylaxis and treatment in both children and adults [28–30]. Our observations may confirm that ondansetron can be safely used in a healthy population, although certain reservations remain regarding its safety in patients with cardiovascular disorders [31]. Additionally, in 2012, the FDA published a warning that 32 mg of ondansetron ‘may affect the electrical activity of the heart (QT interval prolongation), which could pre-dispose patients to develop an abnormal and potentially fatal heart rhythm known as torsades de pointes’ [32]. In our opinion, more extensive studies about the influence of high doses of ondansetron on the electrical function of the heart are needed.
There are some limitations of the present study. As mentioned, this study was performed on patients without any cardiac disorders. It may be possible that in patients with these diseases the influence of the drugs used for cardiac repolarization might be more significant. We decided to include only male patients in our study and exclude women for safety reasons, because they are more prone to torsadogenic drug activities [33].
In conclusion, small doses (1.25 mg) of droperidol, as used in PONV prophylaxis, induced transient QTc prolongation in men without cardiovascular disorders. Neither inter- nor intragroup changes in TDR were detected. This finding allowed us to conclude that the torsadogenic potential of the studied drug is low in men. It must be emphasized that the apparently low risk of the drug applies only in low risk male patients with low pro-QTc score. A further study is needed in women to assess the effects of droperidol and ondansetron on TDR.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Sneyd JR. Droperidol: past, present and future. Anaesthesia. 2009;64:1161–1164. doi: 10.1111/j.1365-2044.2009.06124.x. [DOI] [PubMed] [Google Scholar]
- 2.Habib AS, Gan TJ. Food and Drug Administration black box warning on the perioperative use of droperidol: a review of the cases. Anesth Analg. 2003;96:1377–1379. doi: 10.1213/01.ANE.0000063923.87560.37. [DOI] [PubMed] [Google Scholar]
- 3.Habib AS, Gan TJ. The use of droperidol before and after the Food and Drug Administration black box warning: a survey of the members of the Society of Ambulatory Anesthesia. J Clin Anesth. 2008;20:35–39. doi: 10.1016/j.jclinane.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Faine B, Hogrefe C. News flash: Old Mother Hubbard reports the cupboard is bare … time for the FDA to let droperidol out of the (black) box. Ann Pharmacother. 2012;46:1259–1261. doi: 10.1345/aph.1R156. [DOI] [PubMed] [Google Scholar]
- 5.Habib AS, Gan TJ. The Food and Drug Administration black box warning on droperidol is not justified. Anesth Analg. 2008;106:1414–1417. doi: 10.1213/ane.0b013e31816ba463. [DOI] [PubMed] [Google Scholar]
- 6.Ludwin DB, Shafer SL. The black box warning on droperidol should not be removed (but should be clarified!) Anesth Analg. 2008;106:1418–1420. doi: 10.1213/ane.0b013e3181684e6a. [DOI] [PubMed] [Google Scholar]
- 7.Zienciuk-Krajka A, Kukla P, Pazdyga A, Krajka P, Raczak G. QTc variability. Is a single measurement of QT/QTc interval enough to perform risk stratification and therapeutic decisions in a patient with long QT syndrome? Kardiol Pol. 2013;71:295–299. doi: 10.5603/KP.2013.0046. [DOI] [PubMed] [Google Scholar]
- 8.Antzelevitch C. Role of transmural dispersion of repolarization in the genesis of drug-induced torsades de pointes. Heart Rhythm. 2005;2(Suppl. 2):S9–15. doi: 10.1016/j.hrthm.2004.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigro G, Russo V, Rago A, Papa AA, Carbone N, Marchel M, Palladino A, Hausmanowa-Petrusewicz I, Russo MG, Politano L. Regional and transmural dispersion of repolarisation in patients with Emery-Dreifuss muscular dystrophy. Kardiol Pol. 2012;70:1154–1159. [PubMed] [Google Scholar]
- 10.Owczuk R, Wujtewicz MA, Zienciuk-Krajka A, Łasińska-Kowara M, Piankowski A, Wujtewicz M. The influence of anesthesia on cardiac repolarization. Minerva Anestesiol. 2012;78:483–495. [PubMed] [Google Scholar]
- 11.Apfel CC, Roewer N, Korttila K. How to study postoperative nausea and vomiting. Acta Anaesthesiol Scand. 2002;46:921–928. doi: 10.1034/j.1399-6576.2002.460801.x. [DOI] [PubMed] [Google Scholar]
- 12.Haugaa KH, Bos JM, Tarrell RF, Morlan BW, Caraballo PJ, Ackerman MJ. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–325. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 13.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005. ICH Harmonised Tripartite Guideline. The clinical evaluation of QT/QTc interval prolongation and proarrhytmhic potential for non-antiarrhythmic drugs. Available at http://www.ich.org (last accessed 15 June 2014) [PubMed]
- 14.Benhorin J, Merri M, Alberti M, Locati E, Moss AJ, Hall WJ, Cui L. Long QT syndrome. New electrocardiographic characteristics. Circulation. 1990;82:521–527. doi: 10.1161/01.cir.82.2.521. [DOI] [PubMed] [Google Scholar]
- 15.Cressman WA, Plostnieks J, Johnson PC. Absorption, metabolism and excretion of droperidol by human subjects following intramuscular and intravenous administration. Anesthesiology. 1973;38:363–369. doi: 10.1097/00000542-197304000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J. Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg. 1994;79:983–986. doi: 10.1213/00000539-199411000-00028. [DOI] [PubMed] [Google Scholar]
- 17.Charbit B, Albaladejo P, Funck-Brentano C, Legrand M, Samain E, Marty J. Prolongation of QTc interval after postoperative nausea and vomiting treatment by droperidol or ondansetron. Anesthesiology. 2005;102:1094–1100. doi: 10.1097/00000542-200506000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Charbit B, Alvarez JC, Dasque E, Abe E, Demolis JL, Funck-Brentano C. Droperidol and ondansetron-induced QT prolongation a clinical drug interaction study. Anesthesiology. 2008;109:206–212. doi: 10.1097/ALN.0b013e31817fd8c8. [DOI] [PubMed] [Google Scholar]
- 19.White PF, Song D, Abrao J, Klein KW, Navarette B. Effect of low-dose droperidol onthe QT interval during and after general anesthesia: a placebo-controlled study. Anesthesiology. 2005;102:1101–1105. doi: 10.1097/00000542-200506000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Whyte SD, Booker PD, Buckley DG. The effects of propofol and sevoflurane on the QT interval and transmural dispersion of repolarization in children. Anesth Analg. 2005;100:71–77. doi: 10.1213/01.ANE.0000140781.18391.41. [DOI] [PubMed] [Google Scholar]
- 21.Whyte SD, Sanatani S, Lim J, Booker PD. A comparison of the effect on dispersion of repolarization of age-adjusted MAC values of sevoflurane in children. Anesth Analg. 2007;104:277–282. doi: 10.1213/01.ane.0000252417.23986.6e. [DOI] [PubMed] [Google Scholar]
- 22.Owczuk R, Twardowski P, Dylczyk-Sommer A, Wujtewicz MA, Sawicka W, Drogoszewska B, Wujtewicz M. Influence of promethazine on cardiac repolarisation: a double-blind, midazolam-controlled study. Anaesthesia. 2009;64:609–614. doi: 10.1111/j.1365-2044.2009.05890.x. [DOI] [PubMed] [Google Scholar]
- 23.Mehta D, Sanatani S, Whyte SD. The effects of droperidol and ondansetron on dispersion of myocardial repolarization in children. Paediatr Anaesth. 2010;20:905–912. doi: 10.1111/j.1460-9592.2010.03408.x. [DOI] [PubMed] [Google Scholar]
- 24.Nuttall GA, Malone AM, Michels CA, Trudell LC, Renk TD, Marienau ME, Oliver WC, Ackerman MJ. Does low dose droperidol increase the risk of polymorphic ventricular tachycardia or death in the surgical patient? Anesthesiology. 2013;118:382–386. doi: 10.1097/ALN.0b013e31827dde8d. [DOI] [PubMed] [Google Scholar]
- 25.Litwin JS, Kleiman RB, Gussak I. Acquired (drug-induced) long QT syndrome. In: Gussak I, Antzelevitch C, editors. Electrical Diseases of the Heart. 1st edn. London: Springer-Verlag; 2008. pp. 705–718. [Google Scholar]
- 26.Moss AJ, Schwartz PJ. Delayed repolarization (QT or QTU prolongation) and malignant ventricular arrhythmias. Mod Concepts Cardiovasc Dis. 1982;51:85–90. [PubMed] [Google Scholar]
- 27.Yang P, Kanki H, Drolet B, Yang T, Wei J, Viswanathan PC, Hohnloser SH, Shimizu W, Schwartz PJ, Stanton M, Murray KT, Norris K, George AL, Jr, Roden DM. Allelic variants in long-QT disease genes in patients with drug-associated torsades de pointes. Circulation. 2002;105:1943–1948. doi: 10.1161/01.cir.0000014448.19052.4c. [DOI] [PubMed] [Google Scholar]
- 28.Litke J, Pikulska A, Wegner T. Management of perioperative stress in children and parents. Part II - anaesthesia and postoperative period. Anaesthesiol Intensive Ther. 2012;44:170–174. [PubMed] [Google Scholar]
- 29.Maciejewski D. Guidelines for system and anaesthesia organisation in short stay surgery (ambulatory anaesthesia, anaesthesia in day case surgery) Anaesthesiol Intensive Ther. 2013;45:190–199. doi: 10.5603/AIT.2013.0038. [DOI] [PubMed] [Google Scholar]
- 30.Committee on Quality and Safety in Anaesthesia of the Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesia in ambulatory settings: consensus statement from the Committee on Quality and Safety in Anaesthesia, Polish Society of Anaesthesiology and Intensive Therapy. Anaesthesiol Intensive Ther. 2013;45:183–189. doi: 10.5603/AIT.2013.0037. [DOI] [PubMed] [Google Scholar]
- 31.Hafermann MJ, Namdar R, Seibold GE. Effect of intravenous ondansetron on QT interval prolongation in patients with cardiovascular disease and additional risk factors for torsades: a prospective, observational study. Drug Healthc Patient Saf. 2011;3:53–58. doi: 10.2147/DHPS.S25623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. FDA Drug Safety Communication: new information regarding QT prolongation with ondansetron (Zofran). Available at http://www.fda.gov/Drugs/DrugSafety/ucm310190.htm (last accessed 15 June 2014)
- 33.Średniawa B, Musialik-Lydka A, Jarski P, Kalarus Z, Polonski L. Circadian and sex dependent QT dynamics. Pacing Clin Electrophysiol. 2005;28:211–216. doi: 10.1111/j.1540-8159.2005.00006.x. [DOI] [PubMed] [Google Scholar]


